Abstract
A 405 nm diode laser-based on-line bioaerosol detector, BioScout, was tested and compared with the Ultraviolet Aerodynamic Particle Sizer (UVAPS). Both instruments are based on laser-induced fluorescence of particles. Only a fraction of microbial particles produce enough fluorescence light to be detected by the instruments. This fluorescent particle fraction (FPF) is aerosol and instrument specific. The FPF values for common bacterial and fungal spores and biochemical particles were experimentally determined for both instruments. The BioScout exhibited higher FPF values for all the test aerosols except coenzyme NADH. The difference was higher for smaller particles. The FPF values of fungal spores and bacteria varied between 0.34 to 0.77 and 0.13 to 0.54 for the BioScout and the UVAPS, respectively. The results indicate that the 405 nm diode laser is a useful excitation source for fluorescence-based real-time detection of microbial aerosols. The FPF results of this study can be utilized to estimate the actual concentrations of bacterial and fungal spores in fluorescence-based ambient measurements.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
Bioaerosols such as bacterial and fungal spores can cause adverse health effects for people and animals both in indoor and outdoor environments (Burge and Rogers Citation2000; Peccia et al. Citation2008; Mendell et al. Citation2011). Materials with mold and bacterial growth are typical bioaerosol sources in indoor air. Atmospheric primary bioaerosols, called usually as PBAP (Primary Biogenic Aerosol Particles), have been recognized to have important influence in the climate, acting as cloud condensation nuclei and ice nuclei. Therefore, they contribute to cloud formation and precipitation processes (Vali et al. Citation1976; Bauer et al. Citation2002, Citation2003; Jaenicke Citation2005; Sun and Ariya Citation2006; Jaenicke et al. Citation2007; Andreae and Rosenfeld Citation2008; Pöschl et al. Citation2010; Després et al. Citation2012). Information of concentrations, particle size distributions, and sources of bioaerosols is needed to estimate their health risks and climatic relevance.
Various techniques have been used to collect ambient bioaerosols, such as Andersen impactor and filter sampling (Reponen et al. Citation2011). These require a separate step for analysis before concentration can be determined. LIF (laser-induced fluorescence)-based instruments are modern tools for real-time bioaerosol detection (Pöhlker et al. Citation2012). The LIF technique enables the differentiation of bioaerosols from other particles through their fluorescence ability (Pan et al. Citation2007; Hill et al. Citation2013). Our recent study demonstrated that bacterial and fungal spores may be distinguished from each other through their dissimilar fluorescence spectra (Saari et al. Citation2013). The most well-known real-time LIF instrument is UVAPS (Ultraviolet Aerodynamic Particle SizerTM, TSI Inc., St. Paul, MN, USA), developed by Hairston et al. (Citation1997). It is capable of measuring both the aerodynamic diameter and the autofluorescence intensity of a single particle. The instrument uses a 355 nm pulse laser for fluorescence excitation. Huffman et al. (Citation2012) used the UVAPS and filter sampling technique to measure PBAP size distribution and concentration in the Amazon rainforest region. They found a good correlation between concentration of fluorescent particles (UVAPS) and particles collected on filters and counted under microscope (scanning electron microscope or staining and light microscopy) but concluded that only a fraction of PBAP concentration can be estimated with the UVAPS because some PBAPs cannot be recognized due to weak fluorescence.
All LIF-based instruments have a detection limit for fluorescence intensity sensing. In general, only a fraction of biological particles emit enough fluorescence light to be detected by the instrument. This fraction is named the fluorescent particle fraction (FPF). The FPF should be determined via calibration for each instrument and bioaerosol if actual concentration of biological particles is needed. The performance of the UVAPS against bacterial and fungal spores and common biochemicals (NADH and riboflavin) has been reported by Agranovski et al. (Citation2003, Citation2004) and Kanaani et al. (Citation2007, Citation2008). Their result showed that the FPF varies between 2 and 52% for bacteria and 48 and 99% for fungal spores, depending mainly on the species and physiological stage of the cells and spores.
The FPF-value is bioaerosol specific, but also dependent on the instrument details, such as the excitation source and the detection band. Ho et al. (Citation2001) reported performance of a bioaerosol detector with a 402 nm diode laser as the excitation source. The detector had otherwise the same structure as the UVAPS, except the excitation source. They concluded that by using a small 402 nm diode laser, it is possible to replicate the performance of the 340 to 360 nm light sources in the biological weapons detection applications. However, they did not present detailed FPF values for different types of bioaerosols. These are needed before the instrument can be used for ambient bioaerosol concentration measurements. The commercial version of this instrument is FLAPS-III (TSI Model 3317), and it is focused mainly for military applications but has also been used for atmospheric bioaerosol detection (Jamriska et al. Citation2012). Another 405 nm diode laser-based sensor, instantaneous bioaerosol analysis and collection (IBAC; FLIR/ICx/S3I, Reisterstown, MD, USA), was used to study relationship between biologically fluorescent aerosol and local meteorological conditions (Santarpia et al. Citation2013). Several 405 nm diode laser-based bioaerosol sensors are also described in US patents (Ho Citation2004; Silcott et al. Citation2005; Jiang et al. Citation2012).
Gabey et al. (Citation2011) reported PBAP measurements in Borneo using a LIF-based instrument (WIBS-3) with two excitation wavelengths (280 and 370 nm) and two fluorescence emission bands (310–400 nm and 400–600 nm). They found similar fluorescent particle concentration fractions with both fluorescence bands. This result indicates that deep-UV excitation (280 nm) is not necessary for atmospheric bioaerosol detection and that near-UV excitation wavelengths are equally usable.
Manninen et al. (Citation2009) represented fluorescence excitation and emission maps for typical bacterial spores. The results showed that 405 nm wavelength can be as good as 355 nm to excite autofluorescence from bacterial spores. Pan et al. (Citation2007) found in their excitation-emission studies that dried biological materials, including vegetative bacterial cells, emit fluorescence when excited by light in the 405 nm range. For fungal spores, Saari et al. (Citation2013) reported rather flat excitation spectra, evidently surpassing their instrumentally limited maximum wavelength of 355 nm. These points support the idea that the 405 nm diode laser could be a potential excitation source for a LIF-based bioaerosol detector.
In this study, we introduce a new LIF-based real-time bioaerosol detector called BioScout. A commercial version of the BioScout (ENVI BioScoutTM, Environics Ltd., Mikkeli, Finland) is designed mainly for military applications, but it can be also used for the detection of ambient bioaerosols. The BioScout has a 405 nm diode laser as excitation source and measures autofluorescence and optical particle size of single aerosol particles. The main focus of this article is performance comparison of the BioScout and the widely used UVAPS. In this study, we determined instrument-based FPF values for common biochemical particles as well as for bacterial and fungal spores, and estimated the performance of the instruments in the ambient measurements of airborne microorganisms.
2. MATERIALS AND METHODS
2.1. Description of the Instruments
2.1.1. BioScout
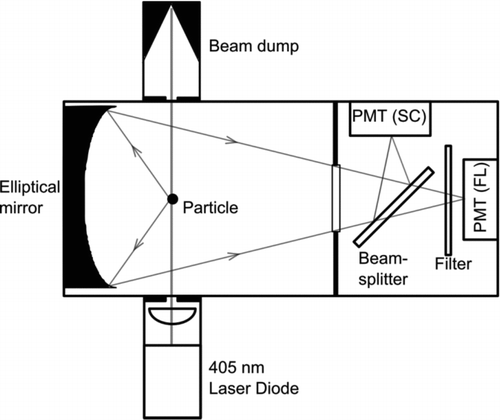
Optical system of the BioScout has been developed at TUT (Tampere University of Technology) and the instrument has been commercialized by Environics Ltd. The operation principle is shown in . The BioScout has a 405 nm continuous wave laser diode with 200 mW optical power to excite autofluorescence from individual microbial particles. Both the autofluorescence and the scattering light are collected by an elliptical mirror and focused on two PMTs (Photomultiplier Tube). The autofluorescence is separated from the scattered light using a beam-splitter and a long-pass filter with a cut-point at 442 nm, and fluorescence is recorded by a PMT and sorted in 16 intensity channels. The scattering light intensity is used to analyze the optical particle size. The data can be recorded at 1 s time resolution. The particle size calibration of the BioScout was made against DOS (di-octyl sebacate) and NaCl particles using the UVAPS (TSI Model 3314) and the SCAR (Single-Charged Aerosol Reference, Yli-Ojanperä et al. Citation2010) as reference instruments. The operative particle size range was optimized between 0.3 and 5 μm.
2.1.2. UVAPS
Hairston et al. (Citation1997) introduced the first prototype of the UVAPS. The current version of UVAPS (TSI Model 3014) measures aerodynamic diameter of particles between 0.5 and 15 μm with 52 channels. It uses the similar double-crest optical system as found in the APS spectrometer (TSI Model 3321). The UVAPS also measures the autofluorescence emission of individual particles using a pulsed 355 nm UV-laser with 30 mJ maximum pulse energy as an excitation source. The autofluorescence is recorded between 430 and 580 nm by a PMT and sorted in 64 channels. In this study, the UV pulse energy and fluorescence PMT gain were set into their default values. The sample time can be adjusted between 1 s and 18 h. According to the instrument manual, the upper concentration limit is 1000 #/cm3, but the upper limit for fluorescent particle concentration has been reported to be 60 #/cm3 (Agranovski et al. Citation2003). The details of the BioScout and the UVAPS are compiled in .
TABLE 1 Detailed comparison of the BioScout and the UVAPS
TABLE 2 Summary of the experiments
2.2. Experiments
2.2.1. Bacterial Spores
Three measurement campaigns were conducted in three different facilities because of the availability of the test aerosol. The first facility was a bioaerosol test chamber at the Finnish Defence Forces Technical Research Centre (FDFTRC). The test chamber volume was about 30 m3. The BioScout and the UVAPS were located in the middle of the chamber and aerosols were generated from the chamber side and then blown by a fan towards the instruments. Each test aerosol was produced by short, 1-min generation periods, which were repeated twice. There was a powerful air exchange system so that the chamber volume could be cleaned in 5 min between the aerosol generation periods. Background levels were measured always before and after the generation periods.
Two bacterial species, Bacillus atrophaeus (also known as B. subtilis var. niger and as B. globigii [BG]) and Bacillus thuringiensis (BT), were used as test aerosols at FDFTRC. Bacterial spores were provided by FDFTRC. These species are common stimulants of biological warfare agents. The BT and BG were grown for 2 weeks on agar plates, scraped, and mixed with distilled water. The growth medium was prepared from tryptone, yeast extract, NaCl, D(+)glucose, manganese sulfate, agar, and water. Both bacterial samples were unwashed.
Three different concentrations of bacteria were mixed to produce various particle sizes in aerosolization process. NaCl-solution was prepared to study fluorescence intensity background from inert particles. The bacterial spores and NaCl particles were aerosolized with sonic nozzle aerosol generator (SNAG, Sonics and Materials Inc., Newtown, CT, USA) at a flow rate of 5 lpm. The sample solution was stirred continuously using a magnetic stirrer and pumped into the SNAG for aerosolizing. The median aerodynamic particle diameters of the generated aerosols are shown in .
2.2.2. Fungal Spores
The second facility was at University of Eastern Finland (UEF) in Kuopio, where spore aerosols from three fungal species were studied: Aspergillus versicolor, Penicillium brevicompactum, and Penicillium expansum. These species are common in indoor air. Fungal species were obtained from the UEF culture collection and grown on malt extract agar plates in room temperature for 1 week. Fungal aerosol was generated directly from the agar surface by the FSSST (Fungal Spore Source Strength Tester; Sivasubramani et al. Citation2004) with a flow rate of 25 lpm and conveyed to the instruments via sampling lines. Each of the species was generated three times. The median aerodynamic particle diameters of the fungal spores are shown in .
2.2.3. Biochemicals
Two biochemicals, ovalbumin (provided by FDFTRC) and l-tryptophan (Merck Chemicals Ltd.) were studied at FDFTRC. Ovalbumin is a general protein that is found, for example, in egg white. Tryptophan is one of the most common amino acids. The ovalbumin and tryptophan samples were prepared by mixing the sample powder with distilled water. Otherwise the test procedure was similar to that of the bacterial spores, described above.
Other two biochemicals, NADH disodium salt (Calbiochem®) and riboflavin (Calbiochem®), were studied at the third facility, Tampere University of Technology (TUT). Coenzyme NADH is related to the metabolism of microbes and usually NADH concentration is high when microbes are active. Riboflavin has a great role in energy metabolism in cells and some fungal species are known to produce riboflavin. Riboflavin and NADH were mixed with NaCl-water solution and aerosolized by a self-made IJAG (ink jet aerosol generator; Bottiger et al. Citation1998) into a flow rate of 5 lpm. Generated aerosol was dried with silica gel tube and then directed to the instruments. Nonfluorescent NaCl was used as a carrier particle, because the desired particle size was above 1 μm due to the UVAPS detection limit. Biochemical mass concentration in a single particle was varied between 6 × 10−17 and 4 × 10−12 g/particle to cover the whole fluorescence signal range of the instruments. The IJAG is a very stable aerosol generator, which produces constant concentration and particle size. Each aerosol type was generated approximately 2 min and background was measured between the generations.
2.3. Data Analysis
The BioScout data were analyzed using MATLAB software. All particles in fluorescence channels 2 to 16 were classified as fluorescent and particles in channel 1 as nonfluorescent. Nonfluorescent NaCl-particles were used to determine the detection limit for fluorescence. The detection limit was adjusted so that less than 1% of NaCl-particles hit to the fluorescence channel 2 or above. After combining the fluorescence data with the optical particle size data, fluorescent particle and total particle size distributions and concentrations were calculated. The UVAPS data were analyzed similarly and all particles in fluorescence channels 2 to 64 were classified as fluorescent. The FPF value was calculated for the all test particles and the results were compared between the BioScout and the UVAPS. Only concentration values under the upper limit of the UVAPS (60 #/cm3) were included in the FPF consideration.
3. RESULTS
3.1. Particle Size Response
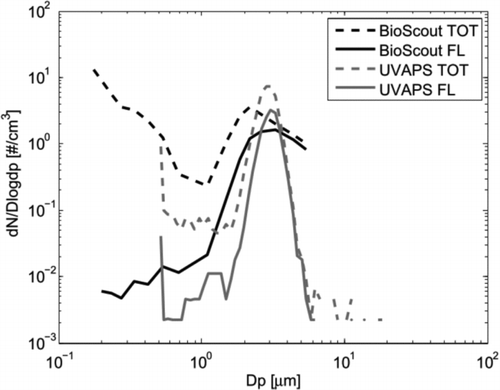
To compare particle size responses of the BioScout and the UVAPS, typical fluorescent and total particle size distributions for fungal spores (Penicillium brevicompactum) are shown in . The size distribution was bimodal: the first mode between 1 and 4 μm represents fungal spores and particles under 1 μm are fungal fragments. The size distributions of the BioScout and the UVAPS have similar main features. The UVAPS has better size resolution, shown here as a narrower spore mode. The BioScout seems to have better particle counting efficiency for smaller particles. One should, however, note that the UVAPS measures particle aerodynamic diameter and the BioScout measures an instrument-specific optical diameter that depends on the refractive index of the particles. The spore mode emitted strong fluorescence, but only a fraction of spores were fluorescent with the BioScout and the UVAPS. The fragment mode seemed to be also weakly fluorescent, but the fragments are not considered in this study due to the limited size range of the UVAPS.
3.2. Fluorescent Particle Fractions
The measured FPFs for both instruments are shown separately for the bacterial and fungal spores ( and ) and biochemicals ( and ). The FPF values of the two instruments for all the particles are collected to . Numerical data of the results are available in the online Supplemental information (Table S1).
FIG. 3 The FPFs of B. atrophaeus (BG) and B. thuringiensis (BT) spores measured by BioScout and UVAPS. The GMD of particles is shown below in parentheses. Error bars represent the standard deviations of three repeats.
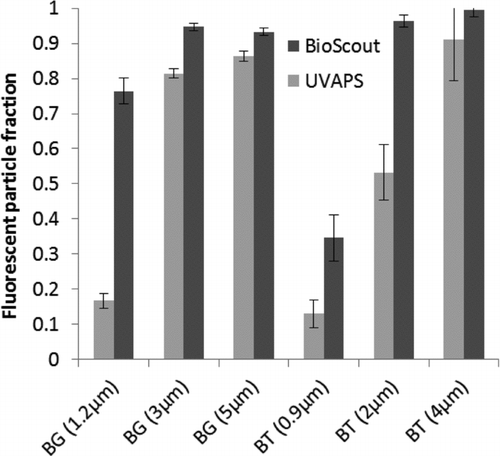
FIG. 4 The FPFs of A. versicolor (A.v.), P. brevicompactum (P.b.), and P. expansum (P.e.) spores measured by BioScout and UVAPS. The GMD of particles is shown below in parentheses. Error bars represent standard deviations of three repeats.
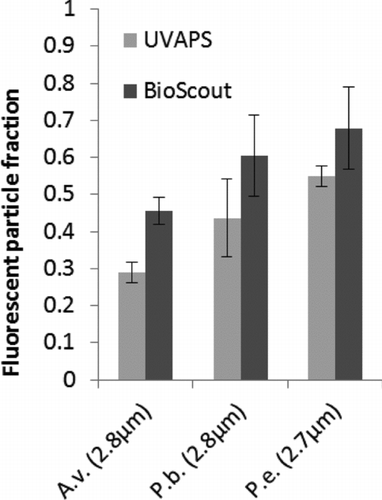
FIG. 5 FPFs of the typtophan (TRP) and ovalbumin (OA) particles measured by BioScout and UVAPS together with background levels and inert NaCl particles. Error bars represent the standard deviations of three repeats. Single particle mass content of the biochemicals, in pg, shown in parentheses.
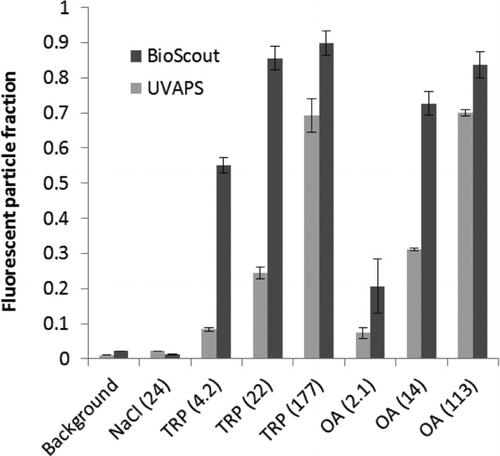
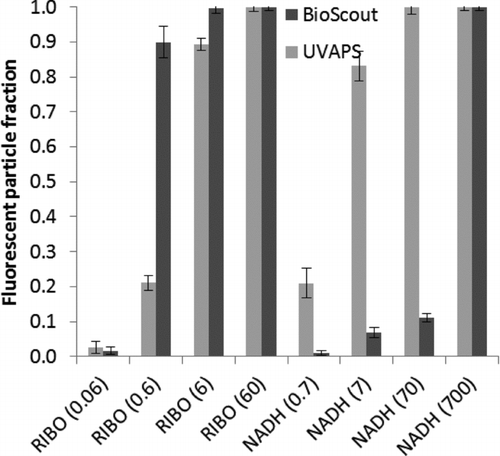
FIG. 6 The FPFs of riboflavin (RIBO)- and NADH-doped NaCl particles measured by BioScout and UVAPS. The single particle biochemical content (fg) in parentheses. Error bars represent the standard deviations of 2 min time series.
3.2.1. Bacterial and Fungal Spores
shows the FPF of bacterial spores. The FPF was high with larger particles that consisted of spore clusters, but dropped down when particle size decreased to the size of single bacterial spores (∼1 μm). The BioScout FPF values were 0.77 for 1.2 μm BG spores and 0.34 for 0.9 μm BT spores, whereas the UVAPS FPF values were 0.17 and 0.13, respectively.
The FPF data of fungal spores are presented in . The FPF varied between the species and ranged from 0.45 to 0.68 with BioScout and from 0.29 to 0.54 with UVAPS. The BioScout FPF values were always higher than those of the UVAPS.
3.2.2. Biochemicals
shows the FPF data of tryptophan and ovalbumin that were measured in the FDFTRC measurement campaign. Background levels and inert NaCl particles were also included in the figure, and they had nearly identical FPF values close to zero. The BioScout detected higher FPF values of tryptophan and ovalbumin particles compared to the UVAPS. The FPF increased as a function of particle size and chemical concentration with both instruments.
The FPF data of riboflavin- and NADH-doped NaCl particles are shown in the . The BioScout detected higher FPF for riboflavin than the UVAPS, whereas the opposite was true for NADH. The FPF increased as a function of particle size and chemical concentration with both instruments.
4. DISCUSSION
The BioScout had higher fluorescent particle detection efficiency for both bacterial and fungal spores and common biochemicals, except NADH, compared to the UVAPS. The fluorescence excitation–emission curves of the tested biochemicals can be found in the papers Putkiranta et al. (Citation2010) and Manninen et al. (Citation2009). Of the biochemicals, riboflavin has similar fluorescence cross sections at the excitation wavelengths of the two instruments, namely 355 nm and 405 nm (Putkiranta et al. Citation2010). The higher FPF values, as shown in , of the BioScout are probably due to the more powerful laser. Dissimilar fluorescence detectors and emission bands of the instruments may also contribute to the observed difference in the fluorescence sensitivity. Fluorescence excitation and emission spectra of tryptophan and ovalbumin particles are nearly identical; absorption peaks are located at 280 nm and fluorescence emission peaks at 330 nm (Manninen et al. Citation2009; Putkiranta et al. Citation2010). They are quite far away from the UVAPS and BioScout spectral range, but still some fluorescence was detected in this study. However, one should note that the tryptophan and ovalbumin concentrations were much higher in this study than in the above-quoted studies. Surrounding environment of the molecules may also cause emission shift to longer wavelengths (Lakowicz Citation2009). The UVAPS showed higher FPF values for NADH than the BioScout (). This is readily explained by the high excitation peak of the NADH at 355 nm. Indeed, the spectral features of the UVAPS are optimized for NADH (Agranovski et al. Citation2003).
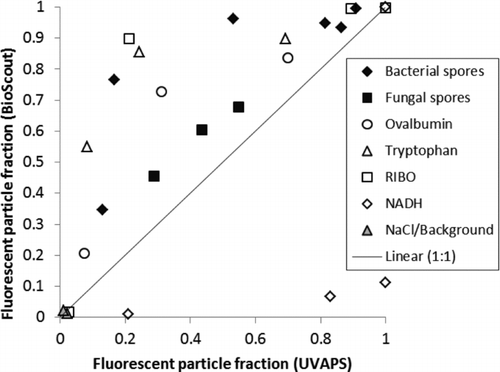
As shown in , with the exception of NADH, all the test aerosols were above the 1:1 line. This means that the 405 nm instrument has a higher sensitivity than the 355 nm instrument for most of the test aerosols. The sensitivity of the 405 nm instrument was much lower for NADH indicating that NADH was not the dominant fluorophore in the tested bacterial and fungal spores. Similar interpretation has been reported earlier (Agranovski et al. Citation2003; Kanaani et al. Citation2007; Raimondi et al. Citation2009; Saari et al. Citation2013). The results indicate that the 405 nm diode laser is a promising excitation source for fluorescence-based real-time detection of airborne microorganisms. In some cases, instruments based on 405 nm excitation may be even more effective bioaerosol sensors than the UVAPS. However, as NADH is known to be present in living cells, the 405 nm instruments may not be able to identify living cells as well as the UVAPS.
The 405 nm instrument was more efficient in the detection of all test bacteria. This difference was most clearly seen for smaller particles. With the BioScout, even single bacterial spores can be detected, which can be important because some references reported significant concentrations of sub-micrometer-sized bioaerosols in the atmosphere (Huffman et al. Citation2012; DeLeon-Rodriguez et al. Citation2013). The present UVAPS FPF results for bacterial and fungal spores are well in line with the findings of Agranovski et al. (Citation2003) and Kanaani et al. (Citation2007, Citation2008). Their result showed that the FPF varies between 2 and 52% for bacteria and 48 and 99% for fungal spores, depending mainly on the species and physiological stage of spores.
Based on this study, only approximately 50% of fungal spores are fluorescent and the FPF value for single bacterial spores is even smaller. If the instrument-specific FPF values of the common bacterial and fungal spores are known, total concentrations of the spores can be estimated in ambient environment dividing the fluorescent particle concentration by the FPF value. This calculation has still some uncertainties, because all factors that can affect the FPF value of spores are not known. For example, the growth conditions, life cycles, oxidation, humidity, and other environmental conditions may affect the fluorescence properties of bacterial and fungal spores. Therefore, it is not clear how well laboratory prepared microbial aerosols represent the fluorescence characteristics of environmental microbial aerosols. Some fluorescent nonbiological particles in real environment, such as particles from incomplete combustion, may be also problematic in this calculation. More studies are needed to answer those questions.
5. SUMMARY
Performance of the 405 nm diode laser-based on-line bioaerosol detector, BioScout, was successfully tested and compared with the widely used TSI UVAPS. The FPF value of the instruments was calibrated for the common bacterial and fungal spores and biochemical particles. The BioScout had higher FPF values for all the other test particles except NADH. The BioScout FPF value varied between 0.34 to 0.77 and 0.45 to 0.68 for single bacterial and fungal spores, respectively, whereas the UVAPS FPF values for bacterial spores were between 0.13 and 0.17 and for fungal spores between 0.29 and 0.54. The FPF values of bioaerosols are important in ambient measurements, therefore they should be well-known and calibrated for each instruments. The results of this study form a foundation for a database that can be utilized to estimate the total concentrations of bacterial and fungal spores in fluorescence-based ambient measurements. However, more studies are still needed to find out all factors that can affect the FPF values of microorganisms.
FUNDING
The work is financially supported by Doctoral School of TUT and TEKES FiDiPro-project named BITEFA. The financial supports of Jenny and Antti Wihuri Foundation and Eemil Aaltonen Foundation are also gratefully acknowledged.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher's website.
Supplementary_Materials.zip
Download Zip (29.4 KB)ACKNOWLEDGEMENTS
The authors would like to thank Dr. Tarmo Humppi and Mrs. Tuula Lehto from FDFTRC and Professor Pertti Pasanen's group from UEF for their help with microbe preparation and aerosolization and Mr. Matti Putkiranta for his help in instrument development and in measurements at FDFTRC.
REFERENCES
- Agranovski , V. , Ristovski , Z. D. , Ayoko , G. A. and Morawska , L. 2004 . Performance Evaluation of the UVAPS in Measuring Biological Aerosols: Fluorescence Spectra from NAD (P) H Coenzymes and Riboflavin . Aerosol Sci. Technol., , 38 ( 4 ) : 354 – 364 .
- Agranovski , V. , Ristovski , Z. , Hargreaves , M. , Blackall , P. and Morawska , L. 2003 . Real-Time Measurement of Bacterial Aerosols with the UVAPS: Performance Evaluation . J. Aerosol Sci., , 34 : 301 – 317 .
- Andreae , M. and Rosenfeld , D. 2008 . Aerosol-Cloud-Precipitation Interactions. Part 1. The Nature and Sources of Cloud-Active Aerosols . Earth-Sci. Rev., , 89 : 13 – 41 .
- Bauer , H. , Giebl , H. , Hitzenberger , R. , Kasper-Giebl , A. , Reischl , G. Zibuschka , F. 2003 . Airborne Bacteria as Cloud Condensation Nuclei . J. Geophys. Res., , 108 : 4658
- Bauer , H. , Kasper-Giebl , A. , Löflund , M. , Giebl , H. , Hitzenberger , R. Zibuschka , F. 2002 . The Contribution of Bacteria and Fungal Spores to the Organic Carbon Content of Cloud Water, Precipitation and Aerosols . Atm. Res., , 64 : 109 – 119 .
- Bottiger , J. , Deluca , P. , Stuebing , E. and VanReenen , D. 1998 . An Ink Jet Aerosol Generator . J. Aerosol Sci., , 29 : S965 – S966 .
- Burge , H. A. and Rogers , C. A. 2000 . Outdoor Allergens . Env. Health Perspect., , 108 ( 4 ) : 653 – 659 .
- DeLeon-Rodriguez , N. , Lathem , T. L. , Rodriguez-R , L. M. , Barazesh , J. M. , Anderson , B. E. Beyersdorf , A. J. 2013 . Microbiome of the Upper Troposphere: Species Composition and Prevalence, Effects of Tropical Storms, and Atmospheric Implications . Proc. Natl. Acad. Scie., , 110 ( 7 ) : 2575 – 2580 .
- Després , V. , Huffman , J. , Burrows , S. , Hoose , C. , Safatov , A. Buryak , G. 2012 . Primary Biological Aerosol Particles in the Atmosphere: A Review . Tellus B , 64 : 29 – 40 .
- Gabey , A. M. , Stanley , W. R. , Gallagher , M. W. and Kaye , P. H. 2011 . The Fluorescence Properties of Aerosol Larger Than 0.8 μm in Urban and Tropical Rainforest Locations . Atm. Chem. Phys., , 11 ( 11 ) : 5491 – 5504 .
- Hairston , P. P. , Ho , J. and Quant , F. R. 1997 . Design of an Instrument for Real-Time Detection of Bioaerosols using Simultaneous Measurement of Particle Aerodynamic Size and Intrinsic Fluorescence . J. Aerosol Sci., , 28 ( 3 ) : 471 – 482 .
- Hill , S. , Pan , Y-L. , Williamson , C. , Santarpia , J. and Hill , H. 2013 . Fluorescence of Bioaerosols: Mathematical Model Including Primary Fluorescing and Absorbing Molecules in Bacteria . Opt. Exp., , 21 : 22285 – 22313 .
- Ho , J. Y. W. 2004 . U.S. Patent No. 6,831,279 , Washington, DC : U.S. Patent and Trademark Office .
- Ho , J. Y. W. , Hairston , P. and Spence , M. 2001 . Biological Detector Performance with a 402 nm Laser Diode (No. DRES-TR-2000-190) , Alberta : Defence Research Establishment Suffield Ralston . Availa- ble online at: http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA394342
- Huffman , J. A. , Sinha , B. , Garland , R. M. , Snee-Pollmann , A. , Gunthe , S. S. Artaxo , P. 2012 . Size Distributions and Temporal Variations of Biological Aerosol Particles in the Amazon Rainforest Characterized by Microscopy and Real-Time UV-APS Fluorescence Techniques During AMAZE-08 . Atm. Chem. Phys., , 12 ( 24 ) : 11997 – 12019 .
- Jaenicke , R. 2005 . Abundance of Cellular Material and Proteins in the Atmosphere . Science , 308 : 73
- Jaenicke , R. , Matthias-Maser , S. and Gruber , S. 2007 . Omnipresence of Biological Material in the Atmosphere . Env. Chem., , 4 : 217 – 220 .
- Jamriska , M. , DuBois , T. C. and Skvortsov , A. 2012 . Statistical Characterisation of Bio-Aerosol Background in an Urban Environment . Atm. Env., , 54 : 439 – 448 .
- Jiang , J. P. , Babico , J. and Morrell , M. 2012 . U.S. Patent No. 8,218,144 , Washington, DC : U.S. Patent and Trademark Office .
- Kanaani , H. , Hargreaves , M. , Ristovski , Z. and Morawska , L. 2007 . Performance Assessment of UVAPS: Influence of Fungal Spore Age and Air Exposure . J. Aerosol Sci., , 38 ( 1 ) : 83 – 96 .
- Kanaani , H. , Hargreaves , M. , Smith , J. , Ristovski , Z. , Agranovski , V. and Morawska , L. 2008 . Performance of UVAPS with Respect to Detection of Airborne Fungi . J. Aerosol Sci., , 39 ( 2 ) : 175 – 189 .
- Lakowicz , J. R. , ed. 2009 . Principles of Fluorescence Spectroscopy , 205 – 231 . Springer . , The Netherlands ISBN 978-0-387-46312-4
- Manninen , A. , Putkiranta , M. , Saarela , J. , Rostedt , A. , Sorvajärvi , T. Toivonen , J. 2009 . Fluorescence Cross Sections of Bioaerosols and Suspended Biological Agents . App. Opt., , 48 : 4320 – 4328 .
- Mendell , M. J. , Mirer , A. G. , Cheung , K. and Douwes , J. 2011 . Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence . Env. Health Perspect., , 119 ( 6 ) : 748
- Pan , Y-L. , Eversole , J. D. , Kaye , P. H. , Foot , V. , Pinnick , R. G. Hill , S. C. 2007 . “ Bio-Aerosol Fluorescence - Detecting and Characterising Bio-Aerosols via UV Light-Induced Fluorescence Spectroscopy ” . In Optics of Biological Particles , 1st ed. , Edited by: Hoekstra , A. , Maltsev , V. and Videen , G. 63 – 163 . (NATO Science Series). Dordrecht: Human Press/Springer, The Netherlands . doi: 10.1007/978-1-4020-5502-7
- Peccia , J. , Milton , D. K. , Reponen , T. and Hill , A. J. 2008 . A Role for Environmental Engineering and Science in Preventing Bioaerosol-Related Disease . Env. Sci. Technol., , 42 ( 13 ) : 4631 – 4637 .
- Putkiranta , M. , Manninen , A. , Rostedt , A. , Saarela , J. , Sorvajärvi , T. Marjamäki , M. 2010 . Fluorescence Properties of Biochemicals in Dry NaCl Composite Aerosol Particles and in Solutions . App. Phys. B, , 99 ( 4 ) : 841 – 851 .
- Pöhlker , C. , Huffman , J. A. and Pöschl , U. 2012 . Autofluorescence of Atmospheric Bioaerosols–Fluorescent Biomolecules and Potential Interferences . Atmos. Measur. Techn., , 5 ( 1 ) : 37 – 71 .
- Pöschl , U. , Martin , S. , Sinha , B. , Chen , Q. , Gunthe , S. Huffman , J. 2010 . Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon . Science, , 329 : 1513
- Raimondi , V. , Agati , G. , Cecchi , G. , Gomoiu , I. , Lognoli , D. and Palombi , L. 2009 . In vivo Real-Time Recording of UV-Induced Changes in the Autofluorescence of a Melanin-Containing Fungus using a Micro-Spectrofluorimeter and a Low-Cost Webcam . Opt. Exp., , 17 : 22735 – 22746 .
- Reponen , T. , Willeke , K. , Grinshpun , S. and Nevalainen , A. 2011 . “ Biological Particle Sampling ” . In Aerosol Measurement, Principles, Techniques, and Applications , 3rd ed. , Edited by: Kulkarni , P. , Baron , P. and Willeke , K. 549 – 570 . John Wiley & Johns, Inc., Hoboken, NJ .
- Saari , S. E. , Putkiranta , M. J. and Keskinen , J. 2013 . Fluorescence Spectroscopy of Atmospherically Relevant Bacterial and Fungal Spores and Potential Interferences . Atm. Env., , 71 : 202 – 209 .
- Santarpia , J. L. , Ratnesar-Shumate , S. , Gilberry , J. U. and Quizon , J. J. 2013 . Relationship Between Biologically Fluorescent Aerosol and Local Meteorological Conditions . Aerosol Sci. Technol., , 47 ( 6 ) : 655 – 661 .
- Silcott , D. B. , Tilley , G. A. , Whitman , B. R. and Pratt , S. J. 2005 . U.S. Patent No. 6,885,440 , Washington, DC : U.S. Patent and Trademark Office .
- Sivasubramani , S. K. , Niemeier , R. T. , Reponen , T. and Grinshpun , S. A. 2004 . Assessment of the Aerosolization Potential for Fungal Spores in Moldy Homes . Indoor Air , 14 ( 6 ) : 405 – 412 .
- Sun , J. and Ariya , P. 2006 . Atmospheric Organic and Bio-Aerosols as Cloud Condensation Nuclei (CCN): A Review . Atm. Env. , 40 : 795 – 820 .
- Vali , G. , Christensen , M. , Fresh , R. , Galyan , E. , Maki , L. and Schnell , R. 1976 . Biogenic Ice Nuclei. Part II: Bacterial Sources . J. Atm. Sci., , 33 : 1565 – 1570 .
- Yli-Ojanperä , J. , Mäkelä , J. M. , Marjamäki , M. , Rostedt , A. and Keskinen , J. 2010 . Towards Traceable Particle Number Concentration Standard: Single Charged Aerosol Reference (SCAR) . J. Aerosol Sci., , 41 ( 8 ) : 719 – 728 .