Abstract
Theoretical and experimental analyses of the steady state, bipolar diffusion charge distribution on nanoparticles are reviewed. This charge distribution plays a critical role in electrical mobility measurements of nanoparticle size distribution functions, where it is approximated via empirical regression equations. While the regression approach has been broadly successful, there remain several unresolved issues related to charge distribution calculations. Specifically, research to date has not revealed a method to reliably calculate nanoparticle-ion collision rates in the presence of strong attractive potentials, charge distribution predictions do not routinely consider the mass and (electrical) mobility distributions of the charging ions, and calculation approaches applicable to both spherical and nonspherical particles have not been compared to experimental data. In light of these issues, we examine the steady-state bipolar charge distribution on gold nanospheres and gold nanorods via tandem differential mobility analysis (TDMA). We compare measurements to regression equations as well as to Brownian Dynamics (BD) simulations, which take ion mobility and mass distributions as inputs. These distributions were measured using a DMA coupled to a mass spectrometer. Both regression equations and BD simulations are found to agree reasonably well with measurements in air, and we find that particle mobility diameter has a much greater influence on charging than particle morphology. Results support the use of BD calculations to predict bipolar charge distributions when ion properties are known. Nevertheless, our work supports continued use of regression equations when such information is not available.
Copyright © 2015 American Association for Aerosol Research
INTRODUCTION
Accurate determination of particle size distribution functions (number-based) using electrical mobility analysis (Knutson and Whitby Citation1975; Hagen and Alofs Citation1983; Talukdar and Swihart Citation2003) is frequently carried out for nanoparticle-laden aerosols (Wiedensohler et al. Citation2012); this approach requires that particles are brought to an accurately calculable charge distribution. The charging of particles is thus of critical importance in nanoparticle measurements. Most frequently, particles are brought to a steady-state charge distribution, arising when particles are exposed to bipolar ions (consisting of approximately equal concentrations of positive and negative ions) for a sufficient amount of time (Fuchs Citation1963). Ions transfer charge to particles during particle-ion collisions, which are brought about through ion thermal motion and potential interactions between particles and ions. This process is termed bipolar diffusion charging, and the steady-state charge distribution attained is commonly referred to as the bipolar charge distribution.
Regression equations developed for nanoparticles in pure N2/air near room temperature and atmospheric pressure by Wiedensohler Citation(1988) have served as a simple tool to calculate bipolar charge distributions. Though use of these regression equations has enabled widespread success of electrical mobility based size distribution measurements, there remain a number of pending issues regarding the physics of bipolar diffusion charging. Resolving these issues could both improve our understanding of particle-ion collisions in the gas phase, and possibly lead to more accurate bipolar charge distribution calculations in unique environments (e.g., high temperature or low pressure environments). Additionally, in many instances, improvements in the accuracy of bipolar charge distributions are necessary. A recent study shows that data inversion techniques can be applied to electrical mobility based size distribution measurement to infer total particle number concentrations which are ±10% accurate in the 20–200 nm size range for atmospheric aerosols. However, uncertainties increase to ±30% outside of this size range (Wiedensohler et al. Citation2012) and larger uncertainties are encountered in nonatmospheric settings. Continued refinement of bipolar charge distribution calculation techniques would lead to potential improvements in measurement accuracy, in particular for environments where the measurement temperature and pressure deviate from 300 K and 1 bar, the temperature and pressure for which regression equations were developed (Lòpez-Yglesias and Flagan Citation2013). Here, the pending issues in the analysis of bipolar diffusion charging are outlined. Following this review and based upon its conclusions, a new comparison of charge distribution measurements to theoretical predictions is presented. The goal of this work is hence two-fold: (1) to provide a succinct review of the progress made in the past several decades in bipolar diffusion charging physics highlighting unresolved issues, and (2) to compare charge distribution calculations and measurements for both spherical and nonspherical particles, with detailed consideration of ion properties.
Review of Prior Bipolar Charge Distribution Predictions and Measurements
In reviewing prior studies of bipolar diffusion charging, we elect to focus primarily on the charging process at the particle level (nano- to microscale), though we note that the design of bipolar chargers requires consideration of fluid flow and the spatial distribution of ions produced (macroscale effects, [Hoppel and Frick Citation1990; Alonso and Alguacil Citation2003; de La Verpilliere et al. Citation2015; Tigges et al. Citation2015]). In addition, we discuss neither the unipolar charging of particles, for which we believe recently developed charging rate expressions (Gopalakrishnan et al. Citation2013b) are accurate for both spherical and nonspherical particles, nor field charging (Lawless Citation1996), applicable to particles in sufficiently strong electric fields.
The bipolar charge distribution can be directly calculated as a function of particle size if the collision rate coefficients between particles and ions are known a priori (Marlow and Brock Citation1975; Adachi et al. Citation1985; Alonso et al. Citation2002). Particle-ion collision rate coefficients are dependent upon particle size and shape, ion mass and (electrical) mobility, and particle material properties, i.e., the collision rate coefficients are dependent upon the physics governing ion motion about a particle. For nanoparticles in atmospheric pressure, room temperature environments, particle-ion collisions need to be described in a different manner than that used for ion-molecule collisions, ion–ion collisions, or supermicrometer particle-ion collisions. Ion-molecule and ion–ion collisions can typically be described by ballistic/free molecular models, wherein the colliding species travel in straight line trajectories in the absence of potential interactions, and follow deterministic equations of motion. There are a number of free molecular models which can accurately describe ion-molecule and ion–ion collisions, including the Langevin model Citation(1905) and modifications to it (Su and Bowers Citation1973; Hsieh and Castleman Citation1981; Eichelberger et al. Citation2003) used in describing chemical ionization, and the orbital motion limited model of Langmuir (Mott-Smith and Langmuir Citation1926; Allen Citation1992) used to describe collisions between ions (e.g., ion recombination [Franchin et al. Citation2015]). Conversely, the motion of ions near supermicrometer particles can typically be described by diffusive mass transfer equations (i.e., Fick's law), permitting ion-large particle collisions to be modeled by continuum model approaches (Gunn Citation1954; Fuchs Citation1964; Northrup et al. Citation1984).
To understand why nanoparticle-ion collisions require a different description than the noted collision processes, we first note that there is a clear contrast between models describing ion-molecule (or ion–ion) collisions and ion-supermicrometer particle collisions. In the former, colliding species move ballistically with respect to one another, while in the latter ions move diffusively. The contrast in models required for accurate collision rate determination was noted by Fuchs Citation(1963) over 40 years ago. It arises because of the difference in diffusive Knudsen number (Dahneke Citation1983) for ion-molecule and ion-large particle collisions. The diffusive Knudsen number is defined as the ratio of the ion's thermal stopping distance (or thermal persistence distance, the average straight line distance traveled by ions due to thermal motion) to the collision length scale (the effective collision radius for the colliding species). Contrary to what has been noted elsewhere (Friedlander Citation2000), the thermal stopping distance is distinct from the gas molecule mean free path; the mean free path is the average distance an entity travels between collisions with gas molecules. The thermal stopping distance is proportional to the ratio of the ion diffusion coefficient to the ion mean thermal speed, while the gas molecule mean free path is proportional to the ratio of the gas kinematic viscosity to gas molecule mean thermal speed (which leads to the consequence that the mean free path to thermal stopping distance ratio is proportional to the Lewis number, and is a function of the background gas properties and temperature). Collisions with large diffusive Knudsen numbers can be modeled via ballistic approaches, while those with small diffusive Knudsen numbers can be modeled via continuum approaches.
In room temperature, atmospheric pressure environments, subnanometer to nanometer sized ions have thermal stopping distances (when colliding with stationary nanoparticles) that are similar in magnitude to the radii of nanoparticles (of order 10 nm). Therefore, the models used in neither the large nor small diffusive Knudsen limits apply for nanoparticle-ion collisions, and the main challenge in modeling bipolar diffusion charging is the development of a collision rate calculation approach which is applicable across the entire diffusive Knudsen number range. At intermediate diffusive Knudsen numbers (the “transition regime” [Filippov Citation1993]), complete analysis of the collision process, even in the absence of potential interactions, has only been attempted in several prior studies (Sahni Citation1966, Citation1983; Loyalka Citation1973). As a much simpler alternative, a flux matching approach (Bricard Citation1962; Fuchs Citation1963; Lushnikov and Kulmala Citation2004; D'yachkov et al. Citation2007) has been frequently invoked for transition regime collision rate calculations, in particular for collisions between ions and nanoparticles. With flux matching, ion motion is modeled as diffusive far from a particle's surface and ballistic within a certain predefined region close to a particle (whose bounds are linked to the ion's thermal stopping distance). In this manner, flux matching enables approximate modeling of a transition regime mass transfer process but in doing so never directly applies equations applicable for the transition regime (i.e., diffusive motion and inertia/ballistic motion are never considered simultaneously).
The flux matching approach of Fuchs Citation(1963), with Hoppel and Frick's Citation(1986) modification to consider a single gas molecule collision with an ion close to the particle surface, was used by Wiedensohler Citation(1988) to develop bipolar charge distribution regression equations. Despite its simplicity and general success in predicting collision rates in the gas phase (Wagner and Kerker Citation1977; Chan and Mozurkewich Citation2001), several studies (Filippov Citation1993; Gatti and Kortshagen Citation2008; Gopalakrishnan and Hogan Citation2012; Ouyang et al. Citation2012), have noted that there are issues with the flux matching approach. These issues manifest themselves in instances where (1) the particle and ion are oppositely charged, (2) the electrostatic potential energy is considerably larger than the thermal energy of the ions, and (3) the diffusive Knudsen number is neither large enough nor small enough for collisions to be described as purely ballistic or purely diffusive (issues arise when criteria 1–3 are all satisfied). This situation leads to a strong, long-range attractive potential interaction between a particle and an ion. The assumption of purely ballistic ion motion close to a particle surface cannot be applied in the presence of strong attractive potentials (Filippov Citation1993; Stommel and Riebel Citation2007; López-Yglesias and Flagan Citation2013), limiting the utility of flux matching in this instance (we note further that the corrections provided in the aforementioned references do not necessarily completely correct flux matching predictions). Issues in calculating particle-ion collision rates in the presence of strong potential interactions are also frequently noted in studies of particle-laden (complex) plasmas (Goree Citation1992; Khrapak et al. Citation2005; Khrapak and Morfill Citation2009); for this reason the flux matching approach is rarely adopted in the plasma sciences (D'yachkov et al. Citation2007). As an alternative to flux matching based regression equations, recently we have developed a Brownian Dynamics (BD) approach for bipolar charge distribution calculations (Gopalakrishnan et al. Citation2013a), which circumvents direct particle-ion collision rate calculations (ion trajectories are instead modeled directly) and is also capable of considering distributions of ion masses and mobilities. However, this model is yet to be compared to appropriate measurements.
There are also differences reported between experimental measurements and flux-matching derived predictions. Many steady-state charge distribution measurements in the diameter ranges of 5–100 nm (Hussin et al. Citation1983; Reischl et al. Citation1983; Adachi et al. Citation1985; Wiedensohler et al. Citation1986) and 500 nm–2 μm (Porstendörfer et al. Citation1984) do agree well with predictions based on flux matching. However, the earlier work of Liu and Pui Citation(1974) notes discrepancies between flux matching predictions and measurements, and a number of other studies (Stober et al. Citation1991; Wiedensohler and Fissan Citation1991; Wiedensohler Citation1988; Reischl et al. Citation1996) show that measured charge distributions cannot be matched as clearly to flux matching predictions in gases other than air. More recent studies (He and Dhaniyala Citation2014; Jiang et al. Citation2014) further show that in certain circumstances, measured charge distributions are in stark disagreement (up to 25% different) with the Wiedensohler Citation(1988) regression-based distribution. While one of these studies suggests that disagreement is attributable to insufficient ion production rates (He and Dhaniyala Citation2014), the other (Jiang et al. Citation2014) suggests that it is due to variations in ion masses and mobilities from the input masses and mobilities in regression equations (which are fixed single values for the masses and mobilities of positive and negative ions) that substantially alter charge distributions. These prior works do not include measurements of ion masses and mobilities. To our knowledge, in only one study (Lee et al. Citation2005) has the influence of ion mobility on the bipolar charge distribution of nanoparticles been examined, and in this study as well, ion masses were not measured. Alternative to ion property measurement, it has been commonplace to either “fit” ion properties in an effort to find the best agreement between flux matching predictions and measurements or utilize ion properties measured in prior studies (Kilpatrick Citation1971; Mohnen Citation1976; Wellisch Citation1909) in different gaseous environments. This has led to inferred ion properties which vary from study to study, and the properties of ions used in bipolar diffusion charging remains an important issue which needs to be resolved for better understanding of the charging process.
Finally, it is important to consider the bipolar diffusion charging of non-spherical entities (Filippov Citation1994), as concerns over combustion generated aggregated particles (Sorensen Citation2011) as well as advances in the synthesis and usage of non-spherical nanomaterials (Eggersdorfer and Pratsinis Citation2014) have generated interest in electrical mobility based size distribution measurements for nonspherical particles. Prior measurements with straight chain iron oxide aggregates with primary particles of diameter 40–80 nm and length to diameter ratios in the range of 10 to 1000 (Wen et al. Citation1984a) as well as bundles of carbon nanotubes with mobility diameters in the range of 200–700 nm (Ku et al. Citation2011) have been compared to continuum regime charging models (Wen et al. Citation1984b). These studies show that simplified continuum regime relationships are not accurate for nonspherical particles. More recent measurements of aggregates (Maricq Citation2008; Xiao et al. Citation2012) as well as carbon nanotubes and fibers (Kulkarni et al. Citation2009; Tanaka et al. Citation2014) in the transition regime show qualitatively that open structures attain a higher fraction of multiply charged states than spheres of the same mobility equivalent diameter. However, beyond the initial results of our BD calculations (Gopalakrishnan et al. Citation2013a), we are not aware of an approach to calculate charge distributions that is equally applicable to spherical and non-spherical particles in the transition regime. Therefore, the charging of non-spherical particles as well remains an open issue.
Pending Issues in Bipolar Diffusion Charging
Despite significant theoretical and experimental advances in bipolar diffusion charging analysis the following issues persist: (1) outside of performing direct ion trajectory calculations it is not presently clear how to calculate particle-ion collision rates for charged particles with ions of the opposite polarity in the transition regime (Gopalakrishnan and Hogan Citation2012), (2) most experimental studies to date have not been used to directly test charging models, as ion properties are often fit to measurements, and (3) an approach to charge distribution calculation equally applicable to spherical and non-spherical particles has not been tested experimentally. In the subsequent portions of this work, we seek to address these three issues by presenting bipolar charge distribution measurements carried out via tandem differential mobility analysis of gold nanospheres and nanorods, with accompanying ion mass-mobility measurements carried out with a high resolution parallel-plate differential mobility analyzer (DMA) coupled to a mass spectrometer (Hogan and Fernandez de la Mora Citation2009; Rus et al. Citation2010). We compare measured charge distributions to charge distribution predictions made using the BD method of Gopalakrishnan et al. Citation(2013a), which takes measured ion mass and mobility distributions as inputs. In using the BD approach, we are also able to account for initial particle size distribution functions, particle shape, and the transfer functions of the DMAs employed in the charge distribution measurements. We demonstrate that the BD-based calculation approach, while more complex than conventional calculation procedures, is a viable approach to charge distribution calculation for nonspherical particles when detailed information on ion mass and mobilities distributions is available.
EXPERIMENTAL AND NUMERICAL METHODS
Aerosol Generation
As test particles of unambiguous chemical composition, gold nanospheres and nanorods were aerosolized from aqueous suspensions (Nanopartz Inc.) using an electrospray aerosol generator (model 3480, TSI Inc., Shoreview, MN, USA) (Kaufman Citation1998). Transmission electron micrograph images of these particles are shown in Figure S1 of the online supplemental information (SI) and their average dimensions (d p: diameter for both spheres and nanorods, lp: nanorod length) are provided in . Measurement of their dimensions is described further in Gopalakrishnan et al. Citation(2015). The as-obtained aqueous suspensions (which were concentrated and stabilized by minimal amounts of α,ω-methacryloylundecyltrimethylammonium bromide (MUTAB) surfactant by the manufacturer) were diluted in 10 mM ammonium acetate for electrospray aerosolization. Silica capillaries of varying inner diameters (50–100 μm) and 360 μm outer diameters were utilized in the electrospray aerosol generator. Particles were electrosprayed into two different gases, ultra-high-purity (UHP) air (99.99% pure, Matheson Inc.), and industrial grade carbon dioxide (99.9% pure, Matheson Inc.), with results for UHP air reported in the main text, and results for CO2 discussed in the SI. When using UHP air, a small amount of carbon dioxide (<2% by volumetric flowrate) was also introduced into the electrospray chamber to prevent corona discharge. The electrospray aerosol generator was operated with a 10 mCi Po-210 bipolar ion source installed downstream, leading to substantial reduction in the charge of the electrospray aerosolized nanoparticles. The charge reduced particles were subsequently passed through a tube furnace (Lindberg TF5503A-1) to evaporate the coating of surfactant on the particle after solvent evaporation (Lenggoro et al. Citation2007). A summary of the electrospray aerosol generator (capillary inner diameter, driving pressure, applied voltage, and measured electrospray current) and furnace (temperature) settings employed for each particle type are provided in Table S1 of the SI. For each particle type, following aerosolization, size distribution functions were measured directly using a TSI 3085® cylindrical DMA (Chen et al. Citation1998) and a TSI 3025A® condensation particle counter (Stolzenburg and McMurry Citation1991) operated in stepping mode.
TABLE 1 Mean dimensions of electrosprayed gold nanoparticles. denotes the mean length to diameter ratio for nanorods.
denotes the mean of the ratio of the orientationally averaged projected area to
, where RS is the Smoluchowski radius. The mobility diameter refers to the mobility equivalent diameter in air at atmospheric pressure and room temperature
Measurement of Charge Distributions
The charge distribution measurement system is depicted in . In it, the electrospray aerosol generator and furnace were used to aerosolize the gold particles. Particles then entered DMA-1 at a flowrate of 1.5 l min−1, which was operated in recirculating mode with fixed sheath flowrate and voltage, corresponding to the peak in the mobility spectrum for each particle type (with settings given in Table S1). The particles transmitted through DMA-1 were then passed through a section of black silicone tubing (composed of a polydimethylsiloxane blend) and into a Teflon chamber containing a new (<1 month old) 10 mCi Po-210 α source that generates bipolar ions. The radioactive intensity of the Po-210 source used here was about a factor of 4 higher than is used in most flow tube geometry bipolar ionizers (He and Dhaniyala Citation2014). The chamber was designed to promote flow recirculation, and gas molecule ionization and particle bipolar diffusion charging occurred simultaneously. Teflon was applied as the housing material to facilitate DMA-MS measurements (described in the subsequent section); ions were directed into the DMA for such measurements electrostatically, hence the housing material was nonconducting (Maisser et al. Citation2015). Shown in the SI, particles were brought to a steady-state charge distribution within the chamber.
FIG. 1. Depiction of the tandem differential mobility analysis system used to infer the fractions of neutral, +1, +2, −1, and −2 gold nanospheres and nanorods, after bipolar diffusion charging within in Po-210 α-irradiation source.
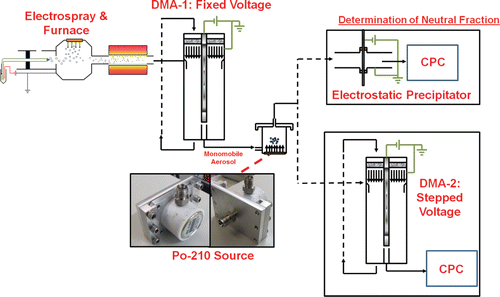
Though methods have been developed for complete charge distribution characterization (Wild et al. Citation2012) and comparison to flux matching predictions (Hogan et al. Citation2009), we opted to characterize the charge distribution through determination of selected charge fractions to simplify comparison between BD calculations and measurements. First, the fraction of neutral (uncharged) particles, f0, was determined by passing particles through an electrostatic precipitator (ESP), in which a potential of 3 kV applied to a tungsten rod relative to ground was found sufficient to collect ∼100% of charged particles for all the particle sizes examined, while leading to negligible collection of neutral particles. With a CPC placed downstream of the ESP, f0 was determined by comparing the CPC number concentration measured with the ESP voltage applied (nON) to the number concentration measured with no voltage applied (nOFF):[1a]
Charged particles were quantified by passing particles from the Po-210 ionizing chamber into DMA-2 (also TSI model 3085®) and then the CPC. Mobility spectra were measured by stepping the DMA-2 voltage. At each voltage, a sufficient number of particles N was counted so that the counting uncertainty (=N−1/2) was less than 1% for the DMA-2 settings of highest number concentration (the peak mobility). For each particle type, measurements of positive and negative particles were made within one hour of each other, and with the electrospray aerosol generator operating stably and repeatably during the measurement period. Specific peaks in number concentration versus electrical mobility (as determined from DMA-2 classification voltage and sheath flow rate) plots were evident, with each peak corresponding to particles which had a net charge of ±1 or ±2. With the stepping procedure employed, ΔZp, the width of the mobility measurement window was approximately constant. The inferred number concentration at each DMA set electrical mobility is directly proportional to the mobility distribution function dnZ/dZp (which can be integrated with bounds over the electrical mobility to give the total number concentration in a specific electrical mobility range). We fit Gaussian distributions for each peak in the measured mobility distributions and integrated these fit distributions to obtain relative concentrations nr,z, where z = +1, −1, +2, or −2. Charge fractions relative to one another were then obtained using the equations:[1b]
[1c]
[1d]
It is through the charge fraction and charge fraction ratios described in (1a—d) that we characterize the charge distribution, and compare to BD calculations.
Ion Mass and Mobility Distribution Measurements
Prior studies have shown that the ions generated by α-irradiation sources likely arise from the tubing used in measurements (Steiner and Reischl Citation2012; Gopalakrishnan et al. Citation2013a; Steiner et al. Citation2014). Therefore, to ensure that mass and mobility measurements were made of ions representative of those employed in particle charging experiments, we used the same silicone tubing upstream of the Teflon chamber (connecting it to a gas cylinder) and directly attached the Teflon chamber to the DMA-MS system. We note that silicone tubing was selected primarily for consistency between the two experimental systems (and because it is commonly encountered in laboratory aerosol measurements) and not in an effort to control the properties of the generated ions. However, it was known a priori that this tubing releases silicones into the vapor phase (Timko et al. Citation2009; Yu et al. Citation2009), hence the presence of polydimethylsiloxane ions was anticipated. The DMA-MS consisted of a high resolution parallel-plate DMA (model P5, SEADM, Boecillo, Spain) attached on the front end of a QSTAR XL mass spectrometer (Applied Biosystems, Waltham, MA, USA). To guide ions into the DMA from the chamber, a voltage of ±2 kV was applied to the Po-210 source itself (relative to the DMA upper electrode, hence the source was floating) and 0.8 l min−1 of gas was passed into the Teflon chamber and then into the DMA. Operation of DMA-MS systems of this type is described in detail in a number of prior studies (Hogan and Fernandez de la Mora Citation2009; Ouyang et al. Citation2013; Fernández-García and Fernández de la Mora Citation2014; Larriba et al. Citation2014) and outside of the use of inlet flow with the Po-210 source, DMA-MS operation was identical to these prior works. DMA calibration was performed via measurement of the (tetraheptylammonium2-bromide)+ ion ((THA2Br)+) in air and the THA+ ion in CO2; the mobilities of these ions at 298 K are reported elsewhere (Ude and Fernandez de la Mora Citation2005; Fernández-García and Fernández de la Mora Citation2014). Temperature correction was not applied during calibration, as a heat exchanger attached to the DMA sheath flow loop enabled measurements at a temperature within 1 K of the calibration measurement temperatures.
Numerical Methods
It is important to note that the charge fractions were not measured for particles of identical dimensions; aerosolization of colloidal particles and mobility classification by DMA-1, which had finite resolving power, led to a distribution of diameters (and lengths for nanorods) entering the Po-210 source and DMA-2. Tandem DMA charge distribution measurements (Jiang et al. Citation2014) can be processed using a data inversion scheme accounting for DMA transmission efficiencies (Stolzenburg and McMurry Citation2008). However, here we apply the converse to this approach, and account for the input size distribution functions, DMA transmission efficiencies (transfer functions), as well as measured ion properties directly in BD simulations, i.e. simulations are performed to mimic experimental measurements as incorporating such complexities does not substantially decrease computation speed. BD simulations were performed as follows: the dimensions of ∼100 particles for each particle type were inferred from transmission electron microscope measurements, as described previously (Gopalakrishnan et al. Citation2015). For each measured particle, its expected singly charged mobility (Zp) was inferred using the equation (Dahneke Citation1973; Zhang et al. Citation2012; Thajudeen et al. Citation2015):[2a] which has been shown to agree with measurements of spheres and rods to within ±10% (Gopalakrishnan et al. Citation2015). In Equation (2), z is the integer charge state (z = ±1 for singly charged particles), e is the unit electron charge, μ is the gas dynamic viscosity, a1, a2, and a3 are the slip correction factor coefficients, λ is the hard sphere mean free path of the gas, RS is the particle's Smoluchowski radius (Gopalakrishnan et al. Citation2011), equated with the hydrodynamic radius as per the Hubbard–Douglas approximation (Hubbard and Douglas Citation1993), and PA is the particle's orientationally averaged projected area. Calculation of the dynamic viscosity and mean free path is described in the SI, and slip correction factor coefficients are also provided in Table S3. For each nanosphere, the relationships RS = dp/2 and PA = πdp2/4 were applied. For nanorods, RS was calculated using the equation (Hansen Citation2004):
[2b] and PA with the equation:
[2c]
In Equation (2b), . The probability (Ω) that each particle would be transmitted through DMA-1 was calculated from the nondiffusing transfer function (Knutson and Whitby Citation1975; Stolzenburg and McMurry Citation2008):
[3a] where QS is the DMA sheath flowrate (Table S1), Qa is the aerosol flowrate (1.5 l min−1), and Zp* is given by the equation:
[3b]
In Equation (Equation3b[3b] ), V is the voltage applied to the DMA (Table S1), L is the classification length, and ri and ro are the inner and outer radii of the DMA, respectively. To commence BD simulations, a TEM-imaged particle (with specific dimensions) was selected at random, with its probability of selection weighted by its Ω value. Subsequently, a measured ion, of a particular mass, mobility, and charge state (+1 or −1) was selected at random, with its probability of selection weighted by the “fraction” values noted in Table S4; these values are based upon signal intensities observed in DMA-MS measurements (presented subsequently). Fraction values are normalized such that there was a 50% probability of selecting a positively charged ion and a 50% probability of selecting a negatively charged ion. The particle and ion selected were then each placed within a spherical simulation domain, and as described by Gopalakrishnan et al Citation(2013a), the ion's trajectory about the particle was monitored using the Ermak and Buckholz Citation(1980) solution to the Langevin equation (Chandrasekhar Citation1943) (assumed reasonably valid for the ion) with the ion's mass (mi), mobility (Zi used to infer a friction coefficient via the equation Zi = e/fi) and charge state taken as inputs to the equations of motion. The ion experienced both Coulomb and image potentials (Brock Citation1970; Filippov Citation1993) while also undergoing thermal diffusion as it moved about the particle, and the simulation domain size was selected such that on the domain surface, the influence of potential interactions on ion motion was negligible relative to thermal influences.
The ion's trajectory was monitored until it either collided with the particle, or left the simulation the domain. In both instances, a new particle was randomly selected (again with a probability proportional to Ω) as was a new ion (with a probability proportional to its fraction of the total ion population). If the ion left the simulation domain, the particle charge was not updated, while if collision occurred, the particle's charge state was increased or decreased according to the colliding ion's charge state (the first selected particle was neutral for all simulations). In total, for each experiment, simulations were performed to monitor ∼20,000 ion-particle collisions (found to be sufficient to achieve convergence of the moments of the charge distribution over time), and over the course of a simulation, the fraction of time spent with the particle attaining a specific charge state was monitored. The neutral fraction, f0, was then calculated as the ratio of the fraction of time the particle was neutral relative to the total simulation time. However, for the charged fractions, it was necessary to consider the influence of the DMA-2 transfer function on results. This was accomplished by recording the particle charge state z (determined from the most recent collision), the particle electrical mobility Zp (calculated with knowledge of the charge state via Equation (Equation2a[2a] )), and the time that the particle under examination t(z, Zp) occupied the simulation domain with this charge state. These values enabled calculation of ΩV2, the probability that the particle would be transmitted through DMA-2 when voltage V2 was applied, via application of Equation (Equation3a
[3b] ). Using t(z, Zp) and ΩV2 values, the detector response after DMA-2, RV2, was calculated as:
[4a] where itot is total number of particle-charge state pairs examined. In Equation (Equation4a
[4a] ), simulated signal intensity is provided in units of simulation time; hence in applying the BD approach we invoked the ergodic hypothesis, i.e., we assumed that the fraction of time a single particle spends with a given charge state is exactly equal to the fraction of particles (in an ensemble, as is measured) with the charge state in question at an instant in time. Similar to measurement results, plots of RV2 versus either DMA-2 applied voltage or mean electrical mobility display specific peaks corresponding to different charge states. Fitting Gaussian curves to each peak and integrating fit distributions (giving values
), enabled calculation of the charge fraction ratios:
[4b]
[4c]
[4d]
Experimentally inferred values (Equation (1)) and numerically inferred values (Equation (4)) can thus be compared directly.
RESULTS AND DISCUSSION
Nanorod and Nanosphere Mobility Measurements
Plots of the CPC measured number concentrations as a function of DMA-1 set electrical mobility (Zp*) are provided in the upper panes of for 50 nm gold nanospheres (left) and mean aspect ratio 2.2 nanorods (right). In the middle and lower panes, similar plots are shown for tandem DMA measurements, with the first DMA set to the electrical mobility of the singly charged peak and with particles passing through the Po-210 chamber before entering DMA-2. In all plots, peaks corresponding to specific charge states are labeled. These results show that the electrospray aerosolization-furnace technique employed enables dispersion of gold nanospheres and nanorods into the gas phase, and that peaks corresponding to specific charge states can be discerned in tandem DMA measurements. In addition, we find that the peak mobilities for gold nanospheres and nanorods are in good agreement with Equation (Equation2a[2a] ) predictions with inputs based upon TEM measurements, as has been reported previously (Gopalakrishnan et al. Citation2015). The exception to this is the average aspect ratio 4.3 nanorods; we were not able to use the furnace to remove surfactant coating from these nanorods without promoting coalescence. Some surfactant hence remained on nanorods in the gas phase, which we accounted for in simulations by considering a coating thickness of ∼3 nm around the entire particle. The mobility diameter for all particles, dm, can be calculated using the equation:
FIG. 2. Number concentration versus DMA centroid electrical mobility (Zp*) for 50 nm nanospheres (left) and lp/dp = 2.2 nanorods. (Top) Measurements obtained by stepping DMA-1 voltage and transmitting particles directly to the CPC. (Middle) Particles selected by DMA-1 operating with fixed voltage and measurements made with DMA-2 operating to select negative particles. Measurements are represented by symbols. Corresponding Gaussian distribution fits for the −1 and −2 peaks are also plotted. (Bottom) Particles selected by DMA-1 operating with fixed voltage and measurements made with DMA-2 operating to selected positive particles. Gaussian distribution fits for the +1 and +2 peaks are also plotted.
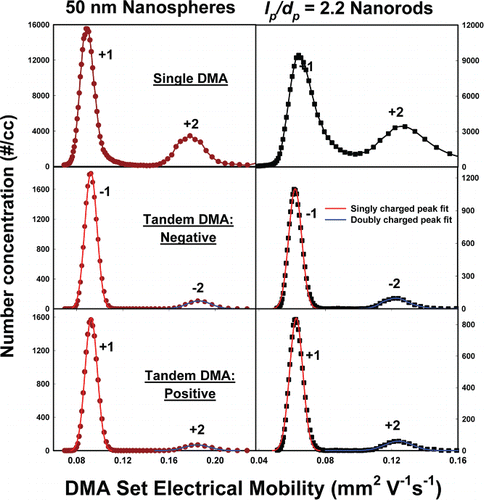
With Zp calculated for UHP air using mean TEM dimensions, dm values for all particles are reported in , with values inferred from measurements (at the peak mobility in spectra) also provided for comparison. With the exception of the aspect ratio 4.3 nanorods (where the measured mobility diameter was 31.6 nm), these values are in excellent agreement with calculations based upon peak Zp values in experiments.
DMA-MS Measurements of Ion Mobility and Mass Distributions
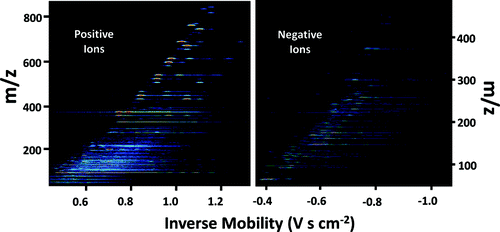
DMA-MS measurements can be represented as contour plots of mass spectrometer measured signal intensity as a function of both inverse mobility (proportional to the DMA voltage and inferred from calibration) and ion mass to charge ratio (Larriba et al. Citation2011; Ouyang et al. Citation2013). For positive and negative ions in UHP air, such contour plots are displayed in and for CO2 contour plots are provided in Figure S5 of the SI. Signal intensity is displayed on a color scale, with color varying logarithmically from blue (least intense) to red (most intense) and black denoting measured signal below a preset threshold. Evident in all contour plots are specific line segments. Each line segment represents an ion of specific chemical composition and mobility, with line segment width governed by mass averaging (over 1 Da to obtain plots), and length determined by the resolving power of the DMA. For the smallest ions detected we find some evidence of collision induced dissociation between the DMA and mass spectrometer; this leads to the appearance of line segments at the inverse of mobility of the parent but the mass or masses of the product ions (Hogan and Fernandez de la Mora Citation2010). Similarly, longer, blue colored line segments in contour plots are evidence of collision based charge exchange in the DMA (Maisser et al. Citation2015); as an ion traverses the DMA it may collide with a vapor molecule (note the sheath gas is of identical composition to the gas entering the chamber) of higher gas phase basicity (for positive charge) or gas phase acidity (for negative charge), leading to charge transfer. Signal is then detected at the mass of the ion receiving charge, and the inverse mobility where this ion appears is a function of the originally charged ion's mobility, its own mobility, and the time at which charge exchange occurred in the DMA. While detailed analysis of the ions detected in DMA-MS measurements require consideration of both collision induced dissociation and exchange, in this study we are mainly interested in inferring the masses and mobilities of the most abundant ions in spectra, and using these values as inputs for BD simulations. We therefore elect not to examine collision induced dissociation and charge exchange in detail here, and report on chemical and structural characterization of ions elsewhere (Maisser et al. Citation2015). For 97 ions in UHP air and 89 ions in CO2 (including isotopes), the masses and mobilities inferred from DMA-MS measurements are provided in Table S4. The peak signal intensities for each ion were used to infer a “fraction” value of each ion, i.e., the fraction of the total ion concentration accounted for by the ion in question. In large part, we find that the detected ion masses are relatively insensitive to gas composition (air versus CO2). This suggests that the detected ions are of identical chemical composition in both gases and that they derive from impurity species found in tubing. Most notably, in positive ion spectra, polydimethylsiloxane ions are present, which have masses in the 300–850 Da range. These ions, found previously to arise via evaporation of polydimethylsiloxane molecules from silicone tubing (Yu et al. Citation2009), are not detected in high concentration in negative spectra, and therefore skew positive ion distributions towards higher masses and lower mobilities than negative ions. Both the measured positive and negative ions are, on average, larger in mass than the assumed ion mass in prior studies, which ranges from 50–200 Da (Reischl et al. Citation1996). Finally, as ion mobility is a function of not only chemical composition but also background gas, each ion detected is found to have very different mobility in air than in CO2 (mobilities are smaller in CO2).
FIG. 3. Mass-mobility contour plots for ions generated in a Po-210 α-irradiation chamber in ultra-high purity air. Data are represented as signal intensity (represented colorimetrically on a log scale) as a function of inverse mobility and mass to charge (m/z) ratio. The visible line segments signify detection of an ion of specific chemical composition; the length of the line segment is dependent upon the DMA resolution.
Experimental and Numerical Charge Ratio Comparison
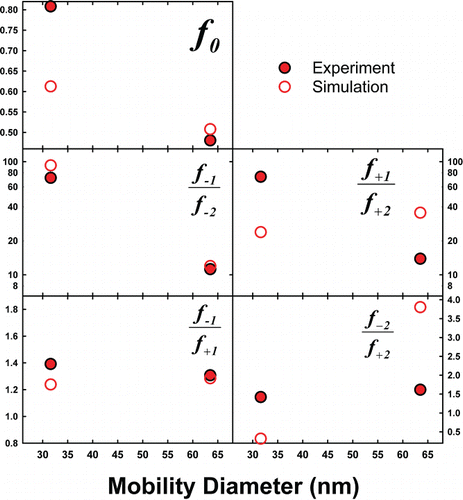
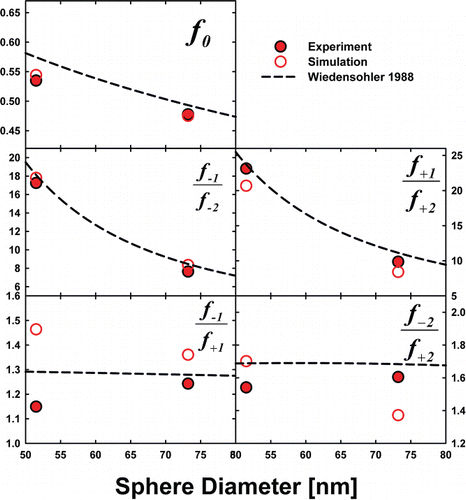
Experiments which show that particles attain a steady-state charge distribution in the Po-210 chamber as well as the validation of simulations are discussed in the SI (including Figures S2–S4). Measurements and simulations of neutral fractions and charge fraction ratios were carried out for spheres, as well as for particles of variable morphology but of similar mobility size. First, for gold nanospheres only, in we display neutral fractions and charge fraction ratios from measurements and simulations in UHP air (with measurements in CO2 shown in Figure S6). Also shown are predicted values from the Weidsenohler Citation(1988) distribution. Immediately apparent in comparing predictions and measurements is that the regression equation predictions, which do not consider the measured ion properties (nor instrument transmission influences), are in reasonable agreement with measurements in air. Measurements hence support continued use of these equations in air near room temperature and atmospheric pressure. At the same time, in UHP air, measurements are in good agreement with BD predictions, and in many instances, BD predictions are in better agreement with measurements than the regression equation. This finding supports the use of the BD approach for bipolar charge distribution calculation when ion properties are known. However, in CO2 (see the SI), larger deviation between measured and simulated results is evident, and all measurement-inferred charge fraction ratios in CO2 are biased towards negative (or less positive) charge states moreso than measurements in air. This finding suggests that in CO2, a low mass, highly mobile negative ion was present in appreciable concentrations to influence charging, but was not detected in DMA-MS measurements (therefore it was not considered in simulations). As future work will be necessary to draw clear conclusions about charging in CO2, we elect not to examine this point further in this study.
FIG. 4. The measured and predicted neutral factions (f0), as well as the charge fraction ratios inferred from both measurements (closed symbols) and simulations (open symbols) for gold nanospheres. For comparison, regression equation derived neutral fractions and charge fraction ratios are plotted as a function of sphere diameter.
To compare and contrast the charge fractions of nanorods, we first remark that the 70 nm spheres and nanorods with mean aspect ratio of 2.2, 11.3, and 14.9 all have expected mobility diameters in UHP air in the 64–73 nm range; thus, they are a set of particles similar in mobility (i.e., mobility equivalent size) yet differing in morphology. Discussed in prior work (Gopalakrishnan et al. Citation2011, Citation2013b), the influences of a particle's structure on mass transfer and charging can be quantified for all shapes via the ratio , which is equal to 1.00 for spheres and decreases with increasing asphericity. Neutral fractions and charge fraction ratios in UHP air, derived from both measurements and simulations, are plotted against
in , with images of each examined particle type also provided to facilitate comparison. With the exception of charge fraction ratios dependent upon f+2, measurement and simulation results are in good agreement with one another, which again supports use of BD simulations for charge distribution calculations. Importantly and in line with prior simulations (Gopalakrishnan et al. Citation2013a), measurement and simulation results are relatively insensitive to
for fixed mobility diameter, suggesting that the influence of particle shape on the bipolar charge distribution is minimal compared to the influence of size. The finding that particle shape does not strongly influence charge fractions is distinct from the conclusions of prior studies on soot aggregates in a similar size range to the particles examine here (Maricq Citation2008; Xiao et al. Citation2012). Both of the noted studies found that non-spherical particle neutral fractions were systematically lower (but only by 5–15%) than was found for spheres of the same mobility diameter. Possible reasons for this discrepancy include potential differences in ion properties for the measurements made of soot aggregates (engine exhaust) as compared to spheres (which were not combustion derived) and the potential for larger, higher aspect ratio aggregates to align in mobility analyzers (Zelenyuk and Imre Citation2007; Shin et al. Citation2010; Li et al. Citation2013; Park et al. Citation2014). This latter phenomenon would lead to an apparent smaller mobility diameter for larger aggregates, and these larger aggregates would have lower neutral fractions than smaller particles.
FIG. 5. Plots of the neutral fractions and charge fraction ratios for gold nanospheres and nanorods in UHP air, inferred from both experiments (E, circles), and simulations (S, squares). Results are plotted as functions of PA/πRS2, the ratio of the orientationally averaged projected area to the effective projected area for a Smoluchowski radius sphere. These values are calculated for 70 nm gold nanospheres, nanorods,
nanorods, and
nanorods, with dimensions reported in . All particles have mobility equivalent diameters in the 64–73 nm range.
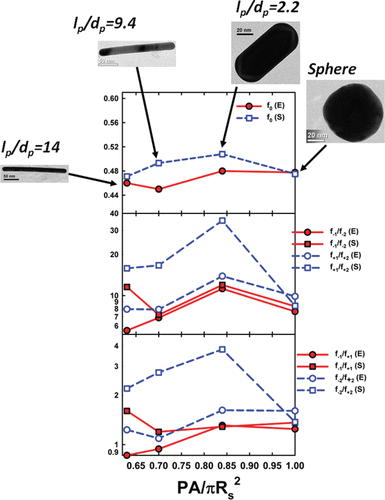
The mean aspect ratio 2.2 and 4.3 rods have nearly identical ratios, and can be used to examine the influence of mobility diameter on non-spherical particle charging. Neutral fraction and charge fraction ratios for these particles in UHP air are plotted in . Good agreement between measurements and theory for most charge fraction ratios, with the exception of those which are a function of f+2 (the smallest fractional value examined and hence the most difficult to obtain). Through this comparison the neutral fraction and ratios of singly to doubly charged particles are again found to vary more strongly with mobility diameter, emphasizing that it is equivalent particle mobility size, not particle morphology, which largely determines the steady state bipolar charge distribution in air near 300 K and atmospheric pressure.
CONCLUSIONS
Through reviewing prior work on the physics of bipolar diffusion charging, and subsequently by carrying out measurements and simulations of the charge distribution on well-defined spherical and cylindrical particles, we make the following conclusions:
Despite the theoretical issues with flux matching discussed here and in prior work, we find that charge distribution predictions based on the Wiedensohler Citation(1988) regression distribution (which derive from flux matching theory) are in good agreement with measurements reported here. This good agreement may arise because the ion properties used in developing these regression equations were selected to force agreement between theory and experimental measurements. We suggest continued use of these regression equations for measurements made in air at room temperature, and atmospheric pressure for submicrometer particles and nanoparticles, when ion properties are not available. However, we note again that discrepancies with regression equations have been observed in prior work (He and Dhaniyala Citation2014; Jiang et al. Citation2014) and that discrepancies will arise when charging ions have extremely different ion properties from those here, when steady-state is not achieved, or in bipolar ion sources with differential losses of positive and negative ions.
We find BD simulations are a viable approach for the calculation of bipolar charge distributions, as they agree as well as regression equation predictions do with experimental measurements, and in some instances are in better agreement with measurements. We anticipate BD simulations will be particularly useful for charge distribution calculations in gases other than air and N2, at reduced or elevated pressure, reduced or elevated temperature, or in the presence of high concentrations of uniquely low or high mass ionizable species. Deviations between BD simulations and measurements appear to arise when the input ion masses and mobilities are not representative of the ions/charge carriers actually present.
While particle shape has an influence on the bipolar charge distribution, its affect is considerably weaker than the influence of mobility equivalent size in air at 300 K and atmospheric pressure.
The literature review and study presented here focus only on a subset of the features of bipolar chargers used in aerosol measurement, i.e., they are limited to the study of the particle-ion collision rate in the absence of external electric fields, and the influence of both ion properties and particle morphology on the collision rate. Improved charge distribution calculations will also require improved macroscopic modeling of the charging process. For example, the effects of ion depletion (Adachi et al. Citation1989), which manifest in higher concentration aerosols, should be incorporated into future charge distribution models. Charge distribution calculations also need to be extended to high ion concentration environments (plasmas) in which particle-ion collisions cannot be accurately modeled without considering many-body potential interactions (Chaudhuri et al. Citation2010; Khrapak and Morfill Citation2009).
While this study and recent related works (Ehn et al. Citation2011; Steiner and Reischl Citation2012; Steiner et al. Citation2014; Maisser et al. Citation2015) show clearly that the mass and mobility distributions of ions generated is a function of the material used in the measurement/sampling system, future studies will need to be carried out to examine the influence of the manner of bipolar ion production has on the bipolar charge distribution. Ionizing radiation from radioactive sources (e.g., Po-201, Am −241, Ni-63 and Kr-85), corona discharges (Stommel and Riebel Citation2005), soft X-rays (Shimada et al. Citation2002), and microplasmas (Manirakiza et al. Citation2013) has been used in bipolar diffusion charging. Such ion generation methods may produce ions of a different chemical composition than Po-210, even within otherwise identical measurement systems.
UAST_1109053_Supplementary_Files.zip
Download Zip (1.2 MB)ACKNOWLEDGMENTS
Numerical simulations were performed with the resources at the University of Minnesota Supercomputing Institute. Ranganathan Gopalakrishnan acknowledges support from a University of Minnesota Doctoral Dissertation Fellowship. The authors thank Dr. Carlos Larriba for his help and guidance in operating the DMA-MS system and Dr. Derek Oberreit for designing the Po-210 charger housing.
SUPPLEMENTAL MATERIAL
A depiction of the gold nanospheres and nanorods used in experiments (Figure S1), a list of the electrospray and DMA settings used in experiments (Table S1) a list of the mobilities of ions used in DMA-MS calibration (Table S2), notes on gas viscosities, a list of the properties of air and CO2 (Table S3), notes on the attainment of the steady state charge distribution within the Po-210 chamber (Figures S2–S4), notes on simulation validation, a summary of the mass and mobilities of Po-210 generated ions in UHP air and CO2 (Table S4 and Figure S5), and neutral and charge fraction ratios for gold nanospheres in CO2 (Figure S6) are included in the supplemental material. Supplemental data for this article can be accessed on the publisher's website.
Funding
The tandem differential mobility analysis experiments were supported by National Science Foundation Grant CBET-1133285. Transmission electron microscopy was carried out at the University of Minnesota Characterization Facility (CharFac), which receives partial support from the NSF through the MRSEC program.
REFERENCES
- Adachi, M., Kousaka Y., and Okuyama K. (1985). Unipolar and Bipolar Diffusion Charging of Ultrafine Aerosol-Particles. J. Aerosol Sci., 16:109–123.
- Adachi, M., Okuyama, K., Kozuru, H., Kousaka, Y., and Pui, D. Y. H. (1989). Bipolar Diffusion Charging of Aerosol Particles Under High Particle/Ion Concentration Ratios. J. Aerosol Sci., 11:144–156.
- Allen, J. E. (1992). Probe Theory - The Orbital Motion Approach. Phys. Scr. 45:497–503.
- Alonso, M., and Alguacil, F. J. (2003). The Effect of Ion and Particle Losses in a Diffusion Charger on Reaching a Stationary Charge Distribution. J. Aerosol Sci., 34:1647–1664.
- Alonso, M., Hernandez-Sierra, A., and Alguacil, F. J. (2002). Diffusion Charging of Aerosol Nanoparticles with an Excess of Bipoloar Ions. J. Phys. a-Mathemat. General, 35:6271–6280.
- Bricard, J. (1962). La Fixation des Petits Ions Atmosphériques Sur les Aérosols Ultra-Fins. Pure Appl. Geophys., 51:237–242.
- Brock, J. R. (1970). Aerosol Charging: The Role of Image Force. J. Appl. Phys., 41:843–844.
- Chandrasekhar, S. (1943). Stochastic Problems in Physics and Astronomy. Rev. Modern Phys., 15:1–89.
- Chan, T. W., and Mozurkewich, M. (2001). Measurement of the Coagulation Rate Constant for Sulfuric Acid Particles as a Function of Particle Size using Tandem Differential Mobility Analysis. J. Aerosol Sci., 32:321–339.
- Chaudhuri, M., Khrapak, S. A., and Morfill, G. E. (2010). Effect of Ionization/Recombination Processes on the Electrical Interactions Between Positively Charged Particles in Highly Collisional Plasmas. Phys. Plasmas, 17:034503.
- Chen, D. R., Pui, D. Y. H., Hummes, D., Fissan, H., Quant, F. R., and Sem, G. J. (1998). Design and Evaluation of a Nanometer Aerosol Differential Mobility Analyzer (Nano-DMA). J. Aerosol Sci., 29:497–509.
- Dahneke, B. E. (1973). Slip Correction Factors for Nonspherical Bodies—III The Form of the General Law. J. Aerosol. Sci., 4:163–170.
- Dahneke, B. E. (1983). Simple Kinetic Theory of Brownian Diffusion in Vapors and Aerosols, in Theory of Dispersed Multiphase Flow, R. E. Meyer, ed., Academic Press, New York.
- de La Verpilliere, J. L., Swanson, J. J., and Boies, A. M. (2015). Unsteady Bipolar Diffusion Charging in Aerosol Neutralisers: A Non-Dimensional Approach to Predict Charge Distribution Equilibrium Behaviour. J. Aerosol Sci., 86:55–68.
- D'yachkov, L. G., Khrapak, A. G., Khrapak, S. A., and Morfill, G. E. (2007). Model of Grain Charging in Collisional Plasmas Accounting for Collisionless Layer. Phys. Plasmas, 14:042102.
- Eggersdorfer, M. L., and Pratsinis, S. E. (2014). Agglomerates and Aggregates of Nanoparticles Made in the Gas Phase. Adv. Powder Technol., 25:71–90.
- Ehn, M., Junninen, H., Schobesberger, S., Manninen, H. E., Franchin, A., Sipilä, M., Petäjä, T., Kerminen, V.-M., Tammet, H., Mirme, A., Mirme, S., Hõrrak, U., Kulmala, M., and Worsnop, D. R. (2011). An Instrumental Comparison of Mobility and Mass Measurements of Atmospheric Small Ions. J. Aerosol Sci., 45:522–532.
- Eichelberger, B. R., Snow, T. P., and Bierbaum, V. M. (2003). Collision Rate Constants for Polarizable Ions. J. Amer. Soc. Mass Spectrom., 14:501–505.
- Ermak, D. L., and Buckholz, H. (1980). Numerical-Integration of the Langevin Equation—Monte-Carlo Simulation. J. Comput. Phys., 35:169–182.
- Fernández-García, J., and Fernández de la Mora, J. (2014). Electrical Mobilities of Multiply Charged Ionic-Liquid Nanodrops in Air and Carbon Dioxide Over a Wide Temperature Range: Influence of Ion-Induced Dipole Interactions. Phys. Chem. Chem. Phys., 16:20500–20513.
- Filippov, A. V. (1993). The Charging of Aerosol in the Transition Regime. J. Aerosol Sci., 24:423–436.
- Filippov, A. V. (1994). Charge-Distribution Among Nonspherical Particles in a Bipolar Ion Environment. J. Aerosol Sci., 25:611–615.
- Franchin, A., Ehrhart, S., Leppä, J., Nieminen, T., Gagné, S., Schobesberger, S., Wimmer, D., Duplissy, J., Riccobono, F., Dunne, E. M., Rondo, L., Downard, A., Bianchi, F., Kupc, A., Tsagkogeorgas, G., Lehtipalo, K., Manninen, H. E., Almeida, J., Amorim, A., Wagner, P. E., Hansel, A., Kirkby, J., Kürten, A., Donahue, N. M., Makhmutov, V., Mathot, S., Metzger, A., Petäjä, T., Schnitzhofer, R., Sipilä, M., Stozhkov, Y., Tomé, A., Kerminen, V. M., Carslaw, K., Curtius, J., Baltensperger, U., and Kulmala, M. (2015). Experimental Investigation of Ion–Ion Recombination Under Atmospheric Conditions. Atmos. Chem. Phys., 15:7203–7216.
- Friedlander, S. K. (2000). Smoke, Dust, and Haze. Oxford University Press, New York.
- Fuchs, N. A. (1963). On the Stationary Charge Distribution on Aerosol Particles in a Bipolar Ionic Atmosphere. Geofis. Pura Appl., 51:185–193.
- Fuchs, N. A. (1964). The Mechanics of Aerosols. Macmillan, New York.
- Gatti, M., and Kortshagen, U. (2008). Analytical Model of Particle Charging in Plasmas Over a Wide Range of Collisionality. Phys. Rev. E, 78:046402.
- Gopalakrishnan, R., and Hogan, C. J. (2012). Coulomb-Influenced Collisions in Aerosols and Dusty Plasmas. Phys. Rev. E, 85:026410.
- Gopalakrishnan, R., McMurry, P. H., and Hogan, C. J. (2015). The Electrical Mobilities and Scalar Friction Factors of Modest to High Aspect Ratio Particles in the Transition Regime. J. Aerosol Sci., 82:24–39.
- Gopalakrishnan, R., Meredith, M. R., Larriba-Andaluz, C., and Hogan, C. J. (2013a). Brownian Dynamics Determination of the Bipolar Steady State Charge Distribution on Spheres and Non-Spheres in the Transition Regime. J. Aerosol Sci., 63:126–145.
- Gopalakrishnan, R., Thajudeen, T., and Hogan, C. J. (2011). Collision Limited Reaction Rates for Arbitrarily Shaped Particles across the Entire Diffusive Knudsen Number Range. J. Chem. Phys., 135:054302.
- Gopalakrishnan, R., Thajudeen, T., Ouyang, H., and Hogan, C. J. (2013b). The Unipolar Diffusion Charging of Arbitrary Shaped Aerosol Particles. J. Aerosol Sci., 64:60–80.
- Goree, J. (1992). Ion Trapping by a Charged Dust Grain in a Plasma. Phys. Rev. Lett., 69:277–280.
- Gunn, R. (1954). Diffusion Charging of Atmospheric Droplets by Ions, and the Resulting Combination Coefficients. J. Meteorol., 11:339–347.
- Hagen, D., E., and Alofs, D. J. (1983). Linear Inversion Method to Obtain Aerosol Size Distributions from Measurements with a Differential Mobility Analyzer. J. Aerosol Sci., 2:465–475.
- Hansen, S. (2004). Translational Friction Coefficients for Cylinders of Arbitrary Axial Ratios Estimated by Monte Carlo Simulation. J. Chem. Phys. 121:9111–9115.
- He, M., and Dhaniyala, S. (2014). Experimental Characterization of Flowrate-Dependent Bipolar Diffusion Charging Efficiencies of Sub-50nm Particles. J. Aerosol Sci., 76:175–187.
- Hogan, C. J., and Fernandez de la Mora, J. (2010). Ion-Pair Evaporation from Ionic Liquid Clusters. J. Amer. Soc. Mass Spectrom., 21:1382–1386.
- Hogan, C. J., Li, L., Chen, D. R., and Biswas, P. (2009). Estimating Aerosol Particle Charging Parameters using a Bayesian Inversion Technique. J. Aerosol Sci., 40:295–306.
- Hogan, C. J., and Fernandez de la Mora, J. (2009). Tandem Ion Mobility-Mass Spectrometry (IMS-MS) Study of Ion Evaporation from Ionic Liquid-Acetonitrile Nanodrops. Phys. Chem. Chem. Phys., 11:8079–8090.
- Hoppel, W. A., and Frick, G. M. (1986). Ion-Aerosol Attachment Coefficients and the Steady-State Charge Distribution on Aerosols in a Bipolar Ion Environment. J. Aerosol Sci., 5:1–21.
- Hoppel, W. A., and Frick, G. M. (1990). The Nonequilibrium Character of the Aerosol Charge-Distributions Produced by Neutralizers. J. Aerosol Sci., 12:471–496.
- Hsieh, E. T.-Y., and Castleman, A. W. Jr (1981). A Reconsideration of the Theory of Capture Cross-Sections for Ion/Molecule Reactions and a Total Energy and Angular Momentum Conserved Average Charge—Dipole Interaction Theory (teams). Int. J. Mass Spectrom. Ion Phys., 40:295–329.
- Hubbard, J. B., and Douglas, J. F. (1993). Hydrodynamic Friction of Arbitrarily Shaped Brownian Particles. Phys. Rev. E, 47:R2983–R2986.
- Hussin, A., Scheibel, H. G., Becker, K. H., and Porstendörfer, J. (1983). Bipolar Diffusion Charging of Aerosol Particles—I: Experimental Results within the Diameter Range 4–30 nm. J. Aerosol Sci., 14:671–677.
- Jiang, J., Kim, C., Wang, X., Stolzenburg, M. R., Kaufman, S. L., Qi, C., Sem, G. J., Sakurai, H., Hama, N., and McMurry, P. H. (2014). Aerosol Charge Fractions Downstream of Six Bipolar Chargers: Effects of Ion Source, Source Activity, and Flowrate. J. Aerosol Sci., 48:1207–1216.
- Kaufman, S. L. (1998). Analysis of Biomolecules using Electrospray and Nanoparticle Methods: The Gas-Phase Electrophoretic Mobility Molecular Analyzer (GEMMA). J. Aerosol Sci., 29:537–552.
- Khrapak, S., and Morfill, G. (2009). Basic Processes in Complex (Dusty) Plasmas: Charging, Interactions, and Ion Drag Force. Contrib. Plasma Phys., 49:148–168.
- Khrapak, S. A., Ratynskaia, S. V., Zobnin, A. V., Usachev, A. D., Yaroshenko, V. V., Thoma, M. H., Kretschmer, M., Höfner, H., Morfill, G. E., Petrov, O. F., and Fortov, V. E. (2005). Particle Charge in the Bulk of Gas Discharges. Phys. Rev. E, 72:016406.
- Kilpatrick, W. D. (1971). An Experimental Mass-Mobility Relation for Ions in Air at Atmospheric Pressure, in Proceedings of the 1s9th Annual Conference on Mass Spectroscopy.
- Knutson, E. O., and Whitby, K. T. (1975). Aerosol Classification By Electric Mobility: Apparatus, Theory, and Applications. J. Aerosol Sci., 6:443–451.
- Ku, B. K., Deye, G. J., Kulkarni, P., and Baron, P. A. (2011). Bipolar Diffusion Charging of High-Aspect Ratio Aerosols. J. Electrostat., 69:641–647.
- Kulkarni, P., Deye, G. J., and Baron, P. A. (2009). Bipolar Diffusion Charging Characteristics of Single-Wall Carbon Nanotube Aerosol Particles. J. Aerosol Sci., 40:164–179.
- Langevin (1905). Une Formule Fondamentale de Theorie Cinetique. Annales de Chimie et de Phys., 5:245–288.
- Larriba, C., Hogan, C. J., Attoui, M., Borrajo, R., Fernandez-Garcia, J., and Fernandez de la Mora, J. (2011). The Mobility-Volume Relationship below 3.0 nm examined by Tandem Mobility-Mass Measurement. J. Aerosol Sci., 45:453–467.
- Larriba, C., Fernandez de la Mora, J., and Clemmer, D. E. (2014). Electrospray Ionization Mechanisms for Large Polyethylene Glycol Chains Studied Through Tandem Ion Mobility Spectrometry. J. Amer. Soc. Mass Spectrom., 25:1332–1345.
- Lawless, P. A. (1996). Particle Charging Bounds, Symmetry Relations, and an Analytic Charging Rate Model for the Continuum Regime. J. Aerosol Sci., 27:191–215.
- Lee, H. M., Kim, C. S., Shimada, M., and Okuyama, K. (2005). Effects of Mobility Changes and Distribution of Bipolar Ions on Aerosol Nanoparticle Diffusion Charging. J. Chem. Eng. Jpn., 38:486–496.
- Lenggoro, I. W., Widiyandari, H., Hogan, C. J., Biswas, P., and Okuyama, K. (2007). Colloidal Nanoparticle Analysis by Nanoelectrospray Size Spectrometry with a Heated Flow. Anal. Chimica Acta, 585:193–201.
- Liu, B. Y. H., and Pui, D. Y. H. (1974). Equilibrium Bipolar Charge Distribution of Aerosols. J. Colloid Interface Sci., 49:305–312.
- Li, M., You, R., Mulholland, G. W., and Zachariah, M. R. (2013). Evaluating the Mobility of Nanorods in Electric Fields. J. Aerosol Sci., 47:1101–1107.
- López-Yglesias, X., and Flagan, R. C. (2013). Ion–Aerosol Flux Coefficients and the Steady-State Charge Distribution of Aerosols in a Bipolar Ion Environment. J. Aerosol Sci., 47:688–704.
- Lòpez-Yglesias, X., and Flagan, R. C. (2013). Population Balances of Micron-Sized Aerosols in a Bipolar Ion Environment. J. Aerosol Sci., 47:681–687.
- Loyalka, S. K. (1973). Condensation on a Spherical Droplet. J. Chem. Physics 58:354.
- Lushnikov, A. A., and Kulmala, M. (2004). Flux-Matching Theory of Particle Charging. Phys. Rev. E, 70:046413.
- Maisser, A., Thomas, J. M., Larriba-Andaluz, C., He, S., and Hogan, C. J. (2015). The Mass-Mobility Distributions of Ions Produced by a Po-210 Source in Air. J. Aerosol Sci., 90:36–50.
- Manirakiza, E., Seto, T., Osone, S., Fukumori, K., and Otani, Y. (2013). High-Efficiency Unipolar Charger for Sub-10 nm Aerosol Particles Using Surface-Discharge Microplasma with a Voltage of Sinc Function. J. Aerosol Sci., 47:60–68.
- Maricq, M. M. (2008). Bipolar Diffusion Charging of Soot Aggregates. J. Aerosol Sci., 42:247–254.
- Marlow, W. H., and Brock, J. R. (1975). Calculations of Bipolar Charging of Aerosols. J. Colloid Interface Sci., 51:23–31.
- Mohnen, V. A. (1976). Formation, Nature, and Mobility of Ions of Atmospheric Importance, in Electrical Processes in Atmospheres, H. Dolezalek, R. Reiter, and H. Landsberg, eds., Steinkopff, Darmstadt, Germany, pp. 1–17.
- Mott-Smith, H. M., and Langmuir, I. (1926). The Theory of Collectors in Gaseous Discharges. Phys. Rev., 28:727–763.
- Northrup, S. H., Allison, S. A., and McCammon, J. A. (1984). Brownian Dynamics Simulation of Diffusion-Influenced Bimolecular Reactions. J. Chem. Phys., 80:1517–1526.
- Ouyang, H., Gopalakrishnan, R., and Hogan, C. J. (2012). Nanoparticle Collisions in the Gas Phase in the Presence of Singular Contact Potentials. J. Chem. Phys., 137:064316.
- Ouyang, H., Larriba-Andaluz, C., Oberreit, D. R., and Hogan, C. J. (2013). The Collision Cross Sections of Iodide Salt Cluster Ions in Air via Differential Mobility Analysis-Mass Spectrometry. J. Amer. Soc. Mass Spectrom., 24:1833–1847.
- Park, M., Li, M., Shin, W. G., Moon, H. J., and Ko, S. H. (2014). Electrical Mobility of Silver Nanowires in Transition and Continuum Regimes. J. Aerosol Sci., 72:21–31.
- Porstendörfer, J., Hussin, A., Scheibel, H. G., and Becker, K. H. (1984). Bipolar Diffusion Charging of Aerosol Particles—II. Influence of the Concentration Ratio of Positive and Negative Ions on the Charge Distribution. J. Aerosol Sci., 15:47–56.
- Reischl, G. P., Mäkelä, J. M., Karch, R., and Necid, J. (1996). Bipolar Charging of Ultrafine Particles in the Size Range Below 10 nm. J. Aerosol Sci., 27:931–949.
- Reischl, G. P., Scheibel, H. G., and Porstendörfer, J. (1983). The Bipolar Charging of Aerosols: Experimental Results in the Size Range Below 20-nm Particle Diameter. J. Colloid Interface Sci., 91:272–275.
- Rus, J., Moro, D., Sillero, J. A., Royuela, J., Casado, A., Estevez-Molinero, F., and Fernandez de la Mora, J. (2010). IMS–MS Studies Based on Coupling a Differential Mobility Analyzer (DMA) to Commercial API–MS Systems. Int. J. Mass Spectrom., 298:30–40.
- Sahni, D. C. (1966). The Effect of a Black Sphere on the Flux Distribution in an Infinite Moderator. J. Nuclear Ener., 20:915.
- Sahni, D. C. (1983). An Exact Solution of Fokker-Planck Equation and Brownian Coagulation in the Transition Regime. J. Colloid Interface Sci., 91:418–429.
- Shimada, M., Han, B. W., Okuyama, K., and Otani, Y. (2002). Bipolar Charging of Aerosol Nanoparticles by a Soft X-Ray Photoionizer. J. Chem. Eng. Jpn., 35:786–793.
- Shin, W. G., Mulholland, G. W., and Pui, D. Y. H. (2010). Determination of Volume, Scaling Exponents, and Particle Alignment of Nanoparticle Agglomerates using Tandem Differential Mobility Analyzers. J. Aerosol Sci., 41:665–681.
- Sorensen, C. M. (2011). The Mobility of Fractal Aggregates: A Review. J. Aerosol Sci., 45:765–779.
- Steiner, G., Jokinen, T., Junninen, H., Sipila, M., Petaja, T., Worsnop, D., Reischl, G. P., and Kulmala, M. (2014). High-Resolution Mobility and Mass Spectrometry of Negative Ions Produced in a Am-241 Aerosol Charger. J. Aerosol Sci., 48:261–270.
- Steiner, G., and Reischl, G. P. (2012). The Effect of Carrier Gas Contaminants on the Charging Probability of Aerosols Under Bipolar Charging Conditions. J. Aerosol Sci., 54:21–31.
- Stober, J., Schleicher, B., and Burtscher, H. (1991). Bipolar Diffusion Charging of Particles in Noble Gases. J. Aerosol Sci., 14:66–73.
- Stolzenburg, M. R., and McMurry, P. H. (1991). An Ultrafine Aerosol Condensation Nucleus Counter. J. Aerosol Sci., 14:48–65.
- Stolzenburg, M. R., and McMurry, P. H. (2008). Equations Governing Single and Tandem DMA Configurations and a New Lognormal Approximation to the Transfer Function. J. Aerosol Sci., 42:421–432.
- Stommel, Y. G., and Riebel, U. (2005). A Corona-Discharge-Based Aerosol Neutralizer Designed for use with the SMPS-System. J. Electrostat., 63:917–921.
- Stommel, Y. G., and Riebel, U. (2007). Comment on the Calculation of the Steady-State Charge Distribution on aerosols <100 nm by Three Body Trapping Method in a Bipolar Ion Environment. J. Aerosol Sci., 41:840–847.
- Su, T., and Bowers, M. T. (1973). Ion-Polar Molecule Collisions - Effect of Molecular-Size on Ion-Polar Molecule Rate Constants. J. Amer. Chem. Soc., 95:7609–7610.
- Talukdar, S. S., and Swihart, M. T. (2003). An Improved Data Inversion Program for Obtaining Aerosol Size Distributions from Scanning Differential Mobility Analyzer Data. J. Aerosol Sci., 37:145–161.
- Tanaka, Y., Higashi, H., Manirakiza, E., Seto, T., Otani, Y., and Hirasawa, M. (2014). Charge Neutralization of Aerosol Carbon Nanofibers. J. Chem. Eng. Jpn. 47:644–650.
- Thajudeen, T., Jeon, S., and Hogan, C. J. (2015). The Mobility of Flame Synthesized Aggregates/Agglomerates in the Transition Regime. J. Aerosol Sci., 80:45–57.
- Tigges, L., Jain, A., and Schmid, H. J. (2015). On the Bipolar Charge Distribution used for Mobility Particle Sizing: Theoretical Considerations. J. Aerosol Sci., 88:119–134.
- Timko, M. T., Yu, Z., Kroll, J., Jayne, J. T., Worsnop, D. R., Miake-Lye, R. C., Onasch, T. B., Liscinsky, D., Kirchstetter, T. W., Destaillats, H., Holder, A. L., Smith, J. D., and Wilson, K. R. (2009). Sampling Artifacts from Conductive Silicone Tubing. J. Aerosol Sci., 43:855–865.
- Ude, S., and Fernandez de la Mora, J. (2005). Molecular Monodisperse Mobility and Mass Standards from Electrosprays of Tetra-alkyl Ammonium Halides. J. Aerosol Sci., 36:1224–1237.
- Wagner, P. E., and Kerker, M. (1977). Brownian Coagulation of Aerosols in Rarified Gases. J. Chem. Phys., 66:638–646.
- Wellisch, E. M. (1909). The Mobilities of the Ions Produced by Rontgen Rays in Gases and Vapours. Philosophic Transac Royal Soci London. Series A, Containing Papers of a Mathematical or Physical Character 209:249–279.
- Wen, H. Y., Reischl, G. P., and Kasper, G. (1984a). Bipolar Diffusion Charging of Fibrous Aerosol-Particles. 1. Charging Theory. J. Aerosol Sci., 15:89–101.
- Wen, H. Y., Reischl, G. P., and Kasper, G. (1984b). Bipolar Diffusion Charging of Fibrous Aerosol-Particles. 2. Charge and Electrical Mobility Measurements on Linear-Chain Aggregates. J. Aerosol Sci., 15:103–122.
- Wiedensohler, A. (1988). An Approximation of the Bipolar Charge-Distribution for Particles in the Sub-Micron Size Range. J. Aerosol Sci., 19:387–389.
- Wiedensohler, A., Birmili, W., Nowak, A., Sonntag, A., Weinhold, K., Merkel, M., Wehner, B., Tuch, T., Pfeifer, S., Fiebig, M., Fjäraa, A. M., Asmi, E., Sellegri, K., Depuy, R., Venzac, H., Villani, P., Laj, P., Aalto, P., Ogren, J. A., Swietlicki, E., Williams, P., Roldin, P., Quincey, P., Hüglin, C., Fierz-Schmidhauser, R., Gysel, M., Weingartner, E., Riccobono, F., Santos, S., Grüning, C., Faloon, K., Beddows, D., Harrison, R., Monahan, C., Jennings, S. G., O'Dowd, C. D., Marinoni, A., Horn, H.-G., Keck, L., Jiang, J., Scheckman, J., Mcmurry, P. H., Deng, Z., Zhao, C. S., Moerman, M., Henzing, B., De Leeuw, G., Löschau, G., and Bastian, S. (2012). Mobility Particle Size Spectrometers: Harmonization of Technical Standards and Data Structure to Facilitate High Quality Long-Term Observations of Atmospheric Particle Number Size Distributions. Atmos. Measurement Tech., 5:657–685.
- Wiedensohler, A., and Fissan, H. J. (1991). Bipolar Charge-Distributions of Aerosol-Particles in High-Purity Argon and Nitrogen. J. Aerosol Sci., 14:358–364.
- Wiedensohler, A., Lutkemeier, E., Feldpausch, M., and Helsper, C. (1986). Investigation of the Bipolar Charge-Distribution at Various Gas Conditions. J. Aerosol Sci., 17:413–416.
- Wild, M., Meyer, J., and Kasper, G. (2012). A fast and Accurate Method of using Electrical Mobility Scans for the Direct Measurement of Aerosol Charge Distributions. J. Aerosol Sci., 52:69–79.
- Xiao, K., Swanson, J. J., Pui, D. Y. H., and Kittelson, D. B. (2012). Bipolar Diffusion Charging of Aggregates. J. Aerosol Sci., 46:794–803.
- Yu, Y., Alexander, M. L., Perraud, V., Bruns, E. A., Johnson, S. N., Ezell, M. J., and Finlayson-Pitts, B. J. (2009). Contamination from Electrically Conductive Silicone Tubing during Aerosol Chemical Analysis. Atmos. Environ., 43:2836–2839.
- Zelenyuk, A., and Imre, D. (2007). On the Effect of Particle Alignment in the DMA. J. Aerosol Sci., 41:112–124.
- Zhang, C., Thajudeen, T., Larriba, C., Schwartzentruber, T. E., and Hogan, C. J. (2012). Determination of the Scalar Friction Factor for Non-spherical Particles and Aggregates Across the Entire Knudsen Number Range by Direct Simulation Monte Carlo (DSMC). J. Aerosol Sci., 46:1065–1078.