Abstract
Vancomycin-sensitive and vancomycin-resistant Enterococcus (VSE and VRE) species have become a significant health problem. CHROMagar medium, which permits direct, color-based identification of target pathogens, could potentially be used to rapidly monitor airborne VSE and VRE. In this study, the efficiency of CHROMagar VRE medium without vancomycin supplementation (CVSE) for collecting airborne vancomycin-sensitive Enterococcus faecalis was evaluated in a chamber study. Subsequently, the performance of bioaerosol samplers combined with CVSE and CHROMagar VRE (CVRE) was evaluated in a hospital environment, a wastewater treatment plant, and a pig-rearing facility. Our results demonstrated that an Andersen impactor was much more effective than a Nuclepore filter for collecting airborne E. faecalis at relative humidity levels of 30% and 55%. In addition, approximately 10% of the isolated environmental Enterococcus strains were vancomycin-resistant. The average sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the colony identification were 58.5%, 81.3%, 5.5%, and 99.1%, respectively, for CVSE and 100%, 88.3%, 8.4%, and 100%, respectively, for CVRE. These findings indicate that the use of CHROMagar might provide a rapid method for detecting airborne VSE or VRE, shortening the detection time to 24–48 h. However, any mauve-colored colonies recovered on CVSE or CVRE by air sampling should be subjected to further identification tests.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
Enterococci are gram-positive cocci that are commonly found in the intestines of humans. Within the genus Enterococcus, E. faecalis, and E. faecium are opportunistic pathogens that are critically important to human health and are frequently identified as the cause of urinary tract, blood stream, and wound infections (Jett et al. Citation1994). Since the widespread introduction of vancomycin into clinical practice, vancomycin-resistant Enterococcus (VRE) strains have emerged worldwide as important nosocomial pathogens (Russell Citation2002). Although VRE is transmitted by close contact with infected persons or with contaminated surfaces (Otter et al. Citation2011), evidence suggests that airborne dispersal and transmission may also be important (Savini et al. Citation2012). Therefore, comprehensive monitoring and accurate and timely identification of airborne VRE are critical for early awareness and preventative measures.
To date, very limited research has been conducted regarding the presence of VRE or vancomycin-sensitive Enterococcus (VSE) in the air. Studies have demonstrated that Enterococcus may be inhaled in hospital wards (Muzslay et al. Citation2012), poultry houses (Brodka et al. Citation2012), swine feeding facilities (Predicala et al. Citation2002), and wastewater treatment plants (Karra and Katsivela Citation2007). The use of chromogenic media has become a key method for the rapid identification of microorganisms in clinical samples. Chromogenic media exploit enzyme substrates that release colored dyes upon hydrolysis by a specific bacterial enzyme, resulting in pigmentation of the target pathogen colonies (Perry and Freydiere Citation2007). Among the various chromogenic media, CHROMagar can be used to detect VRE in clinical stool and rectal swab samples with high sensitivity (98–99%) and specificity (94–99%) (Peltroche-Llacsahuanga et al. Citation2009; Kallstrom et al. Citation2010; Stamper et al. Citation2010). However, the performance of CHROMagar may differ depending on the sample type (i.e., clinical vs. environmental). We previously demonstrated that the sensitivity (71%) and specificity (65%) of CHROMagar for the identification of airborne methicillin-resistant Staphylococcus aureus (MRSA) isolates were significantly lower than for the identification of clinical isolates (Hsiao et al. Citation2012). The differing efficiencies of CHROMagar for clinical and environmental samples may be related to the presence of environmental compounds in the environmental samples, which might interfere with the hydrolytic process and suppress mauve color formation (Hsiao et al. Citation2012). Moreover, the competing effects of other environmental bacterial species may also decrease the performance of this system (Perez et al. Citation2003; Diederen et al. Citation2005).
Although mauve-colored colonies should be verified by further identification processes (Hsiao et al. Citation2012), CHROMagar still shortens the detection time from >3 days to 24–48 h because of its ability to isolate target bacteria from the air and detect antibiotic resistance in a single step. Because of these advantages, the performance of CHROMagar in airborne VRE and VSE sampling is worthy of further analysis. In addition to questions about the relative sensitivity and specificity of CHROMagar, bacterial colony survival rates on different agar media are also of concern. Damaged bacteria may be able to multiply and form colonies on nonselective agar medium but not on selective media containing inhibiting agents or extremely high concentrations of inorganic salts (Stewart et al. Citation1995). Therefore, the resulting impaction stress, which influences the recovery and injury of the collected bacteria, may be different for CHROMagar than for nonselective agar.
The purposes of this study were to evaluate the bacterial colony survival rate on CHROMagar during the collection of airborne vancomycin-sensitive E. faecalis in a chamber study and to evaluate the performance of CHROMagar for identifying airborne VSE and VRE in a hospital environment, a wastewater treatment plant, and a swine confinement-style building. To evaluate the bacterial colony survival rate on CHROMagar compared with other nonselective agar, three agar media, lysogeny broth agar (LB), Mueller Hinton agar (MH), and tryptic soy agar (TSA), were also used in the chamber study. In addition, two different bioaerosol samplers, an Andersen one-stage impactor and a Nuclepore polycarbonate filter, were investigated with the four types of agar medium. An environmental monitoring program for detecting airborne VSE and VRE using the two different samplers was conducted to test the colony identification performance of CHROMagar. After sampling, all colonies recovered using CHROMagar that demonstrated positive (mauve) or negative results were verified using conventional laboratory tests and polymerase chain reaction (PCR).
2. MATERIAL AND METHODS
2.1. Chamber Study
2.1.1. Test Microorganism
Because most clinical Enterococcus isolates are E. faecalis (80–90%) rather than E. faecium (5–10%) (Treitman et al. Citation2005), a single strain of vancomycin-sensitive E. faecalis (ATCC 29212) was used as a surrogate for both VSE and VRE species in the chamber study. An active E. faecalis culture was inoculated in lysogeny broth and incubated for 24 h at 37°C. Following cultivation, the microbe pellets were aseptically washed and resuspended in sterile distilled water to prepare nebulized suspensions.
2.1.2. Aerosol Preparation and Test System
A Collision three-jet nebulizer (BGI Collision Nebulizer, BGI Inc., Waltham, MA, USA) was used to nebulize the E. faecalis stock in deionized water at 20 psi with dry, filtered, compressed laboratory air. The aerosolized suspension was then diluted with filtered, compressed air at 50 l/min. Because the concentration of airborne Enterococcus rarely exceeds 1,000 colony forming units per cubic meter of air (CFU/m3) in the environment (Predicala et al. Citation2002), E. faecalis at 1,000 CFU/m3 was used to simulate field conditions. Preliminary analyses (data not shown) showed that nearly 1,000 CFU/m3 of airborne E. faecalis could be generated by an E. faecalis suspension in a nebulizer at 1,000 CFU/ml. Therefore, the E. faecalis concentration in the nebulizer was approximately 1,000 CFU/ml (coefficient of variation% (CV%) = 5.0%) in each experiment. To determine the concentration of the E. faecalis suspension in the nebulizer, E. faecalis was cultured in a flask and incubated at 37°C until reaching an OD600 of 1.0 (1 × 108 CFU/ml). The E. faecalis culture was then serially diluted to obtain a final concentration of approximately 1 × 103 CFU/ml. Because relative humidity (RH) strongly affects bacterial culturability, collection was performed at three RH levels (30%, 55%, and 85%).
2.1.3. Test Samplers and Sample Processing
To evaluate the bacterial colony survival rate on assorted agar media, an Andersen 1-STG impactor (Andersen Samplers, Inc., Atlanta, GA, USA) and a Nuclepore filter sampler (Nuclepore®, Costar Corp., Cambridge, MA, USA) were used to collect E. faecalis aerosols. The Andersen impactor was operated at a sampling flow rate of 28.3 l/min and a sampling time of 7 min. The Nuclepore filter sampler consisted of a polycarbonate membrane with a 0.4-μm pore size and a 37-mm diameter supported by cellulose pads. The sampling flow rate of the Nuclepore filter sampler was 20 l/min, and the sampling time was 60 min.
Commercially available media for the selective and differential growth of vancomycin-resistant E. faecalis and vancomycin-resistant E. faecium (VRE.faes) that rely on colony color-based identification include CHROMagar VRE (CVRE; CHROMagar Microbiology, Paris, France). According to the manufacturer, CVRE without vancomycin supplementation (CVSE) can distinguish vancomycin-sensitive E. faecalis and vancomycin-sensitive E. faecium (VSE.faes) from other bacterial strains based on colony color. Because E. faecalis was used as a surrogate for VRE in the chamber study, only CVSE (not CVRE) was evaluated for airborne collection of E. faecalis in a chamber. In addition to CVSE, bacterial colony survival rates on several nonselective media such as LB (REF 244520), MH (REF 225250), and TSA agar (REF 236950) (Difco Laboratory, Detroit, MI, USA) were compared. After sampling, the plates of collection medium (CVSE, LB, MH, and TSA) from the Andersen impactor were incubated at 37°C for 24 h. For filter sampling, filters were eluted after sampling by rinsing with 1 ml sterile deionized water. Then, 0.1 ml of eluate was inoculated separately on the various agars and incubated at 37°C for 24 h. Finally, CFU/m3 was calculated based on the dilution ratio, plated volume, sampling time, and flow rate.
2.1.4. Calculation of the Bacterial Colony Survival Rate
Microbial collection and survival in bioaerosol samplers strongly depend on the type of aerosolization, the type of sampler, the sampling time, the sampling flow rate, and the collection medium. Because we generated E. faecalis aerosols using the same method and the same bioaerosol sampler (Andersen impactor or Nuclepore filter sampler) at a constant operating flow rate, sampling time, and RH, the impact stress on the bacterial colony was directly related to the components of the loaded agar (Juozaitis et al. Citation1994; Stewart et al. Citation1995). However, the bacterial colony survival rate on a collection medium can be strongly affected by the culture preparation process (Lin and Li Citation1998, Citation1999; Chen and Li Citation2005; Tseng and Li Citation2005). Therefore, to better understand the colony survival rate on each agar medium evaluated for collecting E. faecalis aerosols, the culturability of microorganisms in the liquid suspension used as the source of the bioaerosols needed to be evaluated (Li et al., Citation2003). To accomplish this, we used a parameter called colony survival (CS) as an indicator to assess the biological performance of the tested sampler for each agar medium:
2.1.5. Dehydration Stress of Filter Sampling
The dehydration stress on E. faecalis induced by the Nuclepore filter sampler was evaluated in a chamber at an RH of 55% according to a previously published method (Wang et al., Citation2001). E. faecalis was cultured overnight to a concentration of 1 × 108 CFU/ml. To determine the effect of dehydration stress on E. faecalis, bacterial cultures were serially diluted to 1 × 105 CFU/ml. The bacterial culture was then spiked onto the Nuclepore filter (approximately 5 × 103 CFU on each filter). All of the filters loaded in the cassettes were simultaneously evaluated by passing clean air through the filters at 20 l/min for sampling times of 0, 30, 60, 90, 120, and 240 min. Immediately after sampling, the filters were removed from each cassette and eluted by rinsing with 1 ml sterile deionized water, which was used for bacterial culture on CVSE, LB, MH, and TSA agar. The dehydration stress on E. faecalis was defined by the ratio C t /C0, where C t and C0 are the bacterial concentrations in the simultaneously collected samples subjected to airflow for t hours and 0 h, respectively.
2.1.6. Statistical Analysis
In the chamber studies, all experiments were performed in triplicate. The CS or C t /C0 values of E. faecalis on the evaluated medium were compared using the nonparametric Kruskal–Wallis test followed by Scheffe's test to evaluate statistically significant differences (p < 0.05). These statistical tests were also used to compare the CS values among the three different RH levels using CVSE or CVRE.
2.2. Field Study
2.2.1. Sampling Location
We collected aerosol samples from the outpatient hall (H) and the wastewater treatment plant (W) of a medical center in Taiwan. At site H, the central air conditioning was supplied by air-handling units. Samples from site W were collected from the influent and effluent tanks. Sampling was also conducted in a swine confinement-style building (S) because Enterococcus species are frequently found in the air of swine facilities (Predicala et al. Citation2002).
2.2.2. Aerosol Sampling
At each sampling site, parallel aerosol samples were collected using an Andersen impactor and a Nuclepore filter sampler. We collected a total of 282 aerosol samples, including 207 impactor samples and 75 filter samples. At site W, we collected aerosol samples at the influent and effluent tanks for wastewater treatment, including the influent and effluent pumping room, through which workers pass. At site S, we selected enclosed environments, such as farrowing and nursery units in the swine building, for air sampling. The air pump and sampling apparatus were placed approximately 1 m from patient seating at site H and in the central walkway at sites W and S. The sampling heights were 1 m above the floor at site H and 1.5 m above the floor at sites W and S, within the breathing zones of the seated patients and occupational workers, respectively. CVSE and CVRE agar media were used in conjunction with each sampler to identify airborne VSE.faes and VRE.faes, respectively. To compare the total bacterial concentrations in the aerosol, the nonselective media LB, MH, and TSA were also investigated. The sample processing was identical to that used in the chamber study. All evaluated agar media (CVSE, CVRE, LB, MH, and TSA) containing the field samples were incubated at 37°C for 24 h.
TABLE 1 Contingency table for air sampling performance of CVSE and CVRE
2.2.3. Bacterial Identification and Antibiotic Susceptibility Test
CVSE and CVRE are selective and differential media for VSE.faes and VRE.faes, respectively. In both cases, the target colonies are mauve in color. After 20–24 h of cultivation, colonies that appeared mauve-positive or mauve-negative were identified using conventional laboratory tests in sequence, including Gram staining, catalase testing, PYR testing, bile-esculin testing, and 6.5% NaCl tolerance testing, to verify the results. A total of 9,532 colonies on both media were subjected to conventional laboratory tests. Enterococcus species are Gram positive, catalase negative, PYR positive, and bile-esculin positive and grow in broth containing 6.5% NaCl.
2.2.4. Molecular Identification of E. Faecalis and E. Faecium from Aerosol Samples
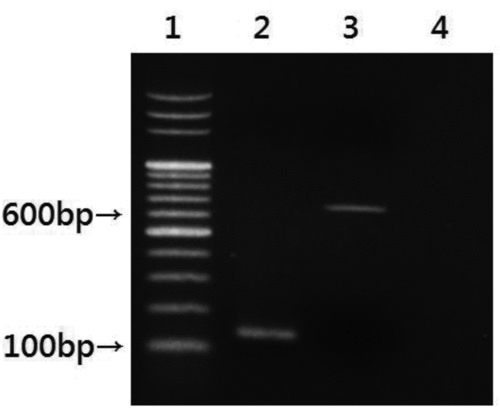
Any sample identified as an Enterococcus species that passed all of the conventional laboratory tests was validated by PCR performed on a DNA thermal cycler GeneAmp PCR system 2700 (Applied Biosystems, Foster City, CA, USA). For DNA extraction, suspensions from airborne samples were heated at 95°C for 15 min and centrifuged for 2 min at 14,000 rpm to sediment the debris. The clear supernatant was used as a DNA template for PCR analysis. To identify E. faecalis, a primer pair (EfF: 5′-CCGAGTGCTTGCACTCAATTGG-3′; EfR: 5′-CTCTTATGCCATGCGGCATAAAC-3′) was used to specifically amplify the E. faecalis 16S rRNA gene, as described previously (Sedgley et al. Citation2005). The primer pair EM1A/EM1B (EM1A: 5′-TTGAGGCAGACCAGATTGACG-3′; EM1B: 5′-TATGACAGCGACTCCGATTCC-3′) was also used to amplify an E. faecium-specific genomic fragment according to a previously described method (Cheng et al. Citation1997). The expected product sizes of the respective PCR amplicons were 138 and 658 bp. The resulting PCR products were analyzed by agarose gel electrophoresis ().
2.2.5. Vancomycin Susceptibility Test
Following verification of E. faecalis or E. faecium, room-temperature MH agar plates were used to evaluate each of the isolates for antibiotic resistance. The vancomycin susceptibilities were assessed using the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI Citation2005) guidelines. To evaluate the performance of CVRE, E. faecalis or E. faecium displaying vancomycin resistance was defined as vancomycin-resistant Enterococcus (VRE).
2.2.6. Data Analysis
The performance of CVSE or CVRE was assessed in a 2 × 2 contingency table format (). The performance statistics reflect sensitivity [a/(a+b)] or [e/(e+f)] and specificity [d/(c+d)] or [h/(g+h)]. Sensitivity refers to the proportion of VSE.faes and VRE.faes with a positive test result (mauve color) using CVSE or CVRE. Specificity refers to the proportion of non-VSE.faes or non-VRE.faes with a negative test result (nonmauve color) using CVSE or CVRE. In addition to sensitivity and specificity, the positive predictive values [PPV; (a/(a+c) or e/(e+g)] and negative predictive values [NPV; d/(b+d) or h/(f+h)] were also calculated. PPV and NPV correspond to the probability that any particular positive (mauve color) or negative (nonmauve color) test result is a true positive or negative result, respectively.
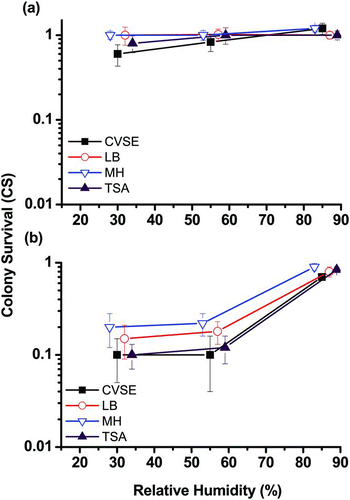
FIG. 2 Effects of RH on colony survival (CS) on four culture media (CVSE, LB, MH, and TSA) when collecting airborne E. faecalis using (a) an Andersen impactor and (b) a Nuclepore filter sampler. The y-axis CS represents the bacterial colony survival rate on different culture media on a logarithmic scale. Experiments were performed in triplicate, and the data shown are the mean ± standard error of the mean.
2.2.7. Statistical Analysis
In field studies, the total concentrations of airborne bacteria at H, W, and S were compared using the nonparametric Kruskal–Wallis test followed by Scheffe's test to evaluate statistically significant differences (p < 0.05). The statistical tests were also utilized to compare the total bacterial concentrations detected using the five different agar media. The nonparametric Wilcox rank-sum (Mann–Whitney) test was utilized to examine the differences in bacterial concentrations between two sampler types or two agar media. The difference in bacterial identification performance between two sampler types or two agar media (CVSE and CVRE) was also analyzed by the same statistical tests.
3. Results
3.1. Chamber Study
3.1.1. Bacterial Colony Survival Rates on the Tested Agar Media
The CS values of the Andersen one-stage impactor for E. faecalis on CVSE, LB, MH, and TSA () ranged from 0.6 to 1.2 at all three RH levels (30%, 55%, and 85%). The RH did not affect the CS of E. faecalis on any of the evaluated agars (p = 0.07). In addition, the CS of E. faecalis on CVSE was only slightly lower than that on LB, MH, or TSA at RHs of 30% or 55%, and the results were not significantly different (p = 0.10). The average CS values of the Nuclepore filter sampler () for E. faecalis on all four agar media did not differ significantly at any of the RH levels (p = 0.21). However, the average CS for E. faecalis on all four agar media at an RH of 85% (from 0.7 to 0.9) was significantly higher than the CS values at RHs of 30% and 55% (from 0.1 to 0.2; p < 0.05).
3.1.2. Dehydration Stress on Closed-Face Filter Cassettes
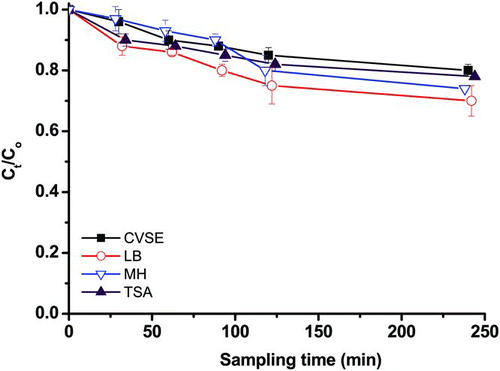
As shown in , all curves representing the C t /C0 values declined with increasing sampling time and decreased gradually after pure air was passed through the filter. The C t /C0 values for all of the evaluated agar media did not differ significantly from each other (p = 0.59). The sampling time for the Nuclepore filter sampler was 60 min in both our chamber and field studies. Following 60 min of sampling, the average C t /C0 values were 0.90 for CVSE, 0.86 for LB, 0.93 for MH, and 0.88 for TSA. Therefore, the recovery rate on E. faecalis collected by a closed-face filter cassette was not less than 0.8 for sampling times of less than 60 min.
FIG. 3 Dehydration stress of E. faecalis recovered on four culture media (CVSE, LB, MH, and TSA) by closed-face filter sampling. The y-axis C t /C0 represents dehydration stress in E. faecalis induced by the Nuclepore filter sampler in arithmetic scale. Experiments were performed in triplicate, and the data shown are the mean ± standard error of the mean.
3.2. Field Study
3.2.1. Total Concentration of Airborne Bacteria at the Three Sampling Sites
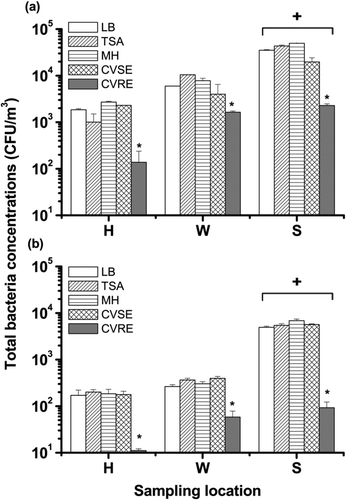
During the sampling period, the mean ambient RHs were 64% in site H, 59% in site W, and 73% in site S. The average bacterial concentrations for the different agar media in an Andersen impactor varied from 138 to 49,638 CFU/m3 (). Site S had the highest total bacterial concentration of the three sampling locations (p < 0.05), while the bacterial concentrations at sites H and W were not significantly different from each other (p = 0.83). The total bacterial concentration measured using CVSE was not significantly different from that detected using the nonselective agars LB, MH, and TSA (p = 0.23); however, the concentration measured using CVRE was significantly lower than that detected using the other three agar types (p < 0.05). In comparison with the Andersen impactor, the Nuclepore filter sampler greatly underestimated the concentration of airborne bacteria (3.5–28.7-fold lower; p < 0.05), regardless of the agar medium used (). Filter sampling also detected the highest total bacterial concentration at site S, and there were no significant differences among the total concentrations measured using CVSE, LB, MH, and TSA (p = 0.13). As with the Andersen impactor, the CVRE medium yielded the lowest bacterial concentration for the Nuclepore filter sampler (p < 0.05).
FIG. 4 Total airborne bacterial concentrations recovered on five culture media (CVSE, CVRE, LB, TSA, and MH) after sampling using (a) an Andersen impactor or (b) a Nuclepore filter sampler in an outpatient hall (H), wastewater treatment plant (W), and swine confinement-style building (S). + p < 0.05 compared to the respective H and W groups. *p < 0.05 compared to the respective LB, TSA, MH, and CVSE groups.
3.2.2. Concentration of Airborne VSE and VRE as Determined Using CVSE and CVRE
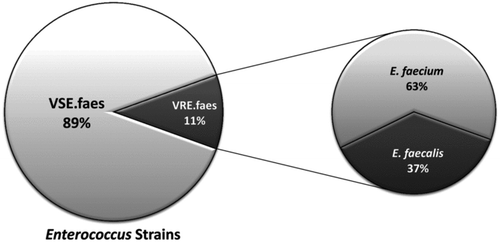
and show the median concentrations of VSE and VRE, which were determined using conventional laboratory tests and PCR on all of the recovered colonies on CVSE or CVRE media. CVSE combined with either the Andersen impactor or the Nuclepore filter sampler demonstrated that the order of VSE concentrations by sampling site was S > W > H. Similarly, the concentration of airborne VRE at site S was also higher than the concentrations at sites H and W. The detected concentrations on CVSE and CVRE media using the Nuclepore filter sampler were 3.1- to 6.0-fold lower than those detected by the Andersen impactor. In addition, several filter samples had no detectable VSE.faes or VRE.faes at site H. Combining the data from the Andersen impactor and the Nuclepore filter sampler, no VSE.faes colonies were detected on the CVRE agar. In addition, there were no significant differences in the VRE.faes concentrations detected by CVSE and CVRE (p = 0.61). As shown in , we observed that approximately 90% of the airborne E. faecalis and E. faecium were VSE, while the remaining 10% of the isolates were VRE. also shows that E. faecium accounted for 63% of the airborne isolates of VRE.faes; E. faecalis accounted for the remaining 37% of isolates.
TABLE 2 Airborne Enterococcus concentrations recovered from different locations by Andersen impactor sampling
TABLE 3 Airborne Enterococcus concentrations recovered from different locations by Nuclepore filter sampling
3.2.3. Performance of CVSE for Air Sampling
The performance of CVSE as a selective differential medium for VSE.faes in air samples was evaluated. As shown in the contingency table (), conventional laboratory tests and PCR were used to evaluate CVSE, and its performance was determined based on sensitivity, specificity, PPV, and NPV. The type of sampler that was used did not affect the performance of CVSE (p = 0.77). Combining the data from the Andersen impactor and the Nuclepore filter sampler to calculate the mean sensitivity, specificity, PPV, and NPV, CVSE exhibited low sensitivity (58.5%) but relatively high specificity (81.3%) for the detection of airborne VSE.faes. Similarly, the PPV was low (5.5%) but the NPV was relatively high (99.1%) for CSA.
TABLE 4 Contingency table for air sampling performance of CVSE using conventional laboratory tests and PCR
3.2.4. Performance of CVRE in Air Sampling
The performance of CVRE as a selective differential medium for VRE.faes in air samples is summarized in . Conventional laboratory tests combined with PCR and vancomycin susceptibility testing were used as comparison tests for CVRE. The performance of CVRE with the Andersen impactor was not significantly different from that with the Nuclepore filter sampler (p = 1.0). Combining the Andersen impactor and the Nuclepore filter sampler data, the mean sensitivity of CVRE (100%) was higher than that of CVSE (58.5%), although the difference was not significant (p = 0.10). Moreover, the mean specificity (88.3%), PPV (8.4%), and NPV (100%) of CVRE were similar to that of CVSE, respectively.
TABLE 5 Contingency table for air sampling performance of CVRE using conventional laboratory tests and PCR
4. DISCUSSION
4.1. Chamber Study
We did not find any significant differences between TSA and CVSE when sampling airborne E. faecalis. This result is surprising given that TSA is considered an excellent general agar for culturing a variety of bacteria. A previous study suggested that impaction onto an agar surface may injure bacteria, particularly when the bacteria are impacted onto a selective agar medium such as CVSE (Stewart et al. Citation1995). It is usually difficult to culture injured bacteria on selective media due to nutritional limitations and the presence of inhibiting agents. Nonselective agars, such as LB or TSA, offer a more effective means for preserving bacterial culturability. However, because CVSE is specifically used for the isolation and direct differentiation of VSE.faes, the components and selective mix that characterize CVSE may help E. faecalis to recover and may enable a CS level similar to that achieved with TSA.
When using the Nuclepore filter, sampling was conducted for 60 min to increase yields; under these conditions, less than 20% of the E. faecalis lost culturability on the filter. If filter sampling was continued for more than 240 min, more than half of the E. faecalis on the filter demonstrated a decrease in culturability during the dehydration process. Previous studies have shown that this is likely due to dehydration, which occurred during the sampling and the extraction processes, and might have caused enough biological stress to reduce the culturability of the bacteria (Chen and Li Citation2005; Tseng and Li Citation2005).
The parameter CS represents the colony survival rate of E. faecalis aerosols on each agar medium; isolates with higher CS values correspond to species that are more resistant to the stress of air sampling. The following levels of resistance to stress from impaction and filtration have previously been reported (listed in descending order): Bacillus subtilis endospores or Penicillium spores (CS = 0.15–0.38) > gram-positive S. aureus (CS = 0.01–0.1) > gram-negative Legionella pneumophila (CS = 0.02) > nonenveloped viruses (CS = 0.01–0.02) > gram-negative Escherichia coli (CS = 0.006) (Li and Lin Citation1999a,Citationb; Li et al. Citation2003; Tseng and Li Citation2005; Chang et al., Citation2010; Hsiao et al. Citation2012). The CS of gram-positive E. faecalis in the present study (CS = 0.6–1.2) was much higher than those reported for S. aureus and E. coli. Enterococcus strains have been reported to survive for at least 1 week and for as long as 4 months on dry surfaces (Wendt et al., Citation2001). Resistance to dry conditions may not only promote the transmissibility of Enterococcus strains but also increase their resistance to sampling stress. Thus, the colony survival rate of a microorganism on an agar medium surface is highly species-dependent. However, collection with the Andersen impactor yielded a higher colony survival rate for airborne E. faecalis than that obtained via the filtration process at RHs of 30% and 55%.
4.2. Field Study
The overall average total bacterial concentration in the swine buildings (site S) measured in the current study using the culture method was 4.9 × 104 CFU/m3, in agreement with the findings of previous studies (Chang et al. Citation2001; Chi and Li Citation2005). Although CVSE and CVRE are selective differential media for VSE.faes and VRE.faes, other bacterial species can still grow on them. However, only the target colonies are mauve-colored. Unlike CVSE, some antibiotic-related supplements are added to CVRE. Therefore, the low total bacterial concentration measured by CVRE is likely due to the inability of antibiotic-sensitive bacteria to grow on CVRE.
and show that the detected concentrations in the CVSE and CVRE media using the Nuclepore filter sampler were lower than those detected with the Andersen impactor. This result, along with our chamber study (), suggests that the loss of E. faecalis culturability in air samples collected using a Nuclepore filter may be related to the stress of impaction and dehydration at a moderate RH. In contrast, demonstrates that the dehydration effect of filter sampling was low when sampling was conducted for 60 min at a RH of 55%. However, the dehydration stress test only accounted for the stress caused by air flow, not for stress resulting from the impaction of bacterial particles onto the filter (Wang et al. Citation2001). The overall stress from filter sampling should be referred to as the sampling indicator CS. This indicator includes the aerosolization of E. faecalis and thus could contain all the stresses (impaction, dehydration, and extraction) that airborne E. faecalis may suffer during aerosolization and filter sampling. The microbial species with higher CSs are also likely more resistant to the overall stress caused by aerosolization and air sampling.
During the sampling period, VRE were widespread in the swine building and were present in the air. Although the swine building had the highest total bacterial concentration, the widespread use of antibiotics there (Gordoncillo et al. Citation2012) may account for the greater concentration of VRE measured at this site. In the absence of antibiotic resistance, VSE.faes are difficult to culture on CVRE medium because the antibiotic-related supplements in CVRE suppress VSE.faes growth. By contrast, both VSE.faes and VRE.faes can grow on CVSE because CVSE cannot distinguish between resistant and sensitive strains of E. faecalis and E. faecium. In the present study, E. faecalis accounted for the majority of airborne VSE.faes isolates, in agreement with previous Enterococcus species identifications from clinical isolates (Treitman et al. Citation2005). However, the concentration of vancomycin-resistant E. faecium was higher than that of E. faecalis. Evidence suggests that the prevalence of vancomycin-resistant E. faecium is increasing and that resistance to vancomycin is far more commonly associated with E. faecium than with E. faecalis (Suppola et al. Citation1998).
There are no previous reports of the utilization of CVSE and CVRE to detect Enterococcus species in environmental samples. Previous studies using clinical specimens demonstrated that the sensitivity and PPV of CVSE were in the range of 84.2–95.9% (Peltroche-Llacsahuanga et al. Citation2009; Kallstrom et al. Citation2010; Stamper et al. Citation2010), compared with the range of 3.8–59.9% observed in the current study. These differences may be related to variations in the tests used for Enterococcus identification. However, the relatively low sensitivity observed in the current study indicates that CVSE is not particularly effective for the recognition of airborne VSE.faes. The low sensitivity of CVSE in field applications is similar to our previous observations for the use of CHROMagar to detect airborne S. aureus (71%). The low sensitivity may be related to environmental interference, bacterial enzyme expression, and competition between different bacterial strains (Perez et al. Citation2003; Diederen et al. Citation2005).
Bacteria form colored colonies on CHROMagar because the media release colored dyes upon hydrolysis by a specific bacterial enzyme. Environmental interference can inhibit PCR amplification of target bacterial sequences from air samples (Chen et al. Citation2009). Therefore, some compounds may interfere with the hydrolytic process and suppress mauve color formation on CHROMagar plates. Moreover, previous studies have demonstrated that bacterial enzymatic expression may vary based on the geographical origin of the species (Perez et al. Citation2003). Some bacterial species recovered from different geographical locations do not express the specific enzyme detected by CHROMagar, decreasing the sensitivity of this medium. Finally, competition between different microbial species on the same CHROMagar plate may also affect its sensitivity (Perez et al. Citation2003; Diederen et al. Citation2005). The specific enzymes expressed by E. faecalis and E. faecium may interfere with other bacterial species and render CVSE unable to detect VSE.faes.
PPV and NPV are two important indicators that have been used to determine whether the mauve and nonmauve colonies on CHROMagar are VSE.faes or VRE.faes. PPV is directly proportional to the percentage of VSE.faes (i.e., the ratio of VSE.faes to total bacteria) in the air. In our study, the percentage of VSE.faes ranged from 0.4 to 2.2%. Therefore, the low percentage of VSE.faes may be reflected in the low PPV of CVSE (5%). As the percentage of VSE.faes in the air increases, it is more likely that a mauve colony will be VSE.faes rather than a false positive (i.e., a mauve colony that is not VSE.faes). False-positive colonies on CHROMagar are most likely to be Candida species, gram-negative rods, Enterococcus species (other than E. faecalis and E. faecium), gram-positive rods, or Streptococcus species (Malhotra-Kumar et al. Citation2008). Although the sensitivity and PPV of CVSE were low, CVSE yielded high specificity and NPV in air sampling (99%). This result suggests that a nonmauve colony recovered using CVSE can be reported as a specimen that is not VSE.faes, while a mauve colony should be further verified.
In addition, our results demonstrated that the sensitivity and specificity of CVRE differ from those of CVSE. Antibiotic supplements, such as vancomycin, are incorporated into CVRE to suppress VSE.faes strains. The selective mixes and antibiotics in CVRE may help to suppress the growth of other bacterial species (particularly vancomycin-sensitive strains) and improve the performance of CVRE in recognizing airborne VRE.faes. The percentage of VRE.faes isolates among the total number of bacteria (0–3.4%) was low in the air. Therefore, the identification of airborne VRE by CVRE also revealed a low PPV (8%). Similar to CVSE, CVRE exhibited a high NPV (100%), suggesting that a nonmauve colony recovered using CVRE in air sampling can be reported as negative, while a mauve colony should be subjected to further identification analyses.
Our study has some limitations. First, vancomycin-sensitive E. faecalis was used as a surrogate for VRE during our chamber experiments for safety reasons. However, for antibiotic-resistant bacteria, the altered binding proteins in the cytoplasmic membranes of VRE strains suggest the possibility of changes in their susceptibility to the sampling stress associated with impaction and filtration. Second, our study was only conducted in limited environments. When CVSE or CVRE are applied in other occupational fields, the enzymatic expression and competing effects of different microbial species may vary between different geographical locations. These factors may affect the performance of CHROMagar in detecting airborne VSE.faes or VRE.faes.
5. CONCLUSIONS
The widespread use of antibiotics has resulted in an increase in the number of antibiotic-resistant bacterial strains. There is an immediate need to develop an environmental monitoring program for the rapid identification of airborne VRE. Based on the similar PPV and NPV results, CVRE may become more valuable than CVSE because CVRE has high sensitivity and specificity. From a health perspective, VRE poses a greater threat to human health than VSE. A high level of sensitivity and specificity in a test is desirable for detecting airborne microbes that cause serious disease. Although the low PPV of CVRE may lead to false-positive results, additional simple tests (such as Gram staining, catalase testing, PYR testing, and bile-esculin testing) can improve the performance of CVRE. In this study, we demonstrated that CVRE could be used in air sampling to isolate VRE.faes without additional susceptibility testing. Therefore, these advantages may enable CVRE to rapidly identify the concentration profiles of airborne VRE.faes in hospital or occupational environments. Although the low PPVs of CVSE and CVRE suggest that a positive result should also be verified using conventional procedures, these media may reduce the detection time of VSE.faes or VRE.faes by 24–48 h, depending on the methods used.
FUNDING
This work was supported by grant NSC 100-2314-B-320-003 from the National Science Council, Republic of China and grant TCMRC-P-101011-01 from Tzu Chi University, Hualien, Taiwan.
REFERENCES
- Brodka , K. , Kozajda , A. , Buczynska , A. and Szadkowska-Stanczyk , I. 2012 . The Variability of Bacterial Aerosol in Poultry Houses Depending on Selected Factors . Int. J. Occup. Med. Environ. Health , 25 : 281 – 293 .
- Chang , C. W. , Chou , F. C. and Hung , P. Y. 2010 . Evaluation of Bioaerosol Sampling Techniques for Legionella pneumophila Coupled with Culture Asay and Quantitative PCR . J. Aerosol Sci. , 41 : 1055 – 1065 .
- Chang , C. W. , Chung , H. , Huang , C. F. and Su , H. J. 2001 . Exposure of Workers to Airborne Microorganisms in Open-Air Swine Houses . Appl. Environ. Microbiol. , 67 : 155 – 161 .
- Chen , P. S. and Li , C. S. 2005 . Sampling Performance for Bioaerosols by Flow Cytometry with Fluorochrome . Aerosol Sci. Technol. , 39 : 231 – 237 .
- Chen , P. S. , Lin , C. K. , Tsai , F. T. , Yang , C. Y. , Lee , C. H. Lia , Y. S. 2009 . Quantification of Airborne Influenza and Avian Influenza Virus in a Wet Poultry Market Using a Filter/Real-Time Qpcr Method . Aerosol Sci. Technol. , 43 : 290 – 297 .
- Cheng , S. , McCleskey , F. K. , Gress , M. J. , Petroziello , J. M. , Liu , R. Namdari , H. 1997 . A PCR Assay for Identification of Enterococcus faecium . J. Clin. Microbiol. , 35 : 1248 – 1250 .
- Chi , M. C. and Li , C. S. 2005 . Fluorochrome and Fluorescent In Situ Hybridization to Monitor Bioaerosols in Swine Buildings . Aerosol Sci. Technol. , 39 : 1101 – 1110 .
- Clinical and Laboratory Standards Institute . 2005 . “ Performance Standard for Antimicrobe Susceptibility Testing; 15th Informantional Supplement ” . Wayne , PA : Clinical and Laboratory Standards Institute .
- Diederen , B. , van Duijn , I. , van Belkum , A. , Willemse , P. , van Keulen , P. and Kluytmans , J. 2005 . Performance of Chromagar MRSA Medium for Detection of Methicillin-Resistant Staphylococcus aureus . J. Clin. Microbiol. , 43 : 1925 – 1927 .
- Gordoncillo , M. J. , Donabedian , S. , Bartlett , P. C. , Perri , M. , Zervos , M. Kirkwood , R. 2012 . Isolation and Molecular Characterization of Vancomycin-Resistant Enterococcus faecium from Swine in Michigan, USA . Zoonoses Public Health , 60 : 319 – 326 .
- Hsiao , P. K. , Chen , W. T. , Chang , K. C. , Ke , Y. J. , Kuo , C. L. and Tseng , C. C. 2012 . Performance of Chromagar Staph aureus and Chromagar MRSA for Detection of Airborne Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus . Aerosol Sci. Technol. , 46 : 297 – 308 .
- Jett , B. D. , Huycke , M. M. and Gilmore , M. S. 1994 . Virulence of Enterococci . Clin. Microbiol. Rev. , 7 : 462 – 478 .
- Juozaitis , A. , Willeke , K. , Grinshpun , S. A. and Donnelly , J. 1994 . Impaction onto a Glass Slide or Agar Versus Impingement into a Liquid for the Collection and Recovery of Airborne Microorganisms . Appl. Environ. Microbiol. , 60 : 861 – 870 .
- Kallstrom , G. , Doern , C. D. and Dunne , W. M. Jr. 2010 . Evaluation of a Chromogenic Agar under Development to Screen for VRE Colonization . J. Clin. Microbiol. , 48 : 999 – 1001 .
- Karra , S. and Katsivela , E. 2007 . Microorganisms in Bioaerosol Emissions from Wastewater Treatment Plants During Summer at a Mediterranean Site . Water Res. , 41 : 1355 – 1365 .
- Li , C. S. and Lin , Y. C. 1999a . Sampling Performance of Impactors for Bacterial Bioaerosols . Aerosol Sci. Technol. , 30 : 280 – 287 .
- Li , C. S. and Lin , Y. C. 1999b . Sampling Performance of Impactors for Fungal Spores and Yeast Cells . Aerosol Sci. Technol. , 31 : 226 – 230 .
- Li , C. S. , Tseng , C. C. , Lai , H. H. and Chang , C. W. 2003 . Ultraviolet Germicidal Irradiation and Titanium Dioxide Photocatalyst for Controlling Legionella Pneumophila . Aerosol Sci. Technol. , 37 : 961 – 966 .
- Lin , W. H. and Li , C. S. 1998 . The Effect of Sampling Time and Flow Rates on the Bioefficiency of Three Fungal Spore Sampling Methods . Aerosol Sci. Technol. , 28 : 511 – 522 .
- Lin , W. H. and Li , C. S. 1999 . Collection Efficiency and Culturability of Impingement into a Liquid for Bioaerosols of Fungal Spores and Yeast Cells . Aerosol Sci. Technol. , 30 : 109 – 118 .
- Malhotra-Kumar , S. , Haccuria , K. , Michiels , M. , Ieven , M. , Poyart , C. Hryniewicz , W. 2008 . Current Trends in Rapid Diagnostics for Methicillin-Resistant Staphylococcus aureus and Glycopeptide-Resistant Enterococcus Species . J. Clin. Microbiol. , 46 : 1577 – 1587 .
- Muzslay , M. , Moore , G. , Turton , J. F. and Wilson , A. P. 2012 . Dissemination of Antibiotic-Resistant Enterococci within the Ward Environment: The Role of Airborne Bacteria and the Risk Posed by Unrecognized Carriers . Am. J. Infect. Control , 41 : 57 – 60 .
- Otter , J. A. , Yezli , S. and French , G. L. 2011 . The Role Played by Contaminated Surfaces in the Transmission of Nosocomial Pathogens . Infect. Control Hosp. Epidemiol. , 32 : 687 – 699 .
- Peltroche-Llacsahuanga , H. , Top , J. , Weber-Heynemann , J. , Lutticken , R. and Haase , G. 2009 . Comparison of Two Chromogenic Media for Selective Isolation of Vancomycin-Resistant Enterococci from Stool Specimens . J. Clin. Microbiol. , 47 : 4113 – 4116 .
- Perez , J. M. , Cavalli , P. , Roure , C. , Renac , R. , Gille , Y. and Freydiere , A. M. 2003 . Comparison of Four Chromogenic Media and Hektoen Agar for Detection and Presumptive Identification of Salmonella Strains in Human Stools . J. Clin. Microbiol. , 41 : 1130 – 1134 .
- Perry , J. D. and Freydiere , A. M. 2007 . The Application of Chromogenic Media in Clinical Microbiology . J. Appl. Microbiol. , 103 : 2046 – 2055 .
- Predicala , B. Z. , Urban , J. E. , Maghirang , R. G. , Jerez , S. B. and Goodband , R. D. 2002 . Assessment of Bioaerosols in Swine Barns by Filtration and Impaction . Curr. Microbiol. , 44 : 136 – 140 .
- Russell , A. D. 2002 . Antibiotic and Biocide Resistance in Bacteria: Introduction . J. Appl. Microbiol. , 92 ( Suppl ) : 1S – 3S .
- Savini , V. , Gherardi , G. , Astolfi , D. , Polilli , E. , Dicuonzo , G. D’Amario , C. 2012 . Insights into Airway Infections by Enterococci: A Review . Recent. Pat. Antiinfect. Drug Discov. , 7 : 36 – 44 .
- Sedgley , C. M. , Nagel , A. C. , Shelburne , C. E. , Clewell , D. B. , Appelbe , O. and Molander , A. 2005 . Quantitative Real-Time PCR Detection of Oral Enterococcus faecalis in Humans . Arch. Oral Biol. , 50 : 575 – 583 .
- Stamper , P. D. , Shulder , S. , Bekalo , P. , Manandhar , D. , Ross , T. L. Speser , S. 2010 . Evaluation of BBL Chromagar Vanre for Detection of Vancomycin-Resistant Enterococci in Rectal Swab Specimens . J. Clin. Microbiol. , 48 : 4294 – 4297 .
- Stewart , S. L. , Grinshpun , S. A. , Willeke , K. , Terzieva , S. , Ulevicius , V. and Donnelly , J. 1995 . Effect of Impact Stress on Microbial Recovery on an Agar Surface . Appl. Environ. Microbiol. , 61 : 1232 – 1239 .
- Suppola , J. P. , Kuikka , A. , Vaara , M. and Valtonen , V. V. 1998 . Comparison of Risk Factors and Outcome in Patients with Enterococcus faecalis vs Enterococcus faecium Bacteraemia . Scand. J. Infect. Dis. , 30 : 153 – 157 .
- Treitman , A. N. , Yarnold , P. R. , Warren , J. and Noskin , G. A. 2005 . Emerging Incidence of Enterococcus faecium among Hospital Isolates (1993 to 2002) . J. Clin. Microbiol. , 43 : 462 – 463 .
- Tseng , C. C. and Li , C. S. 2005 . Collection Efficiencies of Aerosol Samplers for Virus-Containing Aerosols . J. Aerosol Sci. , 36 : 593 – 607 .
- Wang , Z. , Reponen , T. , Grinshpun , S. A. , Gorny , R. L. and Willeke , K. 2001 . Effect of Sampling Time and Air Humidity on the Bioefficiency of Filter Samplers for Bioaerosol Collection . J. Aerosol Sci. , 32 : 661 – 674 .
- Wendt , C. , Wiesenthal , B. , Dietz , E. and Ruden , H. 2001 . Survival of Vancomycin-Resistant and Vancomycin-Susceptible Enterococci on Dry Surfaces . J.Clin. Microbiol. , 36 : 3734 – 3736 .