Abstract
Reusable glass dishes are recommended for use with the six-stage viable impactor for size-fractionated bioaerosol sampling. However, it is not convenient to use glass dishes because they are fragile and heavy, not to mention the time-consuming preparation process prior to bioaerosol sampling. On the other hand, disposable plastic dishes have been widely used in microbiology laboratories. However, plastic materials can retain electrostatic charges and may lead to sampling bias. The objective of this study was to evaluate the sampling bias with the use of plastic dishes when a multistage viable impactor is used for airborne fungi and bacteria sampling for field sampling. Two six-stage viable impactors were placed side-by-side 1 m apart in a 147-m3 room. One was used with plastic dishes and the other with glass dishes. Compared with the concentration data obtained with glass dishes, those collected with the plastic dishes demonstrated a significant difference for both fungi and bacteria. However, there was a strong correlation between the data obtained using glass and plastic dishes, which can be estimated by Cplastic = 0.88 Cglass for airborne fungi and Cplastic = 0.86 Cglass for airborne bacteria. When using plastic dishes fungi and bacteria counts were underestimated by 12% and 14%, respectively.
Copyright 2015 American Association for Aerosol Research
INTRODUCTION
Among commercially available bioaerosol sampling instruments, the six-stage viable Andersen impactor is the most commonly used sampler for the size characterization of airborne viable particles. It has been widely used in a variety of studies to investigate the airborne bacteria and/or fungi in indoor environments. For example, residential buildings (Reponen et al. Citation1989, Citation1994; Lin and Li Citation1996; Górny et al. Citation1999; Lee and Jo Citation2006; Nasir and Colbeck Citation2010), schools (Meklin et al. Citation2002), child care centers (Reponen et al. Citation1994; Kim and Kim Citation2007), hospitals, elderly welfare facility, postpartum nursing center (Kim and Kim Citation2007), and so forth. It has been also employed to characterize bioaerosol emission sources which may pose a health risk to workers and the general public, such as the swine confinement building (Agranovski et al. Citation2004), waste composting plants (Tolvanen Citation2004; Sanchez-Monedero et al. Citation2005; Byeon et al. Citation2008), poultry slaughtering, and processing plants (Heber et al. 2006) and wastewater treatment plants (Sawyer et al. Citation1996). Moreover, some researchers used it to evaluate the effectiveness of bioaerosol control measures, such as UV units (Lai et al. Citation2003), biofiltration (Sanchez-Monedero et al. Citation2003), and floating objects (Hung et al. Citation2010).
An aerosol sampler is intended to give a representative measure of the particle concentration in air. Electrostatic charge has been raised as a factor influencing the accuracy of aerosol sampling when nonconductive plastic materials were used in the sampler. Many types of plastic materials such as polystyrene, polycarbonate, and polyvinyl chloride readily retain certain charge levels. These charges can be incorporated in the bulk plastic during manufacture or accumulated on the surface by handling or contact with other objects (Liu et al. Citation1985). Commonly used for workplace aerosol sampling, the 37-mm plastic filter cassette has been found to exhibit negative sampling biases due to particle losses to the internal walls of the sampler (Baron and Deye Citation1990).
The negative sampling bias has also been observed when freshly laboratory-generated bacteria aerosols were collected with plastic agar plates and the viable cascade impactor. (Andersen Citation1958). Normally, freshly generated aerosols can carry electrostatic charge and then lose it slowly by attracting oppositely charged airborne ions produced by natural radiation. Aerosols can lose most of their charges in time period of about an hour, indicating that only freshly generated aerosols are likely to carry significant charge levels (Hinds Citation1999). In other words, charge levels on viable aerosols in the field may differ from those freshly generated in laboratory due to particle aging.
In fact, bacterial adhesion to external surfaces has been understood in terms of electrostatic interactions (Van Loosdrecht et al. Citation1987). Moreover, electrostatic attraction has been demonstrated to be effective on the collection of airborne microorganisms (Mainelis et al. Citation2001, Citation2002; Lee et al. Citation2004). Normally, bacteria display a negative surface charge under physiological conditions which is associated with the composition of the cell envelope (Van Loosdrecht et al. Citation1987). For example, the surface charge of Mycobacterial cells varied from –22.6 to –29.7 mV (Ayala-Torres et al. Citation2014). Miksch et al. found that deposition rates were slightly higher on surfaces with a positive potential relative to those with a negative potential and this is consistent with reports that charged bacterial aerosols have a slight bias toward a net negative (Miksch et al. Citation2009). Kregiel et al. (Citation2012) demonstrated that phosphorylation of the mannosyl side chains gives yeast (Saccharomyces sp.) its anionic surface charge. However, characteristics of the surface charge of fungi are more diverse than that of bacteria. Holder et al. (Citation2007) found the net surface charge ranging from +4 to −4 mV for blastospores of Beauveria (Cordyceps) bassiana.
The viable cascade impactor is commonly employed to conduct the measurements of airborne fungi and bacteria. Although charge levels carried by different kinds of microorganisms (e.g., bacteria or fungi) may vary. No information regarding the sampling bias of airborne fungi is yet available. To provide information regarding the sampling bias caused by plastic dishes used with the six-stage viable impactor in the field, the present study evaluated the effect of glass versus plastic dishes on the sampling performance of viable cascade impactor for airborne fungi and bacteria.
MATERIALS AND METHODS
The six-stage viable impactor (Graseby Andersen, Atlanta, GA, USA), a multiorifice cascade impactor, is normally employed to measure number concentration and particle size distribution of airborne fungi and bacteria. Each stage contains a plate perforated with 400 holes and immediately below a petri dish of culture medium. Air is drawn through the device at a gas flow rate of 28.3 L/min and the cut-off aerodynamic diameters for the consecutive stages are 7.0, 4.7, 3.3, 2.1, 1.1, and 0.65 μm, respectively.
Malt extract agar (Difco malt extract agar, ref. 211220, Becton, Dickinson and Company, Sparks, MD, USA) and trytic soy agar (Difco tryptic soy agar, ref. 236950, Becton, Dickinson and Company, Sparks, MD, USA) were used for collection and subsequent culture of airborne fungi and bacteria. Media were dispensed in 27-ml aliquots into 90 × 15 mm glass dishes and 45-mL for 85 × 15 mm plastic dishes (GPlus petri dish, 1600-4NGPLUS, Alpha Plus Scientific Co., Taoyuan, Taiwan) to achieve the same jet-to-plate distance of 1 mm. After air sampling, malt extract agar for fungi was incubated at 25°C for 2–5 days while trytic soy agar for bacteria was incubated at 30°C for 2 days. After incubation for the specified time, colonies on each plate were counted and the colony counts from third to sixth stages were corrected for multiple impactions according to the “positive hole” conversion table (Andersen Citation1958).
The experiments were conducted in a 147-m3 room (7 m × 7 m × 3 m height) which is an office with natural ventilation and made of concrete. The air was well mixed by the four ceiling fans for 10 min before each air sampling. Two six-stage viable impactors were placed side-by-side 1 m apart and at a sampling height of 1.5 m. To give a representative measure of airborne microorganisms, the sampling time varied depending on the airborne microbial concentration and the size of colonies formed on the agar plate. In general, the sampling time was 5 min for fungi samples and 10 min for bacteria samples. The temperature ranged from 18 to 24°C during the sampling period and the relative humidity varied from 50% to 90%. In this work, two types of dishes, including plastic and glass dishes, were used and both fungi and bacteria were sampled. For each combination of dish and microorganism, 30 tests were conducted, yielding a total of 120 measurements. These measurements were conducted in seven separate days within 3 months.
The voltages produced by the electrostatic charge on dishes were measured using a non-contacting electrostatic voltmeter (Surface voltmeter, Model SVM2, Salt Lake City, UT, USA). The electrostatic charges of empty plastic dishes were measured right after being taken out from the packaging plastic bag. Generally, the agar plates were prepared and kept at room temperature and humidity 1 day before sampling. The electrostatic charges were measured on these agar plates right before they were used for sampling. After sampling, the electrostatic charge on agar plates were measured as soon as possible because the neutralization of electrostatic charge starts once exposed to the outside air. The measurements were accomplished normally within 30 s.
For an insulator like plastic or glass dish, the meter reading (in volts) is proportional to the dish's charge per area (in coulombs/cm2) on the surface. The dish's charge per area “Q/A” can be estimated by the following equation.[1] where Q is the quantity of charge in coulombs, A is the area of the dish in cm2, V is the meter reading in volts, f is the square root of [1 + D2/4L2], D is the diameter of the sensor disc (2 cm), and L is the sensor-to-dish distance, which was 2.5 cm in this case. The surface potentials for non-conductive plastic dishes were highly variable in polarity and magnitude across their surface. For each type of dishes, 24 dishes were randomly selected and the electrostatic charge measurements were conducted on five fixed locations from each dish. This yielded a total measured area of 15.7 cm2 while the areas are 56.7 and 70.8 cm2 for plastic and glass dishes, respectively.
RESULTS AND DISCUSSION
Concentrations of Airborne Fungi and Bacteria Using Plastic and Glass Dishes
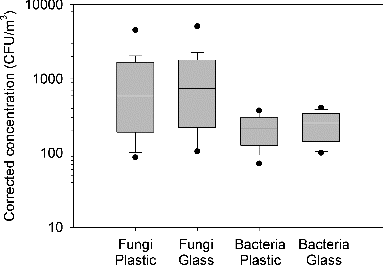
According to the data of side-by-side sampling, the fungal concentrations were 565-5228 CFU (colony forming unit)/m3 for plastic dishes and 707-5131 CFU/m3 for glass dishes. A significant difference was found in the means of the two groups by paired t test (n = 30, p < 0.001). As for airborne bacteria, the concentrations were 95-1329 CFU/m3 for plastic dishes and 106-1452 CFU/m3 for glass dishes. The difference between the bacteria concentration data of the two groups was significant by paired t test (n = 30, p < 0.001). shows the spread of the concentration data for the four groups from left to right in the following order, fungal counts with plastic dishes and glass dishes, bacterial counts with plastic and glass dishes. Both fungal and bacterial samples yielded higher concentrations with glass dishes than with plastic dishes. Moreover, we identified that Cladosporium was the most abundant genus and followed by Aspergillus, Penicillium, and unidentified filamentous fungi. No differences on the types of the fungi were observed between the colocated samples.
FIG. 1. Comparison of fungal and bacterial concentrations using plastic and glass dishes. The top and bottom end of the box represent the 75th and 25th percentiles, respectively, and the line inside the box indicates the median. The bottom and top lines indicate 5th and 95th percentiles. Single points indicate the extremum values.
Size Distributions of Airborne Fungi and Bacteria Using Plastic and Glass Dishes
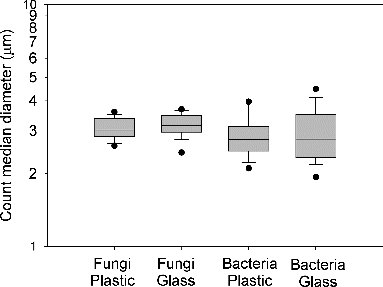
The size distribution patterns of bioaerosols with different kinds of dishes were similar. The count median diameter (CMD) and geometric standard deviation (GSD) were calculated from the concentration data of the six stages for each sample. Results showed that the CMDs of airborne fungi with the use of plastic and glass dishes were 3.1 ± 0.3 and 3.2 ± 0.4 μm, respectively. The GSDs of the two groups were comparable with 1.4 ± 0.1 and 1.4 ± 0.2 for plastic and glass dishes, respectively. Moreover, no significant difference was found for the fungal CMDs of these two groups by paired t test (n = 30, p = 0.22). As for airborne bacteria, the CMDs were 2.9 ± 0.6 and 2.9 ± 0.8 μm for plastic and glass dishes, respectively. The GSDs were 2.0 ± 0.3 and 2.0 ± 0.4 for plastic and glass dishes, respectively. No significant difference was found for the bacterial CMDs of the two dish types by paired t test (n = 30, p = 0.65) and the GSDs of the two groups were comparable. shows the box plot of the CMDs of the four test groups. As can be seen, the spread of CMDs of airborne fungi is less than that of airborne bacteria in the test environment.
FIG. 2. Count median diameters (CMDs) of airborne fungi and bacteria with plastic and glass dishes. The top and bottom end of the box represent the 75th and 25th percentiles, respectively, and the line inside the box indicates the median. The bottom and top lines indicate 5th and 95th percentiles. Single points indicate the extremum values.
Relationship Between Microbial Concentrations with use of Plastic and Glass Dishes
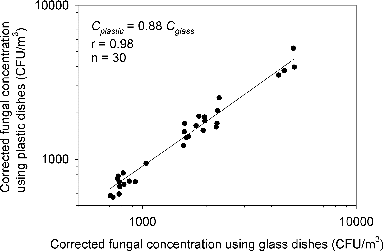
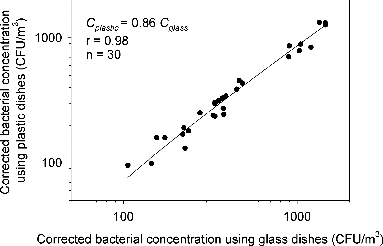
The relationships between microbial concentrations with use of plastic and glass dishes were assessed by Pearson correlation analysis. A good agreement of concentrations with the two groups was found for both fungi and bacteria, as shown in and , respectively. For fungi, the concentrations using plastic dishes can be predicted by Cplastic = 0.88 Cglass (n = 30, p < 0.0001) with r equals to 0.98. The fungal concentrations were underestimated by 12% as plastic dishes were used with the six-stage Andersen viable impactor. As for bacteria, the concentrations using plastic dishes can be estimated by Cplastic = 0.86 Cglass (n = 30, p < 0.0001) with r equals to 0.98. The bacterial concentrations were underestimated by 14% as plastic dishes were used. A difference of 2% was found for the concentration estimation of different kinds of microorganisms (i.e., bacteria and fungi) and this may be attributed to the different constituents and/or surface properties of microbial aerosols as abovementioned in the introduction section.
The experimental results showed that the bacterial concentrations with plastic dishes were underestimated by 14%, which was less than that measured by Andersen (20%) in a laboratory setting (Andersen Citation1958). In Andersen's study, two bacteria (Bacillus subtilis and Serratia marcescens) were freshly generated and the experiments were conducted for nine replicates in the laboratory. On the contrary, the experiments of this study were conducted in a real-world setting with a sample size of 30. The present results provide more realistic estimation on bacteria concentrations with plastic dishes.
Variability Between Two Identical Side-by-Side Samplers on Microbial Concentrations
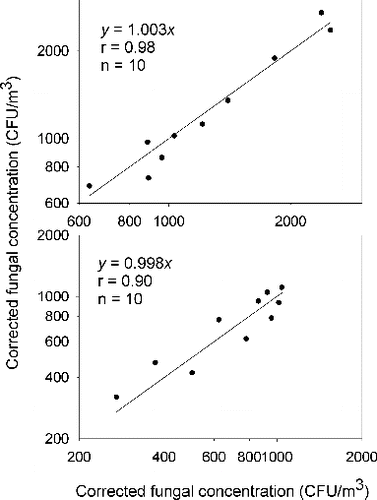
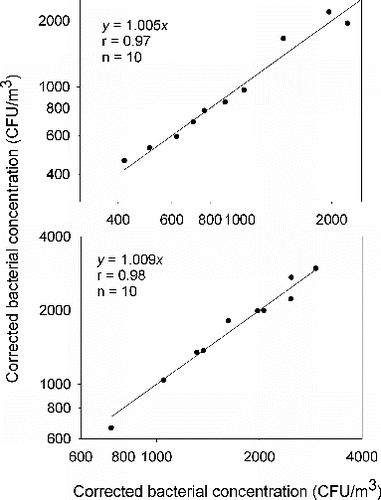
To evaluate the reliability of the correlation of microbial concentrations between plastic and glass dishes, a side-by-side comparison of the same type of dishes were also conducted. represents the fungal concentration data of the side-by-side samplers with plastic and glass dishes, respectively. The concentration data obtained from the colocated samplers with plastic dishes and with glass dishes yielded linear regression lines with slopes of 1.003 and 0.998. represents the bacterial concentration data of the side-by-side samplers with plastic and glass dishes, respectively. The concentration data obtained from the colocated samplers with plastic dishes and with glass dishes yielded linear regression lines with slopes of 1.005 and 1.009. In summary, the experimental data demonstrated good correlations of fungal and bacterial concentration data between the same dish types.
Sampling Bias with the Use of Plastic Dishes
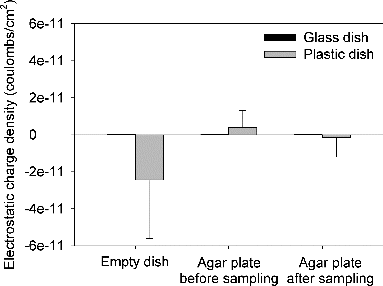
Since the negative sampling bias was probably caused by the electrostatic charge on plastic dishes (Andersen Citation1958), the electrostatic charges carried by plastic and glass dish were measured. shows the electrostatic charge densities of empty dishes, agar plates before and after sampling for 15 min with both dish types. The means and standard deviations of charge densities of glass dishes were 1.4 × 10−13 ± 8.5 × 10−14, 5.1 × 10−13 ± 6.9 × 10−14, and 8.1 × 10−14 ± 1.0 × 10−13 coulombs/cm2 for empty dishes, agar plates before and after sampling, respectively. The means and standard deviations of charge densities of plastic dishes were –2.4 × 10−11 ± 3.2 × 10−11, 3.9 × 10−12 ± 9.1 × 10−12, and -1.5 × 10−12 ± 1.1 × 10−11 coulombs/cm2 for empty dishes, agar plates before and after sampling, respectively. The measurements revealed that the charges on plastic dishes were highly variable in both polarity and magnitude across the surface and between dishes. Moreover, the average charge level on the glass dish was less than that on the plastic dish by 1 or 2 orders of magnitude.
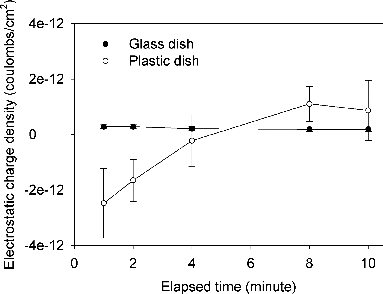
It is noted that the negative electrostatic charges of plastic dishes can be lost as they are exposed to the air. The charges of both glass and plastic agar plate were measured after 15-min sampling. shows decay kinetics of electrostatic charges of glass and plastic dishes. As can be observed from the decay curves, the glass dish was slightly positively charged and remained stable, whereas the electrostatic charges carried by plastic dishes started to decay quickly as soon as the dishes were exposed to the atmosphere.
There are a number of mechanisms that can cause a plastic object to be charged. During manufacturing, electrical charges can be localized in the material of the plastic. After that, the plastic dish may acquire charge generally through triboelectric charge transfer and direct contact with charged objects. In this study, the empty plastic dish was highly negatively charged. However, most of the negative charges carried by the empty plastic dishes were neutralized when the culture medium was filled. Then after sampling for 15 min at RH 60%, the plastic agar plate became slightly negatively charged again. For the multistage viable impactor used in the present study, it was reported that the relative humidity within the sampler increased rapidly from 39% to 88% for the consecutive stages as the air entering the sampler was quite dry (i.e., 23%) (Andersen Citation1958). Moreover, Baron and Deye found that a strong increase in charge and a decrease in aerosol concentration occurred only when the relative humidity was decreased below 15% inside the plastic sampler (Baron and Deye Citation1990). This finding implies that the plastic dishes did not likely gain any charge through triboelectric charge transfer during the sampling process under the humidity conditions (50%–90% RH) in this study.
Another possible reason for the lower concentration obtained with plastic dishes could be the different size of plastic and glass dishes. In this study, the plastic plates were 5 mm smaller in diameter than the glass ones. The impactor stages are 8.2 cm in diameter and the jet-to-plate distances are 1 mm for all stages. The amount of agar was adjusted to achieve the same jet-to-plate distance both for glass and plastic dishes. The air moves toward the inner wall of the plates with an approximate velocity around 180 cm/s. The air velocities through the nozzles of stages 1–6 are 108, 180, 297, 528, 1278, and 2329 cm/s, respectively, at the operating flow rate of 28.3 L/min (Andersen Citation1958). Considering the Stokes number for each impaction stage, particle impaction may occur only in the first stage. Moreover, the microbial recovery was relative low in the first stage as compared to the total recovery based on the CMD and GSD data. Therefore, the different size of the dishes may not significantly contribute to the differences between plate types observed in this study.
In addition, disposable plastic plates may not fit as flatly on the three support posts on the sampling stages as do smooth-bottomed glass dishes, especially those with ridge around the dish bottoms. However, the plastic dishes used in this study can fit flatly on the sampling stages except for the last stage. Nevertheless, this would not cause any significant bias on the results because very few microorganisms were generally found on the impaction plate of the last stage.
CONCLUSIONS
Owing to the electrostatic charge, the use of plastic dish with the viable cascade impactor was demonstrated to give a lower concentration on both airborne fungi and bacteria sampling as compared with the use of glass dish. The fungi and bacteria concentration were underestimated by 12% and 14%, respectively. However, no significant difference was found for the size distributions of airborne fungi and bacteria measured using plastic and glass dishes. With the correction of concentration data, it is feasible to use disposable plastic dishes with multistage viable samplers to save time and labor for viable aerosol sizing in practical applications, especially when the relative humidity is over 50%.
ACKNOWLEDGMENTS
Special thanks to Ping-Cheng Wu, Pei-Fong Ciou, Ting-Chen Tsao, and Yi-Ling Cheng for their assistance with the experimental works.
Funding
This study was partially supported by the National Science Council (Grant No. NSC102-2221-E-273-002) and Ministry of Science and Technology of Taiwan (Grant No. MOST 103-2221-E-273-002-MY2).
REFERENCES
- Ayala-Torres, C., Hernández, N., Galeano, A., Novoa-Aponte, L., and Soto, C. Y. (2014). Zeta Potential as a Measure of the Surface Charge of Mycobacterial Cells. Ann. Microbiol., 64:1189–1195.
- Agranovski, V., Ristovski, Z., Blackall, P. J., and Morawska, L. (2004). Size-Selective Assessment of Airborne Particles in Swine Confinement Building with the UVAPS. Atmos. Environ., 38:3893–3901.
- Andersen, A. A. (1958). New Sampler for the Collection, Sizing, and Enumeration of Viable Airborne Particles. J. Bacteriol., 76:471–484.
- Baron, P. A., and Deye, G. J. (1990). Electrostatic Effects in Asbestos Sampling I: Experimental Measurements. AIHAJ 51:51–62.
- Byeon, J. H., Park, C. W., Yoon, K. Y., Park, J. H., and Hwang, J. (2008). Size Distributions of Total Airborne Particles and Bioaerosols in a Municipal Composting Facility. Bioresour.Technol., 99:5150–5154.
- Górny, R. L., Dutkiewicz, J. J., and Krysińska-Traczyk, E. (1999). Size Distribution of Bacterial and Fungal Bioaerosols in Indoor Air. Ann. Agri. Environ. Med., 6:105–113.
- Hinds, W. C. (1999). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. John Wiley & Sons, Inc., New York, p. 434.
- Holder, D. J., Kirkland, B. H., Lewis, M. W., and Keyhani, N. O. (2007). Surface Characteristics of the Entomopathogenic Fungus Beauveria (Cordyceps) Bassiana. Microbiology, 153:3448–3457.
- Hung, H. F., Kuo, Y. M., Chien, C. C., and Chen, C. C. (2010). Use of Floating Balls for Reducing Bacterial Aerosol Emissions from Aeration in Wastewater Treatment Processes. J. Hazard. Mater., 175:886–871.
- Kim, K. Y., and Kim, C. N. (2007). Airborne Microbiological Characteristics in Public Buildings of Korea. Build. Environment., 42:2188–2196.
- Kregiel, D., Berlowska, J., and Szubzda, B. (2012). Novel Permittivity Test for Determination of Yeast Surface Charge and Flocculation Abilities. J. Ind. Microbiol. Biotechnol., 39:1881–1886.
- Lai, M. H., Moschandreas, D. J., and Pagilla, K. R. (2003). Airborne Bacteria Control Under Chamber and Test-Home Conditions. J. Environ. Eng., 129:202–208.
- Lee, J. H., and Jo, W. K. (2006). Characteristics of Indoor and Outdoor Bioaerosols at Korean High-Rise Apartment Buildings. Environ. Res., 101:11–17.
- Lee, S. A., Willeke, K., Mainelis, G., Adhikari, A., Wang, H., Reponen, T., et al. (2004). Assessment of Electrical Charge on Airborne Microorganisms by a New Bioaerosol Sampling Method. J. Occup. Environ. Hyg., 1:127–138.
- Lin, W. H., and Li, C. S. (1996). Size Characteristics of Fungus Allergens in the Subtropical Climate. Aerosol Sci. Technol., 25:93–100.
- Liu, B. Y. H., Pui, D. Y. H., Rubow, K. L., and Szymanski, W. W. (1985). Electrostatic Effects in Aerosol Sampling and Filtration. Ann. Occup. Hyg., 29:251–269.
- Mainelis, G., Willeke, K., Baron, P., Grinshpun, S. A., and Reponen, T. (2002). Induction Charging and Electrostatic Classification of Micrometer-Size Particles for Investigating the Electrobiological Properties of Airborne Microorganisms. Aerosol Sci. Tech., 36:479–491.
- Mainelis, G., Willeke, K., Baron, P., Reponen, T., Grinshpun, S. A., G’orny, R. L., and Trakumas, S. (2001). Electrical Charges on Airborne Microorganisms. J. Aerosol. Sci., 32:1087–1110.
- Meklin, T., Reponen, T., Toivola, M., Koponen, V., Husman, T., Hyvarinen, A., et al. (2002). Size Distributions of Airborne Microbes in Moisture-Damaged and Reference School Buildings of Two Construction Types. Atmos. Environ., 36:6031–6039.
- Miksch, R. R., Gefter, P., Gehlke, S., Halpin, H. A., Meschke, J. S., Smith, B., et al. (2009). Relationship Between Surface Electrostatic Potential and Deposition of Airborne Bacteria. Industry Applications, IEEE Transactions 45:1068–1073.
- Nasir, Z. A., and Colbeck, I. (2010). Assessment of Bacterial and Fungal Aerosol in Different Residential Settings. Water Air Soil Poll., 211:367–377.
- Reponen, T., Hyvärinen, A., Ruuskanen, J., Raunemaa, T., and Nevalainen, A. (1994). Comparison of Concentrations and Size Distributions of Fungal Spores in Buildings with and Without Mould Problems. J. Aerosol Sci., 25:1595–1603.
- Reponen, T., Nevalainen, A., and Raunemaa, T. (1989). Bioaerosol and Particle Mass Levels and Ventilation in Finnish Homes. Environ. Int., 15:203–208.
- Sanchez-Monedero, M. A., Stentiford, E. I., and Mondini, C. (2003). Biofiltration at Composting Facilities: Effectiveness for Bioaerosol Control. Environ Sci Technol., 37:4299–4303.
- Sanchez-Monedero, M. A., Stentiford, E. I., and Urpilainen, S. T. (2005). Bioaerosol Generation at Large-Scale Green Waste Composting Plants. J. Air .Waste Manage. Assoc., 55:612–618.
- Sawyer, B., Rao, K. C., O’Brien, P., Elenbogen, G., Zenz, D. R., and Lue-Hing, C. (1996). Changes in Bacterial Aerosols with Height Above Aeration Tanks. J. Environ. Eng., 122:368–373.
- Tolvanen, O. K. (2004). Exposure to Bioaerosols and Noise at a Finnish Dry Waste Treatment Plant. Waste Manage. Res., 22:346–357.
- Van Loosdrecht, M. C., Lyklema, J., Norde, W., Schraa, G., and Zehnder, A. J. (1987). Electrophoretic Mobility and Hydrophobicity as a Measured to Predict the Initial Steps of Bacterial Adhesion. Appl. Environ. Microbiol., 53:1898–1901.