Abstract
Aerosols dispersed from the oxidation of various uranium alloys exposed to air and direct flame impingement from combustible substrates are characterized. An apparatus was designed to incorporate desired characteristics of previous experiments on uranium to sample aerosol on a kilogram scale in a laboratory environment. Previous studies involving β-phase stabilized uranium (99.25 wt% U:0.75 wt% Ti) were benchmarked using an identical alloy with identical characteristics of the original specimens. Other studies involving a-phase uranium (100 wt% U) were also benchmarked in this experiment. Unique to this study is the use of γ-phase stabilized uranium (94 wt% U:6 wt% Nb). These three alloys represent the crystallographic range of typical uranium metals, providing a complete spectrum of potential uranium aerosolization. Oxidation rates and extents observed in these experiments were directly comparable to existing data and provided correlation between previous studies. These experiments indicate a distinct order-of-magnitude difference between uranium alloy responses to thermal stress.
INTRODUCTION
This article summarizes the first set of results from a multi-year project undertaken by the National Nuclear Security Administration at the Y-12 National Security Complex in Oak Ridge, Tennessee, USA. The primary purpose of these experiments is to characterize the nature and amount of uranium aerosol dispersed if uranium metal is involved in a fire. The use of uranium as a nuclear fuel continues to increase globally, concurrent with global initiatives to convert research reactors and isotope production facilities from the use of highly enriched uranium to low enriched uranium. The net effect is an increase in the amount of uranium-bearing fuels being manufactured, stored, and transported on a global scale. Understanding the thermal response of uranium metal and alloys is essential to protecting public health, safety, and the environment. Unfortunately, the amount of experimental data that exists on the formation and release of respirable oxide fumes from uranium alloys is minimal.
Two main guidance documents exist for quantifying the release of respirable oxide are the American National Standard for Airborne Release Fractions at Non-Reactor Nuclear Facilities (ANSI Citation1998) and the Department of Energy's Handbook of Airborne Release Fractions/Rates and Respirable Fractions for Nonreactor Nuclear Facilities (DOE Citation1994). Both documents were principally authored by the late Dr. Jofu Mishima using the same sets of reference experiments. The bounding airborne respirable release fraction of 1 × 10−3 for uranium aerosol comes from experiments conducted by Elder and Tinkle on β-phase stabilized depleted uranium specimens containing 0.75 wt% titanium at Los Alamos National Laboratories, Los Alamos, New Mexico, USA (Elder and Tinkle Citation1980). Also reported is a median airborne respirable release fraction of 1 × 10−4 for uranium aerosol from experiments conducted by Carter and Stewart on α-phase uranium at the Atomic Weapons Research Establishment, Berkshire, England (Carter and Stewart Citation1970). These values are an order of magnitude apart indicating a potential difference in alloy-specific responses to thermal stress.
The approach taken in this study of potential accident situations was to measure the fractional airborne release and aerodynamic size distribution differences between categories of uranium metal and alloys. Pure uranium has three normal metallurgical phases, ranging from ductile semi-plastic response to brittle fracture under thermal stress scenarios. Uranium alloys allow specific metallurgical phases to be frozen, preserving desired metallurgical characteristics. By subjecting uranium metal and alloys to identical thermal stress, the relative difference in response of each alloy can be quantified. The resultant differences in fractional airborne release between alloys are important in proper characterization of hazards associated with manufacturing, transportation, and storage of uranium bearing materials. This study looks for distinct order-of-magnitude differences between the dominant alloys representing distinct metallurgical phases.
EXPERIMENTAL METHODS
Caution: uranium is a pyrophoric material but sustains burning only in finely divided form (e.g., dispersed small fragments or fine turnings). A respirable oxide fume of uranium can have significant toxicological effects leading to renal failure. The radiological effects of uranium are dependent on the enrichment level. For depleted uranium, used in this study, there is negligible radiological effect. The influence of nephrotoxicity on urinary excretion of uranium indicates that kidney concentrations of 3 μg uranium per g of kidney correlates to kidney damage in humans (Hodgson et al. Citation2007). The International Commission on Radiological Protection's Publication 2 (ICRP Citation1959) uses a nominal kidney mass of 300 g and a fraction of uranium in the kidneys relative to that in the total body of 0.065. As such, an uptake of 30 mg soluble uranium may lead to renal failure. One of the runs in this experiment (U-Ti run 4) exceeded 30 mg U aerosol in the sampling section, representing a toxicological hazard.
Materials
To represent the α-phase (metallurgical phase) in these experiments, pure depleted uranium consisting of α-annealed uranium with only trace quantities of other elements (i.e., less than 20 ppm) was formed into billets and sheared into nominally 1 inch by 2 inch by 2 inch specimens. This is the same form of uranium used by Carter and Stewart in their experiments (Carter and Stewart Citation1970). To represent the β-phase in these experiments, an alloy of 99.25 wt% uranium with 0.75 wt% titanium was immersion quenched in water from the γ-phase that was subsequently aged in a furnace to produce a pure β-phase stabilized alloy. The water quenching process produces nominally 2 ppm hydrogen during aging, with less than 20 ppm other contaminants. In this study, the use of U-Ti refers specifically to this preparation. This is the same preparation used by National Lead of Ohio to prepare the U-Ti specimens used in the Elder and Tinkle Experiment (Zabielski and Levy Citation1994). To represent the γ-phase in these experiments, an alloy of 94 wt% uranium with 6 wt% niobium was produced by vacuum induction melting depleted uranium around a niobium core, then vacuum arc re-melting this electrode configuration into billets that were then used as electrodes in a second vacuum arc re-melting. This produces a homogenous U‑Nb mixture that is γ‑phase stabilized with less than 20 ppm contaminants. In this study, the use of U-Nb refers specifically to this preparation.
Apparatus
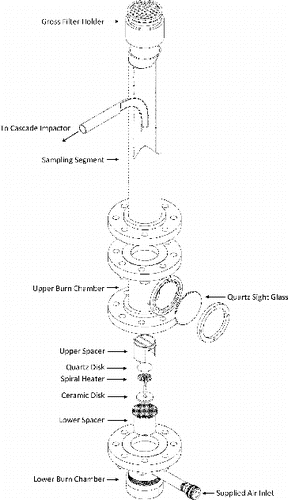
The experimental setup uses a custom designed burn chamber and sampling section incorporating the requirements of ASME NQA‑1 2008 and ANSI/HPS‑N13.1‑1999. An exploded diagram of the apparatus is shown in .
The burn chamber consists of two pieces of nominally a 10 cm (4 inch) diameter, 25 cm (10 inch) long schedule 40 pipe. The lower section contains a 4 inch flange connection on the downstream side and a national pipe thread (NPT) connection on the upstream side with a 4 inch end-cap installed. The lower section has a 1 inch diameter pipe inserted through the sidewall and curving into the centerline of the pipe segment with a curvature ratio (radius of curvature of the bend divided by the tube diameter) of 1.0. The upper section contains a 4 inch flange connection on each side and a 3 inch diameter quartz sight glass welded into the middle of this section in a “T” shape for observation of the uranium piece. Each section is bolted to adjacent sections using a compressible gasket (rated for 1000°C) and standard 304L stainless steel bolts.
The uranium specimen is suspended over the combustible material using a pair of semi-adjustable spacers that are used to situate flame impingement against the specimen and orient the specimen for viewing through the sight glass. The lower spacer is covered by expanded metal mesh and is used to support the heating element and charcoal. Atop the mesh sits a ceramic disk with a spiral resistance heater capable of maintaining temperatures of 1380°C (MC-GAXP-130, MHI GAXP Spiral Microheater, Micropyretics Heaters International, Cincinnati, OH, USA). This heater is used to ignite the charcoal. Between the charcoal and the heater is a small quartz disk that distributes the heat and allows expansion/contraction of the heating element. The charcoal is located beneath the uranium specimen and above the quartz disk. The upper spacer is a nominally 3 inch, 16 gauge stainless steel pipe segment with two 18 gauge tungsten wires spanning the circumference that supports the specimen while not hindering flame impingement on the specimen.
The sampling segment was constructed from a 10 cm (4 inch) diameter, 46 cm (18 inch) long schedule 40 pipe with a 4 inch flange connection on the upstream side and an NPT connection on the downstream side with a 4 inch gross filter holder installed. The sampling segment also has a 1 inch diameter pipe inserted through the sidewall and curving into the centerline of the pipe segment with a curvature ratio (radius of curvature of the bend divided by the tube diameter) of 3.0. The inlet of the sampling nozzle is 6 inches back from the inlet of the sampling section, oriented parallel to the centerline of the pipe. The leading edge of the nozzle has a sharp edge with the external cone angle of 30°. The nozzle is constructed from stainless steel and does not have a rake. To the 1 inch pipe, an eight-stage ambient sampler with 10 μm pre-cutters is attached (Model 20-800 Ambient Cascade Impactor [non-viable], Tisch Environmental, Inc., Cleves, OH, USA). Normal impactor sampling flow rate was 28.3 L/min (1 cfm) at ambient conditions (approximately 775 mb and 20°C) with sensitivity of ±1.5 L/min at 200°C. The sampling section, minus the cascade impactor, was fabricated by the Hi-Q Environmental Products Company (San Diego, CA, USA).
Procedures
The procedure for conducting each run was identical, so that the principal variable between runs was the composition of the uranium specimen. Each run was started with a cleaned apparatus that held less than 0.5 μg residual uranium. A single 4 inch binderless, high-efficiency (HEPA type), high-purity, 100% high-quality borosilicate glass micro fiber filter media, rated for temperatures up to 600°C, with a DOP (dioctyl phthalate) Collection Efficiency of 99.99% for 0.3 μm aerodynamic equivalent diameter (FPAE‑102, Hi-Q Environmental Products Company, San Diego, CA, USA) is held in place across the 102 mm (4 inch) schedule 40 pipe with a screw compression Teflon gasket. Next, 81 mm glass fiber filter media (TE‑20‑301 Glass Fiber Substrate Discs, Tisch Environmental, Cleves, OH, USA) are loaded into each stage of the cascade impactor. Three pieces of Kingsford Original Brand charcoal briquettes (Clorox Company, Oakland, CA, USA), weighing 23.6 ± 0.4 g per briquette, are loaded into the burn chamber atop the quartz disk. The uranium specimen is then loaded across the upper spacer, above the briquettes, and the apparatus is sealed tight.
An airflow rate of 14.2 ± 0.2 scfm is established into the burn chamber, and the vacuum pump pulling 28.3 ± 1.5 L/min is turned on. Next, power is applied to the heating element, which in turn ignites the charcoal. The charcoal is allowed to burn to extinction. One hour after the heating element is turned on, the vacuum pump is turned off. Once the apparatus has cooled to <40°C, airflow to the apparatus is turned off. The apparatus is then disassembled and cleaned. The uranium specimen is hand cleaned of all readily removable oxide. Wipes and filter media from each section of the apparatus are collected for analysis. Residual ash from the apparatus is collected from atop the quartz disk and lower burn chamber for observation and analysis.
Analysis Techniques
Particle size characterization, based on the activity median aerodynamic diameter (AMAD), was obtained using the cascade impactor. In keeping with standard international practices for radiological protection, the AMAD is used for particle sizes for which deposition depends principally on inertial impaction and sedimentation (i.e., typically those greater than about 0.5 μm). For smaller particles, deposition typically depends primarily on diffusion and the activity median thermodynamic diameter (AMTD), which was not measured in this study. The final stage in the cascade impactor has an AMAD of 0.43 μm. For this particle range, the ratio of the Cunningham slip factors are negligible and the ratio between the aerodynamic diameter and the geometric diameter is generally the square root of the density (e.g., [10.92]0.5) divided by an aerodynamic shape factor typically assumed to be 1.0. In this manner, AMADs for uranium are generally three times larger than the corresponding geometric diameter.
The effect of gas temperature on impactor calibration was not corrected for, owing to expected difficulty in measuring gas temperature at each impaction stage in each experiment. The maximum error in the effective aerodynamic cutoff diameter (largest at the pre-cutter and stage 0) would be proportional to (viscosity) where the viscosity of air increases from 185 micropoises (μP) at 25°C (calibration temperature) to 220 μP at 100°C (measured maximum at Stage 1 during the laboratory experiments). The maximum correction factor applied to the pre-cutter and the first few impactor stages in this case would be approximately 1.09 or <10% error in effective aerodynamic cutoff diameter, which is not significant in this instance. The effective aerodynamic cutoff diameter of the pre-cutter is increased from 9–10 μm to 10–11 μm, which skews the fraction of aerosol less than 10 μm in a conservative direction.
All borosilicate glass filter media and ash samples were dissolved in a nitric acid solution. The uranium was extracted from the nitric acid solution by vacuum aspiration over a chromatographic column containing 40% (w/w) diamyl amlyphosphonate on Amberlite XAD-7 or Amberchrom CG-71ms. The column is washed with nitric acid solution to elute most other sample constituents. The uranium is eluted from the column with deionized water, and the elute taken to dryness on a hotplate. The samples were loaded onto rhenium or tantalum filaments and analyzed using a Finnigan Triton 2 Thermal Ionization Mass Spectrometer. The minimum detectable uranium by this method is 0.15 μg.
RESULTS
The masses of uranium oxide collected in sample locations combined with the mass balance on the metal specimen quantify the thermal response for each of the 15 experimental runs summarized in . The initial mass of the specimens was measured in milligrams, whereas aerosolized oxide values are reported in micrograms. Positive values of the mass delta indicate adherent oxide not mechanically separable from the base specimen. All 15 experimental runs utilized the same specific area (i.e., surface area to mass ratio), although the initial mass of the U-Ti is one-third of other alloys. U-Ti alloy was the rarest and most difficult to obtain; therefore, the smaller mass was an attempt to conserve the amount of alloy available.
TABLE 1 Specimen oxidation and oxide collection data
Observation of the oxidation runs in was conducted through the quartz sight glass. Generally, the pure uranium sparked occasionally for 5–7 min while the U-Ti alloy sparked almost continually for a similar duration. The U-Nb alloy was only observed to spark in U-Nb Run 3 where a burr on the edge of the specimen separated from the specimen in a single spark event.
For each of the 15 runs, the filter media from the impactor was analyzed for mass of uranium, as reported in . All values in have been reported to the nearest μg, although measured to the nearest 0.01 μg.
TABLE 2 Mass of uranium collected in each stage of the cascade impactor
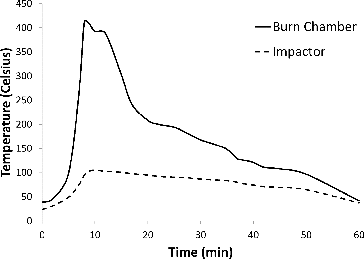
The impactor stages reported in correspond to effective cutoff diameters shown in . The temperature corrections based on the maximum temperatures observed in the sampling section are included in . The air temperatures measured in the burn chamber and at the impactor sampling location are shown in . The temperature measured in the sampling section was used to determine the impact on the effective cutoff diameter of the cascade impactor and to monitor exhaust temperatures.
TABLE 3 Effective aerodynamic cutoff diameters in the cascade impactor
DISCUSSION
Previous experiments assessing the amount of respirable aerosol likely to be released in a fire involving the oxidation of uranium metals have employed the use of geometric means and 95% confidence limits. The maximum values for the dataset in and are compared to the 95% confidence limits and the geometric means of that dataset are reported in for comparison with previous experiments.
TABLE 4 Representative experimental data by alloy
demonstrates a distinctly different thermal response from each of the alloys. The U‑Nb alloy experienced markedly less oxidation and aerosol. The mass delta associated with oxidation of U‑Ti covered the same range as the oxidation of the pure U with markedly higher amounts of aerosol and mechanically separable oxide from the specimen. The experimental data indicates the amount of respirable oxide is highly alloy dependent with nominally order-of-magnitude differences. The representative airborne release fraction (ARF) for the metal is taken as the total mass in the sampler divided by the initial mass of the metal specimen. As used in this study, the respirable fraction (RF) of the aerosol is defined as the total mass collected on stages 1–7 plus the final filter divided by the total impactor mass. As used in this study, the term oxide fume reflects the total mass collected in the sampling section. reflects the ARF, RF, and resultant product reflecting the airborne respirable release fraction (ARF × RF).
TABLE 5 Representative release fractions and limits by alloy
The Elder and Tinkle (Citation1980) experiments have a geometric mean ARF of 1 × 10−4, RF of 50%, and ARF × RF of 7 × 10−5. These values represent the sampling during outdoor burn test #4 in those experiments. The maximum values from Elder and Tinkle outdoor burn test #4 were an ARF of 9 × 10−4, RF of 62%, and ARF × RF of 6 × 10−4. From , the geometric mean values are comparable between these experiments and the Elder and Tinkle outdoor burn test #4. The maximum values with an ARF of 1 × 10−4 and RF of 60% from are also comparable to the Elder and Tinkle experiment. Subsequently, the close alignment between these values indicates appropriate benchmarking of these experiments against those previous studies.
The previous studies by Carter and Stewart (Citation1970) have noted, “It is clear that the sparking phenomenon is the primary source of the fume fraction of the aerosol in all the dynamic experiments, and if the events where sparking was particularly evident are considered as a distinct group, the data fall within fairly well-defined limits.” Carter and Stewart reported geometric mean and 95th percentile fractions of source material releases as aerosols from partial disruption of liquid into droplets. The geometric mean values are 3.5 × 10−3 for Pu and 1.9 × 10−3 for α-phase U, while the 95th percentile values are 1 × 10−2 for Pu and 6 × 10−3 α-phase U.
Acknowledging that temperatures associated with visible sparking are associated with liquid metals, this work directly correlates the aerosol from each of the sparks to the aerosol from partial disruption of liquid droplets. Normalization of the data in to an ash:fume ratio is made for direct comparison to the partial disruption of liquid droplets wherein the total mass in the sampler is divided by the product of the uranium concentration in the ash and the average ash mass (i.e., 71 g ash). As shown in , this normalization indicates that U-Ti (β‑phase U) responds similarly to Pu while α‑phase U and U-Nb (γ‑phase uranium) respond similarly to U in previous experiments. The maximum release for β‑phase U was 1.2 × 10−2 associated with U-Ti Run 4. The maximum release for α‑phase U was 4.7 × 10−2 associated with pure U Run 3, while for γ-phase U was 6.4 × 10−3 associated with U‑Nb Run 3. These values indicate there is a correlation between the U-Ti and Pu from the Carter and Stewart (Citation1970) experiments, while U-Nb and pure U correlate to the α-phase U.
Looking for a correlation between U-Ti and Pu leads to consideration of the crystallographic structure for the alloys. The Pu in the Carter and Stewart (Citation1970) experiments consists of 144 mg metal rods that had been heated to 660°C and were dropped 0.75 m in an Ar atmosphere. As such, the correlation between a liquid Pu and solid U-Ti is not readily apparent. X-ray diffraction studies conducted at Oak Ridge National Laboratory (ORNL) show the crystallographic orientation of the different phase-stabilized alloys tested in this study (Yakel Citation1974). The number of atoms in each unit cell for each of the alloys range from 2 for the γ-phase U-Nb to 30 for the β-phase U-Ti, with pure U having four in the α-phase. Plutonium, being a particularly interesting metal, has many metallurgical phases in the solid state and can have nonlinear response to thermal stress. Lawson describes the number of atoms in a unit cell for plutonium can be 34 for the β-phase up to 58 for the ζ‑phase, which contains at least 10 crystallographically distinct Pu atom types (Lawson Citation2006). Subsequently, if Pu liquid droplets were separated from ζ‑phase Pu, the substrate would have 34 to 68 atoms per unit cell. Consequently, liquid droplets of Pu are comparable to U‑Ti.
The Carter and Stewart experiments also reported airborne respirable release fractions for ignition and burning of metal in air with a geometric mean ARF × RF of 1.1 × 10−4 and a 95% confidence limit ARF × RF of 3.6 × 10−4. The specific areas of the specimens in this study were insufficient to achieve ignition and complete oxidation. However, a comparison to these levels can be made by dividing the amount of material collected in the sampling section by the amount of oxide formed (mass delta for the specimen). This approach results in a geometric mean ARF × RF of 2 × 10−4 and a maximum ARF × RF of 4 × 10−4. Subsequently, the close alignment between these values indicates appropriate benchmarking of these experiments against those previous studies.
Future Work
This work uses specimens with low specific areas and observed that the aerosol fume appeared to be dominated by sparking. Observations related to the ash:fume ratio indicate that the use of high specific area forms (e.g., chips, machine turning, saw fines) will result in complete oxidation of the metal. Complete oxidation of the base metal should maximize the amount of aerosol and determine if the ash:fume ratio observations hold true for ignition conditions. This should allow for direct comparison between uranium values for ignition and burning. As such, future work will employ chips and machine turnings of the three alloys used in this study, as well as any additional alloys identified for study.
The indications that airborne respirable release fractions may be correlated to crystallographic structure will be investigated by using additional alloys of uranium. The U–Mo system with 10 wt% Mo is a γ-phase stabilized alloy with nominally two atoms per unit cell. Other alloys, such as U–Al, will be selected based on availability and number of atoms per unit cell. These alloys will be tested in both the high and low specific area configurations in an attempt to expand the dataset for evaluating the potential correlation between crystallographic structure and aerosol. The calculations for comparison against previous experiments used in the American National Standards Institute (ANSI) and U.S. Department of Energy (DOE) guidance documents will include the same process outlined in this study.
CONCLUSIONS
The apparatus design has performed well in all benchmark tests, including producing data directly comparable to the Elder and Tinkle experiments in 1980 and the Carter and Stewart experiments in 1970. Whereas the previous studies used two distinctly different methods for determining respirable aerosol, this study allows for a single experimental method that incorporates desired attributes of both experiments. The intent of this study was to look for distinct order-of-magnitude differences between the dominant alloys representing distinct metallurgical phases. That objective was accomplished, demonstrating that the thermal response of U-Ti is significantly higher than the thermal response of other uranium alloys. As such, respirable fractions based on that alloy are not representative of pure uranium or other alloys.
The distinct order-of-magnitude differences between dominant alloy types appear to be tied to the observation of sparks and the underlying crystallographic structure with certain structures being more or less prone to sparking than the base metal. As the number of atoms in each unit cell increases from 2 (U-Nb) to 30 (U-Ti), the intensity and duration of sparking also increases from a single event to sustained sparking over a duration of 5–7 min. The number of atoms in a unit cell also impacts how the oxide layer adheres to the base metal, as observed by the adherent oxide for U-Nb compared to the non-adherent oxide produced by U-Ti. These factors impact the overall aerosolization of the metal.
The Elder and Tinkle data used to support the bounding ARF × RF of 1 × 10−3 in DOE guidance appears to be specific to the U–Ti alloy. The Carter and Stewart data supporting the median ARF × RF of 1 × 10−4 in that DOE guidance is appropriate for pure uranium and U–Nb alloys. Both of these values have been benchmarked using the apparatus specifically designed for this study indicating that the apparatus can produce data of sufficient quality for use in regulatory applications. Future studies are planned to investigate demonstrable links between crystallographic structure and respirable aerosol.
SUMMARY
This study identifies that all uranium alloys are not equal and that there appears to be distinct order-of-magnitude differences between the dominant alloys representing distinct metallurgical phases. The Elder and Tinkle data used to support the bounding ARF × RF of 1 × 10−3 in DOE guidance appear to be specific to the U–Ti alloy. The Carter and Stewart data supporting the median ARF × RF of 1 × 10−4 in that DOE guidance is appropriate for pure uranium and U–Nb alloys.
DISCLAIMER
This work of authorship and those incorporated herein were prepared by Consolidated Nuclear Security, LLC (CNS) as accounts of work sponsored by an agency of the United States Government under contract DE‑NA0001942. Neither the United States Government nor any agency thereof, nor CNS, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, use made, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency or contractor thereof, or by CNS. The views and opinions of the author expressed herein do not necessarily state or reflect those of the United States Government or any agency or contractor thereof, or by CNS.
ACKNOWLEDGMENTS
This work is dedicated in the memory of the late Dr. Jofu Mishima, who devoted much time assisting with the initial design and qualification of this work. Dr. Mishima was the author of DOE's Handbook on Airborne Release Fractions/Rates and Respirable Fractions and ANSI/ANS‑5.10‑1998, Airborne Release Fractions at Non-Reactor Nuclear Facilities. The author appreciates the fruitful discussions with and insightful comments from members of the Defense Nuclear Facilities Safety Board staff over the course of this work.
Funding
This research is funded through a combination of the Y-12 National Security Complex's Plant Directed Research and Development and DOE's Nuclear Safety Research and Development programs.
REFERENCES
- ANSI. (1998). American National Standard for Airborne Release Fractions at Non-Reactor Nuclear Facilities, ANSI/ANS-5.10-1998. American Nuclear Society, La Grange Park, Illinois, USA.
- Carter, R. F., and Stewart, K. (1970). On the Oxide Fume Formed by the Combustion of Plutonium and Uranium, in Inhaled Particles III (Proceedings of an International Symposium, British Occupational Hygiene Society, London, 9/14-23/70). Unwin Brothers Limited – The Gresham Press, Old Working, Surrey, England, pp. 819–838.
- DOE. (1994). Airborne Release Fractions/Rates and Respirable Fractions for Nonreactor Nuclear Facilities, Volume I - Analysis of Experimental Data. DOE-HDBK-3010-94. U.S. Department of Energy, Washington, DC.
- Elder, J. C., and Tinkle, M. C. (1980). Oxidation of Depleted Uranium Penetrators and Aerosol Dispersal at High Temperatures. LA-8610-MS. Los Alamos National Laboratory, Los Alamos, NM.
- Hodgson, A., Pellow, P. G. D., and Stradling, G. N. (2007). Influence of Nephrotoxicity on Urinary Excretion of Uranium. HPA-RPD-025. Health Protection Agency, Chilton, Didcot, Oxfordshire, England.
- ICRP. (1959). Report of Committee II on Permissible Dose for Internal Radiation. ICRP Publication 2, Ottawa, Ontario, Canada.
- Lawson, A. (2006). Plutonium Magic. Los Alamos Sci. 30:90–95.
- Yakel, H. L. (1974). A Review of X-Ray Diffraction Studies in Uranium Alloys. in Proceedings of the Physical Metallurgy of Uranium Alloys Conference Sponsored by the AEC Army Material and Mechanical Research Center, Vail, Colorado, Feb. 12–14.
- Zabielski, C. V., and Levy, M. (1994). Fracture Toughness and Stress Corrosion Resistance of U-0.75 wt% Ti. Tri-Service Committee on Corrosion Proceedings. U.S. Army Research Laboratory, Watertown, Massachusetts, USA.