Abstract
Primary biological aerosol particles (PBAP) such as pollen and fungal spores can induce allergenic responses and affect health in general. Conditions such as allergic rhinitis (hay fever) and asthma have been related to pollen concentrations. Likewise some pollen have been shown to induce ice nucleation and cloud condensation at higher temperatures than those associated with some chemical species, thereby affecting planet Earth's albedo and overall radiative balance. Hence, the near real-time (on-line) monitoring of airborne pollen and other PBAP using a variety of spectroscopic and light scattering techniques represents an area of growing development and consequence.
In this study, two separate field campaigns (one at a rural site in Ireland and the other at an urbanized location in Germany) were performed to detect and quantify pollen releases using a novel on-line fluorescence spectrometer (WIBS-4). The results were compared with results obtained using more traditional Hirst-type impactors. Size, “shape,” and fluorescence characteristics of ambient particles were used to determine the concentrations and identity of the PBAP likely to be pollen grains.
The concentration results obtained for both methodologies at both the Irish and German sites correlated very well, with R 2 values >0.9 determined for both campaigns. Furthermore, the sizing data available from the WIBS-4 approach employed in Ireland indicated that pollen grains can be identified in appropriate conditions. WIBS-4 measurements of Yew pollen both in the laboratory and at the rural site indicated almost identical size ranges of 25 to 27 μm. Yew pollen is generally reported to be in this range, but the measurements reported here are the first of their type providing data on the size of in-flight Yew pollen.
Copyright 2014 American Association for Aerosol Research
INTRODUCTION
Primary biological aerosol particles (PBAP) such as pollen and fungal spores can induce allergic responses in humans and affect health in general. Conditions such as allergic rhinitis (hay fever) and asthma have been related to elevated pollen concentrations (Wodehouse Citation2007; Kay Citation2008; Jantunen et al. 2012).
Likewise some pollen have been shown to be involved in ice nucleation (Diehl et al. Citation2001; Després et al. Citation2012) and are known to be more effective than other nonbiological particles such as soot and dust. Thus pollen can affect planet Earth's albedo and overall radiative balance (Pummer et al. Citation2012). However, while the direct and indirect environmental implications of large airborne concentrations of pollen have now begun to be realized, methodologies to achieve their real-time analysis for such purposes have lagged behind chemical particulate matter analysis because of the mainly natural origins of bioaerosols, which are therefore not considered as pollutants.
Current methods employed for pollen detection have remained off-line and traditional in their approach: they commonly use simple impactors such as the Hirst-type volumetric trap and whirling arm traps (Lacey and West Citation2006; Caruana Citation2011). These instruments capture coarse airborne particles on a suitable substrate before they are taken to a laboratory for analysis using optical microscopy (Lacey and West Citation2006). This dual approach is quite laborious and requires highly skilled operators for accurate determinations of number and identities due to the sheer variety of PBAP species present in the environment.
Newer, immunochemical methods of analysis have been used more recently, for example ELISA (enzyme-linked immunosorbent assay), a technique which is used, principally, for the identification of aeroallergens associated with allergenic pollen (Speight et al. Citation1997; Mandrioli et al. Citation2003; Buters et al. 2012; Galan et al. 2013).
Similarly innovative optical methods of PBAP identification for particles such as pollen have begun to be used in ambient locations (Kawashima et al. Citation2007). These instruments use light scattered from particles to define their size and shape. Therefore, they can count particles in the size range appropriate for any specific target particle.
Monitoring intrinsic fluorescence (autofluorescence) is another technique used for the detection of PBAP particles and has been used previously in fluorescent microscopes and spectrometers (Pan et al. Citation1999; Roshchina Citation2008; Mitsumoto et al. Citation2009; Gabey et al. Citation2010; O’Connor et al. Citation2011; Pan et al. Citation2011; Healy et al. Citation2012a; Pöhlker et al. Citation2012; Roshchina Citation2012; Kaliszewski et al. Citation2013; Kiselev et al. Citation2013; Pöhlker et al. Citation2013). One development of this approach, in recent times, is the use of fluorescence for detection by on-line instrumentation such as the Waveband-Integrated Biological Sensor (WIBS), the Ultraviolet Aerodynamic Particle Sizer (UV-APS), and the automated pollen counter (Hairston et al. Citation1997; Kaye et al. Citation2004, Citation2005, 2007; Delaunay et al. Citation2007; Mitsumoto et al. Citation2010; Pan et al. Citation2011). These instruments use combined fluorescence and optical scattering, as a diagnostic to differentiate between fluorescent and nonfluorescent particles present in the atmosphere. This technique offers the potential for rapid number concentration determination of fluorescent biological aerosol particles (FBAP) while being nondestructive and reagent free. PBAP are autofluorescent because of their chemical and biochemical contents. Hence, structural components such as tryptophan, cellulose, chitin, lignin, and “sporopollenin” as well as secondary metabolites such as phenols, NAD(P)H, and terpenoids all can be excited and their fluorescence can be collected over a number of wavelength ranges (Roshchina et al. Citation1995, 2004; Hairston et al. Citation1997; Roshchina et al. Citation1998; Roshchina Citation2003).
Instrumentation like the WIBS employed here and the UV-APS in particular have been used for PBAP detection in both laboratory (Agranovski et al. Citation2003, Citation2004; Agranovski and Ristovski Citation2005; Kanaani et al. Citation2007, Citation2008; Healy et al. Citation2012a; O’Connor et al. Citation2013) and ambient environments recently (Gabey et al. Citation2010; Stanley et al. Citation2011; Huffman et al. 2011, Citation2012; Schumacher et al. Citation2013; Toprak and Schnaiter Citation2013). They have been shown to provide both concentration and limited characterization data. However, the field campaigns have not, as of yet, compared the use of either of these instruments to data from Burkard volumetric traps specifically focused on the identification of pollen grains.
In this study, the conventional method generally employed for PBAP sampling and analysis (automatic volumetric trap) was co-located with a WIBS-4 instrument at a rural site and an urban location. Results from both instrumental methods were compared and contrasted. The sets of data obtained here represent the first to employ the WIBS approach for the detection of biological particles in the super coarse size range 10 to 30 μm. Furthermore, by comparison with the results obtained using traditional impact/optical analysis, it is shown that the evaluation of ambient pollen concentrations can be obtained reliably in near real-time.
MATERIALS AND METHODS
Sampling Site 1: Killarney National Park, Ireland
Particulate sampling was performed in Killarney National Park (KNP), Kerry, Ireland (N 52°01.263′ W 09°30.553′), towards the eastern perimeter of Reenadinna Woods between 24/02/10 and 4/03/10. The Reenadinna Woods area is the most extensive location for Yew Trees in Ireland and covers ∼60 acres (25 hectares). The canopy in this stand is typically strongly dominated by Taxus baccata (Yew) along with Corylus avellana (common Hazel), IIex aquifolium (European Holly), and Fraxinus excelsior (Ash). A list of typical species found in Reenadinna Woods is outlined elsewhere (Kelly Citation1981).
This site offered fairly ideal conditions for the preliminary testing of the WIBS-4 instrument because of its almost pristine air conditions and the dominance of Yew pollen at the time of year selected. There are no sources close to the campaign site of anthropogenic materials, such as diesel particles, which are fluorescent (Pan et al. Citation2003). Specifically there are no landfills, road traffic, animal housing or waste treatment plants in the vicinity of the sampling site. However, even if some interfering airborne chemicals were present, their general size ranges (<1 μm) would preclude them from the WIBS analysis procedure. Larger “dust particles” could also pose possible interference. Some mineral dust is known to fluoresce in the regions targeted by the WIBS-4 and also to have size ranges occurring in the coarse and super-coarse fractions of atmospheric particulates. However, their fluorescence intensities are known to be lower than those of bio-fluorophores and, in fact, this difference could prove useful in the screening of such particles (Pöhlker et al. Citation2012; Toprak and Schnaiter Citation2013). It is of further note that the possibility of smaller chemical pollutants adhering to larger dust particles and then transferring their fluorescent properties could be a concern with regard to potential signal interferences. This possibility and the idea that fluorescent chemicals could “piggyback” bioaerosols (and vice versa) remain little studied phenomena.
The chosen campaign location was also safe and secure. All instruments were housed in a purpose-built mobile laboratory trailer unit which was positioned adjacent to a lawn area in front of “Arthur Vincent House,” KNP, from where a 220 V power supply was obtained. Sample inlets were positioned 2.5 to 3 m in height above ground level and located ∼4 m from the nearest tree. A simple vertical 16 mm inner bore inlet with no size cut off was utilized during the campaign.
Sampling Site 2: Technical University of Munich (TUM), Germany
Sampling was performed at the grounds outside the Centre of Allergy and Environment, Munich, TUM, Germany N 48°09.523′ E 11°35.400′, 510 m above sea level and 1.80 m above the ground between 27/04/10 and 10/05/10. In contrast to KNP, the environment here would be expected to provide a much more complex ambient composition of airborne particulates because the site is located in an urban area, 260 m away from a 70,000 vehicles/day highway and also 1 km away from “The English Gardens.” Hence, this chosen campaign site was set up at a place in which aerosol sources from traffic, domestic heating, and vegetation are likely present. An identical sample inlet to that used in the KNP campaign was used in Munich.
Volumetric Traps
A continuous volumetric Hirst-type pollen and spore trap (Burkard Scientific) was employed for the KNP campaign as a traditional method for PBAP capture. Prepared, silicone-coated sample substrates/tape (Lanzoni, s.r.l., Bologna, Italy) were mounted on the drum within the sampler. The sampler was then set to run for a 7-day period at 10 L/min. The adhesive sampling tape was changed at the end of this period. Upon completion, the tape was dissected into seven 48 mm segments representing 24-h time intervals. Mounting of these segments involved placing them on microscope slides before using a Gelvatol mounting medium, stained with basic fuchsin to adhere a cover lip permanently to the slide. The slides were analyzed at 400× magnification under an optical microscope using the 12-traverse method (Lacey and West Citation2006). Thus a 2-h time resolution for the campaign was employed for the KNP campaign. A similar volumetric trap was used in the TUM campaign although the longitude method of counting was used according the standards set-up by the European Aerosol Society (Frenguelli and Galan 2011). All pollens were counted to a species level with total pollen representing the sum of all pollen species measured.
Ozone Monitoring
A Thermo-Scientific, Model 49i, Ozone Monitor was used for the determination of ambient ozone concentrations during the KNP campaign.
Waveband-Integrated Bioaerosol Sensor, Model 4
The waveband-integrated bioaerosol sensor (WIBS-4) is a single particle, on-line fluorescence spectrometer with the capability to determine the size, “shape,” and fluorescence characteristics of ambient particles at a millisecond time resolution. It achieves this through the use of two xenon flash lamps set to excite at 280 nm and 370 nm, respectively, to gauge the fluorescent intensities of individual particles. It also uses a 635 nm diode laser to establish their size and “shape” in terms of an asymmetry, AF, factor. The AF values are obtained by using the ratio of scattered light falling on a four quadrant detector, more detailed descriptions of this process have been discussed in great detail elsewhere (Gabey et al. Citation2010; Healy et al. Citation2012a). Effectively, a numerical value between 0 and 100 is returned by the WIBS, with zero defining a perfect sphere while values closer to 100 represent rod-shaped particles. When equal amounts of light fall on each of the four quadrants, a low asymmetry value is calculated by the WIBS-4 thus indicating a spherical morphology. Should two of the quadrants receive more light then the other two, a higher AF value is calculated, indicative of a rod-like particle. Varying intensities of light over each quadrant are representative of irregularly shaped particles such as dust, for example.
Individual particle fluorescence is evaluated using three detector channels, termed FL1, FL2, and FL3. These channels record the total fluorescence over two wavelength ranges, namely, FL1 = 310 to 400 nm and both FL2 and FL3 = 420 to 650 nm. Each particle is excited at both 280 nm (FL1 and FL2) and 370 nm (FL3). The WIBS-4 device used for the campaigns discussed here is similar to those that have been described previously (Kaye et al. Citation2005; Gabey et al. Citation2010; Healy et al. Citation2012a, Citationb). The WIBS-4 instrument does differ in important respects from its predecessor (WIBS-3) as noted in those reports (Healy et al. Citation2012a), especially as WIBS-4 allows the user to designate the size fraction of the ambient air upon which to focus sampling and subsequent data analysis. This feature is related to two sensitivity settings in WIBS-4: High Gain (HG) and Low Gain (LG). HG allows particles between 0.5 and 12 μm to be evaluated whilst LG allow particles from 3 to 31 μm to be analyzed (Healy et al. Citation2012a,Citationb). Given that the target particles (pollen) for the KNP and TUM campaigns described here are generally considered to be of sizes >15 μm, the LG mode was selected. These sizes are also, of course, far larger than highly fluorescent chemical particulates such as diesel particles from combustion or secondary organic particles originating from atmospheric processes. These are known to emit light because they contain fluorescing components such as polycyclic aromatic hydrocarbons (PAHs) (Beltran et al. Citation1998; Pan et al. Citation2003).
Very large data sets were created during the two field campaigns undertaken in this on-line pollen detection study. Thus various filters were applied to the data sets in order to allow the particles of interest to be more easily recognized and counted. The limits of detection for each of the three fluorescence channels FL1, FL2, and FL3 of the WIBS-4 were obtained by placing the WIBS-4 in forced trigger mode. This causes the xenon flash lamps to discharge in the absence of a triggering particle. The WIBS-4 was allowed to sample in this mode until a test data set with 1000 points was established. Mean values plus three times the standard deviation from the test data from each of the three fluorescent channels were then used as the thresholds between particles that were deemed to be biological or nonbiological. This procedure is similar to that described previously (Gabey et al. Citation2010; Healy et al. Citation2012a). A fluorescence intensity threshold value of 1000 was used for the FL2 and FL3 detection channels and a size range above 15 μm was used to discriminate between pollen and possible interferences from large fungal spores or fluorescent nonbiological particles. Large test particles >10 μm have been observed to give fluorescent signals that were below the instrumental limit of detection discussed above. Thus it was assumed that this detection limit was viable for larger particles. Data were averaged over both 24 and hourly periods for use in the analyses.
Weather Station
Meteorological parameters were collected using a Casella Nomad Weather Station at 5-min time resolution. The data were subsequently averaged to 2 h intervals for more appropriate comparison with the results obtained from both the counting/identification methods used in the KNP Campaign.
RESULTS AND DISCUSSION
KNP Campaign
Using WIBS-4, a large number of fluorescent, spherical particles (>15 μm) were counted throughout the KNP campaign. Over 50% of the FBAP monitored in the Killarney campaign were of this type of particle. Such information alone is suggestive that pollen was indeed sampled given the size, shape, and fluorescence data obtained. In general, it was found that the PBAP detected, in flight, was 24 to 27 μm in diameter and to be spherical in morphology. Daily concentration comparisons using results obtained from both the traditional volumetric trap and the WIBS-4 were clearly in full agreement as shown in . The plot shows excellent daily correlations, with a computed R 2 correlation value, 0.93.
FIG. 1 (a) Daily concentration data for the pollen and FBAP > 15 μm (m−3) obtained with the SporeWatch/microscope-dashed (red) line and the WIBS-4 solid (blue) line, respectively. (b) Image plot obtained from the WIBS-4 data showing the size distribution for all fluorescent particles sampled >5 μm in size for the campaign with 1 h resolution. The scale indicates fluorescent particles (m−3).
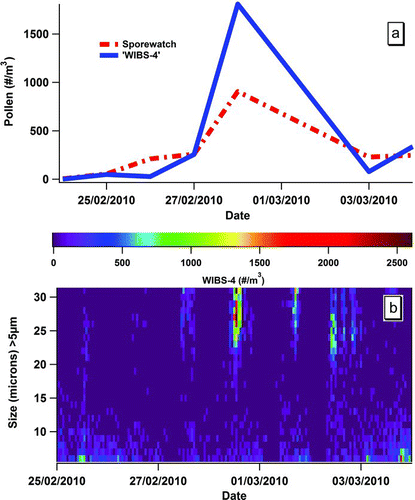
It can be seen that the counting data obtained from both approaches track each other well as shown in and b. However, shows some of the added advantages for analysis that are apparent using the WIBS-4 technique. Thus particle (pollen) data are available at much higher time resolution than is traditionally possible with impaction/microscopy because of the labor-intensive nature of analysis. For example displays the data at an hourly resolution, although much shorter time scales are also possible to analyze. Specific features such as that shown for 28/02/10 are readily apparent from the size distribution color-coded data. Clearly, this date represents that on which the peak period of pollen release was found. Additional information such as the fact that large releases of fluorescent particles (>20 μm) occurred after midday on a number of occasions during the campaign is also apparent. The phenomenon was investigated in more detail as outlined below.
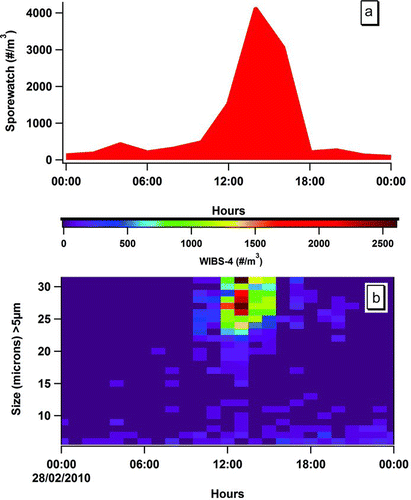
shows a comparison between the SporeWatch and WIBS-4 data obtained for 28/02/10. In , the pollen counts are expressed in grains per m3 using the traditional method, whereas in time-resolved (30 min) number–size distribution profiles are shown. The color-coding scheme further highlights one of the main advantages of the WIBS-4 technique: its far higher sampling time-resolution capability. The major peak in the large fluorescent particle count arose at ca. 13:00 (red color). This measurement correlates well with the concentration obtained with the traditional pollen sampling method, which also peaked at about this time, albeit at a lower, bi-hourly, resolution (). Indeed, the pollen peak determined by the SporeWatch/microscopy method was identifiable with Yew pollen grains alone. The size and shape information obtained using the WIBS-4 is in full agreement with such identification and provides an indication of the potential for this on-line, automated technique.
FIG. 2 Comparison between the SporeWatch and WIBS-4 data obtained for 28 February 2010 where in (a), the pollen counts are expressed in grains per m3 using impaction/microscopy. In (b), time-resolved number–size distribution profiles are shown (see scale). Note only FBAP ≥4.9 μm are presented for the purpose of illustration.
Comparing the size profiles of the pollen grains sampled during the WIBS-4 field (ambient) campaign with that of Yew samples freshly harvested at the site in Killarney, and investigated in the laboratory in an earlier study, (Healy et al. Citation2012a) show very little deviation between the two. The agreement is shown in .
FIG. 3 Average number–size distribution for Yew pollen introduced in the laboratory to the WIBS-4 solid (blue) trace, left y-axis (data from Healy et al. Citation2012a) as compared to fluorescent particles detected in the KNP campaign dashed (red) trace, right y-axis.
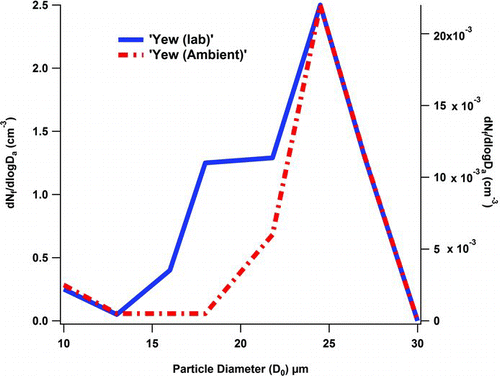
Both the laboratory data (blue) and the ambient data (red) tracked well at the peak maximum. One significant difference between the two trends is the appearance of a “shoulder” for particles with a size D o ∼ 18 to 20 μm as sampled in the laboratory. However, this slight divergence is most likely due to desiccation or the break-up of the source material because of the aerosolization process, as previously described. (Healy et al. Citation2012a). While both pollen samples were recorded in the same size range by the WIBS-4, it is also of interest that they were also seen in the same size fraction when investigated under a light microscope. The pollen grains were thereby shown to be of sizes between 20 and 30 μm (centered at 25–27 μm). Thus, the WIBS-4 designation of “size” would appear to be consistent with the physical diameter evaluated optically.
Given the high time resolution of the WIBS-4 instrument, the potential for monitoring unique correlations between particle releases and meteorological parameters would also appear to be a distinct possibility. shows treatments of this type where the average release of Yew pollen as a function of time over the full campaign and the corresponding meteorological parameters associated with dispersal are plotted.
FIG. 4 Diel cycles comparing windspeed, temperature, relative humidity, solar radiation, ozone, and Yew pollen concentrations (see color scale) as detected by WIBS-4 (presented as hourly median values vs. local time of day) averaged for the complete campaign.
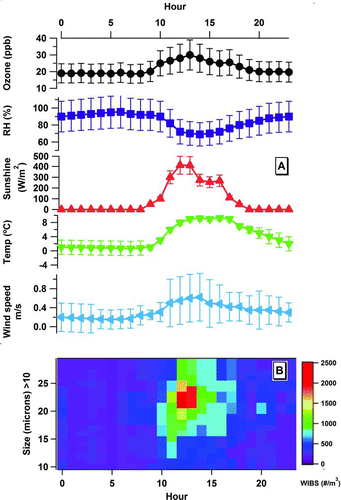
From , it can be determined that associations between temperature, relative humidity (RH), and windspeed with Yew pollen release appear to exist. In fact, over the campaign period increasing in-flight Yew pollen concentrations were observed as RH values decreased. Although not shown in , it was also noted that atmospheric pressure had to be >995 mb for Yew pollen to be detectable, in flight. Finally, mean temperatures of between 5 and 8°C were shown to coincide with peak sampling of the Yew pollen as also indicated in .
TABLE 1 Descriptive statistics of optical size and shape charactistics for “pollen,” total particles, and fluorescent particles sampled by WIBS-4 at “Killarney and Munich sites”
In addition shows correlations between light levels experienced as well as ozone mixing ratios monitored with Yew pollen concentration. Again both solar radiation and ozone appear to peak in coincidence with pollen release as noted throughout the campaign. The ozone relationship is particularly interesting and requires more detailed investigation. However, all that is stated here is that pollen release was observed only at ozone levels >20 ppbv. This observation is perhaps not unexpected though given the increased solar radiation levels monitored at these time periods; the ozone levels themselves most likely have little impact on pollen release.
TUM Campaign
The campaign at TUM (Munich) was undertaken in a more urbanized environment than that provided by KNP. Again a traditional Hirst-type impaction sampler was co-located with the WIBS-4 and again very good daily correlations between the concentrations of pollen monitored using both approaches was obtained. The results of the study are shown in .
FIG. 5 Daily Burkard, dashed (red) trace, volumetric impactor counts vs. WIBS-4 “large” fluorescent particle counts, solid (blue) trace.
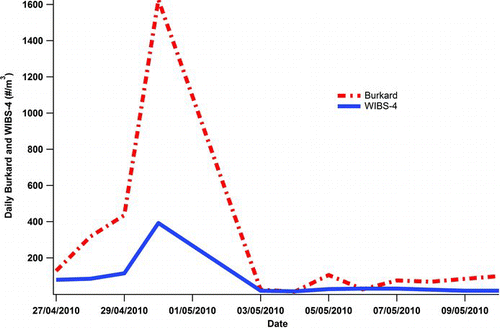
The correlation between the on-line and off-line techniques to determine pollen concentrations at TUM are remarkable displaying an R 2 value, 0.98. Clearly possible chemical interfering signals caused by nonbiological particles would appear to be negligible at this urbanized site. However, the finding is not surprising as chemical pollutants, such as primary combustion particulates like diesel particles and other secondary organic aerosol, SOA, particles, which are known to fluoresce upon excitation at wavelengths used by the WIBS-4 method are found airborne typically in the sub-micron size range. Therefore they are far smaller than pollen (Pan et al. Citation2003). Although of little apparent relevance to the TUM results discussed here, the prospect that airborne chemical particulate matter could adhere to the surface of pollen grains and thereby alter their size, shape, and fluorescent properties remains a little explored phenomenon that deserves more experimental attention.
shows the descriptive statistics for the size and “shape” of likely “pollen” grains, total particles, and fluorescent particles detected by the WIBS-4 instrument for both campaigns. It can be seen that the the interquartile ranges span between 22 and 31 μm and 24 and 29 μm for TUM and KNP, respectively. Saturation of the WIBS-4 sizing detector occurs at 30 to 31 μm and the 99th and 75th percentiles are therefore observed at this value for the TUM campaign. The “shape” of the “pollen” particles of interest exhibit median values of ∼20 and 18 AF units for both of the campaigns. It has been shown previously that AF < 25 can be considered to be roughly spherical in nature with the degree of roundness increasing at lower AF values (Healy et al. Citation2012a). Given that Birch and Platanus were the most prominent pollen types indicated by the impactor/microscopy technique over the course of the TUM campaign, AF values in the 12 to 42 range, but with a median value of 20, could be considered to be similarly assignable because of the mainly globular morphologies associated with these pollen types. Yew pollen are also classed as spherical so the low AF values measured in the KNP campaign would also suggest that the values of the WIBS-4 correspond to the optical microscope meaurements.
The shape values for the fluorescent particle ranges for both campaigns were very similar. They did, however, deviate with respect to size, with the TUM samples exhibiting larger size ranges. This is not suprising given that the spore types, such as Alternaria, were present in TUM but absent in KNP. Total particle size distributions again provided slight differences, with the particles measured to be of bigger size in the TUM campaign as well as possessing higher AF values. Hence, the TUM data collection showed larger, more irregularly sized particles than that obtained at KNP.
In summary both the size and “shape” data obtained by the WIBS-4 regarding the in flight pollen detected in the TUM and KNP campaigns are consistent with the size ranges and morphologies that are known for the pollen species present at both campaign sites. Birch is generally of diameter 19 to 22 μm, while Platanus pollen is commonly found between 17 and 19 μm. (Winkler et al. 2001) The larger size ranges observed for the TUM data are more likely due to the presence of Oak pollen which was also monitored in significant qualities over the campaign. Oak pollen is noted for its size range between 26 and 34 μm being of more elliptical shape (Wodehouse Citation2007). For the KNP campaign, Yew pollen was, by far, the most dominant airborne particle. Yew pollen exhibits a range between 19 and 31 μm with average grains measured typically with size 25 and 27 μm (Wodehouse Citation2007). Yew pollen grains are also quite spherical, which again corroborates the WIBS-4 results.
Limitations of the Study
While very good correlations were attained for both campaigns shown here, it should be noted that the relatively short periods over which they occurred weaken the conclusions of any of the findings. However, the campaigns were mounted as a “proof of principle” of the WIBS-4 approach rather than a determination relevant to all circumstances. The KNP campaign also was quite unusual, although not for early Spring in KNP, because pollen was the dominant PBAP species found to be airborne there. Dependant on the time of the year fungal spores would be generally considered to be far more prevalent, certainly in the Autumn season. Thus later in the year interferences from larger fluorescent fungal spores may represent a real problem for the sampling of pollen using an autofluorescence instrument, although generally fungal spores are smaller than pollen (Eduard 2009).
Sampling in the KNP campaign was undertaken very close to the release location of the Yew pollen. This approach was taken to ensure that pollen, in this earliest field study, would indeed be sampled over the course of the campaign. However, it is unlikely that such conditions would be prevalent should the WIBS system be incorporated into a large-scale sampling network. Thus at other locations, in alternative seasons, there may be limitations for determining overall pollen concentrations especialy at higher time resolutions. Again this possibility remains to be seen.
It is also of note that given the relatively low flow rate of the WIBS-4 instrument, meterological condtions such as strong wind speeds or gust speeds may adversely affect the ability of the WIBS to successfully sample pollen. This is due to the increased momentum of the particles imparted on them by the wind. Similarly larger pollen types such as pine or even grass pollen may be undersampled due to increased wall losses.
While the use of fluorescence signals appears to have been helpful in classifing certain ambient particles as pollen, the fluorescent characteristics as monitored by the WIBS-4 of these FBAP appear to be very similar. All “pollen” classifed in this study and in previous laboratory work were found to be highly fluorescent in all three detection channels but in particular FL2 and FL3 (Healy et al. Citation2012a). Indeed, all size distributions for the three detection channels were identical. Thus the fluorescent fraction for pollen with the WIBS-4 in its current configuration could be considered to represent a 100% sampling efficiency. Therefore, in principle, the use of the WIBS-4 to differentiate between pollen species is unlikely, especially in an environment with a number of different pollen species with overlaping size ranges. However, there are a number of discrimiating features that are potentially available for WIBS analyses: FL1, FL2, FL3 data (along with their ratios) as well as size and “shape.” Indeed in other studies, measurements on pollen fluorescence have been conducted that focuses on the blue/red fluorescence ratio in the visible spectrum. This approach thereby allowed for the differentation between pollen species when linked with size information (Mitsumoto et al. Citation2009, Citation2010). If the FL2 and FL3 detection channels in the WIBS instrument could be split up into more channels, then more potenial information on particle identity could be measured and thereby provide inceased information on speciation.
Finally, there is the possibility that large nonfluorescent particles such as dust particles could be contaminated by smaller fluorescent particles. This could be particularly problematic in urban environments with increased anthropogenic sources of potentially fluorescent aerosols, such as diesel particles and SOA. Such a possibility could lead to an over estimation of the pollen content of the ambient surrounding. While large nonfluorescent, >15 μm particles were monitored throughout both campaigns they did not correlate with the large-fluorescent particles designated as pollen. It should also be noted that these large nonfluorescent had far smaller mean and median size ranges and more irregular-shaped morphologies.
CONCLUSION
The WIBS-4 instrument was sited at two contrasting locations, one rural and the other urbanized in order to evaluate its potential for detecting, counting, and identifying pollen. In order to obtain meaningful information, the traditional impactor/optical microscopy techniques of pollen collection and analysis were also employed. The concentration results correlated very well with R 2 values >0.9 determined at both campaign sites. Furthermore, the sizing data available from the WIBS-4 approach employed here indicates that pollen grains can be identified in appropriate conditions. Hence, Yew pollen was sampled both in the laboratory and in the field at KNP for this study: the results indicated almost identical size ranges. In fact Yew pollen is generally reported to be between 25 and 27 μm in diameter but the measurements reported here are the first of their type providing data on the size of in-flight Yew pollen. The number, size, and “shape” ranges of PBAP determined by use of the WIBS-4 in the TUM campaign were also in good agreement with the results obtained using the traditional off-line technique.
The campaigns performed were only of a relatively short duration but they do show that the counting ability of the near real-time WIBS-4 method provides excellent agreement with the labour intensive, time-consuming impaction/microscopy methodology. Additionally, because of the high time resolution of the WIBS-4 instrument correlations between pollen release levels and parameters such as ozone levels and RH can be readily visualized as virtual “snapshots.”
The overall study should be taken as a proof of principle of the WIBS-4 approach to pollen counting. More detailed assessments would require comparison studies to be performed over full pollen seasons and the potential interferences posed by some of the larger spores such as Alternaria, Epiccocum, and Polythrincium should be investigated. In fact few of these fungal spore types were found to be present during both the KNP and TUM campaigns but their contributions later in the calender year may become more apparent.
FUNDING
The authors would like to thank the Irish EPA for financial support with two separate grants: 2007 CCRP Project 4.4.6.b. (BioCheA) and a Doctoral Scholarship to David J. O’Connor.
ACKNOWLEDGMENTS
The help of Sebastian Öder and Ingrid Weichenmeier for the Munich campaign was greatly appreciated.
REFERENCES
- Agranovski , V. and Ristovski , Z. D. 2005 . Real-Time Monitoring of Viable Bioaerosols: Capability of the UVAPS to Predict the Amount of Individual Microorganisms in Aerosol Particles . J. Aerosol Sci. , 36 : 665 – 676 .
- Agranovski , V. , Ristovski , Z. D. , Ayoko , G. A. and Morawska , L. 2004 . Performance Evaluation of the UVAPS in Measuring Biological Aerosols: Fluorescence Spectra from NAD (P) H Coenzymes and Riboflavin . Aerosol Sci. Technol. , 38 : 354 – 364 .
- Agranovski , V. , Ristovski , Z. , Hargreaves , M. , Blackall , J P. and Morawska , L. 2003 . Performance Evaluation of the UVAPS: Influence of Physiological Age of Airborne Bacteria and Bacterial Stress . J. Aerosol Sci. , 34 : 1711 – 1727 .
- Beltran , J. , Ferrer , R. and Guiteras , J. 1998 . Multivariate Calibration of Polycyclic Aromatic Hydrocarbon Mixtures from Excitation–Emission Fluorescence Spectra . Ana. Chim. Acta , 373 : 311 – 319 .
- Caruana , D. J. 2011 . Detection and Analysis of Airborne Particles of Biological Origin: Present and Future . Analyst , 136 : 4641 – 4652 .
- Delaunay , J.-J. , Sasajima , H. , Okamoto , Y. and Yokota , M. 2007 . Side-by-Side Comparison of Automatic Pollen Counters for use in Pollen Information Systems . Ann. Allergy, Asthma & Immunol. , 98 : 553 – 558 .
- Després , V. R. , Huffman , J. A. , Burrows , S. M. , Hoose , C. , Safatov , A. S. , Buryak , G. , Fröhlich-Nowoisky , J. , Elbert , W. , Andreae , M. O. and Pöschl , U. 2012 . Primary Biological Aerosol Particles in the Atmosphere: A Review . Tellus B , 64 : 15598 doi: 10.3402/tellusb.v64i0.15598
- Diehl , K. , Quick , C. , Matthias-Maser , S. , Mitra , S. K. and Jaenicke , R. 2001 . The Ice Nucleating Ability of Pollen Part I: Laboratory Studies in Deposition and Condensation Freezing Modes . Atmos. Res. , 58 : 75 – 87 .
- Eduard , W. 2009 . Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting . Crit. Rev. Toxicol. , 39 ( 10 ) : 799 – 864 .
- Frenguelli , G. and Galan , C. 2011 . Minimum requirements to manage aerobiological monitoring stations included in a national network involved in the EAN . International Aerobiology Newsletter , 72 : 1
- Gabey , A. , Gallagher , M. , Whitehead , J. , Dorsey , J. , Kaye , P. and Stanley , W. 2010 . Measurements and Comparison of Primary Biological Aerosol Above and Below a Tropical Forest Canopy using a Dual Channel Fluorescence Spectrometer . Atmos. Chem. Phys. , 10 : 4453 – 4466 .
- Galan , C. , Antunes , C. , Brandao , R. , Torres , C. , Garcia-Mozo , H. , Caeiro , E. , Ferro , R. , Prank , M. , Sofiev , M. , Albertini , R. , Berger , U. , Cecchi , L. , Celenk , S. , Grewling , Ł. , Jackowiak , B. , Jäger , S. , Kennedy , R. , Rantio-Lahtimäki , A. , Reese , G. , Sauliene , I. , Smith , M. , Thibaudon , M. , Weber , B. , Weichenmeier , I. , Pusch , G. and Buters , J. T. 2013 . Airborne olive pollen counts are not representative of exposure to the major olive allergen Ole e 1 . Allergy , 68 : 809 – 812 .
- Hairston , P. P. , Ho , J. and Quant , F. R. 1997 . Design of an Instrument for Real-Time Detection of Bioaerosols using Simultaneous Measurement of Particle Aerodynamic Size and Intrinsic Fluorescence . J. Aerosol Sci. , 28 : 471 – 482 .
- Healy , D. A. , O’Connor , D. J. , Burke , A. M. and Sodeau , J. R. 2012a . A Laboratory Assessment of the Waveband Integrated Bioaerosol Sensor (WIBS-4) using Individual Samples of Pollen and Fungal Spore Material . Atmos. Environ. , 60 : 534 – 543 .
- Healy , D. A. , O’Connor , D. J. and Sodeau , J. R. 2012b . Measurement of the Particle Counting Efficiency of the “Waveband Integrated Bioaerosol Sensor” Model Number 4 (WIBS-4) . J. Aerosol Sci. , 47 : 94 – 99 .
- Huffman , J. , Sinha , B. , Garland , R. , Snee-Pollmann , A. , Gunthe , S. , Artaxo , P. , Martin , S. , Andreae , M. and Pöschl , U. 2012 . Size Distributions and Temporal Variations of Biological Aerosol Particles in the Amazon Rainforest Characterized by Microscopy and Real-Time UV-APS Fluorescence Techniques During AMAZE-08 . Atmos. Chem. Phys. , 12 : 11997 – 12019 .
- Jantunen , J. , Saarinen , K. and Rantio-Lehtimäki , A. 2012 . Allergy symptoms in relation to alder and birch pollen concentrations in Finland . Aerobiologia , 28 ( 2 ) : 169 – 176 .
- Kaliszewski , M. , Włodarski , M. , Bombalska , A. , Kwaśny , M. , Mularczyk-Oliwa , M. , Młyńczak , J. and Kopczyński , K. 2013 . “ The Application of Semiconductor Based UV Sources for the Detection and Classification of Biological Material ” . In Tenth Symposium on Laser Technology , 870305 – 870305-8 . International Society for Optics and Photonics .
- Kanaani , H. , Hargreaves , M. , Ristovski , Z. and Morawska , L. 2007 . Performance Assessment of UVAPS: Influence of Fungal Spore Age and Air Exposure . J. Aerosol Sci. , 38 : 83 – 96 .
- Kanaani , H. , Hargreaves , M. , Smith , J. , Ristovski , Z. , Agranovski , V. and Morawska , L. 2008 . Performance of UVAPS with Respect to Detection of Airborne Fungi . J. Aerosol Sci. , 39 : 175 – 189 .
- Kawashima , S. , Clot , B. , Fujita , T. , Takahashi , Y. and Nakamura , K. 2007 . An Algorithm and a Device for Counting Airborne Pollen Automatically using Laser Optics . Atmos. Environ. , 41 : 7987 – 7993 .
- Kay , A. B. 2008 . Allergy and Allergic Diseases. Volume 1, The Scientific Basis of Allergy [Online] , Malden, Massachusetts : Blackwell . Available at: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=267056. (accessed 1 November 2012)
- Kaye , P. H. , Aptowicz , K. , Chang , R. K. , Foot , V. and Videen , G. 2007 . Angularly Resolved Elastic Scattering from Airborne Particles . Opt. Biol. Particles (II. Mathematics, Phys. Chem. , 238 : 31 – 61 .
- Kaye , P. H. , Hirst , E. , Foot , V. E. , Clark , J. M. , Baxter and Year , K. 2004 . “ A Low-Cost Multi-Channel Aerosol Fluorescence Sensor for Networked Deployment ” . In Proceedings of SPIE 5617, Optically Based Biological and Chemical Sensing for Defence 388 London , UK doi: 10.1117/12. 578283
- Kaye , P. , Stanley , W. , Hirst , E. , Foot , E. , Baxter , K. and Barrington , S. 2005 . Single Particle Multichannel Bio-Aerosol Fluorescence Sensor . Opt. Express , 13 : 3583 – 3593 .
- Kelly , D. L. 1981 . The Native Forest Vegetation of Killarney, South-West Ireland: An Ecological Account . J. Ecol. , 69 : 437 – 472 .
- Kiselev , D. , Bonacina , L. and Wolf , J.-P. 2013 . A Flash-Lamp Based Device for Fluorescence Detection and Identification of Individual Pollen Grains . Rev. Sci. Instruments , 84 : 033302
- Lacey , M. and West , J. S. 2006 . The Air Spora a Manual for Catching and Identifying Airborne Biological Particles [Online] , Dordrecht : Springer . Available online at: http://site.ebrary.com/id/10159305
- Mandrioli , P. , Caneva , G. and Sabbioni , C. 2003 . Cultural Heritage and Aerobiology : Methods and Measurement Techniques for Biodeterioration Monitoring , New York : Kluwer Academic Publishers .
- Mitsumoto , K. , Yabusaki , K. and Aoyagi , H. 2009 . Classification of Pollen Species using Autofluorescence Image Analysis . J. Biosci. Bioeng. , 107 : 90 – 94 .
- Mitsumoto , K. , Yabusaki , K. , Kobayashi , K. and Aoyagi , H. 2010 . Development of a Novel Real-Time Pollen-Sorting Counter using Species-Specific Pollen Autofluorescence . Aerobiologia , 26 : 99 – 111 .
- O’Connor , D. J. , Healy , D. A. and Sodeau , J. R. 2013 . The On-Line Detection of Biological Particle Emissions from Selected Agricultural Materials using the WIBS-4 (Waveband Integrated Bioaerosol Sensor) Technique . Atmos. Environ. , 80 ( 0 ) : 415 – 425 .
- O’Connor , D. J. , Iacopino , D. , Healy , D. A. , O’Sullivan , D. and Sodeau , J. R. 2011 . The Intrinsic Fluorescence Spectra of Selected Pollen and Fungal Spores . Atmos. Environ. , 45 : 6451 – 6458 .
- Pan , Y. L. , Hartings , J. , Pinnick , R. G. , Hill , S. C. , Halverson , J. and Chang , R. K. 2003 . Single-Particle Fluorescence Spectrometer for Ambient Aerosols . Aerosol Sci. Technol. , 37 : 628 – 639 .
- Pan , Y. L. , Hill , S. C. , Pinnick , R. G. , House , J. M. , Flagan , R. C. and Chang , R. K. 2011 . Dual-Excitation-Wavelength Fluorescence Spectra and Elastic Scattering for Differentiation of Single Airborne Pollen and Fungal Particles . Atmos. Environ. , 45 : 1555 – 1563 .
- Pan , Y. , Holler , S. , Chang , R. K. , Hill , S. C. , Pinnick , R. G. , Niles , S. and Bottiger , J. R. 1999 . Single-Shot Fluorescence Spectra of Individual Micrometer-Sized Bioaerosols Illuminated by a 351-or a 266-nm Ultraviolet Laser . Opt. Lett. , 24 : 116 – 118 .
- Pummer , B. , Bauer , H. , Bernardi , J. , Bleicher , S. and Grothe , H. 2012 . Suspendable Macromolecules are Responsible for Ice Nucleation Activity of Birch and Conifer Pollen . Atmos. Chem. Phys. , 12 : 2541 – 2550 .
- Pöhlker , C. , Huffman , J. , Förster , J.-D. and Pöschl , U. 2013 . Autofluorescence of Atmospheric Bioaerosols–Spectral Fingerprints and Taxonomic Trends of Native Pollen . Atmos. Meas. Tech. Discuss. , 6 : 5693 – 5749 .
- Pöhlker , C. , Huffman , J. and Poschl , U. 2012 . Autofluorescence of Atmospheric Bioaerosols-Fluorescent Biomolecules and Potential Interferences . Atmos. Meas. Tech. , 5 : 37 – 71 .
- Roshchina , V. V. 2003 . Autofluorescence of Plant Secreting Cells as a Biosensor and Bioindicator Reaction . J. Fluoresc. , 13 : 403 – 420 .
- Roshchina , V. V. 2008 . Fluorescing World of Plant Secreting Cells , Science Publishers . Plymouth, New Jersey
- Roshchina , V. V. 2012 . Vital Autofluorescence: Application to the Study of Plant Living Cells . Int. J. Spectros , 2012 : 124672 – 14p .
- Roshchina , V. V. , Melnikova , E. V. , Mit’kovskaya , L. V. and Kharnaukhov , V. N. 1998 . Microspectrofluorimetry for the Study of Intact Plant Secreting Cells . Zh. Obshch. Biol. , 59 : 531 – 554 .
- Roshchina , V. V. , Melnikova , E. V. , Spiridonov , N. A. and Kovaleva , L. V. 1995 . Azulenes are Blue Pigments of Pollen . Dokl. Akademii Nauk , 340 : 715 – 718 .
- Roshchina , V. V. , Yashin , V. A. and Kononov , A. V. 2004 . Autofluorescence of Developing Plant Vegetative Microspores Studied by Confocal Microscopy and Microspectrofluorimetry . J. Fluoresc. , 14 : 745 – 750 .
- Schumacher , C. , Pöhlker , C. , Aalto , P. , Hiltunen , V. , Petäjä , T. , Kulmala , M. , Pöschl , U. and Huffman , J. 2013 . Seasonal Cycles of Fluorescent Biological Aerosol Particles in Boreal and Semi-Arid Forests of Finland and Colorado . Atmos. Chem. Phys. Discuss. , 13 : 17123 – 17158 .
- Speight , S. E. , Hallis , B. A. , Bennett , A. M. and Benbough , J. E. 1997 . Enzyme-Linked Immunosorbent Assay for the Detection of Airborne Microorganisms used in Biotechnology . J. Aerosol Sci. , 28 : 483 – 492 .
- Stanley , W. R. , Kaye , P. H. , Foot , V. E. , Barrington , S. J. , Gallagher , M. and Gabey , A. 2011 . Continuous Bioaerosol Monitoring in a Tropical Environment using a UV Fluorescence Particle Spectrometer . Atmos. Sci. Lett. , 12 : 195 – 199 .
- Toprak , E. and Schnaiter , M. 2013 . Fluorescent Biological Aerosol Particles Measured with the Waveband Integrated Bioaerosol Sensor WIBS-4: Laboratory Tests Combined with a One Year Field Study . Atmos. Chem. Phys. , 13 : 25 – 243 .
- Winkler , H. , Ostrowski , R. and Wilhelm , M. 2001 . Pollenbestimmungsbuch der Stiftung Deutscher Polleninformationsdienst Paderborn , , Takt-Verlag
- Wodehouse , R. P. 2007 . Hayfever Plants , Read Books .
