Abstract
For infants born with respiratory distress syndrome (RDS), liquid bolus delivery of surfactant administered through an endotracheal tube is common practice. While this method is generally effective, complications such as transient hypoxia, hypercapnia, and altered cerebral blood flow may occur. Aerosolized surfactant therapy has been explored as an alternative. Unfortunately, past efforts have led to disappointing results as aerosols were generated outside the lungs with significant pharyngeal deposition and minimal intrapulmonary instillation. A novel aerosol generator (Microjet™) is evaluated herein for intrapulmonary aerosol generation within an endotracheal tube and tested with Curosurf and Infasurf surfactants. Compared with other aerosol delivery devices, this process utilizes low air flow (range 0.01–0.2 L min−1) that is ideal for limiting potential barotrauma to the premature newborn lung. The mass mean diameter (MMD) of the particles for both tested surfactants was less than 4 μm, which is ideal for both uniform and distal lung delivery. As an indicator of phospholipid function, surfactant surface tension was measured before and after aerosol formation—with no significant difference. Moreover, this device has an outside diameter of <1 mm, which permits insertion into an endotracheal tube (of even 2.0 mm). In the premature infant where intravenous access is either technically challenging or difficult, aerosol drug delivery may provide an alternative route in patient resuscitation, stabilization, and care. Other potential applications of this type of device include the delivery of nutrients, antibiotics, and analgesics via the pulmonary route.
Copyright 2015 American Association for Aerosol Research
INTRODUCTION
An estimated 1% of newborns in the US (i.e., about 40,000 neonates per year) develop respiratory distress syndrome (RDS) at birth (Rodriguez and Martin Citation1999). Such newborns are normally given surfactant therapy in the delivery room, or shortly thereafter, in the neonatal intensive care unit. Treatment with exogenous surfactant improves gas exchange and optimizes survival in newborn babies with RDS (Jobe Citation1993; Mercier and Soll Citation1993; Suresh and Soll Citation2002). Currently, surfactant is administered as a liquid bolus in the central airways via an endotracheal tube followed by gradual distal pulmonary dissipation (Rodriguez and Martin Citation1999). Up to 19% of infants require repeat dosing due to RDS progression (Verder et al. Citation1994). Although this has improved survival, current therapy may result in several complications. Specifically, rapid intratracheal administration of a large volume of surfactant solution may cause transient hypoxia, hypercapnia, and altered cerebral blood flow, and increase the incidence of intraventricular hemorrhage (Cowan et al. Citation1991; Halliday and Robertson Citation1992; Gunkel and Banks Citation1993). This is especially relevant in the very premature infant and the critically unstable neonate. Therefore, it would be desirable to deliver surfactant using a gentler modality that more readily allows for an effective and uniform intra-pulmonary distribution. To this end, several animal experiments and clinical trials have been conducted to deliver surfactant as an aerosol. Results of these trials have not been promising and may preclude viable clinical application (Lewis et al. Citation1991; Lewis and McCaig Citation1993; Fok et al. Citation1998; Berggren et al. Citation2000). For instance, in a study by Jorch et al. Citation(1997), only 10% of aerosolized surfactant was delivered in the pharyngeal tubing resulting in a modest gain in lung function. More recently, the synthetic surfactant Aerosurf has been clinically evaluated for aerosolized delivery with variable outcomes (Finer et al. Citation2010).
To our understanding, the above studies with natural surfactants were primarily limited by the tested aerosol devices. Previous clinical work has been based on generating aerosols outside the lung with subsequent delivery into the lung using various breathing support devices (Mazela et al. Citation2007). These methods deliver less than 1% of surfactant to the lungs (Fok et al. Citation1998; Berggren et al. Citation2000). Further, these devices were designed to aerosolize and deliver drugs using high air flow (of several liters/minute) into the lungs of adults under relatively stable conditions (Berggren et al. Citation2000). Such required air flow would almost certainly induce barotrauma in the neonatal or infant lung. A low flow device developed to match neonatal tidal volume and inspiratory phase with efficient surfactant delivery would be an ideal solution to mitigate the above issues. Here, we present a novel device of narrow gauge (OD <1 mm) to be inserted into an endotracheal tube that should allow intra-pulmonary aerosol generation and near complete drug delivery while maintaining low air pressure and flow.
MATERIALS AND METHODS
Catheter-Based Aerosolization Device
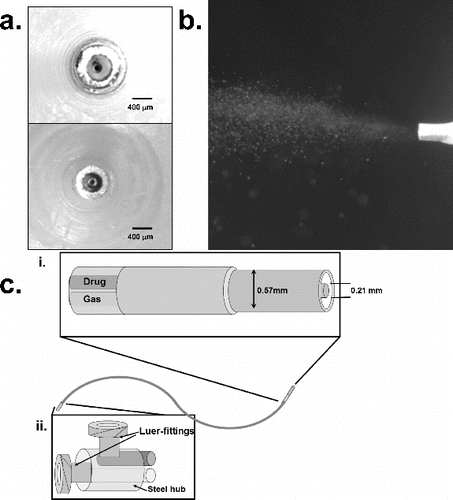
A catheter-based aerosolization device (Microjet™) developed by Powerscope Inc. (now part of MSP Corporation, MN, USA) was tested in this study. The proof-of-concept experiments were conducted with a prototype device that consists of two concentric needles at the delivery tip (). Gas is injected through the inner needle allowing for entrainment of surfactant through the gap between the two needles. The gap between the concentric needles is designed to control particle size (The needle pair in the upper image in would generate larger drops compared to the pair in the lower image). In our current study, this device was used to aerosolize surfactant into droplets of approximately 4 μm in diameter dispersed in a cone with an approximately 30 degree vertex angle (). The proximal orifices of the needles are attached to standard needle hubs that are combined into an assembly, which is then used to infuse liquid drug (surfactant) and compressed air in the manner described above. Adaptation of this device for in vivo delivery is illustrated in , where the hub is connected to a dual-tip flexible catheter for intra-tracheal instillation. The flow of liquid and the particle size were controlled by the rate of air flow and the gap between the needles, respectively.
FIG. 1. Design of needle-based aerosol generator (Microjet). (a) End-on view of needle tip showing concentric needles with inner needle for air flow and outer cavity for liquid flow. Two different outer needle sizes are shown with a gap of 200 μm and 100 μm. (b) Image of aerosol spray from the tip of the needle at 40 psi air pressure. (c) Schematic of catheter-based device (diameter 1.5 mm) with needle tip and flexible plastic tubing to supply air and liquid.
Measurement of Surfactant Aerosol
Two commercially available surfactants, Curosurf (Chiesi Farmaceutici, Parma, Italy)—derived from porcine lung—and Infasurf (ONY Inc., NY, USA)—derived from calf lung—were selected for their different phospholipid concentrations (Curosurf: 106 mg mL−1, Infasurf: 51 mg mL−1; Chiesi Farmaceutici Citation2011). For all measurements, surfactants were injected into the Microjet and aerosolized within 30 min. An oil-free diaphragm air compressor (CO2MAC, Freeman Tools) with a range of 0–120 psi was used to evaluate device performance. An air flow meter (0–0.5 L min−1 Rotameter, Dwyer) was used to measure resultant flow. At a given air pressure, the rate of aerosol formation was documented. The delivery time for a known volume of liquid was used to quantify surfactant flow. The same protocol was used for both surfactants at increasing air pressures. At each pressure point, the aerosol was collected in a test-tube for surface tension measurements.
Particle Characterization of Surfactant Aerosol
Surfactants aerosolized with the Microjet device were characterized using a Phase Doppler Interferometer (PDI, Artium Technologies, CA, USA), which takes in situ measurements of droplets crossing the intersection region of two laser beams to infer drop size. Specifically, the Microjet was mounted on a dual-axis micrometer and positioned to spray downward with the spray needle tip 1 cm above the PDI, which was centered in the spray field. For each applied air flow, count, velocity, and size distribution of the particles were measured. This procedure was repeated with distilled water as a control. Curosurf and Infasurf surfactants were tested at room temperature.
Surface Tension Quantification of Surfactant Aerosol
The surface tensions of water, liquid surfactant, and previously aerosolized surfactant were measured at room temperature (∼25°C). The modified hanging drop method was used for the measurement of surface tension. The test solution was ejected from a blunt 1.26 mm diameter needle of a 1 cc syringe (see later in the article). Using Equation Equation(1)(1) (Harkins and Humphrey Citation1916) surface tension (γ) was calculated for each solution with water that was used as a control. In Equation Equation(1)
(1) , (m) is the mass of an individual droplet, (g) is gravitational acceleration, and (d) is the diameter of the blunt needle tip.
(1)
STATISTICS
At least three or more repeat measurements (≥3) were undertaken with values presented as mean±SD. Statistical analysis was performed in Graphpad PRISM®. ANOVA was performed with Tukey post-hoc analysis to calculated differences at p < 0.05. Significant differences are shown with paired symbols.
RESULTS
Pressure and Flow Characteristics
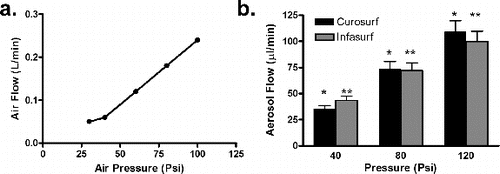
The Microjet nebulizer was tested with compressed air pressures of 25 to 100 psi with corresponding air flows from 0.05 to 0.24 L min−1. shows the relationship between the flow rate and the air pressure. At three compressed air pressures (40, 60, and 80 psi), surfactants (Curosurf and Infasurf) were aerosolized and the rate of aerosol flow was measured. shows the aerosol flow rates of these two surfactants. No significant differences were observed between the test surfactants at any given air pressure. There was a significant increase in aerosolized surfactant flow rate with increased applied air pressure. The resultant surfactant flow rate ranged from 35 to 108 μL min−1.
Aerosolized Surfactant Characterization
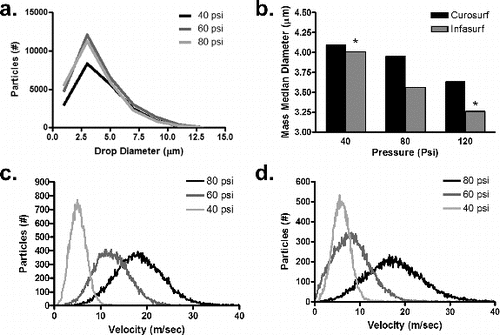
Surfactant aerosol size distribution was akin to a log-normal distribution, with tail elongation towards larger particles (). The mass mean surfactant droplet size decreased with increased air flow for both test surfactants (). Specifically, the particle size for Curosurf did not have a statistically significant change in the tested range, while the mean particle size for Infasurf decreased significantly with increasing air flow (40 psi vs. 120 psi, p < 0.05). In addition, the particles were normally distributed with respect to velocity, with a broader range at higher air flow ( for Curosurf and for Infasurf). Average particle velocity ranged from 5 m s−1 to 18.6 m s−1, at 1.0 cm from the needle tip.
FIG. 3. Aerosol characterization of surfactant using a Phase Doppler Interferometer (Artium Techologies, CA, USA). (a) Aerosol particle size distribution profile for Curosurf. (b) Average MMD as a function of air pressure. Symbol * indicates significant difference between paired groups (p < 0.05). (c) Velocity profile of Curosurf aerosol. (d) Velocity profile of Infasurf aerosol at 40, 60, and 80 psi back-pressure.
Aerosolization Causes No Change in Functional Properties of Surfactant
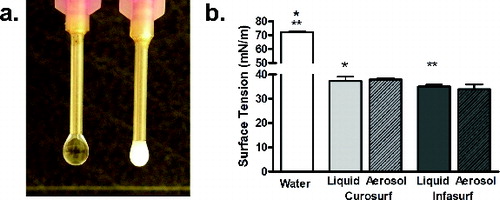
Aerosolized surfactant particles were collected and evaluated for surface tension characteristics. shows the modified hanging drop test using previously aerosolized test surfactants. Water was used as control with a surface tension of 72.2 mN m−1. At room temperature (∼25°C), the surface tension of Curosurf and Infasurf liquids were 37.2 mN m−1 and 34.9 mN m−1, respectively. No significant deterioration in surface tension of either surfactant was observed after aerosolization through the Microjet device ().
FIG. 4. (a) Image of the modified hanging drop method for measuring surface tension. (b) Surface tension of Curosurf and Infasurf before (liquid, solid color bars) and after aerosolization (Aerosol, hash-line bars) with water as a control (white bars). Symbols * and ** represent significant differences between Curosurf and Infasurf compared to water. No significant difference was noted pre and post aersolization for both surfactants.
DISCUSSION
Surfactant aerosol therapy may be a viable alternative to liquid bolus therapy, which has certain inherent complications. To date, several animal and clinical trials have been attempted with extracorporeal generation of surfactant aerosol and delivery through an endotracheal tube. These studies have resulted in limited success (Lewis et al. Citation1991; Jorch et al. Citation1997; Fok et al. Citation1998; Berggren et al. Citation2000; Sun et al. Citation2009; Finer et al. Citation2010). Although an animal model showed promise, Berggren et al. Citation(2000) found no significant benefit in a clinical trial of neonatal RDS. Moreover, actual pulmonary deposition could not be measured accurately. Factors that may compromise clinical utility include aerosol loss via deposition in the pharyngeal space, which curtails an extracorporeal aerosol generation approach. Variable compliance in diseased lung segments may also limit or alter distal pulmonary aerosol delivery. An obstacle in translating aerosol therapy to the neonatal patients has been a lack of an appropriate device (Mazela and Polin Citation2011). For instance, the majority of aerosol nebulizer devices are designed to operate with an air flow of 5–8 L min−1, which may be a suitable air flow for an adult with a lung tidal volume of 500 mL and a high inspiratory to expiratory ratio (I:E of 3:5). However, these devices cannot be safely applied to an infant with an average tidal volume of 50 mL and an I:E ratio of 1:5 (Dolovich Citation2002). Further, commercially available devices have high environment and pharyngeal losses as shown by Fok et al. Citation(1998), where more than 99% of surfactant was lost in the device chamber and ventilator tubing. Similarly, commercial nebulizers designed for adults demonstrate low efficiency when applied to neonates (Tal et al. Citation1996; Amirav et al. Citation2002; Amirav et al. Citation2003). More recently, a vibrating mesh device with low flow has been tested using Aerosurf, a synthetic surfactant developed by Discovery Lab (not currently approved by the FDA; Finer et al. Citation2010). It remains to be seen if a vibrating mesh nebulizer can be applied to a wide array of surfactant products without clogging the mesh.
Given the need for a low flow aerosol generator, the Microjet device was evaluated. The Microjet is a narrow bore (<1 mm OD) device with concentric needle tips that allows for air shearing of liquid particles to generate a fine aerosol. The small size of the air needle (33 gauge) allows the device to generate a high air velocity without requiring a high air flow, which is especially attractive for neonatal application. Moreover, the PDI flow meter system measured an almost complete decline in air velocity within 2 cm from the needle tip. By eliminating the impactor dome found in a majority of commercially available jet nebulizers, this device also avoids potential impaction damage to large molecules like surfactants, proteins, and plasmids (unpublished data). In this study, aerosol testing was performed with Curosurf (porcine) and Infasurf (bovine) surfactants, which were chosen for their wide variation in total phospholipid concentrations (Chiesi Farmaceutici Citation2011).
Microjet was evaluated at operating air pressures up to 80 psi with a corresponding air flow of <0.2 L min−1, yielding a surfactant aerosol flow of 0.11 mL min−1. Based on these conditions, 1 mL of surfactant can be delivered in less than 10 min. For the pre-term neonate, a dose of 100 mg kg−1 would be required (Mazela et al. Citation2007). Based on a 100 mg mL−1 concentration of Curosurf and a 50 mg mL−1 concentration of Infasurf, total therapy would require 10–20 min. Due to its lack of dead space and intra-tracheal aerosol generation for drug delivery, this device may even enable lower drug dosing. Further studies are required for clinical feasibility. Theoretically, low air flow complements pressure support ventilation and continuous positive pressure support, without exceeding normal neonatal tidal volumes. Moreover, it may be coordinated to match inspiratory cycles on a ventilator. Resultant surfactant particles had a mass mean diameter (MMD) of <4 µm. This is attractive, as prior research has shown uniform lung distribution with particles of <4 µm (Raabe et al. Citation1988; Asgharian et al. Citation2003).
Another catheter-style aerosol device was tested by Murgia et al. Citation(2011) using Curosurf. The air flow with this catheter was greater than 1 L min−1 and aerosol particle size ranged from 8 to 30 μm. Although this catheter design also allows for endotracheal delivery, the greater air velocity may cause epithelial trauma, and with larger aerosol particles generated, may compromise distal lung delivery (Raabe et al. Citation1988). Of benefit, Microjet has an air flow of less than 0.2 L min−1, but it is unclear what air velocity is safe to minimize epithelial damage during intra-tracheal surfactant aerosol generation.
Drug formulation may also affect surfactant delivery (Fok et al. Citation1998). At varied air pressures, Infasurf (with a lower phospholipid concentration), but not Curosurf (with a higher phospholipid concentration), yielded variable particle sizes (). Fok et al. Citation(1998) showed that a synthetic surfactant (Exosurf, GlaxoSmithKline, UK) with a low phospholipid concentration had higher deposition in an animal model than a natural surfactant (Survanta, Abbot Pharma, USA) with a higher phospholipid concentration. Both Curosurf and Infasurf had velocity distribution profiles that changed with increased air pressure. The ability to adjust particle size and velocity for a given drug or formulation may allow for targeted lung distribution.
In conclusion, this study tested Microjet, an aerosol device that operates at low air flow, generates small aerosol particles, and maintains tested surfactant surface tensions. This device may overcome challenges faced by current nebulizer technology (Fok et al. Citation1998). Future preclinical and clinical studies will be required to determine if this provides a safe and efficacious treatment option for RDS and other lung diseases. The potential of intra-pulmonary drug aerosol generation and delivery is an especially attractive clinical application in premature and neonatal patients and warrants further development and testing.
ACKNOWLEDGMENTS
The authors would like to thank the University of Minnesota Fairview Children's Hospital pharmacy for providing Curosurf and Infasurf and Zehraa Cheaib with manuscript assistance.
FUNDING
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 and NIH SBIR 5R44EY016229-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
DISCLOSURE STATEMENT
Dr. Zeeshan Syedain was affiliated with Powerscope Inc. and the University of Minnesota at the time of this research. Dr. Amir Naqwi had ownership in Powerscope Inc. Drs. Myrna Dolovich and Arif Somani have no financial support from Powerscope. Dr. Somani has a collaborative grant submitted (R43 Sept. 2013) with MSP Corp.
REFERENCES
- Amirav, I., Balanov, I., Gorenberg, M., Groshar, D., and Luder, A. S. (2003). Nebuliser Hood Compared to Mask in Wheezy Infants: Aerosol Therapy without Tears! Arc. Dis. Child., 88:719–723.
- Amirav, I., Balanov, I., Gorenberg, M., Luder, A. S., Newhouse, M. T., and Groshar, D. (2002). Beta-Agonist Aerosol Distribution in Respiratory Syncytial Virus Bronchiolitis in Infants. J. Nucl. Med., 43:487–491.
- Asgharian, B., Kelly, J. T., and Tewksbury, E. W. (2003). Respiratory Deposition and Inhalability of Monodisperse Aerosols in Long-Evans Rats. Toxicol. Sci., 71:104–111.
- Berggren, E., Liljedahl, M., Winbladh, B., Andreasson, B., Curstedt, T., Robertson, B., and Schollin, J. (2000). Pilot Study of Nebulized Surfactant Therapy for Neonatal Respiratory Distress Syndrome. Acta. Paediatr., 89:460–464.
- Cowan, F., Whitelaw, A., Wertheim, D., and Silverman, M. (1991). Cerebral Blood Flow Velocity Changes after Rapid Administration of Surfactant. Arch. Dis. Child., 66:1105–1109.
- Dolovich, M. B. (2002). Assessing Nebulizer Performance. Respir. Care, 47:1290–1301; discussion 1301–1304.
- Farmaceutici, C. (2011). CUROSURF® (poractant alfa) Intratracheal Suspension Information. Chiesi Farmaceutici, Parma, Italy.
- Finer, N. N., Merritt, T. A., Bernstein, G., Job, L., Mazela, J., and Segal, R. (2010). An Open Label, Pilot Study of Aerosurf(R) Combined with nCPAP to Prevent RDS in Preterm Neonates. J. Aerosol. Med. Pulm. Drug. Deliv., 23:303–309.
- Fok, T. F., al-Essa, M., Dolovich, M., Rasid, F., and Kirpalani, H. (1998). Nebulisation of Surfactants in an Animal Model of Neonatal Respiratory Distress. Arch. Dis. Child. Fetal Neonatal. Ed., 78:F3–F9.
- Gunkel, J. H., and Banks, P. L. (1993). Surfactant Therapy and Intracranial Hemorrhage: Review of the Literature and Results of New Analyses. Pediatrics, 92:775–786.
- Halliday, H., and Robertson, B. (1992). Cerebral Blood Flow Velocity Changes after Rapid Administration of Surfactant. Arch. Dis. Child., 67:470.
- Harkins, W. D., and Humphrey, E. C. (1916). The Drop Weight Method for the Determination of Surface Tension. J. Am. Chem. Soc., 38:228–236.
- Jobe, A. H. (1993). Pulmonary Surfactant Therapy. New Engl. J. Med., 328:861–868.
- Jorch, G., Hartl, H., Roth, B., Kribs, A., Gortner, L., Schaible, T., Hennecke, K. H., and Poets, C. (1997). Surfactant Aerosol Treatment of Respiratory Distress Syndrome in Spontaneously Breathing Premature Infants. Pediatr. Pulmonol., 24:222–224.
- Lewis, J. F., Ikegami, M., Jobe, A. H., and Tabor, B. (1991). Aerosolized Surfactant Treatment of Preterm Lambs. J. Appl. Physiol., 70:869–876.
- Lewis, J. F., and McCaig, L. (1993). Aerosolized Versus Instilled Exogenous Surfactant in a Nonuniform Pattern of Lung Injury. Am. Rev. Respir. Dis., 148:1187–1193.
- Mazela, J., Merritt, T. A., and Finer, N. N. (2007). Aerosolized Surfactants. Curr. Opin. Pediatr., 19:155–162.
- Mazela, J., and Polin, R. A. (2011). Aerosol Delivery to Ventilated Newborn Infants: Historical Challenges and New Directions. Eur. J. Pediatr., 170:433–444.
- Mercier, C. E., and Soll, R. F. (1993). Clinical Trials of Natural Surfactant Extract in Respiratory Distress Syndrome. Clin. Perinatol., 20:711–735.
- Murgia, X., Gastiasoro, E., Mielgo, V., Alvarez-Diaz, F., Lafuente, H., Valls-i-Soler, A., Gomez-Solaetxe, M. A., Larrabe, J. L., and Rey-Santano, C. (2011). Surfactant and Perfluorocarbon Aerosolization by Means of Inhalation Catheters for the Treatment of Respiratory Distress Syndrome: An In Vitro Study. J. Aerosol. Med. Pulm. Drug. Deliv., 24:81–87.
- Raabe, O. G., Al-Bayati, M. A., Teague, S. V., and Rasolt, A. (1988). Regional Deposition of Inhaled Monodisperse Coarse and Fine Aerosol Particles in Small Laboratory Animals. Ann. Occup. Hyg., 32:53–63.
- Rodriguez, R. J., and Martin, R. J. (1999). Exogenous Surfactant Therapy in Newborns. Respir. Care Clin. N. Am., 5:595–616.
- Sun, Y., Yang, R., Zhong, J. G., Fang, F., Jiang, J. J., Liu, M. Y., and Lu, J. (2009). Aerosolised Surfactant Generated by a Novel Noninvasive Apparatus Reduced Acute Lung Injury in Rats. Crit. Care, 13:R31.
- Suresh, G. K., and Soll, R. F. (2002). Lung Surfactants for Neonatal Respiratory Distress Syndrome: Animal-Derived or Synthetic Agents? Paed. Drug., 4:485–492.
- Tal, A., Golan, H., Grauer, N., Aviram, M., Albin, D., and Quastel, M. R. (1996). Deposition Pattern of Radiolabeled Salbutamol Inhaled from a Metered-Dose Inhaler by Means of a Spacer with Mask in Young Children with Airway Obstruction. J. Pediatr., 128:479–484.
- Verder, H., Robertson, B., Greisen, G., Ebbesen, F., Albertsen, P., Lundstrom, K., and Jacobsen, T. (1994). Surfactant Therapy and Nasal Continuous Positive Airway Pressure for Newborns with Respiratory Distress Syndrome. Danish-Swedish Multicenter Study Group. New Engl. J. Med., 331:1051–1055.