Abstract
Adhesion between dust particles and indoor surfaces can lead to negative effects on human health by triggering allergic and asthmatic reactions. In this study, adhesion forces of indoor office dust and activated carbon (AC, as model soot) particles to four common indoor materials (Al, Cu, PVC, and glass) were measured by colloidal probe atomic force microscopy. Chemical analysis of office dust shows it is largely made up of oxygenated hydrophilic organic carbon material. Both metal surfaces experienced weaker dust and AC adhesion than PVC or glass by up to 2–12 times lower primarily due to the presence of attractive electrostatic forces in the latter two (non-conducting) surfaces. Dust and AC adhesion were also highly sensitive to surface roughness, with an inverse relationship between adhesion force and roughness due to the reduction in contact area between the particle and a rougher material surface. Capillary forces play only a minor or negligible role in dust and AC surface adhesion. Adhesion models utilizing a purely van der Waals approach such as the simple Hamaker model and modified Rumpf's model are insufficient to determine the actual particle-surface contact radii and requires the accounting of non-van der Waals forces to adhesion.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
The indoor air quality of air-conditioned homes and offices is critical to human respiratory health since residents in urban areas spend the bulk of their daily hours inside (Hess-Kosa Citation2011). The World Health Organization has suggested that up to 30% of new and remodeled buildings globally subject their occupants to health hazards related to poor indoor air quality (1991). The indoor air pollutants most commonly observed include chemicals such as volatile organic compounds including formaldehyde from adhesives, upholstery, carpeting, computers, copy machines, and cleaning agents. Indoor respirable solid particles (known colloquially as dusts) varying widely in size from <2.5 μm (PM2.5) to >0.1 mm originate from copy machines, soot from motor vehicle exhausts through air intake vents/windows/doors, plants (pollen), fungi (mold spores), dust mites, and human activities such as smoking, vacuuming, and cooking.
Indoor airborne particulate matter can deposit onto indoor surfaces over time if air circulation and ventilation is poor. The deposition rate is dependent on the particle size, surface type (e.g., plastic vs. metal, wet vs. dry), orientation (horizontal or vertical surface), and air flow conditions. Furthermore, when human or animal (e.g., pets and pests) activity is present, the deposited particles can get detached and resuspended from the surface back to the air. Deposited particles can also be moved from one surface to another by contact transfer, e.g., a person rests his arm on a table and some deposited dust particles are transferred from the table onto his clothing (McDonagh et al. Citation2012).
In the study of indoor particle transport, it is important to recognize that particle size plays an important role in determining whether the particles stay airborne or get deposited on various surfaces (Hess-Kosa Citation2011). In general, the particle size spectrum can be broadly classified into two categories according to the particle diameter (Liu et al. Citation2004): fine (<2.5 μm) and coarse (>2.5 μm). Fine particles were typically found to be a mixture of solid carbon from incomplete fuel combustion and secondary particulates generated by chemical reactions in the atmosphere (acid condensates, sulfates, and nitrates) (Ormstad Citation2000). Coarse particles are generally of organic material origin and larger soot aggregates (Ormstad et al. Citation1997). Coarse particles have been found to be significantly linked to decline in lung function, respiratory and cardiovascular diseases and deaths, while fine particles are strongly correlated with deaths from cardiopulmonary disease and lung cancer (Berico et al. Citation1997).
In addition, human activities' indoors and entry from or exit to the doors can introduce relatively large airborne particulates to the indoor environment which is air-filtered. Transport and fate of such indoor aerosols have been investigated extensively (Thatcher and Layton Citation1995; Nazaroff Citation2004), including that of deposited particle transport via contact transfer (McDonagh et al. Citation2012). To date, the quantitative assessment of the adhesion forces of dust particles by atomic force microscopy (AFM) to common indoor surfaces has not been reported in scientific literature. A previous study by (Hu et al. Citation2008) used the electrostatic detachment method to measure the adhesion force distribution of micron-sized particles to common indoor flooring materials such as vinyl and rubber. This present study aims to determine the adhesion of collected indoor office dust and powdered activated carbon, chosen as a model for soot generated from cooking/smoking, to common indoor material surfaces such as glass, copper, aluminum and polyvinyl chloride (PVC) using colloid probe AFM. The dust and activated carbon powder particles were affixed to tipless AFM cantilevers prior to the adhesion force measurements. We also measured the static contact angles of the four different indoor surfaces and their surface roughness (both ‘‘smooth’’ and ‘‘roughened’’) to determine the effects of surface chemistry and morphology respectively on dust adhesion.
The objective of these experiments is to quantify the key forces at play in the fate and transport of indoor dust particles in ambient air-conditioned environments via their adhesion to indoor surfaces. Understanding dust adhesion to these common surfaces will aid in the development of better indoor dust cleaning and removal technologies as well as methods to minimize the resuspension and spreading of dust particles.
2. MATERIALS AND METHODS
2.1. Samples and Preparation
Polyvinyl chloride (PVC) sheet, microscope glass slide (glass), aluminum foil (Al), and copper (Cu) tape were chosen as model substrate materials to represent the three major classes of commonly used indoor materials (plastic, glass, and metal). Roughened samples of the four types of materials were also prepared by rubbing their surfaces with a 1000Cw silicon carbide electro-coated waterproof abrasive paper (Eye brand, Solingen, Germany).
The original-‘‘smooth’’ and roughened PVC, glass, Al, and Cu samples were first rinsed with deionized (DI) water followed by ethanol and again with DI water. The substrates were then dried using Kimwipes disposable wipers (Kimberly Clark) to remove all traces of dirt and impurities on the sample surfaces prior to use for the contact angle, surface roughness, and adhesion force measurements.
2.2. Attachment of Dust and Activated Carbon Particles to Tipless AFM Cantilevers
Activated carbon (AC) powder of 100-mesh particle size (Sigma–Aldrich) was selected to model soot, a major component of indoor dust (Morawska and Salthammer Citation2003; Balasubramanian and Lee Citation2007). Actual dust particles from the Singapore University of Technology and Design (SUTD) administration office were collected via vacuuming and deposited on clean microscope glass slides before attachment to AFM cantilevers. Single particulates of activated carbon (AC) and dust were attached to tipless rectangular cantilevers with nominal spring constants 0.6–3.7 N/m (FORTA, Applied Nanostructures, Inc., Mountain View, CA, USA) using a small amount of epoxy glue and a micromanipulator (NMN-21, Narishige, Japan) viewed through a binocular microscope (Ducker et al. Citation1991).
2.3. Contact Angle Measurements
Static water contact angles were measured on both the original and roughened PVC, glass, Al and Cu surfaces using a USB digital microscope (Dino-Lite Premier). A 10 μL drop and 20 μL drop of deionized water were dispensed onto each substrate sample surface using a 1 mL syringe. Two sets of measurements were conducted for each drop volume.
2.4. Surface Roughness Measurements
A Nanosurf easyScan AFM (Nanosurf AG, Liestal, Switzerland) with rectangular silicon cantilevers of nominal spring constant 0.29 N/m (SICONA, Applied Nanostructures, Inc., Mountain View, CA, USA) was used to measure the average surface area (S a) and root-mean-square (RMS) roughness of the original and roughened material samples. For each sample, five random 50 μm × 50 μm scans of the sample surface were done and the mean value of the roughness measurements was calculated.
2.5. Adhesion Force Measurements
Adhesion pull-off force measurements were carried out by means of an AFM (Nanosurf easyScan, Liestal, Switzerland) in a room where the temperature and relative humidity (RH) were measured to be 25°C and 50% respectively using a thermohygrometer (HI9564, Hanna Instruments). The colloidal probe AFM technique works by bringing the dust- and AC particle-tipped AFM cantilevers into contact with the sample indoor materials’ surfaces and then retracting. The deflection of the cantilevers during the approach and retraction are monitored and recorded electronically. Vertical position of the tip and deflection of the cantilever are then converted to force-versus-distance curves which can be used to analyze the adhesion behavior of the particle with the surface (Butt et al. Citation2005). For each set of the tip-sample surface combination, 20 deflection–displacement curves were obtained randomly from three different 50 μm × 50 μm areas of the substrate's surface. The actual spring constants of the dust- and activated carbon (AC)-tipped cantilevers were determined directly by the Burnham method (Burnham et al. Citation2003) using a Dimension 300 AFM (Veeco, Digital Instruments). The adhesion forces were calculated by Hooke's Law using the cantilevers’ deflection values and their measured spring constant values. Two dusts and two AC-tipped AFM cantilevers were used for the adhesion force measurements. The spring constants for the four cantilevers (dust 1, dust 2, AC 1, and AC 2) are measured to be 0.157, 0.149, 0.137, and 0.154 N/m, respectively. The applied load during the measurement was kept at minimum to a negligible value, similar to actual indoor situations when a dust particle lands or is attracted to a surface. Statistical analyses using Minitab 16 software are based on the experimental adhesion force data obtained from the four particles (two dusts and two AC, chosen as model soot) in contact with the four indoor materials. The adhesion force datasets were first tested for Gaussian distribution by employing the Anderson–Darling test (Anderson 1954) with a significance level α = 0.05. A p-value greater than 0.05 would indicate normality. The adhesion forces of the various tip-surface combinations were then analyzed by two-way analysis of variance (ANOVA) at 95% confidence level. For two-way ANOVA, any p-value smaller than 0.05 indicates a statistically significant difference in adhesion between two tip-surface combinations.
2.6. Scanning Electron Microscopy (SEM)
The dust and activated carbon AFM probes were characterized by scanning electron microscopy (SEM, JEOL JSM-7600F) at an accelerating potential of 5.0 kV after all the adhesion force measurements were completed. The cantilevers were mounted on metal stubs using copper tapes and sputtered with gold before fixing onto a sample holder for SEM imaging.
2.7. High Resolution-Aerosol Mass Spectrometer (HR-AMS) of SUTD Office Dust
Samples of SUTD office dust were collected and sent to the University of California-Riverside Center for Environmental Research and Technology Aerosol Characterization Facility. We added 40 mg of the consolidated dust sample to 250 mL of ultrapure Millipore water (<18 mΩ and <4 ppb dissolved organic carbon). The sample was then sonicated and stirred for 40 min. The solution was atomized and the wet droplets dried using two consecutive silica drying units. The composition of the atomized sample was characterized with an Aerodyne High Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS or HR-AMS; Jimenez et al. Citation2003; DeCarlo et al. Citation2006). Data were recorded every 2.25 min for over an hour. Unit mass resolution (UMR) data discern differences in mass-to-charge (m/z) ion fragmentation. The elemental composition and oxidation state was examined with high-resolution (HR) data analysis (Aiken et al. Citation2007, Citation2008).
The HR-AMS provides information on the following elemental oxygen, hydrogen, sulfur, and nitrogen to carbon ratios (O/C, H/C, S/C, and N/C). O/C has been considered an important factor in characterizing the oxidation state of secondary organic aerosols (SOA) and particle hygroscopicity (Jimenez et al. Citation2009). The organic mass to organic carbon ratio (OM/OC) is also reported. The fraction of oxygen to carbon (O/C ratio) is an indication of the oxygenated and potential hydrophilic nature of the particles. Aerosol that contain long chained hydrophobic materials have low O/C ratios typically less than 0.5. For hydrophobic material, the majority of mass spectra in high-resolution datasets are hydrocarbon fragments and oxygenated compounds are not observed in datasets. Ambient oxidized aerosols have O/C values ≥0.3 can be hygroscopic. Increased O/C ratios obtained from mass fragmentation correlate to increased hygroscopicity of the measured aerosol (Jimenez et al. Citation2009).
2.8. Factorial Experimental Design on Dust-Surface Interactions
Factorial experiments are used to study the effect of factors such as particle type, surface type, and surface roughness on the adhesion force of dusts to common indoor surfaces. A factorial analysis was done using the method demonstrated by (Hu et al. Citation2008). Four different types of surfaces, i.e., Al, Cu, PVC, and glass were studied in the factorial analysis. The particles that were studied include two single dust particles (Dust 1 and Dust 2) and two single activated carbon particles (AC 1 and AC 2). Each particle was attached to a tipless cantilever and tested for the adhesion force between the particle and the surface using the AFM. The effects of surface roughness, i.e., ‘‘smooth’’ and ‘‘roughened’’ surfaces of the four material substrates were also considered.
For each set of particle–surface combination, 20 adhesion force data were collected from three different 50 μm × 50 μm areas of the substrate's surface. Thus, a total of 640 adhesion force data were collected from 32 sets of particle–surface combination, for the study on four particles and eight smooth and roughened material surfaces.
This is a three factor factorial design whereby the adhesion force is taken as the response.
3. RESULTS AND DISCUSSION
3.1. Characterization of SUTD Office Dust
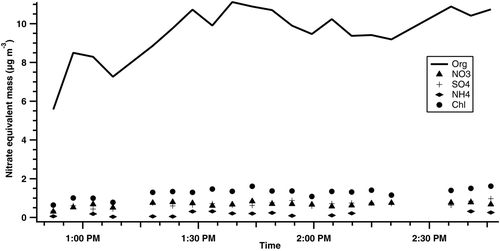
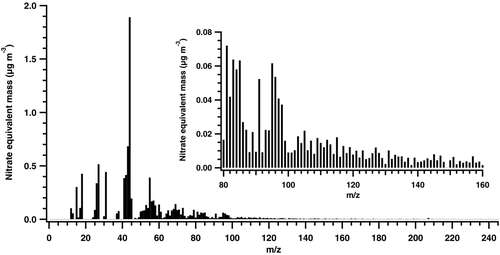
The dusts collected from SUTD were re-aerosolized and analyzed using the Aerosol Mass Spectrometer (AMS) to determine the average chemical composition of soluble aerosol components below 10 μm. The bulk (∼70%) of SUTD office dust is found to be organic in origin (). The organic mass fragments from UMR data show large peaks at m/z 44. Si and Si-based compounds were not observed in the AMS spectrum (Figures S1 and S2 in the online supplementary information). The m/z 44 fragment ion is mostly CO+ 2 from organic material. Significant fragments also exist at m/z fragments greater than 100 (). HR-AMS data also confirm the presence of Cl—, SOx—, and NH3—containing fragments (). However, these fragments are small in number compared to the C x H y O z species in the aerosol. Table S1 shows the elemental ratio composition of the SUTD office dust. Organic carbon constitutes ∼ 50% of the organic mass (Table S1, OM/OC ratio ∼2). Dust contains oxygenated organic material (O/C ∼0.68); other hydrophilic elements (S, N) are negligible. The O/C value of organic material is greater than 0.3 (Figure S3) and this suggests that the non-refractory SUTD dust contains significant amounts of hydrophilic organic components.
3.2. Adhesion of Dust to Indoor Surfaces
Eight different sample indoor surfaces (‘‘smooth’’ and roughened) surfaces of four common indoor materials (Al, Cu, PVC, and glass) were used to study and compare the effect of surface roughness and surface chemistry on the adhesion of dust to a surface.
Given the relatively high content of organic compounds present in the dust sample, AC was chosen as a model for soot to compare with these carbon-containing dust particles. shows the dust and AC AFM tips that were prepared and used for the adhesion force measurements.
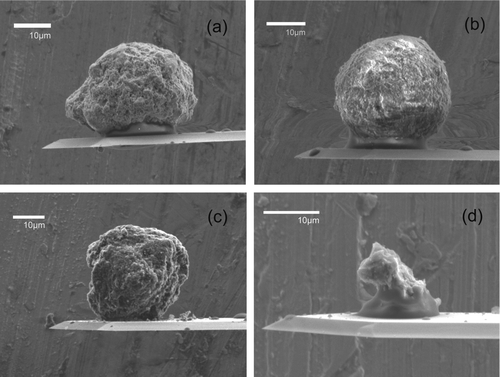
FIG. 3 SEM images of single selected particulates attached to the end of tipless AFM cantilevers: (a) dust tip 1, (b) dust tip 2, (c) activated carbon tip 1, and (d) activated carbon tip 2.
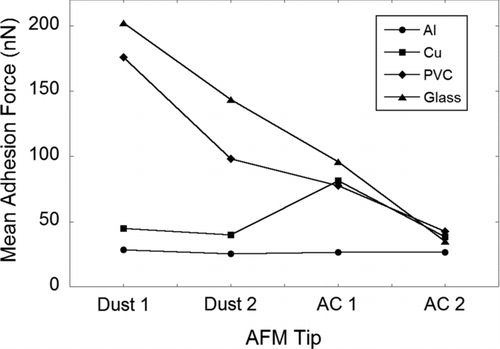
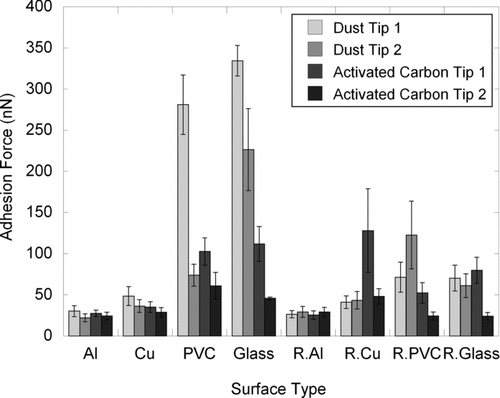
Adhesion forces of the various tip-surface combinations in air are shown in . Both dust tips have the strongest adhesion with the ‘‘smooth’’ glass substrate surface at ∼330 and ∼230 nN (dust tip 1 and dust tip 2, respectively) followed in decreasing order by PVC, Cu, and Al. Adhesion forces of dust with Al surface are weakest at ∼30 and ∼25 nN. Similar trends in adhesion forces are observed when the dust tips were brought into contact with the roughened substrate surfaces although it is the roughened PVC surface which has the strongest adhesion with dust (∼75 and ∼130 nN). Adhesion trends of the two AC tips with the ‘‘smooth’’ substrate surfaces mirror the results of the dust tips and are of the same order of magnitude although the strongest adhesion with the smooth glass surface is ∼120 nN or about a third of the dust–glass adhesion force.
FIG. 4 Adhesion forces of the different tip–surface combinations. R.Al, R.Cu, R.PVC, and R.Glass are roughened substrate surfaces. Error bars are 95% confidence intervals.
3.2.1. Factorial Analysis
The individual adhesion force data sets were Gaussian (Anderson–Darling test, p-values shown in Table S2). A two-way analysis of variance (ANOVA) was carried out using statistical software (Minitab 16) to analyze the adhesion force results. The surface roughness and the particle type (AFM tip) were set as the two factors, while the adhesion force was set as the response. Statistical analysis results showed that there are no significant differences (p > 0.05) in adhesion between the four tips (two dusts and two AC) with ‘‘smooth’’ and roughened Al surfaces (). This corroborates with our experimental results in and , showing that the adhesion forces between the four tips with ‘‘smooth’’ and roughened Al were comparable to one another. On the other hand, the p-values for Cu, PVC, and glass are smaller than 0.05 (), meaning that there are significant differences in the adhesion between the four tips with ‘‘smooth’’ and roughened Cu, PVC, and glass. An interaction plot of the mean adhesion force () also showed that the decrease in adhesion forces from glass to PVC to Cu and to Al is smaller for both AC tips than with dust. The non-conducting surfaces (glass and PVC) have higher adhesion forces with dust/AC AFM tips compared to the conducting metal surfaces (Cu and Al). One possible reason for the smaller adhesion between the AC tips and the surfaces compared to dust tips is the absence of polar (permanent dipole-permanent dipole/Keesom and permanent dipole-induced dipole/Debye) interactions for the former. Polar forces play a significant role in contact adhesion and can be as large as or greater in magnitude than the third and final component (London dispersion forces) of the total van der Waals interactions (Israelachvili Citation1992). We have shown in the previous section that the SUTD office dust contains an elevated amount of oxygenated hydrophilic organic groups. The presence of these oxygenated hydrocarbons suggests the contribution of polar forces in the dust tips’ adhesion to the various surfaces, which are not present for the AC tips.
TABLE 1 Statistical analysis for particle–surface adhesion force
Metals and metal oxides (the surface of Al typically has a thin layer of alumina, Al2O3 with thickness of about 3.5 nm (Campbell et al. Citation1999) in general have larger Hamaker constants than organics or quartz (Israelachvili Citation1992). The particle–surface forces present most likely to be relevant to this work are van der Waals forces, capillary forces, and electrostatic forces (Israelachvili Citation1992). If we assume that van der Waals forces are the primary interaction between the various tips and material surfaces, then adhesion for the dust/AC–metal combinations would be the strongest compared to the dust/AC–PVC/glass interactions given that van der Waals forces scale linearly with Hamaker constants. Our experimental results showed otherwise, indicating that capillary and/or electrostatic forces may play an important role in contributing to the tip–surface adhesion. Furthermore, measured adhesion forces may also be dependent on the effects of surface roughness. These three critical factors could explain the wide variations in tip–surface adhesion and would have to be decoupled from van der Waals interactions in order to understand the discrepancies. Further discussion on the effects of these factors on the adhesion forces is given below.
TABLE 2 Average contact angle (at 95% confidence interval) of various material surfaces
3.2.2. Effects of Surface Roughness on Hydrophobicity and Adhesion Force
shows the contact angles of drops of DI water on the different original-‘‘smooth’’ and roughened common indoor materials, which indicate the surfaces’ wettability and hydrophobicity. Cu has the highest contact angle with water drops at 94/91°, followed by PVC at 82/83°, Al at 76/81°, and glass at 40/45°. Roughened surfaces for the representative indoor materials have a higher contact angle than their corresponding ‘‘smooth’’ counterparts, although we observed the reverse happening in the water contact angle for Cu. This anomaly in the variation of contact angle can be explained by the fact that the original-‘‘smooth’’ Cu surface is rougher than after it is being rubbed with an abrasive paper (Figure S4a). Our contact angle results suggest that surface roughness plays a role in controlling the wettability (and by extension, hydrophobicity) of a material surface. According to Papadopoulos et al. (Citation2013), one of the requirements to create a superhydrophobic surface is to have a topography with micro- or nanoscale roughness. Furthermore, by using the Wenzel and Cassie model, Yang et al. (Citation2006) showed that surface roughness can enhance materials’ hydrophobic behavior. The Wenzel model considers that the surface roughness increases the contact area, resulting in an increase in the effective free energy at the solid–liquid interface, causing an increase in the hydrophobicity of the surface (Yang et al. Citation2006). On the other hand, the Cassie model assumes that air is trapped between the asperities of the rough surface and a droplet that is placed on top, resulting in increased hydrophobicity (Yang et al. Citation2006; Papadopoulos et al. Citation2013).
FIG. 6 Adhesion force versus surface area roughness between the different AFM tips and various sample surfaces. (a) Dust tip 1, (b) dust tip 2, (c) activated carbon tip 1, and (d) activated carbon tip 2. Error bars are 95% confidence intervals.
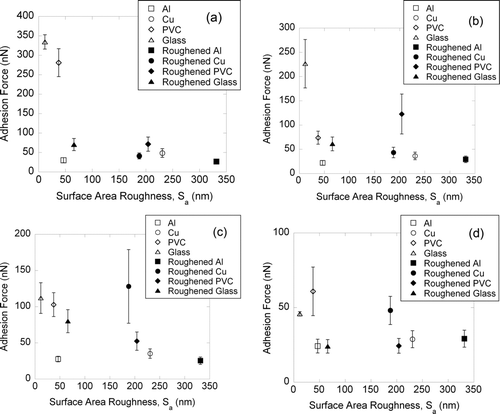
show the relationship between the measured adhesion forces (pull-off forces) using the dusts and AC-tipped AFM cantilevers on the material surfaces as a function of S a. In general, particle–surface adhesion forces decrease as surface roughness increases except for a few outliers in each graph. We define the unevenness of a surface as peaks and valleys. A perfectly smooth sample surface is considered flat (S a = 0 nm) while a rough surface would have peaks and valleys. When a dust particle lands on a surface, three possible scenarios can happen (Figures S5a–c) depending on the particle size and roughness.
If the dust size is much greater than the scratch width, the particle is unable to fit into the space between the peaks and it will just rest above the peaks (Figure S5a). The area of contact is only at the tip of the peaks, which is smaller than if the particle is on a perfectly smooth surface (Figure S5c) and thus adhesion is expected to decrease. Conversely, if the particle is smaller than the scratch width, it is possible for the particle to be trapped in between the peaks. In this case, the contact area between the particle and the material surface is larger (Figure S5b) than if the material is flat (Figure S5c) leading to an increase in adhesion forces.
An ultra-fine abrasive paper with an average grit size of 10.3 μm was used in our study to alter the original “smooth” sample surfaces by introducing scratches. Figure S6 shows the surface topography of all eight sample surfaces used for this dust adhesion study. The resulting scratch width (peak-to-peak distance) on the material surface is assumed to be similar to that of the grit size of the abrasive paper used. The dust and AC particles used for our colloidal probe AFM adhesion study range between 11 and 30 μm () in diameter, which are greater than the scratch width. The general decrease in particle–surface adhesion forces is likely directly linked to the reduction in contact area between the particle and material surface when S a increases and is in agreement with previous studies showing an inverse relationship between particle–surface adhesion forces and surface roughness (Meine et al. Citation2004; Benz et al. Citation2006; Ramakrishna et al. Citation2011).
S a is essentially a function of the height deviations on our sample surface (Peltonen et al. Citation2004). Reviewing , we observed that the adhesion forces decrease as the surface roughness increase, except for a few outlier cases where electrostatic interactions dominate. An increase in the surface roughness represents an increase in the distances (height) of the peaks and valleys. The adhesion forces between the particles and indoor material surfaces are primarily dependent on van der Waals forces, which are short-range forces where their effect is dominant at distances less than ∼10 nm (Israelachvili Citation1992; Thio and Meredith Citation2007). Therefore, at any particle–surface separations that is beyond 10 nm, the van der Waals forces (F = AR/6d 2, Hamaker model) decrease inversely square with distance, resulting in no significant difference in the adhesion forces regardless of the height differences whether it is 50 nm, 75 nm, or 100 nm, etc.
3.2.3. Presence of Electrostatic Interactions
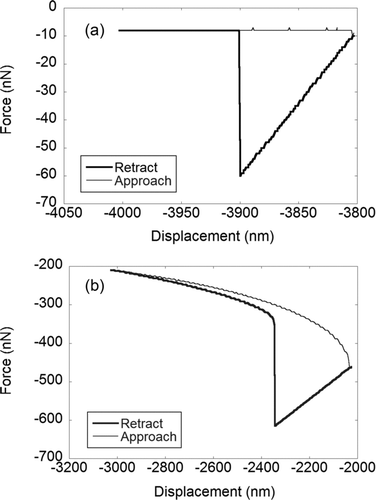
During the adhesion force measurements, typical force– displacement curves (represented by ) were obtained for most tip–surface combinations except for the case of dust tip interactions with PVC and glass surfaces (). Two artifacts (Millet et al. Citation2013) stand out in the force–displacement curve when comparing between : the presence of long-range forces which lead to an earlier snap-in at a tip-surface distance separation of ∼1 μm versus 10 nm, and also a higher cantilever deflection before the snap-in (∼2 μm vs. 20 nm). The absolute value of the adhesion force (upon retraction) is also much larger by about an order of magnitude for than . The large adhesive force indicates a strong adhesion (75–330 nN; ) of the dust tips to PVC and glass surfaces compared to <50 nN for dust–Al and dust–Cu interactions. Transfer of charges between non-conductors (Horn and Smith Citation1992) can happen when an AFM tip is brought into contact and separated from a material surface, resulting in electrostatic forces between the two surfaces (a long ranged interaction) (Israelachvili Citation1992). As glass and PVC are non-conductors, their ability to dissipate any electrical charges generated is poor. This leads to electrostatic charge build up on the glass and PVC sample surfaces and to a stronger adhesion to dust/AC. On the other hand, Cu and Al are conductors and are able to dissipate any charge that is accumulated on the surface effectively. Our force–displacement curves data suggest that there were electrostatic forces present during the dust-tipped AFM force measurements with the PVC and glass surfaces. Artifacts on the force–displacement curves for the AC-tips with PVC and glass surfaces are not as distinct as in but the forces upon retraction are larger compared to the AC–tip interactions with Al and Cu surfaces.
3.2.4. Effect of Capillary Forces
Ambient air-conditioned offices have a typical temperature range and RH values of about 22–25°C and 40–60%, respectively (Cheong and Chong Citation2001). In our study, room conditions were at 25°C and 50% RH. It is possible that a thin layer of water can form on the indoor surfaces due to condensation and contribute to AFM–tip adhesion to surfaces via capillary forces. The critical question is whether capillary forces play a significant role in the tip–surface adhesion. We estimate the magnitude of capillary forces present using the Kelvin equation (Thio and Meredith Citation2007).
3.2.5. Modeling of Adhesion Force Data (van der Waals Approach) to Calculate Contact Radius
In air, the significant particle–surface interactions when the AFM tip comes into contact with a surface are van der Waals forces, electrostatic and capillary forces (Tabor Citation1977; Israelachvili Citation1992) depending on humidity levels and type of materials present. For interactions where van der Waals forces dominate, the Hamaker model is one of the most widely used approach to calculate the magnitude of the adhesion (Israelachvili Citation1992). The simplest form is given by
Rumpf's model (Rumpf Citation1990) is commonly employed to describe van der Waals adhesion for particles to surfaces with nanoscale roughness. The model is based on the interaction between a single hemispherical asperity of radius r on a surface with a larger spherical adhering particle R along a line normal to both surfaces connecting their centers. This model incorporates two terms to describe the total van der Waals interaction. The first represents the interaction of the particle in contact with the asperity, while the second is the “non-contact” force between the particle and the flat surface separated by the asperity (Prokopovich and Starov Citation2011). Unfortunately, the radius r of the asperity cannot be easily measured and the asperity may not even be hemispherical. Rabinovich et al. (Citation2000b) then modified the Rumpf's model by substituting r for the RMS roughness of the material surface (Figure S4b), which is termed as the modified Rumpf's model:
Hamaker constants of dissimilar but known materials, such as AC with the four model material surfaces in air, were estimated using the method of combining relations (Israelachvili Citation1992),
shows the average contact radii of the dust and AC tips with the original and roughened Al and Cu surfaces calculated using Equations (2) and (3). PVC and glass surfaces are excluded from the van der Waals approach as we have shown earlier in Section 3.2.3 the generation of electrostatic charges when the tips were brought into contact with these two nonmetallic surfaces. The contact radii estimated using the simple Hamaker model (Equation (2)) lie in the range ∼13–63 nm, while the modified Rumpf model (Equation (3)) shows radii larger than the Hamaker model's calculations by three times to five orders of magnitude. While it is not possible to measure exactly the particle contact radius at the exact moment of impact, the values obtained using (Equation (3)) is unrealistic. For example, the dust tip 1 contact radius with Al was calculated to be 8.25 mm which is three orders of magnitude larger than the dust particle itself (). The contact radii using (Equation (2)) are likely to be an underestimation as the surface microtopography of both particle and surface are not factored into the equation.
TABLE 3 Mean contact radius (uncertainties at 95% confidence interval) of dust/AC particle—Al and Cu interactions derived from measured adhesion forces using simple Hamaker and modified Rumpf models
There are several major drawbacks with these theoretical adhesion models in determining particle–rough surface contact radius for van der Waals interactions. The models assume a nanoscopically smooth particle surface, do not consider the possible elastic deformation of surface (and particle) asperities (Prokopovich and Starov Citation2011) and the contribution to adhesive force by friction (Figures S5b and S5d) in addition to van der Waals forces. The presence of any of these factors can lead to an underestimation of adhesion. While there are several multi-asperity models which accounts for both asperity deformation and stretching/friction when a pull-off force is applied (Dundurs et al. Citation1973; Adams and Nosonovsky Citation2000; Nosonovsky and Bhushan Citation2007), they are mathematically too complex to be solved analytically and require the use of numerical methods which is beyond the scope of this study. We also note that under real indoor conditions as observed in our present work contact interactions between particles and surfaces will involve a complex interplay of the different types of forces (i.e., van der Waals, capillary, and electrostatic) depending on the type materials present, humidity and temperature (Israelachvili Citation1992), and the appropriate adhesion model has to be comprehensive and able to account for all the different types of forces.
4. CONCLUSIONS
We report the use of colloidal probe AFM to measure the adhesion of dust and simulated soot (AC) particles to four model surfaces (Al, Cu, PVC, and glass) representing materials commonly found indoors in an air-conditioned room environment. The fundamental forces involved in adhesion of dust and AC particles to the indoor surfaces are primarily van der Waals forces, with negligible capillary forces and significant electrostatic forces if the materials are non-electrical conductors. The materials’ surface roughness has a strong effect on particle adhesion, with a sharp decrease in particle adhesion forces when surface roughness is increased due to the reduction in real contact area between the particle probe and the substrate surface. Our results show the adhesion forces of the particles with glass and PVC were higher than with Al and Cu. The enhancement of adhesion in the particle–PVC and glass contact interactions can be attributed to the generation of electrostatic charges (triboelectric effect) through analyses of the AFM force–displacement curves. Similarly, while we cannot rule out condensed water–forming liquid bridges at the particle–surface junction, the AFM curves show no evidence of the presence of capillary forces contributing to adhesion when the particles approach or get retracted from a surface. Using a simple Hamaker model and a modified Rumpf model we estimated the radius of contact of the individual particles with Al and Cu surfaces as these are interactions governed primarily by van der Waals forces. The calculated lengthscale of the particles’ contact radii ranged mostly from nanometres in the former up to millimetre in the latter, which likely under- and overestimated the true particle–surface contact radii for the two models, respectively. These approaches do not adequately represent contacts between real surfaces under actual indoor air conditioned environments, which require more complex models to account for the effects of surface irregularities and the contribution of non-van der Waals forces to adhesion.
FUNDING
This work was supported in part by the Singapore University of Technology and Design (SUTD) – Zhejiang University Research Collaboration Pilot Grant SUTD-ZJU/PILOT/03/2012. Shaokai Gao and Akua Asa-Awuki would like to thank the CE-CERT Packard Post-Doctoral Fellowship for financial support of Shaokai Gao.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher's website.
Supplemental_file_Adhesion_of_Dust_Particles_to_Common_Indoor_Surfaces.zip
Download Zip (2 MB)ACKNOWLEDGMENTS
We are grateful to Adeline Chua and Eileen Tan-Tay of Hwa Chong Institution for providing access to the Nanosurf AFM, Dr. Dong Gui and Professor Mohideen at the University of California, Riverside for assistance with the spring constant measurements of the modified AFM cantilevers. We would also like to thank Ashley Vizenor and Professor Roya Bahreini at the University of California, Riverside for the assistance in the additional mass spectral characterization of ultrapure water blank samples.
REFERENCES
- Adams , G. G. and Nosonovsky , M. 2000 . Contact Modeling: Forces . Tribol. Int. , 33 : 431 – 442 .
- Aiken , A. C. , DeCarlo , P. F. and Jimenez , J. L. 2007 . Elemental Analysis of Organic Species with Electron ionization High-resolution Mass Spectrometry . Anal. Chem. , 79 : 8350 – 8358 .
- Aiken , A. C. , Decarlo , P. F. , Kroll , J. H. , Worsnop , D. R. , Huffman , J. A. Docherty , K. S. 2008 . O/C and OM/OC Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-resolution Time-of-flight Aerosol Mass Spectrometry . Environ. Sci. Technol. , 42 : 4478 – 4485 .
- Anderson , T. W. and Darling , D. A. 1954 . A Test of Goodness of Fit . J. Am. Stat. Assoc. , 49 : 765 – 769 .
- Balasubramanian , R. and Lee , S. S. 2007 . Characteristics of Indoor Aerosols in Residential Homes in Urban Locations: A Case Study in Singapore . J. Air Waste Manage. Assoc. , 57 : 981 – 990 .
- Benz , M. , Rosenberg , K. J. , Kramer , E. J. and Israelachvili , J. N. 2006 . The Deformation and Adhesion of Randomly Rough and Patterned Surfaces . J. Phys. Chem. B , 110 : 11884 – 11893 .
- Berico , M. , Luciani , A. and Formignani , M. 1997 . Atmospheric Aerosol in an Urban Area: Measurements of TSP and PM10 Standards and Pulmonary Deposition Assessments . Atmos. Environ. , 31 : 3659 – 3665 .
- Burnham , N. A. , Chen , X. , Hodges , C. S. , Matei , G. A. , Thoreson , E. J. Roberts , C. J. 2003 . Comparison of Calibration Methods for Atomic-force Microscopy Cantilevers . Nanotechnology , 14 : 1 – 6 .
- Butt , H. J. , Cappella , B. and Kappl , M. 2005 . Force Measurements with the Atomic Force Microscope: Technique, Interpretation and Applications . Surface Sci. Rep. , 59 : 1 – 152 .
- Campbell , T. , Kalia , R. K. , Nakano , A. , Vashishta , P. , Ogata , S. and Rodgers , S. 1999 . Dynamics of Oxidation of Aluminum Nanoclusters using Variable Charge Molecular-dynamics Simulations on Parallel Computers . Phys. Rev. Lett. , 82 : 4866 – 4869 .
- Cheong , K. W. and Chong , K. Y. 2001 . Development and Application of an Indoor Air Quality Audit to an Air-conditioned Building in Singapore . Build. Environ. , 36 : 181 – 188 .
- DeCarlo , P. F. , Kimmel , J. R. , Trimborn , A. , Northway , M. J. , Jayne , J. T. Aiken , A. C. 2006 . Field-deployable, High-resolution, Time-of-flight Aerosol Mass Spectrometer . Anal. Chem. , 78 : 8281 – 8289 .
- Ducker , W. A. , Senden , T. J. and Pashley , R. M. 1991 . Direct Measurement of Colloidal Forces Using an Atomic Force Microscope . Nature , 353 : 239 – 241 .
- Dundurs , J. , Tsai , K. C. and Keer , L. M. 1973 . Contact between Elastic Bodies with Wavy Surfaces . J. Elasticity , 3 : 109 – 115 .
- Fuller , K. N. G. and Tabor , D. 1975 . The Effect of Surface Roughness on the Adhesion of Elastic Solids . Proc. R. Soc. London, A: Math. Phys. Sci. , 345 : 327 – 342 .
- Hess-Kosa , K. 2011 . Indoor Air Quality: The Latest Sampling and Analytical Methods , 2nd ed. , Boca Raton, FL : CRC Press .
- Horn , R. G. and Smith , D. T. 1992 . Contact Electrification and Adhesion between Dissimilar Materials . Science , 256 : 362 – 364 .
- Hu , B. , Freihaut , J. D. , Bahnfleth , W. P. and Thran , B. 2008 . Measurements and Factorial Analysis of Micron-sized Particle Adhesion Force to Indoor Flooring Materials by Electrostatic Detachment Method . Aerosol Sci. Technol. , 42 : 513 – 520 .
- Israelachvili , J. N. 1992 . Intermolecular and Surface Forces , Academic Press, New York .
- Jimenez , J. L. , Canagaratna , M. R. , Donahue , N. M. , Prevot , A. S. H. , Zhang , Q. Kroll , J. H. 2009 . Evolution of Organic Aerosols in the Atmosphere . Science , 326 : 1525 – 1529 .
- Jimenez , J. L. , Jayne , J. T. , Shi , Q. , Kolb , C. E. , Worsnop , D. R. Yourshaw , I. 2003 . Ambient Aerosol Sampling using the Aerodyne Aerosol Mass Spectrometer . J. Geophys. Res.-Atmos. , 108(D7) doi: 10.1029/2001JD001213
- Liu , Y. S. , Chen , R. , Shen , X. X. and Mao , X. L. 2004 . Wintertime Indoor Air Levels of PM10, PM2.5 and PM1 at Public Places and their Contributions to TSP . Environ. Int. , 30 : 189 – 197 .
- McDonagh , A. , Sextro , R. G. and Byrne , M. A. 2012 . Mass Transport of Deposited Particles by Surface-to-surface Contact . J. Hazardous Mater. , 227–228 : 370 – 377 .
- Meine , K. , Kloss , K. , Schneider , T. and Spaltmann , D. 2004 . The Influence of Surface Roughness on the Adhesion Force . Surf. Interface Anal. , 36 : 694 – 697 .
- Millet , G. , Lécuyer , A. , Burkhardt , J. M. , Haliyo , S. and Régnier , S. 2013 . Haptics and Graphic Analogies for the Understanding of Atomic Force Microscopy . Int. J. Human-Computer Studies. , 71 : 608 – 626 .
- Morawska , L. and Salthammer , T. 2003 . Indoor Environment: Airborne Particles and Settled Dust , Weinheim Great Britain : Wiley-VCH .
- Nazaroff , W. W. 2004 . Indoor Particle Dynamics . Indoor Air , 14 : 175 – 183 .
- Nosonovsky , M. and Bhushan , B. 2007 . Multiscale Friction Mechanisms and Hierarchical Surfaces in Nano- and Bio-tribology . Mat. Sci. Eng. R. , 58 : 162 – 193 .
- Ormstad , H. 2000 . Suspended Particulate Matter in Indoor Air: Adjuvants and Allergen Carriers . Toxicology , 152 : 53 – 68 .
- Ormstad , H. , Gaarder , P. I. and Johansen , B. V. 1997 . Quantification and Characterisation of Suspended Particulate Matter in Indoor Air . Sci. Total Environ. , 193 : 185 – 196 .
- Papadopoulos , P. , Mammen , L. , Deng , X. , Vollmer , D. and Butt , H. J. 2013 . How Superhydrophobicity Breaks Down . PNAS , 110 : 3254 – 3258 .
- Peltonen , J. , Jarn , M. , Areva , S. , Linden , M. and Rosenholm , J. B. 2004 . Topographical Parameters for Specifying a Three-Dimensional Surface . Langmuir , 20 : 9428 – 9431 .
- Prokopovich , P. and Starov , V. 2011 . Adhesion Models: From Single to Multiple Asperity Contacts . Adv. Colloid Interface Sci. , 168 : 210 – 222 .
- Rabinovich , Y. I. , Adler , J. J. , Ata , A. , Singh , R. K. and Moudgil , B. M. 2000a . Adhesion between Nanoscale Rough Surfaces - II. Measurement and Comparison with Theory . J. Colloid Interface Sci. , 232 : 17 – 24 .
- Rabinovich , Y. I. , Adler , J. J. , Ata , A. , Singh , R. K. and Moudgil , B. M. 2000b . Adhesion between Nanoscale Rough Surfaces - I. Role of Asperity Geometry . J. Colloid Interface Sci. , 232 : 10 – 16 .
- Ramakrishna , S. N. , Clasohm , L. Y. , Rao , A. and Spencer , N. D. 2011 . Controlling Adhesion Force by Means of Nanoscale Surface Roughness . Langmuir , 27 : 9972 – 9978 .
- Rumpf , H. 1990 . Particle Technology , London : Chapman & Hall .
- Tabor , D. 1977 . Surface Forces and Surface Interactions . J. Colloid Interface Sci. , 58 : 2 – 13 .
- Thatcher , T. L. and Layton , D. W. 1995 . Deposition, Resuspension, and Penetration of Particles within a Residence . Atmos. Environ. , 29 : 1487 – 1497 .
- Thio , B. J. R. and Meredith , J. C. 2007 . Measurement of Polyamide and Polystyrene Adhesion with Coated-tip Atomic Force Microscopy . J. Colloid Interface Sci. , 314 : 52 – 62 .
- 1991 . Sick Building Syndrome , Washington , DC : United States Environmental Protection Agency . U.S. Environmental Protection Agency
- Yang , C. , Tartaglino , U. and Persson , B. N. J. 2006 . Influence of Surface Roughness on Superhydrophobicity . APS Phys. Rev. Lett. , 97 : 116103(116101) – (116104) .