Abstract
This study reports on the effects of BaTiO3—a high dielectric constant additive—addition on charging and filtration properties of meltblown polypropylene (PP) electret filters. Since electrostatic capture efficiency of electret filters is mainly dependent on electrical forces, surface potential and aerosol filtration properties were analyzed and compared. Due to quasi-permanent nature of electret property, stability of charging and filtration performance was also investigated via following an isothermal charge decay procedure. Addition of BaTiO3 did not alter fiber morphology significantly. Particularly, the stability of electrostatic filtration performance was found to be promising with the addition of BaTiO3. Possible microstructural changes after addition of BaTiO3 were investigated via wide angle X-ray diffraction. Changes in crystal structure of PP upon addition of BaTiO3 did not deteriorate electrostatic properties.
Copyright 2015 American Association for Aerosol Research
1. INTRODUCTION
Electret filters have gained significant importance in air filtration and personal protective equipment (PPE) market over the past few decades. Uncharged (mechanical) filters separate particulates from air streams through well-known mechanisms of impaction, interception, and Brownian diffusion. However, significant portion of filtration efficiency of electret filter media can be attributed to electrostatic capture mechanism, whereas contribution of mechanical filtration can be as low as 30–50% of total filtration efficiency (Romay et al. Citation1998; Ji et al. Citation2003). Studies by Barrett and Rousseau Citation(1998) showed that electret media exhibits very low resistance (3–6 mmH2O) compared to glass filter media at similar capture efficiencies. Mechanical filter composed of glass fibers can possess high collection efficiency (>99%) for submicron particles, but it also has too high resistance (25–40 mmH2O). Such pressure range against airflow increases the energy and infrastructural costs in buildings (Raynor et al. Citation2008). It is not therefore surprising that electret filters are used in high-efficiency filtration because they are effective at low pressure drops, which leads to huge energy savings in HVAC systems. Low flow resistance is also one of the critical metrics in PPE design, since it will directly affect breath comfort.
However, electret filter performance is largely dependent on the strength of the electric field within the fibrous filter media. Besides, charging stability is also critical since the loss of electrostatic property will result in significant reductions in total filtration efficiency. (Angadjivand et al. Citation1996; Eitzman and Rousseau Citation2002; Rousseau et al. Citation2003). One strategy to achieve high charge density and stability is incorporation of additives into polymeric filter media. There is currently no special class of polymer additive that is intentionally designed for electret purposes; however, nucleating agents, antioxidants, light stabilizers, fluorochemicals, and inorganic ceramic ferroelectrics were investigated. Nucleating agents either provide smaller spherulites whose boundaries are traps for space charges, or generate microvoids that prohibit charge migration (Behrendt et al. 2006; Mohmeyer et al. 2006, 2007). Antioxidants and light stabilizers can act as nucleators and also provide deep charge traps (Cartwright et al. Citation1996; Rousseau et al. Citation2003). Prohibiting formation of conductive liquid film during filtration, fluorine-based surface modification improve charge stability (Jones and Rousseau Citation1995a,Citationb). On the other side, inorganic additives have some distinct behaviors that are needed to be considered. For instance, BaTiO3 as nonlinear dielectric owes high polarizability due to the rotation of central Ti atoms between two equivalent equilibrium positions. Thus, transformation of tetragonal unit cell into cubic paraelectric at the so called Curie temperature causes dielectric constants of 104 or more (Hench and West Citation1990; McNeal et al. Citation1998). Effects of grain size and composition on its dielectric properties were largely discussed in the literature (Arlt et al. Citation1985; Hennings Citation1987). In our previous study (Kilic et al. Citation2013), it was shown that charge density and stability of polypropylene (PP) filaments were improved significantly after incorporation of BaTiO3. High dielectric constant particles increase polarizability of electret fiber and enhance space charge formation over the fiber surface. Electrostatic aerosol particle capture performance would be another indicator to analyze charge retention of fibrous mats. In this study, aerosol filtration properties of BaTiO3/PP meltblown webs were analyzed, and results were checked against surface potential measurements.
2. MATERIALS AND METHODS
2.1. Materials
Achieve 6936G1 PP polymer resin with a melt flow rate of 1550 dg min−1 was kindly provided from ExxonMobil. Barium titanate (219-6A) in powder form was purchased from Ferro Electronics (OH). Ninety percent of the particles had diameters below 2.1 μm and 10% of them were smaller than 0.8 μm according to manufacturer data. A room temperature dielectric constant of 2300 was reported for the powder. To ensure uniform blending, PP masterbatch was prepared at 20%(w/w) additive by Techmer PM. Subsequently, the masterbatch was blended with pure PP resin during extrusion to achieve concentrations of 0.1 and 1, and 10%(w/w).
2.2. Meltblown Web Production
Meltblown (MB) filter media were produced with the mini MB line in the Nonwovens Institute (Raleigh). The unit has a 12.5 cm die with a total of 125 capillaries. Polymer pellets were melted and pressurized using a 3.2 cm C.W. Brabender extruder, mounted horizontally. The heating in the extruder involves four zone temperature controls. The die was manufactured by the Reifenhauser Reicofil GmbH & Co. KG. The air plate angle is 60° with an air gap of 0.3 mm and the molten polymer exit (die tip) outsets the die face by 1 mm. Polymer temperature was increased from 190 to 250°C stepwise inside extruder with four zones, whereas blowing air was measured around 260°C. Several process parameters can be used to control the fibers and the structures produced by meltblowing. By varying die/air temperature, polymer throughput, air pressure, die-collector distance, and belt velocity, one can produce samples having different fiber diameter distributions and solidity (Shambaugh Citation1988; Milligan et al. Citation1992; Wang and Ke Citation2006). Process parameters such as die-collector distance and zone temperatures were kept constant. Belt speed and throughput was adjusted to the desired basis weight (25 ± 2gm−2). The most defect free and uniform webs were found to be produced at a throughput around 0.2–0.25 g hole−1 min−1 and air pressure of 20–30 psi.
2.3. Basic Web Properties
Important properties that control aerosol filtration such as basis weight, thickness, fiber diameter and distribution, and solidity were measured. Basis weight measurements were done via weighing five specimens of 100 cm2 area and averages were calculated thereof. Web thickness was measured according to ASTM D 5729 procedure where thickness testing gage (AMES, BG1110- 04) with a presser foot diameter of 25.4 mm at pressure of 4.14 kPa was used. From measured basis weight and thickness, solidity was calculated from the following equation:[1]
Samples were examined with Phenom FEI scanning electron microscope (SEM) operating at 5 kV accelerating voltage. To reduce charging effect, samples were coated with a thin layer (1–3 nm) of gold. Fiber diameter distributions were analyzed from 100 measurements taken from 15 images.
2.4. Filtration Properties
Filtration properties of MB webs were evaluated with dioctyl phthalate (DOP) aerosols in 0.02–0.3 µm range at a face velocity of 5.3 cm s−1. DOP aerosols were generated by a collision type atomizer and then evaporated through a membrane dryer. Then particles were selected in a long differential mobility analyzer (DMA, TSI Model 3081, MN, USA) and neutralized by a Kr-85 radioactive source. The neutralized DOP aerosols were fed into a filter holder with 25.81 cm2 of effective area and their number concentrations at upstream and downstream positions were measured by using two condensation particle counters (CPCs, TSI Model 3760A, MN, USA). The flow rate and the resistance were measured by a mass flow meter (TSI Model 4043, MN, USA) and an electronic manometer (TSI Model 220, MN, USA). DOP loading is particularly preferred since such oily aerosols are more challenging for electrostatic capture performance. Quality factor (QF = –lnP/Δp) that relates aerosol particle capture efficiency by the pressure drop across the media was also calculated. To analyze charging stability, we performed isothermal decay tests at elevated temperatures to accelerate the process. Charged samples were placed in the oven at 80°C and filtration tests were repeated after 24 h decay time.
2.5. Charging and Surface Potential Analysis
Charging of the samples was carried out with a corona charger (Mystic Marvels, Model NIP-7E). The applied voltage was set at 9 kV where maximum output direct current of the emitter needle was about 160 μA. Samples were charged for 10 min from a distance of 3 cm. For thermally charging, procedure described in our early work (Kilic et al. Citation2013) was followed. Samples were heated to 130°C, then charged for 10 min and cooled down to room temperature instantly. The surface potential was measured with noncontact electrostatic voltmeter (Monroe 244 Model with 1017AE electrostatic voltage probe) according to procedure explained in our previous work. Surface potential of 16 distinct points were measured from both front and back sides and potential maps were drawn accordingly. After that, absolute difference between front and back values were calculated as the electric field over particles will be close to that value. Detailed explanation of the procedure can be found in our previous work (Kilic et al. Citation2014).
2.6. Crystal Structure
Crystal structure was investigated using Omni Instrumental X-ray diffractometer. The diffractometer was equipped with Be-filtered CuKα radiation with a wavelength of 1.54 Å generated at 35 kV and 25 mA. The samples were scanned from 2Ѳ range from 10° to 60° at an increment of 0.1°.
3. RESULTS AND DISCUSSIONS
3.1. Web Properties
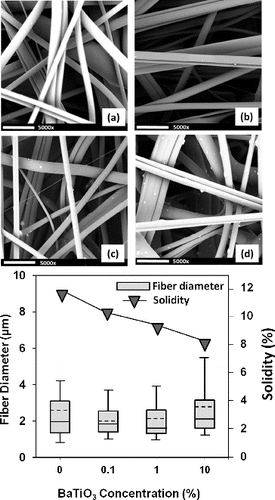
Since web structures, in particular, fiber diameters, and their distribution significantly alter particle capture efficiency, the effects of BaTiO3 on MB web structures were evaluated. SEM images () demonstrate that addition of BaTiO3 has not adversely affected MB fiber formation process. Even at 10% concentration, MB fabric defects such as agglomeration and bead formation have not been observed. These agree well with our previous works on filaments that showed BaTiO3 particles were uniformly distributed within PP fibers without compatibilizer use (Kilic et al. Citation2013). Fiber diameter and its distribution were not found to be largely deviated upon BaTiO3 addition. At 1 and 10% concentrations, particles can be differentiated on the fiber surface. Though barium titanate diameter is comparable to that of MB fiber, particles on the fiber surfaces were relatively small (0.5–1.0 μm). Despite not effecting fiber morphology significantly, addition of BaTiO3 resulted with more fluffy webs. This was proved via solidity analysis, which showed nearly 22% and 35% reduction in solidity of 1% and 10% BaTiO3/PP webs compared to reference mat.
FIG. 1. SEM images of BaTiO3 containing MB webs. Scale bar corresponds to 10 μm. Polymer flowrate was kept 0.25 g/hole/min at an air pressure of 25 psi. (a) Reference, (b) 0.1% BaTiO3/PP, (c) 1% BaTiO3/PP, (d) 10% BaTiO3/PP (solid lines are median values, whereas dashed lines correspond to mean values).
3.2. Filtration Properties
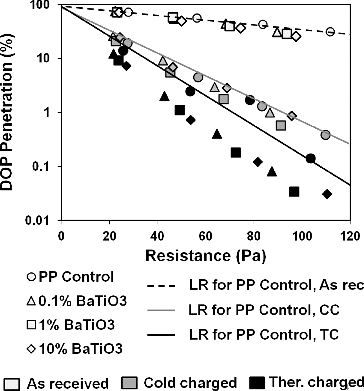
As shown in trend for mechanical filtration efficiencies were quite correlated (uncharged sample, white-colored plots), hence electrostatic filtration efficiencies depending on additive presence would be compared. Corona charging resulted with significant improvement in filtration properties of all samples. DOP capture efficiency of MB media ranges 25–30% with an expense of pressure drop around 23–27 Pa. BaTiO3 incorporation little changes filtration performance of uncharged samples. Difference between PP control and PP/BaTiO3 samples were small and variations probably are caused by differences in web structures discussed previously. Upon cold charging, DOP penetrations drop greatly at a similar trend, notwithstanding on the presence of BaTiO3. DOP penetration of cold charged filter media was 19–25%. Compared to linear regression curve for cold charged reference sample (gray line) addition of BaTiO3 little improved filtration performance of cold charged filter media, though BaTiO3 exhibits high polarizability even at room temperatures. There is a small increase in efficiency of 1% BaTiO3/PP sample, which is not substantial compared to thermally charged samples. It was clearly shown that filter media charged at higher temperature consistently showed lower DOP penetration compared to that of cold charged media. This is more apparent for BaTiO3 containing samples. For the case of 10% BaTiO3/PP meltblown media, thermally charging reduced DOP penetration from 14% to 7.3%. Even though one should consider minor differences in web structures, it is apparent BaTiO3 plays a role in better filtration performance of thermally charged samples through enhanced charge holding capability. HEPA-level efficiency, which is 99.97% for 0.3 µm particle size, was found to be reached at an expense of 100 Pa resistance. Following the linear regression lines, at least 12 layers of uncharged media should be laid to reach the same efficiency. Increase in electrostatic capture performance did not cause an increase in pressure drop, which is basically the advantage of electret filters.
FIG. 2. Penetration versus resistance curves of samples (dp: 0.3 μm, u: 5.3 cm/s). LR indicates linear regression on semilog plot, As rec stands for as received, CC for cold charged, and TC for thermally charged (charged for 10 min from a distance of 3 cm).
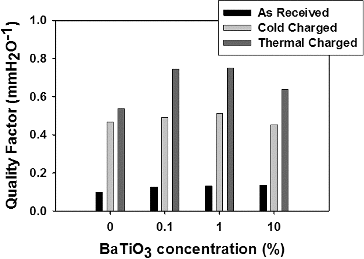
The negative slope of semilog penetration-pressure drop plot relates filter's efficiency to its resistance to the air flow. These are two of the most important filter performance indicators, often referred to quality factor (or filtration index). summarizes the quality factor values after cold and thermally charging. It is an expected result that reference samples also exhibited a significant improvement upon charging at high temperatures. This was explained with accumulation of charges through deep traps (Xia et al. Citation1992). Also polarization of dipoles coming from any kind of impurities within PP will be more effective at high temperatures because of high molecular mobility. However, the increase in electrostatic capture efficiency of BaTiO3 containing samples is larger due to additional polarization. Most probably at room temperatures, the applied electric field (3 kV/cm) was not efficient for the polarization of BaTiO3. However, dielectric permittivity and atomic scale mobility increases largely at Curie temperature, which may explain the enhancement in electrostatic capture performance.
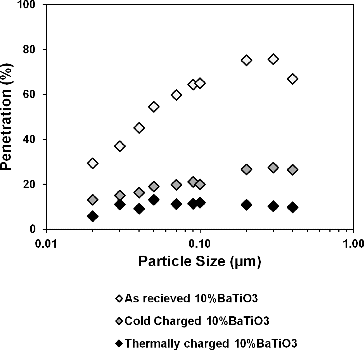
For high-efficiency electret filter media, it is known that maximum penetrating particle size (MPPS) shifts to lower diameters (Bałazy et al. Citation2006). To analyze MPPS for modified media, we performed full fractional efficiency test for 10% BaTiO3/PP webs. As shown in , without charging nearly 20% penetration was obtained for particles around 0.3 µm. However, MPPS shifted to 50 nm for the same media when thermally charged and penetration decreased to less than 5% for 0.3 µm particles. We can conclude neutral DOP particles were induced and captured more efficiently within thermally charged webs.
3.3. Charging Properties
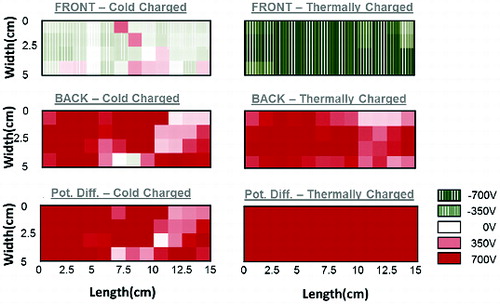
Obtained enhancement in electrostatic capture efficiency might be watched via analyzing surface potential of the charged webs. As shown in , thermally charged sample exhibited highly uniform potential values on both face and back. Hence, the potential difference through web, which is roughly related with electric field towards aerosol particles, is larger and more uniform compared to cold charged sample. Weakly charged regions on cold charged sample would act as defective regions for electrostatic particle capture process. The efficiency of 10 min thermally charged 1% BaTiO3/PP sample was observed as 90.8%, whereas it was 78.9% for cold charged sample, which is thought to be due to more uniform and effective charging ().
FIG. 5. Potential map for cold charged and thermally charged 1%BaTiO3/PP web (charging distance: 3 cm, charging time 10 min).
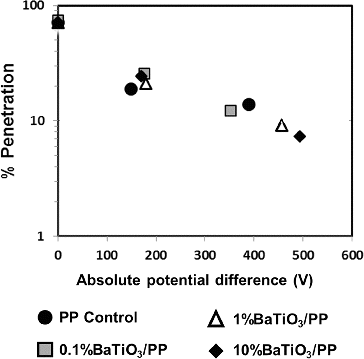
Either charged or neutral aerosol particles will be subjected to an electric field acting between charged fibers of electret filter. Since depth filtration is the major mechanism in aerosol filters, the total electric forces will be roughly related with potential difference between the front and back of the electret filter. depicts relation between absolute surface potential and percent aerosol penetrations. Generated electric field between the fibers provided quite large electrostatic capture performances (over 70%). This approach might be intriguing for future works on predicting performance of electret filters.
3.4. Filtration Properties After Isothermal Potential Decay
One other concern on electret filters is the stability of charges and the resultant filtration properties. For this purpose, we designed a simple accelerated decay method at elevated temperatures. At higher temperatures, molecular motions will lead to ease of decay. After measuring the initial filtration efficiencies, samples were kept in the oven at 80°C for 24 h and same testing protocol was applied again. Charge decay is connected to four mechanisms:
Charge/polarized species mobility within bulk
Charge/polarized species mobility on surface
Contamination with air ions (Jonassen Citation2002)
Captured particles leading to neutralization and screening of charges (Brown et al. Citation1988; Walsh and Stenhouse Citation1998)
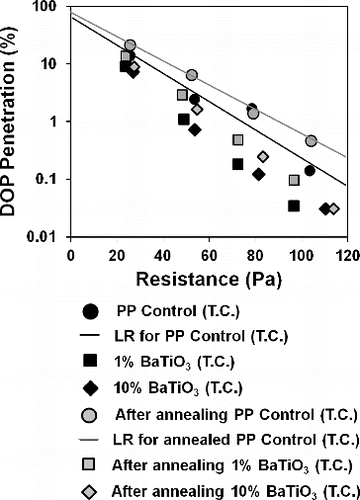
However, it should be noted that contamination with air ions in the oven may not be as high as during filtration. When compared to real situation, the decaying effect caused by captured particles will be relatively small since the samples were loaded for a few minutes during tests. Due to that reasons accelerated decay test would be useful especially for storage conditions. shows stability of electrostatic capture performance for thermally charged samples. The increase in penetration is apparent especially for the case of reference sample. For 10% BaTiO3/PP sample filtration efficiency dropped only 0.01%, with a minor change in resistance, which might be due to thermal shrinkage upon holding samples at 80°C for 24 h. All BaTiO3/PP samples exhibited higher particle capture performances than control PP even after decay procedure.
3.5. Microstructural Properties of BaTiO3/PP Webs
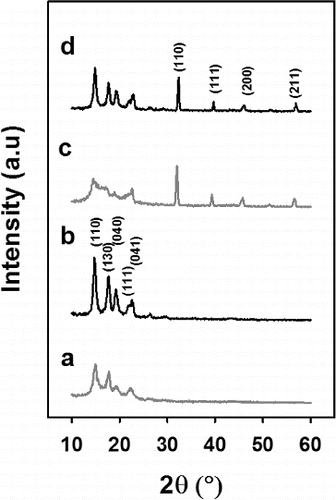
X-ray diffraction analysis showed significant changes in morphology upon thermally charging. As shown in , PP control fibers exhibited distinct diffraction peaks at 2θ = 14.1°, 17.0°, and 18.6°, which correspond to (110), (040), and (130) planes in α-monoclinic crystal of iPP, respectively. Corresponding peak angles for PP crystals shifted to left when compared to the data in literature (Lee and Wadsworth Citation1992), which indicates a small increase in d-spacing between atomic layers. On the other side, 10% BaTiO3/PP MB fibers show a mesomorphic structure before thermally charging (Yan et al. Citation1993). However, similar to PP control fibers, α-form PP crystals became clearer upon charging at 130°C. Mesomorphic structure of 10% BaTiO3/PP sample might be due to changes in temperature profile of solidifying molten jet. BaTiO3 might have caused early solidification, due to its higher heat transfer coefficient than that of PP (He Citation2004; Weidenfeller et al. Citation2004). Diffraction peaks in the range of 30–60° mainly correspond to perovskite barium titanate crystals (Wang et al. Citation2002; Yuh et al. Citation2005). Apparently, change in BaTiO3 structure was much less. Improved crystallinity should have been effective on charge stability of the webs, which was seen on filtration tests upon isothermal potential decay test.
4. CONCLUSIONS
The effects of BaTiO3 addition on electrostatic charging and particle capture performance of PP MB webs were analyzed. Widely investigated as a nonlinear dielectric, BaTiO3 is a promising additive to improve charging properties of polymeric fibers, which will enhance particle capture performance. However, additive properties should be considered to design proper charging mechanism. Here thermally charging around Curie temperature of BaTiO3 (∼130°C) provided superior and stable electret filtration for composite fibrous webs. Uniformly charged samples having high potential values exhibited high particle capture performance. Accelerated decay test was applied to analyze charge stability. Above 1% BaTiO3 concentration, filtration efficiency and stability increased significantly. Penetration of 0.3 μm DOP particles was measured nearly 20% for uncharged four-layer 10% BaTiO3/PP web, whereas it dropped to 0.7% after cold charging and 0.03% after thermal charging. For properly charged 10% BaTiO3/PP webs, HEPA-level efficiency (99.97% for 0.3 μm particles) was reached at an expense of 95 Pa pressure drop. Even after isothermal potential decay test, filtration performance did not change significantly for webs containing over 1% BaTiO3. Results showed that barium titanate is solely an effective electret additive, despite giving minor changes in crystal structure.
ACKNOWLEDGMENTS
Thanks are due to ExxonMobil Co. for supplying polypropylene, Ms. Birgit Andersen (TECS, NCSU) for analytical assistance, and Angela Thornhill (Techmer PM, TN, USA) for masterbatch production.
Funding
The authors gratefully acknowledge the Nonwovens Cooperative Research Center (NCRC) for financial support to this work.
REFERENCES
- Angadjivand, S. A., Jones, M. E., and Meyer, D. E. (1996). Webs of Thermoplastic Microfibers and Water Drops for Filtration. (US Patent 5,496,507).
- Arlt, G., Hennings, D., and de With, G. (1985). Dielectric Properties of Fine-Grained Barium Titanate Ceramics. J. Appl. Phys., 58:1619–1625.
- Bałazy, A., Toivola, M., Reponen, T., Podgórski, A., Zimmer, A., and Grinshpun, S. A. (2006). Manikin-Based Performance Evaluation of N95 Filtering-Facepiece Respirators Challenged with Nanoparticles. Ann. Occup. Hyg., 50:259–269.
- Barrett, L. W., and Rousseau, A. D. (1998). Aerosol Loading Performance of Electret Filter Media. Am. Ind. Hyg. Assoc. J., 59:532–539.
- Behrendt, N., Mohmeyer, N., Hillenbrand, J., Klaiber, M., Zhang, X., Sessler, G. M., Schmidt, H. W., and Altstädt, V. (2006). Charge Storage Behavior of Isotropic and Biaxially-Oriented Polypropylene Films Containing α- and β-Nucleating Agents. Journal of Applied Polymer Science, 99:650–658.
- Brown, R. C., Wake, D., Gray, R., Blackford, D. B., and Bostock, G. J. (1988). Effect of Industrial Aerosols on the Performance of Electrically Charged Filter Material. Ann. Occup. Hyg., 32:271–294.
- Cartwright, G. A., Davies, A. E., Swingler, S. G., and Vaughan, A. S. (1996). Effect of an Antioxidant Additive on Morphology and Space-Charge Characteristics of Low-Density Polyethylene. IEEE Proc. Sci. Measurem. Technol., 143:26–34.
- Eitzman, P. D., and Rousseau, A. D. (2002). Method and Apparatus for Making a Fibrous Electret Web Using a Wetting Liquid and an Aqueous Polar Liquid. (US Patent 20020190434).
- He, Y. (2004). Heat Capacity, Thermal Conductivity, and Thermal Expansion of Barium Titanate-Based Ceramics. Thermochim. Acta, 419:135–141.
- Hench, L. L., and West, J. K. (1990). Principles of Electronic Ceramics. Wiley, New York, p. 244.
- Hennings, D. (1987). Barium Titanate Based Ceramic Materials for Dielectric Use. Int. J. High Technol. Ceram., 3:91–111.
- Ji, J. H., Bae, G. N., Kang, S. H., and Hwang, J. (2003). Effect of Particle Loading on the Collection Performance of an Electret Cabin Air Filter for Submicron Aerosols. J. Aerosol Sci., 34:1493–1504.
- Jonassen, N. (2002). Electrostatics, Springer, Dordrecht, pp. 163–165.
- Jones, M. E., and Rousseau, A. D. (1995a). Oily Mist Resistant Electret Filter Media and Method for Filtering. (US Patent 5,411,576).
- Jones, M. E., and Rousseau, A. D. (1995b). Oily Mist Resistant Electret Filter Media. (US Patent 5,472,481).
- Kilic, A., Shim, E., Pourdeyhimi, B., and Yeom, B.-Y. (2014). Aerosol Filtration Properties of Nucleating Agent Containing Electret Filters. Polym. Eng. Sci., 54:1533–1539.
- Kilic, A., Shim, E., Yeom, B. Y., and Pourdeyhimi, B. (2013). Improving Electret Properties of PP Filaments with Barium Titanate. J. Electrostat., 71:41–47.
- Lee, Y., and Wadsworth, L. C. (1992). Effects of Melt-Blowing Process Conditions on Morphological and Mechanical Properties of Polypropylene Webs. Polymer, 33:1200–1209.
- McNeal, M. P., Jang, S.-J., and Newnham, R. E. (1998). Size Effects on the Dielectric Properties of Barium Titanate (BaTiO3) at Microwave Frequencies. Ferroelectrics, 211:153–164.
- Milligan, M. W., Lu, F., Buntin, R. R., and Wadsworth, L. C. (1992). The Use of Crossflow to Improve Nonwoven Melt-Blown Fibers. J. Appl. Polym. Sci., 44:279–288.
- Mohmeyer, N, Behrendt, N., Zhang, X., Smith, P., Altstädt, V., Sesslerd, G. M., and Schmidt, H. W. (2007). Additives to Improve the Electret Properties of Isotactic Polypropylene. Polymer 48:1612–1619.
- Mohmeyer, N., Schmidt, H. W., Kristiansen, P. M., Altstadt, V. (2006). Influence of Chemical Structure and Solubility of Bisamide Additives on the Nucleation of Isotactic Polypropylene and the Improvement of Its Charge Storage Properties. Macromolecules 39:5760–5767.
- Raynor, P., Kim, B., Ramachandran, G., Strommen, M., Horns, J., and Streifel, A. (2008). Collection of Biological and Non-Biological Particles by New and Used Filters Made from Glass and Electrostatically Charged Synthetic Fibers. Indoor Air, 18:51–62.
- Romay, F. J., Liu, B. Y. H., and Chae, S. J. (1998). Experimental Study of Electrostatic Capture Mechanisms in Commercial Electret Filters. Aerosol Sci. Technol., 28:224–234.
- Rousseau, A. D., Jones, M. E., and Angadjivand, S. A. (2003). Fibrous Webs Having Enhanced Electret Properties. (US Patent 5,908,598).
- Shambaugh, R. L. (1988). A Macroscopic View of the Melt-Blowing Process for Producing Microfibers. Ind. Eng. Chem. Res., 27:2363–2372.
- Walsh, D. C., and Stenhouse, J. I. T. (1998). Parameters Affecting the Loading Behavior and Degradation of Electrically Active Filter Materials. Aerosol Sci. Technol., 29:419–432.
- Wang, M.-C., Hsiao, F.-Y., Hsi, C.-S., and Wu, N.-C. (2002). Crystal Structure and Ferroelectricity of Nanocrystalline Barium Titanate Thin Films. J. Crystal Growth, 246:78–84.
- Wang, X., and Ke, Q. (2006). Experimental Investigation of Adhesive Meltblown Web Production Using Accessory Air. Polym. Eng. Sci., 46:1–7.
- Weidenfeller, B., Höfer, M., and Schilling, F. R. (2004). Thermal Conductivity, Thermal Diffusivity, and Specific Heat Capacity of Particle Filled Polypropylene. Composites Part A, 35:423–429.
- Xia, Z.-F., Yang, G.-M., and Sun, X.-M. (1992). Charge Dynamics in Mylar Films Corona-Charged at Various Temperatures. IEEE Trans. Electr. Insulat., 27:702–707.
- Yan, R. J., Li, W., Li, G., and Jiang, B. (1993). Structure of Mesomorphic Form of Isotactic Polypropylene. J. Macromol. Sci. Part B, 32:15–31.
- Yuh, J., Nino, J. C., and Sigmund, W. M. (2005). Synthesis of Barium Titanate (BaTiO3) Nanofibers via Electrospinning. Mater. Lett., 59: 3645–3647.