Abstract
A methodology for studying the deagglomeration performance and emptying behavior of micronized mannitol powder from two commercial capsule-based dry powder inhalers (DPIs), the low- and high-resistance RS01®, is presented. Mathematical modeling played a key role in the interpretation of the powder release behavior from these two DPI systems. Non-linear regression models, which were characterized from the aerosol obscuration versus time profiles obtained from laser diffraction particle sizing data, were used to estimate rate constants for emptying of mannitol powder. The effects of device resistance and associated pressure drops, sampling flow rate, rates of powder emptying, and the presence of capsule on the dispersion characteristics were studied. The presence of a capsule significantly improved the aerosolization performance of mannitol powder from both inhalers, which may be due to the extended powder–air–device interactions within the device. It is important to consider the stochastic nature of movement and physical state of the capsule when assessing the aerosolization mechanisms and dispersion performance from these complex delivery systems. The methodology set out in this study has the capacity to provide a greater level of detail in the study of aerosol plume characteristics from capsule-based DPIs.
Copyright 2015 American Association for Aerosol Research
1. INTRODUCTION
Dry powder inhalers (DPIs) for inhalation therapy provide an alternative to the traditional pressurized metered dose inhaler (pMDI) for the treatment of localized lung diseases (Suarez and Hickey Citation2000). To ensure effective drug delivery into the deep lungs, DPIs rely on patient's inspiratory flow to overcome device resistance and aerosolize powder formulation to form an air/powder dispersion (Cegla Citation2004). Unlike pMDI, the design of each DPI is unique. Through careful construction of turbulence generating/particle impaction grids and air pathways, there is potential to improve the dispersion behavior of powder from the entrained airflow (Islam and Cleary Citation2012). The structural design of DPIs determines the intrinsic resistance to airflow, and have been categorized as low- (LR), medium- (MR), or high-resistance (HR) DPIs (Hindle and Byron Citation1995). The resistance of device has been reported to have fundamental consequences on aerosolization performance from these delivery systems. For instance, an increase in device resistance due to reduction in the inhaler air inlet dimension causes higher flow velocities that can increase particle impaction forces with the device walls and result in higher flow turbulence levels sufficient to deagglomerate powder formulation (Coates et al. Citation2006). Therefore, attempts to better understand the powder dispersion behavior and delivery characteristics from complex DPI systems as a function of device resistance have been explored (Hira et al. Citation2012).
While less complex than their pMDI counterparts, DPIs still require a successful combination of components to work well, and can be categorized as either single-unit dose (capsule-based) or multi-unit (blister or reservoir) devices. One factor that has been addressed very little in the published literature on the design aspects of capsule-based DPIs, is the overall capsule interaction with the device and its influence on powder aerosolization and delivery performance. With different commercial inhalers having various designs and capsule chambers, which influence the airflow and consequently control the motion of capsule and degree of powder release, the working principles following powder aerosolization coexisting within single-unit dose DPIs can differ significantly (Behara et al. Citation2014). For instance, in the Rotahaler® DPI (Glaxo-SmithKline, UK), the capsule is split open by a twisting mechanism and the powder is completely released into the airstream. Conversely, with the Dinkihaler® DPI (Aventis, USA), the capsule ends are punctured by a piercing mechanism during actuation and the powder is released from the pierced ends upon inhalation, with the capsule spinning and rattling inside the inhaler (Chew and Chan Citation1999). A comparative study between these devices demonstrated that variations in generated pressure drops and powder emptying period are due to differences in inhaler design, presence of capsule, and dispersion mechanisms (Chew et al. Citation2002).
Inertial impaction apparatuses, such as the multi-stage liquid impactor and cascade impactors, have been widely used for particle size measurement of the emitted aerosol dose into aerodynamic size classes that are relevant to deposition in the respiratory tract. However, details of the powder release behavior cannot be established from these analytical techniques and therefore the kinetics of powder deagglomeration is poorly understood (de Boer et al. Citation2002). Real-time laser diffraction analysis, which provides a direct way of capturing the plume duration and powder emptying behavior, can be used to obtain fundamental details of powder deagglomeration and powder release as reported by a number of authors (Kippax and Morton Citation2008; Behara et al. Citation2011; Behara et al. Citation2012). Empirically, it is known that the capsule significantly improves the powder deagglomeration of pharmaceutical aerosols (Chew et al. Citation2002). However, these observations have never been demonstrated via a quantitative approach. Therefore, the major objective of the present study was to assess the aerosol generation mechanisms of capsule and the effect of device resistance and flow rate on the powder deagglomeration process by adopting a rapid screening method to characterize the release behavior of pharmaceutical powder formulation. The approach used in this study is similar to that described by Behara et al. (Citation2011), however, with attention concentrated on the influence of capsule on powder dispersion behavior.
2. MATERIALS AND METHOD
2.1. Materials
Micronized mannitol powder was supplied by Pharmaxis (NSW, Australia), and was employed as the model drug in this study. Mannitol powder was stored in a desiccator over silica gel until use. The inhalers, LR and HR RS01® DPIs (Plastiape S.p.A, Osnago-Lecco, Italy) were used to disperse the powder formulation. Both DPIs are equivalent in shape and size, consisting of two tangential air inlets, with the inlet area of the HR inlet being one-third of the LR inhaler.
2.2. Methodology
To assess the influence of capsule on the powder deagglomeration process, mannitol powder was either weighed directly on the base of the inhaler while keeping it in an upright position or preloaded into gelatin capsules (size 3, Capsugel®, NSW, Australia) and placed into the device. The DPIs were actuated via a piecing mechanism, using the pins (one at each side) located at the base of the inhaler to produce one hole at each end of the capsule.
2.2.1. Pressure Drop
Flow through both inhalers was produced using a vacuum pump (Westech Scientific Instruments, UK) and recorded using a digital flow meter (Series 4000, TSI Inc., Shoreview, MN, USA) tested over a range of airflow rates (30 to 100 L min−1). The device resistance was calculated as the slope of the plot of the square root of the measured pressure drop over the flow rate as reported by Clark and Hollingworth Citation(993). The pressure drop readings across each device were measured with and without capsule to assess the effect of capsule on the resistance of both DPIs.
2.2.2. Primary Particle Size Analysis
The primary particle size distribution of the micronized mannitol powder was determined using a wet dispersion apparatus by means of light scattering (Mastersizer Hydro MV, Malvern Instruments, Worcestershire, UK). Prior to the measurement, approximately 15.0 mg of mannitol powder was sonicated in 15.0 mL of cyclohexane. Sonicating the suspension for a period of 5 min was enough to completely disperse mannitol agglomerates into their primary size ranges. The suspension was immediately transferred to the automated dispersion unit where automated particle sizing was performed. The refractive indices of mannitol and cyclohexane were set to 1.333 and 1.426, respectively (Zhu et al. Citation2015). The volume median diameter of mannitol was measured to be 4.96 ± 0.10 µm (n = 3; mean ± SD).
2.2.3. Aerosolization Performance
The aerosolization performance of mannitol powder dispersed from the LR and HR inhalers was assessed using laser diffraction for particle size distribution measurements (Spraytec®, Malvern Instruments, Worcestershire, UK). Approximately 20.0 ± 1.0 mg of mannitol powder was directly loaded, with and without capsule, and dispersed using both inhalers at 30, 60, and 90 L min−1 for a standard total inhalation volume of 4.0 L. Airflow rates were monitored using the TSI digital flow meter (Series 4000, TSI Inc., Shoreview, MN, USA) and controlled by varying the valve of vacuum pump (Westech Scientific Instruments, UK). The setup was disassembled after each run and cleaned with deionized water to remove any possible mannitol particles that could obscure lenses. Measurements were performed in triplicate to obtain mean values.
2.2.4. Powder Release Behavior
As mannitol powder passes through the measurement cell at different concentrations in the Spraytec® apparatus, the laser detector was able to record changes in laser intensity as a function of time (laser obscuration profile). The measurement was automatically triggered once the laser transmission intensity fell below 99%, capturing data at an acquisition rate of 2.5 kHz for each run. The obscuration profiles were modeled in GraphPad Prism 6.0 (GraphPad Software, Inc., CA, USA) using a non-linear least squares approach, details of which are presented in Sections 3.4 and 3.5.
2.2.5. Statistical Analysis
Statistical analysis using unpaired two-tailed t-tests was performed in GraphPad Prism 6.0. Significance was determined at 95% confidence interval (CI), with p-values less than 0.05 indicating the values of significance.
3. RESULTS
3.1. Pressure Drop
displays the plot of the square root of pressure drop versus flow rate for both LR and HR with and without capsule. The mean inhaler resistance for LR and HR DPIs without capsule was measured and found to be 0.021 and 0.034 kPa½ (L min−1)−1, respectively. The corresponding pressure drop values for LR and HR at each tested flow rate, with and without capsule, are summarized in . These measured values were in general agreement with the values presented in the previous study by Elkins et al. Citation(2014). Such resistances were expected due to the differences in air inlet dimensions, as discussed by Coates et al. Citation(2006). Interestingly, resistances for LR and HR were significantly lower when the capsule was present in the device, recording mean values of 0.019 and 0.030 kPa½ (L min−1)−1, respectively. It has been demonstrated by Coates et al. Citation(2005b) that the presence of capsule in Aerolizer® DPI, which is similar to RS01® DPI, significantly reduced the overall turbulence levels. This explains significant reduction in resistance to airflow from both HR and LR devices when the capsule was present (p ≤ 0.006).
FIG. 1. Relationship between airflow rate and generated pressure drop for low- (LR) and high-resistance (HR) RS01® inhalers, with and without a capsule. Coefficient of correlation values were greater than 0.996 for all regression lines.
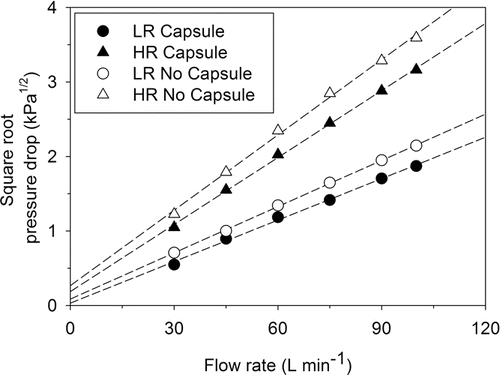
TABLE 1 Pressure drop measurements, with and without capsule, for low-resistance (LR) and high-resistance (HR) RS01® inhalers at 30, 60, and 90 L min−1 flow rate
3.2. Emitted Dose
The emitted dose (ED) from both inhalers was determined by subtracting the amount of mannitol remaining in the DPI from the initial mass of mannitol loaded. The influence of pressure drop and the presence of capsule on emitted dose for LR and HR devices are illustrated in . In general, an increase in emitted dose was evident in both cases where mannitol was loaded directly into inhaler and capsule, and was more prominent at lower pressure drops. This behavior was expected due to an increase in energy associated with higher pressure drops, as reported by Coates et al. Citation(2005a).
FIG. 2. Emitted dose as a function of pressure drop for low- and high-resistance RS01® inhalers (n = 5; mean ± SD).
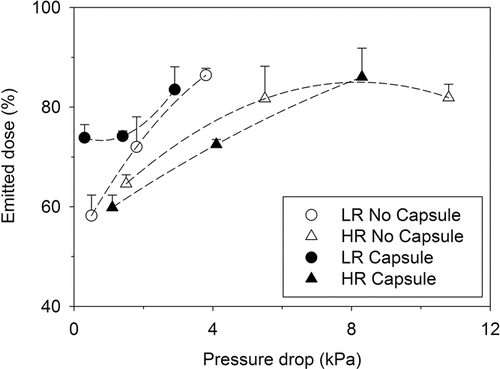
When comparing the effect of capsule at each tested flow rate, the emitted dose was found to be generally comparable at most flow rates. When the LR device was operated at 30 L min−1, mannitol powder was found to remain at the base of the device for experiments without capsule, which explains decrease in emitted dose from 73.8% (with capsule, 0.30 kPa) to 58.1% (without capsule, 0.50 kPa). In this scenario, the presence of capsule enabled the entrained air to empty powder from two pierced ends in a more efficient manner. Conversely, the presence of capsule at 60 L min−1 significantly decreased the emitted dose from the HR device from 81.7% (without capsule, 5.50 kPa) to 72.5% (with capsule, 4.10 kPa), respectively. When comparing emitted dose from both devices, HR device was found to perform less efficiently, requiring a higher pressure drop to deliver approximately the same amount of powder from the inhaler.
3.3. Aerosolization Performance
The aerosolization performance of mannitol powder dispersed with and without capsule from LR and HR inhalers at different flow rates is illustrated in . To fully differentiate the extent of powder deagglomeration, the corresponding volume medium diameter (X50) of the emitted dose, with and without capsule, at each tested flow rate is tabulated in . No significant change in X50 was observed with increasing flow rates when mannitol powder was dispersed without capsule. As expected, a substantial improvement in the aerosolization performance was evident when mannitol powder was dispersed with capsule, resulting in a shift in the particle size distribution curve to the finer region ().
FIG. 3. Influence of flow rate and presence of a capsule on the mean cumulative volumetric particle size distributions of aerosolized mannitol powder measured from Malvern Spraytec® at (a) 30, (b) 60, and (c) 90 L min–1 (n = 3).
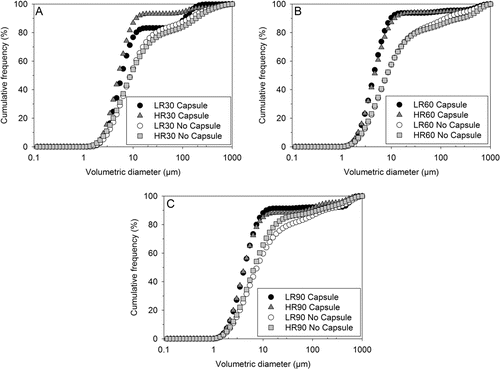
TABLE 2 Corresponding volume medium diameter (X50) of aerosolized dose measured from Malvern Spraytec® (n = 3; mean ± SD) without and with capsule
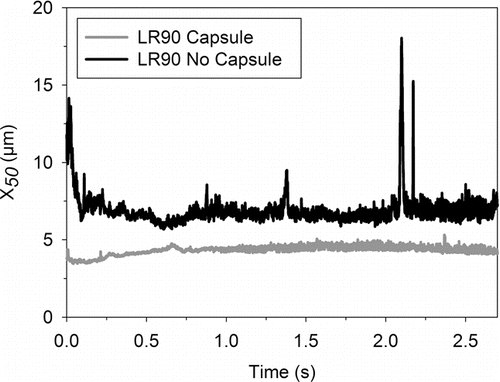
It is evident for experiments without a capsule that the device delivers powder of large particle sizes throughout the aerosolization process, particularly at the early stages of powder release. This effect is demonstrated in , which compares the change in X50 as a function of time from LR at 90 L min−1 with and without a capsule. However, by loading mannitol powder into the capsule, large agglomerates are required to first disintegrate into smaller particles and exit the pierced ends of the capsule. It has been reported by Coates et al. (2005a) that the shearing of mannitol agglomerates in the capsule could have facilitated the deagglomeration mechanism, which could be the case here. Similarly, X50 transmission profiles were evident for the dispersion of mannitol from both inhalers at all tested flow rates. Generally, a significant reduction in X50 was evident at increasing flow rates for dispersion with capsule except at 90 L min−1. However, it is important to note that at this flow rate the shattering of capsule occurred, which significantly enlarged the pierced ends, resulting in high variations in measured X50 values from run to run. It is claimed that the shattering of capsule did not prevent larger powder agglomerates from exiting the capsule, which is reflected by discrete steps in the particle size distribution (). Therefore, it is postulated that the degree of deagglomeration of mannitol powder from LR and HR devices not only depend on the resistance of the device, flow rate, and the presence of capsule but may also be dependent on how the powder is metered from the capsule.
3.4. Powder Emptying Profiles
The Spraytec® laser diffraction apparatus determines the concentration of the aerosolized sample in the measurement cell by measuring the laser light intensity reduction caused by the aerosolized powder, and is given as a percentage. Therefore, by recording changes in laser obscuration as a function of aerosolization time, the powder emptying behavior of aerosolized mannitol powder from LR and HR inhalers can be obtained, which fundamentally could not be achieved using conventional inertial impaction techniques. shows the obscuration profiles for the dispersion of mannitol powder with and without a capsule from LR at 90 L min−1. The obscuration profiles for LR and HR at all tested flow rates were similar in shape to that shown in . Qualitatively, the behavior of powder release shows a rapid rise to a maximum obscuration value (initial powder release), followed by a decaying phase (final powder release). The parameters derived from the obscuration profiles–the peak obscuration percentage (%PO), and the time taken to reach peak obscuration, tPO, characterize the rate at which mannitol powder is emptied. The values of these parameters were obtained from each run, and are presented in .
FIG. 5. Typical example showing the obscuration profiles of mannitol powder dispersed with and without capsule from low resistance at 90 L min–1.
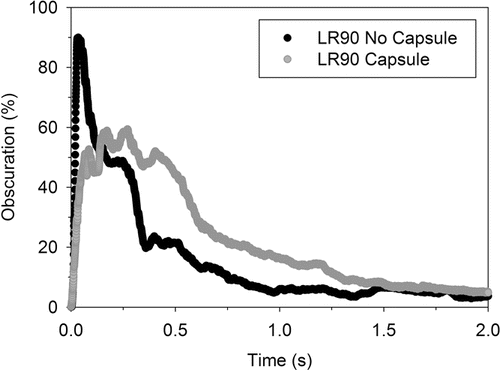
TABLE 3 Powder obscuration data tabulating %PO and tpo parameters (n = 3; mean ± SD)
In the case where mannitol powder was dispersed without a capsule, a significant increase in powder release was observed with flow rate as %PO values increased and corresponding tPO values decreased significantly. Presence of a capsule played a crucial role in the emptying behavior of mannitol from both inhalers. First, the dispersion of powder from a capsule significantly decreased the %PO values at all flow rates and increased the time taken to reach peak obscuration in most cases (with the exception of HR operated at 30 and 90 L min−1). It was reported by Jiang et al. Citation(2012) that increase in powder residence time inside the inhaler increases the interaction between the dispersed powder, air stream, and device, which could potentially aid in the powder deagglomeration process, as in our experiment. Second, it is important to note significant variability in the powder emptying behavior for the dispersion of mannitol powder with a capsule. This variability was expected as the powder had to be first released from the two ends as the capsule continuously spun and affected the walls of the device. The above findings reveal the highly complex nature of emptying behavior of capsule, the importance of considering the motion and physical state of capsule, and how it influences the powder release behavior and subsequent dispersion performance. Therefore, in order to assess the importance of capsule on powder deagglomeration, modeling of obscuration profiles to attain the rates of empting is undertaken.
3.5. Rates of Emptying
Data for the obscuration profiles were fitted using two independent mathematical models. The initial powder release (from the start of transmission to reaching tPO) displayed a sigmoidal behavior and was modeled using the following Boltzmann sigmoidal modified function:[1] where %PO′ is the peak calculated obscuration value in percentage form, t50 is the time taken to reach half of %PO′ in seconds, and tempty describes the slope of the function, with a larger tempty value indicating a slower emptying behavior of inhaler. Fitting of transmission profile to Equation Equation(1)
[1] was tested for all triplicates.
The final decay phase of the obscuration profile was fitted to a single-decay rate constant according to the following equation:[2] where A0 is the difference in obscuration percentage from %PO to baseline reading; B0 is the baseline; and Kdm corresponds to the single-decay rate constant and has units in s−1. Modeling of the final powder release behavior using Equation Equation(2)
[2] was performed for all triplicate experiments and was fitted from tPO to the end of the aerosolization period.
To evaluate the overall appropriateness of each model to the experimental data, an assessment of parameter dependency was carried out. The concept of dependency describes the degree to which the estimated parameter is dependent upon other parameters within the mathematical model (Knott and Shrager Citation1972). For instance, if a change in any one parameter of the model results in changes in other parameters such that the overall functional curve remains unchanged or results in minor changes, the parameters are regarded to be highly dependent on one another as reported by Knott and Shrager Citation(1972). The statistical software GraphPad Prism 6.0 quantifies this degree of dependency from 0.0 (parameters are entirely independent) to 1.0 (parameters being entirely dependent), with dependency values greater than 0.99 being undesirable. The correlation coefficient for each estimated parameter is tabulated in for both sigmoidal and single-decay rate models. Correlation coefficient (R2) was greater than 95% for most cases. With dependency values being less than 0.99, the proposed models provide acceptable approximations to the experimental data. Thus, the models can be used as a starting point for the analysis of the relationships between pressure drop, powder release behavior, and dispersion performance.
TABLE 4 Dependency values and coefficient correlation for each estimated parameter for the initial release and single-decay rate models (n = 3; mean ± SD)
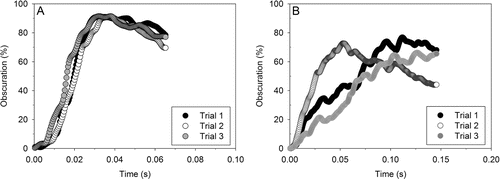
Convergence in the fitting of the sigmoidal model could only be obtained for all experiments without a capsule, and in some cases for experiments with a capsule. As such, the tempty parameter could be calculated for most flow rates, but not all (). A likely explanation is the variable nature of rattling motion of capsule inside the inhaler, as described by Sim et al. Citation(2014). compares the individual initial release behavior of mannitol powder for all triplicate experiments with and without capsule from the LR sampled at 90 L min−1. Greater variations in obscuration profiles at initial phase between each capsule experiment () were observed as opposed to the experiment without capsule. These results show that the presence of capsule plays an integral role in the mechanisms of powder release and aerosolization due to the chaotic movement of capsule and its impaction onto the walls of inhaler.
FIG. 6. Initial obscuration profiles of individual experiments from the low-resistance RS01® at 90 L min–1 (a) without, and (b) with a capsule.
The influence of pressure drop and the presence of a capsule on the emptying behavior tempty and Kdm are illustrated in . Generally, tempty decreases with increasing pressure drop, which indicates faster release behavior of mannitol powder. The presence of capsule significantly increased tempty as expected, as mannitol powder has to first exit the ends of the pierced holes from the capsule. Despite having non-converged fits for some capsule experiments, increase in tPO values, as shown in , reflects this prolonged nature of powder release due to the presence of capsule.
FIG. 7. Influence of pressure drop on the estimated rate constant parameters for (a) time constant, tempty, and (b) single-decay rate constant, Kdm (n = 3; mean ± SD).
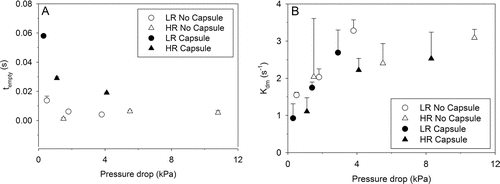
The emptying behavior during the decay phase also showed interesting behavior. First, regardless of whether the mannitol was contained in capsule or not, an increase in Kdm was observed with increasing pressure drop, especially at lower pressure drops. No significant differences in Kdm were observed when comparing dispersion with and without a capsule at each tested flow rates; however Kdm significantly decreased when mannitol was dispersed using the LR with capsule (0.30 kPa) at 30 L min−1.
An alternative method for examining the relationship between the kinetics of powder release and the dispersion performance of mannitol powder is to create scatter plots of the data. displays the scatter plots partnering each estimated parameter with their corresponding volume medium diameter obtained from the data collected with the Spraytec.
FIG. 8. Scatter plots showing the effect of estimated parameters (a) tempty, and (b) Kdm on X50 from the low- and high-resistance RS01®.
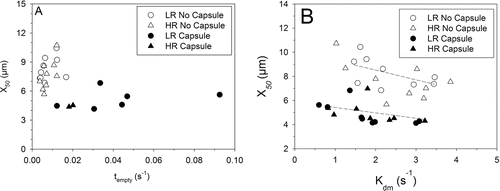
shows the relationship between tempty and X50 values. The sample data concerning the dispersion of mannitol with a capsule displayed less variation in X50 and a wider scattering of points over the entire range of tempty values when compared with experiments without a capsule. Regression analysis of both capsule-free and capsule experiments revealed the slope value to be significantly close to zero at 95% CI, indicating the dispersion performance of mannitol powder to be independent of initial emptying kinetics (p ≥ 0.0661).
On the contrary, the slopes of both capsule-free and capsule experiments were significantly different from zero at the 95% CI level (p ≤ 0.0276), revealing an inverse relationship between Kdm and X50 () This gives compelling evidence that the deagglomeration potential of mannitol powder is statistically dependent on the final powder release kinetics regardless of whether a capsule is present or not, as opposed to the initial release behavior.
4. DISCUSSION
Many types of DPIs have been developed for delivering capsule-based products. These devices are reasonably cheap to manufacture, relatively simple in design, and effective in use. In this study, factors such as device resistance, sample flow rate, rates of powder emptying, and presence of a capsule were found to influence the degree of deagglomeration of mannitol powder, with some factors having a more pronounced effect than others do. It was found that not only the structural design (i.e. air inlet area) influences the device resistance to airflow and associated pressure drops but the presence of a capsule also influences these. It has been reported that the pressure drop across the device should be sufficient to generate enough turbulence within the device to deagglomerate powder formulation, and should provide a reproducible emitted dose across a wide range of airflow rates. Although many conflicting views still exist on the actual dispersion mechanism of dry powder formulations, such details are not the focus in this study. Instead, the purpose of this study is to emphasize how the supplied energy from the airflow translates into the aerosolization process of powder deagglomeration, i.e. how it influences the motion and physical state of capsule for capsule-based DPIs. It has been noted in this study that the shattering of capsule or significant enlargement in capsule-aperture size occurred for all experiments in the HR DPI at 90 L min−1, which could have potentially changed deagglomeration and emptying behavior. In addition, if fragments of the capsule exit the device, they will be inhaled by the patient and could cause irritation in their throats. Therefore, ensuring proper metering and free-flowing properties of powder from the capsule ends as well as maintaining the structural integrity of capsule can potentially warrant better control and less variability in the emitted dose and particle size distribution of the dose discharged from capsule-based DPI. Hence, this study demonstrates the need to consider the physical influences of capsule on both release behavior and deagglomeration performance of capsule-based DPIs.
5. CONCLUSIONS
As powder dispersion from DPI systems is a time-dependent process, the results presented in this study, obtained by laser diffraction, indicate that mannitol powder released from an inhaler with the presence of capsule behaves differently than the inhaler without capsule. The shape and intensity of obscuration profiles can give critical insight into the release of powder, which has not been achieved with conventional impaction methods. A modeling technique to quantitatively compare powder release behavior was presented and applied to the study of the influence of capsule on the aerosolization performance and release behavior of micronized mannitol powder from two different capsule-based DPIs.
REFERENCES
- Behara, S. R., Farkas, D. R., Hindle, M., and Longest, P. W. (2014). Development of a High Efficiency Dry Powder Inhaler: Effects of Capsule Chamber Design and Inhaler Surface Modifications. Pharm. Res., 31(2):360–372.
- Behara, S. R. B., Kippax, P., Larson, I., Morton, D. A. V., and Stewart, P. (2011). Kinetics of Emitted Mass – A Study with Three Dry Powder Inhaler Devices. Chem. Eng. Sci., 66(21):5284–5292.
- Behara, S. R. B., Larson, I., Kippax, P., Stewart, P., and Morton, D. A. V. (2012). Insight into Pressure Drop Dependent Efficiencies of Dry Powder Inhalers. Eur. J. Pharm. Sci., 46(3):142–148.
- Cegla, U. H. (2004). Pressure and Inspiratory Flow Characteristics of Dry Powder Inhalers. Respir. Med., 98(Supplement 1(0)):S22–S28.
- Chew, N. Y., and Chan, H. K. (1999). Influence of Particle Size, Air Flow, and Inhaler Device on the Dispersion of Mannitol Powders as Aerosols. Pharm. Res., 16(7):1098–1103.
- Chew, N. Y., Chan, H. K., Bagster, D. F., and Mukhraiyab, J. (2002). Characterization of Pharmaceutical Powder Inhalers: Estimation of Energy Input for Powder Dispersion and Effect of Capsule Device Configuration. J. Aerosol Sci., 33:99–1008.
- Clark, A. R., and Hollingworth, A. M. (1993). The Relationship Between Powder Inhaler Resistance and Peak Inspiratory Conditions in Healthy Volunteers – Implications for in vitro Testing. J. Aerosol. Med., 6(2):99–110.
- Coates, M. S., Chan, H. K., Fletcher, D. F., and Raper, J. A. (2005a). Influence of Air Flow on the Performance of a Dry Powder Inhaler Using Computational and Experimental Analyses. Pharm. Res., 22(9):1445–1453.
- Coates, M. S., Chan, H. K., Fletcher, D. F., and Raper, J. A. (2006). Effect of Design on the Performance of a Dry Powder Inhaler Using Computational Fluid Dynamics. Part 2: Air Inlet Size. J. Pharm. Sci., 95(6):1382–1392.
- Coates, M. S., Fletcher, D. F., Chan, H. K., and Raper, J. A. (2005b). The Role of Capsule on the Performance of a Dry Powder Inhaler Using Computational and Experimental Analyses. Pharm. Res., 22(6):923–932.
- de Boer, A. H., Gjaltema, D., Hagedoorn, P., Schaller, M., Witt, W., and Frijlink, H. W. (2002). Design and Application of a New Modular Adapter for Laser Diffraction Characterization of Inhalation Aerosols. Int. J. Pharm., 249(1–2):233–245.
- Elkins, M. R., Anderson, A. D., Perry, C. P., Daviskas, E., and Charlton, B. (2014). Inspiratory Flows and Volumes in Subjects with Non-CF Bronchiectasis Using a New Dry Powder Inhaler Device. Open Respir. Med. J., 8:8–13.
- Hindle, M., and Byron, P. R. (1995). Dose Emissions from Marketed Dry Powder Inhalers. Int. J. Pharm., 116(2):169–177.
- Hira, D., Okuda, T., Ichihashi, M., Mizutani, A., Ishizeki, K., Okada, T., et al. (2012). Influence of Peak Inspiratory Flow Rates and Pressure Drops on Inhalation Performance of Dry Powder Inhalers. Chem. Pharm. Bull. (Tokyo), 60(3):341–347.
- Islam, N., and Cleary, M. J. (2012). Developing an Efficient and Reliable Dry Powder Inhaler for Pulmonary Drug Delivery – A Review for Multidisciplinary Researchers. Med. Eng. Phys., 34(4):409–427.
- Jiang, L., Tang, Y., Zhang, H., Lu, X., Chen, X., and Zhu, J. (2012). Importance of Powder Residence Time for the Aerosol Delivery Performance of a Commercial Dry Powder Inhaler Aerolizer((R)). J. Aerosol. Med. Pulm. Drug Deliv., 25(5):265–279.
- Kippax, P., and Morton, D. A. (2008). Unlocking the Secrets of the Dry Powder Inhaler Plume. Drug Deliv. Tech., 8(1):53–58.
- Knott, G., and Shrager, R. (1972). On-Line Modeling by Curve-Fitting. SIGGRAPH Comput. Graph. 6(4):138–151.
- Sim, S., Margo, K., Parks, J., Howell, R., Hebbink, G. A., Orlando, L., et al. (2014). An Insight Into Powder Entrainment and Drug Delivery Mechanisms from a Modified Rotahaler. Int. J. Pharm., 477(1–2):351–360.
- Suarez, S., and Hickey, A. J. (2000). Drug Properties Affecting Aerosol Behavior. Respir. Care, 45(6):652–666.
- Zhu, B., Young, P. M., Ong, H. X., Crapper, J., Flodin, C., Qiao, E. L., et al (2015). Tuning Aerosol Performance using the Multibreath Orbital(R) Dry Powder Inhaler Device: Controlling Delivery Parameters and Aerosol Performance via Modification of Puck Orifice Geometry. J. Pharm. Sci., 104(7):2169–2176.