Abstract
Secondary condensation of organic material onto primary seed particles is one pathway of particle growth in the atmosphere, and many properties of the resulting mixed particles depend on organic volume fraction. Environmental chambers can be used to simulate the production of these types of particles, and the optical, hygroscopic, and other properties of the mixed particles can be studied. In the interpretation of the measured properties, the probability density function p(ϵ;d) of volume fraction ϵ of the condensing material for particle diameter d in the outflow of the chamber is typically needed. In this article, analytic equations are derived p(ϵ;d) for condensational growth in a continuously mixed flow reactor. The equation predictions are compared to measurements for the condensation of secondary organic material on quasi-monodisperse sulfate seed particles. Equations are presented herein for discrete, Gaussian, and triangular distribution functions for the seed particle number–diameter distributions, including generalization to any linearly segmented distributions. The analytic equations are useful both for the interpretation of laboratory data from environmental chambers, such as the construction of probability density functions for use in interpretation of hygroscopic growth data, cloud–condensation–nuclei data, or other laboratory data sets dependent on organic volume fraction, as well as for understanding atmospheric processes at times that condensational growth processes prevail.
Copyright 2014 American Association for Aerosol Research
1. INTRODUCTION
Atmospheric particles have significant effects on air quality and climate, and an important source of particulate material is the production of secondary organic material (Seinfeld and Pandis Citation2006). A common atmospheric particle type observed by microscopy consists of a primary particle coated by secondary organic material (Pöschl et al. Citation2010). Secondary organic material is produced by multiple pathways (Hallquist et al. Citation2009), and for certain reaction conditions condensational growth can be the dominant pathway (Seinfeld et al. Citation2003; Kuwata and Martin Citation2012). The production pathways of secondary organic material have been widely simulated in laboratory environmental chambers in efforts to understand and quantify the involved chemistry and associated environmental effects (Hallquist et al. Citation2009). One common configuration is to operate the chamber as a continuously mixed flow reactor (CMFR; Kleindienst et al. Citation1999; Shilling et al. Citation2008; King et al. Citation2009; Mentel et al. Citation2009; Drozd et al. Citation2013). The CMFR can be maintained at steady state for weeks at a time, allowing long times for signal integration for online measurements (Shilling et al. Citation2008) or for sufficient sample collection for offline analysis (You et al. Citation2012; Renbaum-Wolff et al. Citation2013; Shrestha et al. Citation2013). Inorganic seed particles are typically used to suppress oscillatory behavior in the chamber (McGraw and Saunders Citation1984) and/or to simulate internally mixed atmospheric particles (Seinfeld and Pandis Citation2006). As a result, mixed inorganic–organic particle populations, similar in microscopy images to those observed for collected atmospheric particles, are produced. In the interpretation of the measured properties, the probability density function p(ϵ;d) of volume fraction ϵ of the condensing material for particle diameter d in the outflow of the chamber is typically needed.
For mixed inorganic–organic particle populations produced by condensational growth in a CMFR, particles of a single diameter d in the CMFR outflow have an associated probability density function p(ϵ) of organic volume fraction ϵ arising from a distribution of individual particle residence times in the CMFR (Davis and Davis Citation2003). For instance, particles grown on smaller seeds but having longer residence times can have the same final diameter in the CMFR outflow as particles grown on larger seeds but having shorter residence time. Knowledge of p(ϵ) at a specific diameter in the CMFR outflow is important because a size slice of the outflow aerosol is often selected for investigation of additional particle properties, such as hygroscopic growth (Swietlicki et al. Citation2008), phase transitions (Smith et al. Citation2011, Citation2012, Citation2013), or cloud activation potential (King et al. Citation2009, Citation2010). Interpretation of those data sets requires knowledge of p(ϵ) at the analyzed diameter.
TABLE 1. Equations for the distribution functions f(d,t), f(d,d0), f(d0,t), f(d,ϵ), and f(d,z)
In previous treatments, p(ϵ) was simulated numerically by a sectional model (King et al. Citation2009, Citation2010; Smith et al. Citation2011, Citation2012, Citation2013). Herein, we develop an analytical expression for p(ϵ) in the outflow from a CMFR. The developed expression is more concise and accurate than the previously used sectional models. The equations are useful both for the interpretation of laboratory data from environmental chambers and for understanding atmospheric processes at times that condensational growth processes prevail.
2 THEORY
2.1 Definition
The theoretical development considers a particle population that begins as monodisperse seed particles of diameter d0 of a first material upon which a second material condenses to a variable extent on each seed particle (e.g., because of variable exposure times for different seed particles). For an individual particle of diameter d in the population, the volume fraction ϵ(d,d0) of the second material is given by the following equation:
[1]
Both the initial seed particle and the grown particle are taken as effectively spherical. EquationEquation (1)[1] implies that the volume fraction of a growing particle rapidly converges on unity irrespective of the initial seed diameter (e.g., ϵ = 0.98 for d/d0 = 3.68) (Figure S1). An additional term z, which is the inverse of the diameter growth factor, is useful in presenting succinct equations and is given as follows:
[2] The following useful derivative is obtained:
[3]
2.2 Definitions f(d,t), f(d,d0), f(d0,t), f(d,ϵ), and f(d,z) and Relationships to N, n(d), and p(ϵ)
The particle population in the reactor outflow has a total number concentration N. There are distribution functions f(d,t), f(d,d0), f(d0,t), f(d,ϵ), and f(d,z) of the number count of the particle population with respect to particle diameter d, particle residence time t, seed particle diameter d0, volume fraction ϵ of the second material, and inverse diameter growth factor z (). The relationship of these distribution functions to the number–diameter distribution n(d) in the reactor outflow is as follows:
[4] The distribution n(d) cannot be obtained directly from f(d0,t) and instead requires a path integral along d through f(d0,t) (i.e., EquationEquation (6)
[6] as one possible example) or a transformation of variables (i.e., thereby recovering one of the forms of EquationEquation (4)
[4] ). The total particle number concentration N is given by N = ∫∞0n(d) dd.
The probability density function p of volume fraction ϵ for particle diameter d, abbreviated as p(ϵ) or written in full form as p(ϵ;d), is given by the following equation:
[5] for which n(d) serves as the normalization term of f(d,ϵ) such that the integral across p(ϵ) is unity. The foregoing equation, as well as several others noted below, is derived in the Supplemental Information. The objective of this study is to provide analytic formulations for p(ϵ), implying by EquationEquation (5)
[5] that analytic formulations are also needed for f(d,ϵ) and n(d).
2.3 Condensational Growth Rate
The main application considered in the present study is diameter growth by condensation of organic molecules from the gas phase onto the surfaces of sulfate seed particles. Mixed organic-sulfate particles are the most prevalent submicron particle in anthropogenically affected continental regions of the world (Zhang et al. Citation2007). For this application, the term ϵ corresponds to the organic volume fraction.
As described in Seinfeld et al. (Citation2003) and Kuwata and Martin (Citation2012), the condensational diameter growth rate I(d), describing dd/dt, is assumed to be adequately described by I(d) = β/(d + λ). Terms include a mean free path λ for the condensing species and a gas-phase concentration parameter β that is proportional to the difference between partial pressure and vapor pressure of the condensing species. Additional considerations related to the physical interpretation of terms λ and β are discussed in Seinfeld et al. (Citation2003) and Kuwata and Martin (Citation2012). These studies have shown that even with the myriad compounds of secondary organic aerosol, the number–diameter distributions of actual data sets, such as those of α-pinene or β-caryophyllene ozonolysis, are adequately represented by I(d) = β/(d + λ). The main intellectual justification for this success is limited variability of mean free paths among different organic molecules.
Integration of the equation for I(d) results in a relationship by which knowledge of any two of d, d0, and t is sufficient to obtain the third, as follows:
[6] This equation defines implicit functions for d(t,d0) and d0(d,t) and an explicit function for t(d,d0). Parameters λ and β are taken as known. In this case, by EquationEquation (6)
[6] any two of d, d0, and t are sufficient to find the third. Furthermore, for fixed β and λ the equation implies that d(t) for different initial diameters constitute a set of non-intersecting lines. EquationEquation (6)
[6] omits any treatment of size-dependent behavior, such as by the Kelvin effect.
2.4 Continuously Mixed Flow Reactor
For the case under consideration in this study, the number–diameter distribution n*0(d) of the seed particles in the inflow to the CMFR is measured or otherwise known. The term n0(d) describes the seed particle number–diameter distribution in the outflow from the reactor and differs from n*0(d) because of particle wall loss. The term n(d) describes the number–diameter distribution in the CMFR outflow (i.e., after condensational growth on the seed particles). Coagulation processes affecting n(d) and n0(d) are not included in the present analysis because for typical environmental chambers they are important only for high number concentrations (>1010 m3). The possibility of new particle formation is also not included in the model because for typical environmental chambers nucleation is not observed in the presence of seed particles. Possible competing losses of gas-phase species to chamber walls or other surfaces is not relevant in the present treatment because the growth rate law I(d) underlying EquationEquation (6)[6] is prescribed.
Condensational growth occurs onto the seed particles during their distribution of residence times t in the CMFR. The distribution of residence times in a CMFR is described by Poisson statistics. There is a mean reactor residence time τ of the particle population, including loss both by outflow from the CMFR and by deposition on the reactor walls. Particle diameter increases by condensational growth that takes place in the reactor. The distribution of residence times in the CMFR implies a distribution of particle diameters in the reactor outflow.
For the stated conditions and assumptions, the distribution function f(d,t) in the reactor outflow is bivariate in particle diameter d and particle residence time t. It is written as follows for the growth rate law of EquationEquation (6)[6] , as follows (see the online supplemental information [SI]):
[7] for
. For orientation to the reader, shows an example of a density plot of f(d,t). and show cross-sections in constant diameter and constant residence time, respectively, through the density plot. Plots for f(d,d0) and f(d0,t) are shown in Figures S2 and S3, respectively.
![FIG. 1. Distribution function f(d,t). (a) Density plot of f(d,t) as a function of grown particle diameter d and particle residence time t (EquationEquation (7)[7] ). Parameter values τ, β, λ, and n0(d) are as follows: 10,000 s, 10 nm2 s−1, 100 nm, and ∧(10–90 nm) with NT = 1 m−3, respectively. Contours of constant organic volume fraction are shown. The line with arrow shows the forward trajectory of 50-nm seed particles through time. (b) Cross-sections through f(d,t) for fixed d and variable t. The sections are shown for five diameters ranging from 50 to 450 nm. The dashed line in this and other figures is always associated in some manner with 50 nm, which is the mode diameter of the seed particles: n0(d) = ∧(10–90 nm). (c) Cross-sections through f(d,t) for fixed t and variable d. The sections are shown for nine particle residence times ranging from 0(×103) to 16(×103) s. In this panel, the line for t = 0 showing ∧(10–90 nm) represents n0(d) / τ.](/cms/asset/b874b7f4-ef41-4714-9495-43b56ea52e2d/uast_a_931564_f0001_oc.jpg)
After a transformation of variables, functions f(d, ϵ) and f(d, z) are obtained (see the SI):
[8]
[9] These expressions, as well as f(d,d0) and f(d0,t), are summarized in . shows density plots of EquationEquation (8)
[8] scaled linearly () as well as logarithmically (). Cross-sections through the density plot are shown in and . Panels e and f of the figure show probability density functions of organic volume fraction for several different diameter cross-sections (Section 2.5).
The probability density function p(ϵ) at a diameter d is obtained by combining Equations (4), (5), and (9), as follows (see the SI):
[10] for the cumulative distribution function c(ϵ;d). The dependence z(ϵ) is given by EquationEquation (2)
[2] .
![FIG. 2. Distribution function f(d,ϵ). (a) Density plot of f(d,ϵ) as a function of grown particle diameter d and for organic volume fractions ϵ of up to 0.9 (EquationEquation (8)[8] ). Parameter values are as for Figure 1. Contours of constant particle residence time ranging from 2(×102) to 20(×102) s are shown. (b) Cross-sections through f(d,ϵ) for fixed d and variable ϵ. The sections are shown for four diameters ranging from 50 to 125 nm. (c) Cross-sections through f(d,ϵ) for fixed ϵ and variable d. The sections are shown for five organic volume fractions ranging from 0.0 to 0.8. (d) Density plot of log10 f(d,ϵ) for organic volume fractions approaching close to unity. Contours of constant particle residence time ranging from 125 to 8000 s are shown. (e) Probability density function p(ϵ;d) of organic volume fraction ϵ for fixed particle diameter d (EquationEquation (5)[5] ). This panel shows p(ϵ;d) for four smaller values of d, ranging from 50 to 125 nm. (f) Same as panel (e) but for five larger values of d, ranging from 50 to 250 nm.](/cms/asset/8b1320ca-02f2-4786-bab4-1af9a62e1817/uast_a_931564_f0002_oc.jpg)
As a reference point, in the limiting case of a monodisperse seed particle population of number concentration N with a distribution defined by a Dirac delta function centered at d0 (i.e., n0(d) = N δ(d − d0)), EquationEquation (10)[10] evaluates to the following (see the SI):
[11]
2.5 p() for Discretized n0(d)
A number–diameter distribution n0(d) for the seed particles can be discretized into k monodisperse bins of monotonically increasing diameters {d0,1, d0,2, …, d0,k} having respective number concentrations {N1, N2, … Nk}. The relationship holds that N = ΣjNj for j = [1,k]. Each bin corresponds to a Dirac delta function centered at d0,j (i.e., n0,j(d) = Nj δ(d − d0,j)) and makes a partial contribution to n0(d) as n0(d) = Σj n0,j(d) for j = [1,k]. For this case, EquationEquation (10)[10] develops as follows (see the SI):
[12]
The index q in the last line for the denominator corresponds to the index of the last element of {d0,1, d0,2, …, d0,k} that satisfies d − d0, j > 0.
The cumulative distribution function c(ϵ) is then given as follows (see the SI):
[13] in which r is the index of the first element of {d0,1, d0,2, …, d0,k} that satisfies zd − d0, j < 0.
2.6 p() for Continuous n0(d)
2.6.1 Gaussian Distribution
For many CMFRs, prior to entering the chamber polydisperse seed particles are classified in unipolar electric mobility by passage through a differential mobility analyzer (DMA) (Knutson and Whitby Citation1975). A distribution of organic volume fractions arises because of a combination of factors, including Poisson statistics that regulate the residence times of individual particles, the finite width of the DMA transfer function, and the multimodes of seed particles in diameter space (i.e., +1, +2, +3 charges). In diameter space, each charge q contributes one mode to the seed particle population. The mode can be approximately described by a Gaussian distribution characterized by a mode diameter , a variance σ2q, and a concentration Nq. The associated equation for n0(d) is as follows:
[14]
Although the upper limit of EquationEquation (14)[14] is represented as infinity for completeness, in practice |q| ∈ { + 1, +2, +3} is sufficient. By EquationEquation (10)
[10] , the following expression is obtained for p(ϵ;d) (see the SI):
[15] for a normalization factor ξ given by
[16]
Terms in the equations include: γ0 = β τ, γq = γ0 − σ2q, , y(x1, x2, x3) = (x1 − x3) + x2(λ + x3), and y(x1) = y(x1, 1, 0) = x1 + λ. The cumulative distribution function is given as follows:
[17]
In some cases, identification of p(ϵ; d)|Gauss for each particle type q is desirable, as p(ϵ; d, q)|Gauss. The individual probability density function of each particle type is as follows:
[18] for an individual normalization factor ξq given by
[19]
2.6.2 Linearly Segmented Distribution
The seed particle distribution of a DMA can sometimes be approximated by linearly segmented distributions. As the simplest form, triangular functions can describe a seed particle distribution, as follows:
[20] for a matrix d of having rows of {start diameter, center diameter, stop diameter} that define a triangular function, including the possibility for skewness. The variables are transformed for succinctness to slopes m and intercepts b, as follows: mj1 = 2(dj3 − dj1)− 1(dj2 − dj1)− 1, bj1 = −dj1mj1, mj2 = 2(dj3 − dj1)− 1(dj2 − dj3)− 1, and bj2 = −dj3mj2.
For the triangular distribution of EquationEquation (20)[20] , EquationEquation (10)
[10] evaluates as follows (see the SI):
[21] for a normalization factor ξ given by
[22]
The term gj,k is given by . The cumulative distribution function is given as follows:
[23]
The term hj,k is given by .
More generally, any seed particle distribution broken into linear segments can be treated similarly as represented by Equations (20) and (22) for a triangular function. Any seed distribution can be approximated for sufficiently short linear segments. The distribution p(ϵ;d) can then be obtained.
3 APPLICATIONS
Kuwata and Martin (Citation2012) previously compared the number–diameter distributions obtained by Equations (4) and (6) to observed distributions exiting the Harvard Environmental Chamber. The model was based on condensational growth described by Equations (6) and (7) in a CMFR (Seinfeld et al. Citation2003; Kuwata and Martin Citation2012).The model could describe the data for secondary organic material produced by ozonolysis of α-pinene and β-caryophyllene. The interpretation is that condensational growth can be considered as the principal mechanism for change in particle diameter in these experiments. By comparison, the distributions observed for isoprene photo-oxidation could not be described. The implication could be that, in the case of ozonolysis, the rate of particle growth is largely governed by the steady condensation of reaction products from the gas phase to the particle surfaces whereas, in the case of photo-oxidation, the rate of particle growth is significantly influenced by particle-phase chemistry and subsequent diameter changes that occurred on a timescale that was of the same order as the particle residence time in the reactor. Kuwata and Martin (Citation2012) explained theoretically how EquationEquation (6)[6] can be modified to accommodate additional processes and test growth mechanisms. Shiraiwa et al. (Citation2013) provided a recent practical example of using number–diameter distributions to discriminate among mechanisms.
Smith et al. (Citation2011, Citation2012, Citation2013) required a description of p(ϵ;d) for aerosol particles in the outflow of the Harvard Environmental Chamber. Smith et al. focused on interpretation of data sets of hygroscopic growth related to phase transitions of efflorescence, deliquescence, and liquid–liquid phase separation. King et al. (Citation2009, Citation2010) required a description of c(ϵ;d) for chamber experiments related to cloud activation potential. Both Smith et al. and King et al. used numerical sectional models based on the underlying equations of Seinfeld et al. (Citation2003).
An important exercise is to compare the solutions of the analytical equations of this study for .p(ϵ; d)|Gauss of EquationEquation (15)[15] or .c(ϵ; d)|Gauss of EquationEquation (17)
[17] to the numerical results of the prior studies. Parameters for EquationEquation (14)
[14] were obtained by numerical simulation using the AerosolCalculator (http://www.seas.harvard.edu/AerosolCalculator, version 1.8). For a nondiffusing transfer function of a DMA (TSI Inc., model 3081) operated with an aerosol-to-sheath flow ratio of 10:1 and set to pass particles of an electric-field mobility diameter of 75 nm (+1 charge), the parameters for q ∈ { + 1, +2, +3} are as follows:
nm and σq = {1.84, 2.88, 3.84} nm. Statements of uncertainty on these parameter values are omitted because our purpose here is numerical simulation. The small difference between the simulated
and the setpoint value of 75 nm is explained by the finite bin resolution of the AerosolCalculator, which was set to 256 bins for the seed particle population. The population prior to entering the DMA was defined by a lognormal distribution having a geometric mean diameter of 200 nm, a geometric standard deviation of 2, and total particle concentration of 1010 m−3. The concentrations exiting the DMA, assuming bipolar equilibrium charging prior to the DMA, were as follows: Nq = 106 {23.6, 8.9, 2.1} m−3. shows a plot of EquationEquation (14)
[14] using these parameter values. The concentrations represent the inflow to the CMFR and must be scaled by τ/τ0 to represent the concentrations of n0(d) in the CMFR because of the difference between the mean residence time τ0 of an inert gas and the particle mean residence time τ that accounts for particle wall loss (Appendix A1 of Kuwata and Martin [Citation2012]). This constant scaling factor, however, is unimportant for the objectives of the present study and for simplicity is omitted from the analysis (i.e., effectively assuming that there is no particle wall loss). shows the resulting .f(d, ϵ)|Gauss and .p(ϵ; d)|Gausscorresponding to . Figure 4 shows that the results of the analytical and numerical solutions are nearly identical.
![FIG. 3. Simulated seed particle number-diameter distribution exiting the DMA and representing n0(d) within a constant scaling factor (see main text). The three modes are represented by normal distributions having the following parameter values with respect to EquationEquation (14)[14] : q ∈ { + 1, +2, +3}, nm, σq = {1.84, 2.88, 3.84} nm, and Nq = 106 {23.6, 8.9, 2.1} m−3. The sheath-to-sample flow was 10:1.](/cms/asset/a9fe5a31-bad1-46d1-8d15-943125b6578e/uast_a_931564_f0003_b.gif)
![FIG. 4. Distribution function .f(d, ϵ)|Gauss for .n0(d0)|Gaussrepresented in Figure 3. (a) Density plot of .f(d, ϵ)|Gauss as a function of grown particle diameter d and for organic volume fractions ϵ of up to 0.9 (Equations (8) and (14)). Parameter values τ, β, and λ are as for Figure 1. Contours of constant particle residence time ranging from 2(×102) to 20(×102) s are shown. (b) Density plot of .log 10f(d, ϵ)|Gauss for organic volume fractions approaching close to unity. Contours are shown of constant particle residence time, ranging geometrically from 125 to 8000 s. (c) Probability density function .p(ϵ; d)|Gauss of organic volume fraction ϵ for fixed particle diameter d (EquationEquation (15)[15] ). Plots are shown for four diameters ranging from 50 to 125 nm.](/cms/asset/7a3f6a35-8c44-42b3-9a0f-ee5e542523f1/uast_a_931564_f0004_oc.jpg)
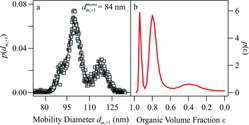
An example of the application of EquationEquation (15)[15] for .p(ϵ; d)|Gauss is shown in for a data set collected by a hygroscopic tandem differential mobility analyzer (HTDMA) (Smith et al. Citation2011, Citation2012, Citation2013). A particle population described by some f(d,t) was grown in the Harvard Environmental Chamber by the dark ozonolysis of α-pinene in the presence of ammonium sulfate seed particles. The seed distribution n0(d) was measured by an SMPS. The mean reactor residence time τ was 14,100 s. The grown distribution n(d) was measured by an SMPS. The parameters β and λ (7.6 nm2 s−1 and 170 nm, respectively) were obtained by optimization of between the measured n(d) and that calculated by Equation (B1) of Kuwata and Martin (Citation2012). For the HTDMA experiment shown in , a particle subpopulation at a dry mobility diameter (+1 charge) of 84 nm was selected by a first DMA. For this subpopulation, .p(ϵ; d)|Gauss was calculated by EquationEquation (15)
[15] . In the HTDMA experiment, the subpopulation was grown hygrocopically to 84% RH. shows the resulting number–diameter distribution that was measured (points). The dry mode of 84 nm resolves into three modes at higher RH. plots .p(ϵ; d)|Gauss, showing three modes of organic volume fraction. These three modes explain the presence of three modes in the measurement at 84% RH (panel a) because the hygroscopic growth factor decreases for increasing organic volume fraction (note the reversal of the abscissa in panel b). The lines in panel a show the modeled number–diameter distribution at 84% RH based on calculated .p(ϵ; d)|Gauss and the hygroscopic growth factors of mixed particles of sulfate and α-pinene SOM (Smith et al. Citation2011). There is excellent agreement between the measured (points) and modeled (lines) number–diameter distributions at 84% RH, supporting the validity to the calculated .p(ϵ; d)|Gauss.
In addition to the equations developed herein for a focus on condensational growth in a CMFR, the Appendix presents a generalized form of p(ϵ;d) applicable to any steady-state flow through reactor (e.g., laminar-flow reactors, plug-flow reactors of variable dispersion, or any reactor of arbitrary but known distribution for particle residence times) in which particles undergo condensational growth according to any governing equation on a number–diameter distribution n0(d) of seed particles.
Applications of the generalized equations can also be used to approximate atmospheric processes under particular applicable scenarios, such as large urban regions that emit primary particles that are subsequently mixed and coated during downwind transport by secondary gas-to-particle processes. For this case, the probability density function of particle residence time follows from the coupling of atmospheric eddies with Lagrangian transport, corresponding to a plug-flow-reactor with dispersion (Dragoescu and Friedlander, 1989). In the case that growth rates are known, effective residence times (i.e., time zero at urban emission of the primary particle) are obtained by inverting size-resolved chemical measurements. Alternatively, effective residence times are obtained by the known decay rates of measured gas-phase species.
FUNDING
This study was supported by the Office of Science (BES), U.S. Department of Energy, Grant No. DE-SC0006677, and by the U.S. National Science Foundation, Grant No. AGS-1249565. Mikinori Kuwata was supported by the Japan Society for the Promotion of Science (JSPS) postdoctoral fellowship for research abroad.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the .
Supplementary Material
Download Zip (1.6 MB)REFERENCES
- Davis, M.E., and Davis, R.J. (2003). Fundamentals of Chemical Reaction Engineering. McGraw-Hill, New York.
- Drozd, G.T., Woo, J.L., and McNeill, V.F. (2013). Self-Limited Uptake of α-Pinene Oxide to Acidic Aerosol: The Effects of Liquid–Liquid Phase Separation and Implications for the Formation of Secondary Organic Aerosol and Organosulfates from Epoxides. Atmos. Chem. Phys., 13:8255–8263. doi:10.5194/acp-13-8255-2013.
- Hallquist, M., Wenger, J.C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., et al. (2009). The Formation, Properties and Impact of Secondary Organic Aerosol: Current and Emerging Issues. Atmos. Chem. Phys., 9:5155–5236. doi:10.5194/acp-9-5155–2009.
- King, S.M., Rosenoern, T., Shilling, J.E., Chen, Q., and Martin, S.T. (2009). Increased Cloud Activation Potential of Secondary Organic Aerosol for Atmospheric Mass Loadings. Atmos. Chem. Phys., 9:2959–2971.
- King, S., Rosenoern, T., Shilling, J., Chen, Q., Wang, Z., Biskos, G., et al. (2010). Cloud Droplet Activation of Mixed Organic-Sulfate Particles Produced by the Photooxidation of Isoprene. Atmos. Chem. Phys., 10:3953–3964.
- Kleindienst, T.E., Smith, D.F., Li, W., Edney, E.O., Driscoll, D.J., Speer, R.E., et al. (1999). Secondary Organic Aerosol Formation from the Oxidation of Aromatic Hydrocarbons in the Presence of Dry Submicron Ammonium Sulfate Aerosol. Atmos. Environ., 33:3669–3681.
- Knutson, E.O., and Whitby, K.T. (1975). Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications. J. Aerosol Sci., 6:443–451.
- Kuwata, M., and Martin, S.T. (2012). Particle Size Distributions Following Condensational Growth in Continuous Flow Aerosol Reactors as Derived from Residence Time Distributions: Theoretical Development and Application to Secondary Organic Aerosol. Aerosol Sci. Technol., 46:937–949. doi:10.1080/02786826.2012.683204.
- McGraw, R., and Saunders, J.H. (1984). A Condensation Feedback Mechanism for Oscillatory Nucleation and Growth. Aerosol Sci. Technol., 3:367–380. doi:10.1080/02786828408959025.
- Mentel, T.F., Wildt, J., Kiendler-Scharr, A., Kleist, E., Tillmann, R., Dal Maso, M., et al. (2009). Photochemical Production of Aerosols from Real Plant Emissions. Atmos. Chem. Phys., 9:4387–4406.
- Pöschl, U., Martin, S.T., Sinha, B., Chen, Q., Gunthe, S.S., Huffman, J.A., et al. (2010). Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon. Science, 329:1513–1516.
- Renbaum-Wolff, L., Grayson, J.W., Bateman, A.P., Kuwata, M., Sellier, M., Murray, B.J., et al. (2013). Viscosity of α-Pinene Secondary Organic Material and Implications for Particle Growth and Reactivity. Proc. Natl. Acad. Sci., 110:8014–8019. doi:10.1073/pnas.1219548110.
- Seinfeld, J.H., Kleindienst, T.E., Edney, E.O., and Cohen, J.B. (2003). Aerosol Growth in a Steady-State, Continuous Flow Chamber: Application to Studies of Secondary Aerosol Formation. Aerosol Sci. Technol., 37:728–734.
- Seinfeld, J.H., and Pandis, S.N. (2006). Atmospheric Chemistry and Physics. Wiley, New York.
- Shilling, J.E., Chen, Q., King, S.M., Rosenoern, T., Kroll, J.H., Worsnop, D.R., et al. (2008). Particle Mass Yield in Secondary Organic Aerosol Formed by the Dark Ozonolysis of Alpha-Pinene. Atmos. Chem. Phys., 8:2073–2088.
- Shiraiwa, M., Yee, L.D., Schlling, K.A., Loza, C.L., Craven, J.S., Zuend, A., et al. (2013). Size Distribution Dynamics Reveal Particle-Phase Chemistry in Organic Aerosol Formation. Proc. Natl. Acad. Sci.110(29):11746–11750.
- Shrestha, M., Zhang, Y., Ebben, C.J., Martin, S.T., and Geiger, F.M. (2013). Vibrational Sum Frequency Generation Spectroscopy of Secondary Organic Material Produced by Condensational Growth from α-Pinene Ozonolysis. J. Phys. Chem. A, 117:8427–8436. doi:10.1021/jp405065d.
- Smith, M.L., Bertram, A.K., and Martin, S.T. (2012). Deliquescence, Efflorescence, and Phase Miscibility of Mixed Particles of Ammonium Sulfate and Isoprene-Derived Secondary Organic Material. Atmos. Chem. Phys., 12:9613–9628. doi:10.5194/acp-12-9613-2012.
- Smith, M.L., Kuwata, M., and Martin, S.T. (2011). Secondary Organic Material Produced by the Dark Ozonolysis of Alpha-Pinene Minimally Affects the Deliquescence and Efflorescence of Ammonium Sulfate. Aerosol Sci. Technol., 45:244–261. doi:10.1080/02786826.2010.532178.
- Smith, M.L., You, Y., Kuwata, M., Bertram, A.K., and Martin, S.T. (2013). Phase Transitions and Phase Miscibility of Mixed Particles of Ammonium Sulfate, Toluene-Derived Secondary Organic Material, and Water. J. Phys. Chem. A, 117:8895–890. doi:10.1021/jp405095e.
- Swietlicki, E., Hansson, H.C., Hameri, K., Svenningsson, B., Massling, A., McFiggans, G., et al. (2008). Hygroscopic Properties of Submicrometer Atmospheric Aerosol Particles Measured with H-TDMA Instruments in Various Environments - A Review. Tellus B, 60:432–469. doi:10.1111/j.1600-0889.2008.00350.x.
- You, Y., Renbaum-Wolff, L., Carreras-Sospedra, M., Hanna, S.J., Hiranuma, N., Kamal, S., et al. (2012). Images Reveal That Atmospheric Particles Can Undergo Liquid–liquid Phase Separations. Proc. Natl. Acad. Sci., 109:13188–13193. doi:10.1073/pnas.1206414109.
- Zhang, Q., Jimenez, J.L., Canagaratna, M.R., Allan, J.D., Coe, H., Ulbrich, I., et al. (2007). Ubiquity and Dominance of Oxygenated Species in Organic Aerosols in Anthropogenically-Influenced Northern Hemisphere Midlatitudes. Geophys. Res. Lett., 34:L13801. doi:10.1029/2007gl029979.
APPENDIX
A generalized form of p(ϵ;d) can be developed for any steady-state flow through reactor having a known or measured distribution pt(t) of particle residence times. The equation is as follows (see the SI):
[A1]
This equation is a generalized version for any growth law and any residence time distributions for different types of steady-flow flow-through reactors. Particle growth occurs on a number–diameter distribution n0(d) of seed particles by gas-phase condensation, heterogeneous interfacial chemistry, or any other mechanism so long as I(d) > 0 for all d. A condition for use of this equation is that the residence time distribution pt(t) must not depend on d0. Further governing conditions and assumptions are discussed in more detail in Kuwata and Martin (Citation2012).
As a check in Equation (A1), use of EquationEquation (6)[6] for t, i.e.,
for condensational growth (cf. EquationEquation (1)
[1] of Kuwata and Martin (Citation2012) and of
for a CMFR (EquationEquation (4)
[4] Kuwata and Martin [Citation2012]) transforms Equation (A1) into the following:
[10]
This result reproduces EquationEquation (10)[10] .
