Abstract
Aerosol particles deposit onto human body surfaces in indoor environments. However, the relative importance of this pathway is poorly characterized. In this study, an improved three-layer model was developed; it incorporates Brownian and turbulent diffusion, gravitational settling, turbophoresis, thermophoresis, and diffusiophoresis to predict particle deposition velocities onto human body surfaces. The model was preliminarily evaluated with manikin-based experiments, conducted in an 8 m3 stainless steel chamber for particles ranging from 0.01 μm to 5 μm. Both standing and sitting manikins with heat dissipation ranging from 50 w to 100 w were used. Following comparisons with the experimental results, the model was used to estimate particle deposition velocities onto the body surfaces of standing and sitting humans for three normal scenarios (transition season, summer, and winter). For particles from 0.01 μm to 3 μm deposition velocities were the highest in summer and the lowest in winter. For particles larger than 3 μm the trend was inversed. The modeled results suggest that direct deposition onto human body for particles ranging from about 0.05 μm to 0.5 μm is a relatively unimportant exposure pathway for standing and sitting human beings. However, for particles smaller than 0.05 μm and larger than 0.5 μm, direct deposition onto standing and sitting human beings may be an important exposure pathway.
Copyright 2013 American Association for Aerosol Research
INTRODUCTION
Deposition is one of the aerosol dynamic features of airborne particles, which deposit not only onto indoor surfaces but also onto human body surfaces. Lots of attention has been paid to particle deposition in indoor environments and ventilation ducts in previous works, and related models have been established to predict particle deposition velocities in such environments for different conditions (Lai and Nazaroff Citation2000; Zhao and Wu Citation2006a,Citationb; You and Zhao Citation2013). However, particle deposition onto human body surfaces has received less attention so far.
Deposition of indoor airborne particles onto exposed skin may contribute to exposure via the dermal pathway. For example, exposure to several kinds of semivolatile organic compounds (SVOCs), a group of air pollutants, which can adsorb onto airborne particles due to their low vapor pressure, generally ranging from 10−9 Pa to 10 Pa (Weschler and Nazaroff Citation2008) were found to bring about severe health risks for human beings in previous studies: high dermal exposure to polycyclic aromatic hydrocarbons (PAHs) is responsible for the increasing risk of skin cancer (Boffetta et al. Citation1997); chronic exposure to some phthalates was regarded as rodent carcinogens and reproductive toxicant by toxicological studies (Latini Citation2005). In addition to SVOCs, heavy metals are another typical group of atmospheric pollutants. Heavy metals, such as cadmium and lead, can exist in the particle phase and enter into human body via different exposure pathways. The detrimental implications on health of exposure to cadmium are kidney damage as well as bone fractures. Exposure to lead may result in neurotoxic effects, especially for children (Jarup Citation2003).
Dermal exposure as a consequence of particle deposition onto skin surfaces may be a potentially significant exposure pathway for human beings. Rodes et al. (Citation2001) experimentally studied the particle transfer factor to skin by contact events. In addition to contact events, airborne particles can directly deposit onto human body surfaces. Moreover, deposition onto human body surfaces can be a removal pathway for indoor particles; it may affect not only the size distribution but also the fate of airborne particles indoors.
Ge et al. (Citation2013), Tang et al. (Citation2011) and Salmanzadeh et al. (Citation2012) studied the particles' pollution levels as well as the fluid around human bodies through computational simulations and experiments, but they did not focus on particle deposition onto human body surfaces. Schneider et al. (Citation1994) studied particle deposition onto facial skin as well as on the eyes and established a semiempirical model that accounted for the effect of electric fields on predicting particle deposition velocity (vd). Andersson et al. (Citation2006) measured the deposition velocity (vd) onto specific anatomic parts of human beings of particles with certain diameters. The influence of electrophoresis, moisture, temperature, and physical movement on particle deposition velocity onto human skin was also analyzed in that work. Both of these studies mainly concerned particle deposition velocity onto a specific part of human body instead of the whole body. Moreover, other mechanisms affecting particle deposition velocities onto human body surfaces, such as thermophoresis and diffusiophoresis were not theoretically studied. Weschler and Nazaroff (Citation2012) employed the particle deposition velocity (vd) derived from the general particle deposition rate (K) onto indoor surfaces such as walls and furniture to estimate the net mass transport of SVOC-bound particles onto human body surfaces. No characteristic of the human skin was taken into account in this estimation. It should be noted that, as the deposition rate is a parameter depending on the area of deposition surfaces and on the volume of the room, the skin of different people located in various indoor environments can experience different particle deposition rates (K). Compared with particle deposition rates (K), particle deposition velocity (vd) is a built-in physical parameter, which is independent from the shaping parameters of the envelope. Therefore, the objective of this article is to explore the deposition velocity of airborne particle onto human body surfaces, which quantitatively scales the transfer rate of airborne particles onto human body surfaces. In this study, particle deposition velocity was experimentally measured using manikins in an 8 m3 stainless steel chamber. An improved three-layer model, which accounts for Brownian and turbulent diffusion, gravitational settling, turbophoresis, thermophoresis, and diffusiophoresis was established to estimate particle deposition velocity onto human body surfaces. The experimental results were used to evaluate the model, which was then utilized to calculate particle deposition velocities onto human body surfaces for different scenarios.
MODEL
Lai and Nazaroff (Citation2000) employed a three-layer model to estimate particle deposition velocity, considering Brownian and turbulent diffusion, as well as gravitational settling. Zhao and Wu (Citation2006a) improved this three-layer model by incorporating turbophoresis to predict particle deposition velocity in ventilation ducts. In the case of particle deposition onto human body surfaces, there is a temperature difference between the human body surfaces and the ambient air. In addition, the humidity of the skin may be different from that of the air around. Under such circumstances, the effects of thermophoresis and diffusiophoresis on particle deposition velocity must be accounted for. The particle flux onto a flat surface with the characteristics of human skin can be described by
[1]
The first, second, and third terms on the right side of EquationEquation (1)[1] represent the particle deposition flux caused by Brownian and turbulent diffusion, gravitational settling, and turbophoresis, respectively. The fourth term is the particle deposition flux caused by thermophoresis (defined as the particle transport due to the temperature gradient at the human body surface). The thermophoretic velocity (vth) can be estimated using the following equation (Talbot et al. Citation1980):
[2] where the thermal creep coefficient Cs is 1.17, the temperature jump coefficient Ct is 2.18, and the velocity slip coefficient Cm is 1.14. Km is calculated with ka/kp. kp is set as 4.2 W·m−1·K−1, which is the thermal conductivity of carbon, the major chemical composition of airborne particles in Beijing (Cao et al. Citation2012). τp is the particle relaxation time, expressed as
[3]
The fifth term on the right side of EquationEquation (1)[1] represents the particle deposition flux caused by the combined effects of diffusiophoresis (defined as the particle transport due to the mass diffusion transfer) and the Stefan flow. The humidity of human skin may not be equivalent to that of the ambient air all the time. In consequence, water vapor can evaporate or condense at the surfaces of the human body, in which case, suspended particles in the vicinity of the skin surface experience the combination of a diffusiophoretic force as well as an aerodynamic flow of air, which is diffusiophoresis and Stefan flow, respectively. The resultant velocity can be simply estimated by the following equation (Hinds Citation1982):
[4]
Then the particle deposition velocity is described as
[5]
The turbulent diffusivity, ɛp, was estimated by its relationship with fluid turbulent diffusivity, νt, proposed by Hinze (Citation1975). The fluid turbulent diffusivity was calculated by Johansen's correlations (Citation1991). The drag coefficient was obtained using Stoke's law when Re < 1 and using empirical equation cited by Hinds (Citation1982) when 1 < Re < 1000. The air wall normal fluctuating velocity intensity was calculated with the correlation by Guha (Citation1997). The details of how to estimate or model these terms could be found in Zhao and Wu (Citation2006a). Using the above elements, the dimensionless particle deposition velocity (vd+) onto a flat surface of a certain inclination with the characteristics of human skin can be expressed as
[6]
The influence of skin roughness on particle deposition velocity onto human body surfaces may also not be negligible: when the surface, onto which particles deposit is rough, there is a shift of the boundary layer that should be considered. Thus, the boundary conditions of EquationEquation (6)[6] are correspondingly as follows:
[7] k+ is the dimensionless roughness height of skin defined as k+ = ku*/ν. e+ is the dimensionless shifted distance of the virtual origin of the velocity profile from the wall. Zhao and Wu (Citation2006b) fitted the correlation between k+ and e+ within different regions from measured data:
[8]
The definitions of above dimensionless parameters and details on how to solve EquationEquation (6)[6] can be found in Zhao and Wu (Citation2006a).
MANIKIN-BASED EXPERIMENTS
Experimental Design
It should be noted that real human bodies are not only a sink of particles due to particle deposition, but also a source of particles due to human exhalation. Secondary organic aerosol (SOA) generating from the reaction between ozone and skin surface lipids and exfoliation from the skin can be other potential particle sources of human beings. Experiments using a manikin can avoid the uncertain and unstable particle source effects encountered in living human beings. Although manikin-based experiments cannot fully account for the real conditions of particle deposition onto living human beings, the manikins may be used to study major characteristics shared with real human body surfaces, including the influence of the complex shape of the body as well as the effect of thermophoresis. As a first step to investigate particle deposition onto human body surfaces, manikins were chosen for the experiments in this study. Two manikin postures—standing and sitting—were investigated to simulate daily life general conditions. The surfaces of the manikins were uniformly covered with resistance wire so that they could be heated homogeneously. The manikins were hairless and not clad. Comparisons using infrared photography between the heated manikin and a real person were made. For comparisons, the manikin heat dissipation was set as 75 W to simulate normal heat dissipation of living human beings. The infrared images of the manikin and living human are shown in Figure S1 (see the online supplemental information). It can be seen that the largest temperature difference between human body and manikin for a given part is less than 2°C, which is inside the range of interpersonal skin temperature levels between different humans. To simulate differences in the heat generation intensity of human beings, three scenarios of heat dissipation, 50 W, 75 W, and 100 W were performed on the manikin for both postures by adjusting the corresponding input voltage.
The manikin-based experiments were conducted in a cubic stainless steel chamber. The dimension of the chamber is 2 m (length) × 2 m (width) × 2 m (height). The chamber was sealed during each experiment. The air exchange rate of the closed chamber measured by the CO2-decay method was lower than 0.04 ACH, and the chamber was thus considered to be well sealed during the experiment. A fan was located at the center beneath the ceiling to mix the air within the chamber. To make the experiments representative of real world conditions, ambient air particles, ranging from 0.01 μm–5 μm were measured.
The experiments were conducted in two steps. First, the average particle deposition rate in the empty chamber was measured. Second, the manikin was introduced and then the average particle deposition rate in the chamber with the manikin inside was measured. The deposition velocity onto the manikin surfaces was obtained through result comparison. The details of the experimental process are as follows.
The natural decay approach was employed to measure the particle deposition rate. Indoor airborne particle concentrations in the chamber were continuously monitored for three hours. The concentrations of particles ranging from 0.01 μm to 0.4 μm were measured using a nanoscan SMPS model 3910 (TSI, Inc., St. Paul, MN, USA), which measures the electrical mobility diameter distributions, while particles ranging from 0.5 μm to 5 μm were measured using an aerodynamic particle sizer (APS) model 3321 (TSI, Inc., St. Paul, MN, USA), which measures the aerodynamic diameter distributions. To avoid the impact of particle coagulation on the measured deposition rate, we fitted the deposition rate on the particle concentration decay curve when the initial particle concentration is relatively lower. During the regression, the initial concentrations measured by the aerodynamic particle sizer were smaller than 800 pt·cm−3 and the initial concentrations measured by the nanoparticle sizer were smaller than 8000 pt·cm−3. The initial coagulation rates of particles were estimated to be smaller than 20% of the corresponding total loss rate, which decreased as time elapses because of the decreasing particle concentrations.
The measurement error of the aerodynamic particle sizer was 10% after calibration by TSI. Indoor airborne particle concentrations in the chamber naturally decreased due to the particle deposition onto all the surfaces within the chamber, which can be described as:
[9]
The particle deposition rate, K, was estimated by regression of the measured airborne particle concentration decay curve. In step one, K corresponded to the particle deposition rate of the empty chamber (denoted as K1), under which circumstances particle concentrations decreased because of deposition onto the inner surfaces of the chamber. In step two, K represented the particle deposition rate of the chamber with the manikin inside (denoted as K2), and particle concentrations decreased because of deposition both onto the inner surfaces of the chamber and the manikin surface. As the heat dissipation of the manikin was relatively small, and the indoor temperatures of the chamber with and without the manikin were almost identical (the difference of the indoor temperature with and without the manikin was about 1°C based on the monitored results during experiments), the increase of surfaces due to the presence of the manikin was considered as the only reason of the variance of K. The particle deposition velocity onto the manikin surfaces vd was calculated using the following formula:
[10]
Several parameters were also measured during the experiments for the purpose of validating the theoretical model. The temperature gradient at the manikin surface must be known to calculate the thermophoretic effect. The heat balance between heat conduction and heat convection at the manikin surface was established to obtain the temperature gradient adjacent to the surface:
[11]
For these experiments, the surface temperature of the manikin, tsk, was monitored using a K-thermocouple, which was calibrated with a mercurial thermometer. The measurement error of the K-thermocouple is 0.2°C. A temperature and humidity recorder (TJHY, Model WSZY-1) was located in the chamber to measure the indoor air temperature ta. The measurement error of the temperature and humidity recorder is 0.1°C. McIntyre (Citation1980) summarized the correlations for getting the coefficients of heat convection near the human body under different conditions. For these experiments, the correlation for forced ventilation was as follows:
[12]
The air speed at the surface of different parts of the manikin was measured with a hot-wire anemometer (LSI-LASTEM) to determine the faced-weighted average free stream air speed (u∞) near the manikin. The measurement error of the hot-wire anemometer is 0.1 m·s−1. With EquationEquations (11)[11] and Equation(12)
[12] , and the measured tsk, ta, u∞ the temperature gradient at the manikin surface was solved for each experiment.
The friction velocity u* is another important input parameter of the model: it can be calculated using its relationship to the air velocity gradient at the surface (Lai and Nazaroff Citation2000):
[13]
In this article, the velocity gradient at the manikin surface was estimated using the following correlation (Schlichting Citation1979):
[14]
Thus, using EquationEquations (13)[13] and Equation(14)
[14] , and the measured value for u∞, the friction velocity (u*) of each experiment was obtained. The calculation-related measured parameters of friction velocity are listed in .
Table 1 Friction velocity related parameters measured for the experiments
Lastly, the roughness height k caused by the resistance wire on the manikin was also measured to define the boundary condition corresponding to the experiments. The indoor air temperature as well as the roughness height of manikin surfaces for each experiment is shown in .
Table 2 Parameters relative to the experiments
Table 3 Areas of the surfaces making up the simplified manikins
Measured Data and its Comparison with the Modeled Results
For the purpose of calculating the particle deposition velocity onto the manikin surfaces with the improved three-layer model, the manikin of each posture was simplified to a combination of several flat surfaces with certain inclinations. The corresponding inclination angles of the manikin surfaces were measured to build up the simplified-models of the manikins, which are shown in Figure S2 (available in the online supplemental information). The areas of surfaces with certain inclination angles, which make up the simplified models, are shown in . The particle deposition velocity of a certain diameter onto each surface i (vdi) was calculated with EquationEquation (6)[6] . The average particle deposition velocity of certain diameter onto the manikin surfaces can then be expressed as
[15] where Ai is the area of each surface.
There is no obvious difference in particle deposition velocity onto the standing manikin surfaces between cases with different heat dissipation rate. It may be due to the fact that the temperature differences between the manikin and the ambient air were similar in the three cases, with a largest and smallest difference 6.33°C and 2.22°C, respectively. Therefore, the measured and modeled results onto standing and sitting manikin with heat dissipation rate of 100 W were analyzed as examples. However, the SMPS measured data and the APS measured data cannot be merged directly, because the particle of a wide range of mobility diameter may correspond to the same aerodynamic diameter (Park et al. Citation2008). In this study, the spherical particle of 1000 kg·m−3 was utilized in the modeling analysis. Consequently, the electric mobility diameter measured by SMPS should be converted to aerodynamic diameter so that the comparison between the modeled and experimental particle deposition velocity is reasonable. To combine the electric mobility diameter and the aerodynamic diameter, the bulk average particle density and dynamic shape factor were set as 900–1500 kg·m−3 and 1–1.9, respectively (Hinds Citation1982; Kulkarni et al. Citation2011), as such densities and dynamic shape factors may be representative for the ambient particles. The relationships between the electric mobility diameter dm, the aerodynamic diameter da, and the volume equivalent diameter dve were described by the following equations where ρ0 = 1000 kg·m−3 (Seinfeld and Pandis Citation2006; Buonanno et al. Citation2009):
[16]
[17] with which the SMPS and the APS size distributions can be merged to compare with the modeled results.
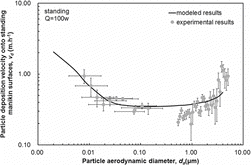
The deposition velocities of particles with diameters ranging from 0.01 μm to 5 μm onto the standing and sitting manikins with heat dissipation rate of 100 W obtained from the experiments, and the improved three-layer model, are shown in and , respectively. There are two parts of uncertainty for measuring the particle deposition velocity in this study. One is the systematic uncertainty of the measurements utilized in the experiments. For APS, the uncertainty of the measured particle number concentration is combined with the counting statistical uncertainty, the time uncertainty, the aerosol flow rate uncertainty, and the sample efficiency factor uncertainty. In this study, a total error of ±10% is assumed according to the manual book of TSI 3321. For SMPS, the uncertainty of the measured particle number concentration is combined with the counting statistical uncertainty, the time uncertainty, the aerosol flow rate uncertainty, the dilution factor uncertainty, and the sample efficiency factor uncertainty. The other is the uncertainty for fitting the decay rate with the particle concentration. We have compared these two parts of uncertainty with each other. The result turned out that the former uncertainty is smaller than the later one. Consequently, the 95% confidence intervals when fitting the concentration decay rate of the particles was chosen as the vertical error bars. The uncertainty of converting the electric mobility diameter to aerodynamic diameter was chosen as the horizontal error bars.
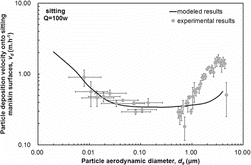
Table 4 Parameters related to model calculation for different scenarios
As shows, the measured deposition velocities onto the standing manikin with heat dissipation rate of 100 W for particles from 0.01 μm to 5 μm were between 0.16 m·h−1 and 1.28 m·h−1. The particle deposition velocity decreased as the diameter increased for particles from 0.01 μm to 0.2 μm, while it increased along with the diameter for particles from 0.2 μm to 5 μm. This is mainly because diffusion is the dominant deposition mechanism for particles smaller than 0.2 μm, while gravitational settling becomes dominant for larger particles deposition. Both effects are not substantial for particles with a diameter around 0.2 μm. The modeled particle deposition velocities onto the standing manikin with heat dissipation rate of 100 W for particles from 0.01 μm to 5 μm were between 0.35 m·h−1 and 0.87 m·h−1. For particles smaller than 0.2 μm, the modeled particle deposition velocities were in reasonable agreement with the experimental data. For particles larger than 0.2 μm, although there was some discrepancy between the predicted and modeled deposition velocities, both results were of the same order of magnitude. Furthermore, the modeled results displayed a similar trend to the experimental data; both increased with the corresponding particle diameter. The simplification of the shape of the manikin may cause deviations from real conditions. We resolved the human body surface into a collection of flat surfaces with certain inclination angles so that the three-layer model can be utilized. Nevertheless, the application of a set of discrete flat surfaces to represent the complex human body can lead to discrepancies between the measured and predicted particle deposition velocities. Such discrepancies are more pronounced for coarse particles because the inclination of the surface influences deposition velocity more significantly for such particles.
As shows, the measured deposition velocities onto the sitting manikin with heat dissipation rate of 100 W for particles from 0.01 μm to 5 μm were between 0.18 m·h−1 and 1.65 m·h−1. The particle deposition velocity also decreased as the diameter increased for particles from 0.01 μm to 0.2 μm and increased with the diameter for particles from 0.2 μm to 5 μm. The modeled deposition velocities onto the sitting manikin for particles from 0.01 μm to 5 μm were between 0.34 m·h−1 and 0.98 m·h−1. Also the modeled deposition velocities matched experimental data well for particles smaller than 0.2 μm. The difference between the modeled and experimental results for particles larger than 0.2 μm may be due to the use of a simplified manikin-model too. Particle deposition velocities onto the sitting manikin surfaces were larger than those onto the standing manikin for both experimental and modeled results. This is mainly because the particle deposition velocity onto face-up surfaces is larger than that onto vertical surfaces and face-down surfaces. Face-up surfaces occupied a larger proportion of the surfaces of the sitting manikin than of those of the standing manikin; particle deposition velocities were larger for the former.
MODELING ANALYSIS
Using the partially validated model presented above, particle deposition velocities onto human body surfaces under various scenarios can be estimated. In this study, several normal exposure scenarios, including the transition season, summer and winter were studied. It is noteworthy that the modeled scenarios were quite different from the experimental scenarios. During the experiments, which were conducted in summer, the friction velocity was relatively large due to the mixing fan while the temperature difference between the skin and the air was relatively small. For model analysis, the friction velocity was relatively small due to the smaller air velocity in normal indoor environments. The temperature difference between the skin and the air was relatively larger in transition season and winter than the experimental scenarios. The procedure to estimate particle deposition velocities onto human body surfaces was as follows.
Step 1. Estimate the Friction Velocity
The typical indoor air free stream speed was assumed to be 0.15 m·s−1, and this value was used to calculate the friction velocity. The measured indoor temperature of each scenario from an earlier experiment conducted to study the thermal comfort of human beings in the transition season, summer, and winter (Zeng Citation2008) was used to determine this value. Using the free stream air speed as well as related parameters of the air, the friction velocity along the human body can be obtained with EquationEquations (13)[13] and Equation(14)
[14] . Parameters used in the calculation and the estimated friction velocities are listed in .
Step 2. Estimate the Thermophoretic Velocity
Skin temperature varies with different ambient environments. The skin temperatures corresponding to the three scenarios were also measured in the experiment mentioned above. The following correlation was used to estimate the coefficient of heat convection near the human beings for natural ventilation:
[18]
With the skin and air temperature, the temperature gradient at the skin surface can be calculated with EquationEquations (11)[11] and Equation(18)
[18] . Then, the thermophoretic velocity can be obtained by EquationEquation (2)
[2] . All the parameters related to the estimation of thermophoretic velocity are shown in .
Step 3. Estimate the Diffusiophoretic Velocity
The combined diffusiophoretic velocity resulted from diffusiophoresis and Stefan flow can be calculated with EquationEquation (4)[4] . The water vapor pressure gradient at the skin surface is determined by the air partial water vapor pressure and the skin surface partial water vapor pressure. Considering there is no measured or calculated partial water vapor pressure at the skin surface in previous studies, the saturated water vapor pressure at the skin surface, Psk, was used to calculate the partial water vapor pressure gradient, with which the effect of diffusiophoresis and Stefan flow cannot be underestimated. The saturated water vapor pressure at the skin surfaces linearly correlates with the skin temperature, which is described as (Zhu Citation2011):
[19]
The air partial water vapor pressure was determined using the indoor air temperature and the relative humidity, which was set as 50% to represent a moderately humid indoor environment. It was assumed that the water vapor pressure varied linearly with the distance from the surface within the concentration boundary layer of the skin. The thickness of the concentration boundary layer can be calculated by y = y+ν/u*, where y+ was assumed to be equal to 30 (Bejan Citation1995). The water vapor pressure gradient at the skin surface was calculated using the air partial water vapor pressure, the skin surface partial water vapor pressure, as well as the thickness of the concentration boundary layer. The diffusiophoretic velocity was then estimated using EquationEquation (4)[4] . The calculation-related parameters are shown in .
Step 4. Identify the Skin Roughness Height
Skin roughness height is an important parameter influencing the boundary condition when calculating particle deposition velocity onto human body surfaces with the improved three-layer model. For human beings, the skin roughness height varies with several parameters. Manuskiatti et al. (Citation1998) measured the skin roughness height in different anatomic sites of people from a variety of ethnic groups and with different ages. The average skin roughness height in their measured results was used as the input parameter for our estimation; it can be found in .
Step 5. Calculate the Particle Deposition Velocity onto Human Body Surfaces
Using the friction velocity, thermophoretic velocity, diffusiophoretic velocity as well as skin roughness height, deposition velocities of particles from 0.01 μm to 10 μm onto human body surfaces can be calculated with EquationEquation (6)[6] . shows the particle deposition velocities onto standing and sitting humans for three normal scenarios. It can be seen that, for particles ranging from 0.01 μm to 3 μm, both for standing and sitting humans, the largest particle deposition velocities corresponded to the summer scenario, the intermediate particle deposition velocities were found in the transition season scenario, and the smallest particle deposition velocities corresponded to the winter scenario. This is mainly because when the skin temperature is higher than the air temperature, the thermophoretic velocity of particles is oriented from the skin toward the air, which counteracts the particle deposition velocity. Larger temperature differences between air and skin lead to larger temperature gradient at the skin surface. Consequently, a larger temperature gradient at the skin surface results in a larger thermophoretic velocity of particles, and the particle deposition velocity is concomitantly smaller. The seasonal trend for particles ranging from 3 μm to 10 μm is not apparent as the figure shows. For particles smaller than 2 μm, the deposition velocity difference between different scenarios of particles with a given diameter was more significant than for larger particles. As for particles of 10 μm, the deposition velocities in different scenarios were almost identical. For all three scenarios, the deposition velocity decreased as the diameter increased for particles between 0.01 μm and 0.2 μm, and it increased with the diameter for particles between 0.2 μm and 10 μm. Deposition velocities smaller than 1 × 10−5 m·h−1 was small enough to be neglected. For the standing human, the largest deposition velocity was 1.40 m·h−1 for particles of 10 μm in winter, while the smallest deposition velocity was 2.65 × 10−5 m·h−1 for particles of 0.6 μm in summer. For the sitting human, the largest deposition velocity was 2.15 m·h−1 for particles of 10 μm in winter while the smallest deposition velocity was 9.22 × 10−5 m·h−1 for particles of 0.6 μm in summer.
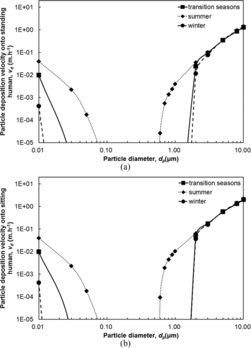
Table 5 Comparisons of different deposition mechanisms
DISCUSSION
Comparisons of Different Deposition Mechanisms
We utilized three different models, the model by Lai and Nazaroff (Citation2000), the model by Zhao and Wu (Citation2006b), and our present model, to calculate particle deposition velocity in order to compare the relative significance of different mechanisms, such as turbophoresis, thermophoresis, and diffusiophoresis. The results of the comparison are shown in . It was found that for particles smaller than 1 μm, thermophoresis is a significant mechanism for deposition; as a consequence, particle deposition velocities onto human body surfaces are quite different from deposition velocities onto typical indoor surfaces. Turbophoresis is the main mechanism influencing deposition for particles of 10 μm when the friction velocity is 0.1 m·s−1. Diffusiophoresis is a relatively important mechanism of deposition for particles smaller than 1 μm when the friction velocity and thermophoresis are not significant.
Limitations of Present Study
In the model validation, manikins were used as a substitute for real human beings to avoid the uncertain source effect of living humans, but manikin cannot imitate the humid condition of real skin. Thus, the effect of diffusiophoresis was not taken into account in the experiment. As the results showed, diffusiophoresis does not influence particle deposition onto human body surfaces significantly, and the experimental results are credible. Besides, the fact that humans tend to be substantially clothed and hairy is without a doubt a very important characteristic that influences particle deposition onto the human body surfaces. Thus exposure levels must be corrected for the fraction of exposed skin. The pathway is more important for a man wearing only shorts on a hot day than for that same man fully clothed on a cold day. The simplified models for real-shape manikins or human bodies applied in this study were adopted from the manikin used in the validation experiment. However, different human body shapes should correspond to different simplified models, with which circumstances particle deposition velocities may be different. In our simplification, the vertical surface occupies the majority of the total skin surface. As a result, differences in particle deposition velocities cannot be substantial between different human body shapes. Further studies about the shape of the human body should be conducted to account for representative shapes of humans of different ages and genders.
Particle deposition velocities onto human body surfaces can be quite different in different indoor environments and for different skin conditions. Factors such as friction velocity, thermophoresis, diffusiophoresis, and skin roughness height can vary over a substantial range in realistic conditions. Considering that the improved three-layer model was only partially validated over a limited range of input parameters, caution must be exercised in extending this model to a larger range of input parameters. The particle deposition velocities onto human body surfaces obtained for different exposure scenarios using the improved three-layer model should be viewed as initial estimates only. Further experiments, conducted under more varied circumstances, are necessary to evaluate the sensitivity of the improved three-layer model to various input parameters.
CONCLUSIONS
An improved three-layer model was established to calculate particle deposition velocities onto human body surfaces. Brownian and turbulent diffusion, gravitational settling, thermophoresis as well as diffusiophoresis were taken into account. The model was partially validated with manikin-based experiments. The following conclusions can be made based on the studied scenarios:
The modeled particle deposition velocities onto standing and sitting manikins were in reasonable agreement with the experimental results. The model may be used to understand the general characteristics of particle deposition onto human body surfaces as an initial approach, but its limitations should be recognized.
Particle deposition velocities onto standing and sitting human body surfaces decreased as the diameter increased for particles from 0.01 μm to 0.2 μm, and increased with the diameter for particles from 0.2 μm to 10 μm. Generally speaking, particle deposition velocity decreased from summer, through the transition season, to winter. The largest particle deposition velocity onto the standing human body surfaces was 1.40 m·h−1 for particles of 10 μm in winter, while the smallest was under 1 × 10−5 m·h−1 for particles with a diameter of around 0.2 μm. The largest particle deposition velocity onto the sitting human body surfaces was 2.15 m·h−1 for particles of 10 μm in winter, while the smallest was under 1 × 10−5 m·h−1 for particles with a diameter of about 0.2 μm.
Thermophoresis is the main mechanism for the deposition of particles smaller than 1 μm. Turbophoresis is quite important for particles of 10 μm with a large friction velocity. The results suggest that direct deposition onto human body of particles ranging from about 0.05 μm–0.5 μm is a relative unimportant exposure pathway for standing and sitting human beings. However, for particles smaller than 0.05 μm and larger than 0.5 μm, direct deposition onto standing and sitting human beings may be an important exposure pathway.
NOMENCLATURE
| A | = | area of human body surface (m2) |
| C | = | indoor particle concentration (μg·m−3) |
| Cc | = | Cunningham coefficient |
| C∞ | = | particle concentration out of the boundary layer (μg·m−3) |
| C+ | = | dimensionless indoor particle concentration |
| D | = | Brownian diffusivity of the particle (m2·s−1) |
| da | = | particle aerodynamic diameter (μm) |
| dm | = | particle electric mobility diameter (μm) |
| dp | = | particle diameter (μm) |
| dve | = | particle volume equivalent diameter (μm) |
| e+ | = | dimensionless shifted distance of velocity boundary layer |
| hc | = | coefficient of heat convection at the human body surfaces (W·m−2·K−1) |
| J | = | particle mass flux (μg·m−2·s−1) |
| K | = | particle deposition rate (h−1) |
| k | = | skin roughness height (μm) |
| ka | = | air thermal conductivity (W·m−1·K−1) |
| kp | = | particle thermal conductivity (W·m−1·K−1) |
| Kn | = | Knudsen number, 2λ/dp |
| k+ | = | dimensionless skin roughness height |
| mp | = | particle mass (μg) |
| P | = | partial vapor pressure (kPa) |
| Pa | = | air partial vapor pressure (kPa) |
| Psk | = | skin surface partial vapor pressure (kPa) |
| r+ | = | dimensionless particle radius |
| Sc | = | Schmidt number |
| t | = | time (s) |
| ta | = | air temperature (°C) |
| Ta | = | air temperature (K) |
| tsk | = | skin temperature (°C) |
| Tsk | = | skin temperature (K) |
| u* | = | friction velocity (m·s−1) |
| u∞ | = | free stream air speed (m·s−1) |
| V | = | bulk of the chamber (m3) |
| vd | = | particle deposition velocity (m·h−1) |
| vdif | = | particle diffusiophoretic velocity (m·s−1) |
| v+d | = | dimensionless particle deposition velocity |
| vs | = | particle gravitational settling velocity (m·s−1) |
| v+s | = | dimensionless particle gravitational settling velocity |
| vt | = | particle turbophoretic velocity (m·s−1) |
| vth | = | particle thermophoretic velocity (m·s−1) |
| = | dimensionless air wall normal fluctuating velocity intensity | |
| y | = | vertical distance from the skin surface (m) |
| y+ | = | dimensionless vertical distance from the skin surface |
| = | Greek Symbols | |
| ɛp | = | particle eddy diffusivity in the boundary layer (m2·s−1) |
| θ | = | surface inclination angel (°) |
| μ | = | air dynamic viscosity (N·s·m−2) |
| ν | = | air kinetic viscosity (m2·s−1) |
| vt | = | air turbulent viscosity (m2·s−1) |
| ρ | = | air density (kg·m−3) |
| ρp | = | particle density (kg·m−3) |
| τL | = | Lagrangian timescale of the air (s) |
| τp | = | particle relaxation time (s) |
| τ+ | = | dimensionless particle relaxation time |
| χ | = | dynamic shape factor of particles |
Supplementary_Information.zip
Download Zip (572.5 KB)REFERENCES
- Andersson, K.G., Roed, J., Byrne, M.A., and Hession, H. (2006). Deposition of Contaminant Aerosol on Human Skin. J. Environ. Radioactiv., 85:182–195.
- Bejan, A. (1995). Convection Heat Transfer.2nd ed. Wiley, New York.
- Boffetta, P., Jourenkove, N., and Gustavsson. P. (1997). Cancer Risk from Occupational and Environmental Exposure to Polycyclic Aromatic Hydrocarbons. Cancer Cause Control, 8:444–472.
- Buonanno, G., Dell’Isola, M., Stabile, L., and Viola, A. (2009). Uncertainty Budget of the SMPS-APS System in the Measurement of PM1, PM2.5 and PM10. Aerosol. Sci. Tech., 43:1130–1141.
- Cao, J.J., Shen, Z.X., Chow, J.C., Watson, J.G., Lee, S.C., Tie, X.X., et al. (2012). Winter and Summer PM2.5 Chemical Compositions in Fourteen Chinese Cities. J. Air. Waste. Manage., 62(10): 1214–1226.
- Ge, Q.J., Li, X.D., Inthavong, K., and Tu, J.Y. (2013). Numerical Study of the Effects of Human Body Heat on Particle Transport and Inhalation in Indoor Environment. Build. Environ., 59:1–9.
- Guha, A. (1997). A Unified Eulerian Theory of Turbulent Deposition to Smooth and Rough Surfaces. J. Aerosol. Sci., 28, 1517–1537.
- Hinds, W.C. (1982). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley, New York.
- Hinze, J.O. (1975). Turbulence.2nd ed. McGraw-Hill, New York.
- Jarup, L. (2003). Hazards of Heavy Metal Contamination. Brit. Med. Bull., 68:167–182.
- Johansen, S.T. (1991). The Deposition of Particles on Vertical Walls. In. J. Multiphase Flow., 17:355–376.
- Kulkarni, P., Baron, P.A., and Willeke, K. (2011). Aerosol Measurement: Principles, Techniques, and Applications.3rd ed. John Wiley & Sons, New York.
- Lai, A.C. K., and Nazaroff, W.W. (2000). Modeling Indoor Particle Deposition from Turbulent Flow onto Smooth Surfaces. J. Aerosol. Sci., 31:463–476.
- Latini, G. (2005). Monitoring Phthalate Exposure in Humans. Clin. Chim. Acta, 361:20–29.
- Manuskiatti, W., Schwindt, D.A., and Maibach, H.I. (1998). Influence of Age, Anatomic Site and Race on Skin Roughness and Scaliness. Dermatology, 1998(196):401–407.
- McIntyre. (1980). Indoor Climate. Applied Science Publisher, London.
- Park, K., Dutcher, D., Emery, M., Pagels, J., Sakurai, H., Scheckman, J., et al. (2008). Tandem Measurements of Aerosol Properties—A Review of Mobility Techniques with Extensions. Aerosol. Sci. Tech., 42(10): 801–816.
- Rodes, C.E., Newsome, J.R., Vanderpool, R.W., Antley, J.T., and Lewis, G.L. (2001). Experimental Methodologies and Preliminary Transfer Factor Data for Estimation of Dermal Exposures to Particles. J. Expo. Anal. Epid., 11:123–139.
- Salmanzadeh, M., Zahedi, G., Ahmadi, G., Marr, D.R., and Glauser, M. (2012). Computational Modeling of Effects of Thermal Plume Adjacent to the Body on the Indoor Airflow and Particle Transport. J. Aerosol. Sci., 53:29–39.
- Seinfeld, J.H., and Pandis, S.N. (2006). Atmospheric Chemistry and Physics.2nd ed. John Wiley & Sons, New Jersey.
- Schlichting, H. (1979). Boundary-Layer Theory.7th ed. McGraw-Hill, New York.
- Schneider, T., Bohgard, M., and Gudmutndsson, A. (1994). A Semiempirical Model for Particle Deposition onto Facial and Eyes—Role of Air Currents and Electric-Fields. J. Aerosol. Sci., 25:583–593.
- Talbot, L., Cheng, R.K., Schefer, R.W., and Willis, D.R. (1980). Thermophoresis of Particles in a Heated Boundary-Layer. J. Fluid. Mech., 101:737–758.
- Tang, J.W., Noakes, C.J., Nielsen, P.V., Eames, I., Nicolle, A., Li, Y., et al. (2011). Observing and Quantifying Airflows in the Infection Control of Aerosol-and Airborne-Transmitted Diseases: An Overview of Approaches. J. Hosp. Infect., 77(3):213–222.
- Weschler, C.J., and Nazaroff, W.W. (2008). Semivolatile Organic Compounds in Indoor Environments. Atmos. Environ., 42:9018–9040.
- Weschler, C.J., and Nazaroff, W.W. (2012). SVOC Exposure Indoors: Fresh Look at Dermal Pathway. Indoor Air., 22:356–377.
- You, R., and Zhao, B. (2013). A Simplified Method for Assessing Particle Deposition Rate in Aircraft Cabins. Atmos. Environ., 67:80–84.
- Zeng, L.L. (2008). Experiment Study on the Response of Indoor Thermal Environment Based on Skin Temperature. Master Degree of Engineering Dissertation, Chongqing Univerisity, Chongqing, China (in Chinese).
- Zhao, B., and Wu, J. (2006a). Modeling Particle Deposition from Fully Developed Turbulent Flow in Ventilation Duct. Atmos, Environ., 40:457–466.
- Zhao, B., and Wu, J. (2006b). Modeling Particle Deposition onto Rough Walls in Ventilation Duct. Atmos. Environ., 40:6918–6927.
- Zhu, Y.X. (2011). Building Environment. China Architecture and Building Press, Beijing, P. R. China . (in Chinese).