Abstract
The direct transfer of flame-synthesized aerosols of silica nanoparticles into aqueous suspensions is investigated. Silica nanoparticle aerosols with production rates of 0.5 g/h and different mean diameters and degrees of agglomeration are transferred into liquid suspensions by means of a novel wet electrostatic precipitator. Particle collection efficiencies above 99.999% were measured. The influence of the transfer on the particle size distribution was investigated by comparison of aerosol and suspensions size measurements. Aerosol sizes were measured with the scanning mobility particle sizer (SMPS), and suspension size measurements were conducted by dynamic light scattering (DLS) and by SMPS measurements of the aerosolized suspension employing a novel nebulizer. Depending on the aerosol and stabilization conditions, particle transfer with nearly no influence on the particle size distribution is possible. Suspensions generated from the same particle aerosol by direct transfer and by sonication of the respective powder were compared. In contrast to the direct transfer, the aerosol particle size distribution could not be restored by ultrasonication.
Copyright © 2015 American Association for Aerosol Research
INTRODUCTION
Gas phase synthesis is highly suitable for the mass production of nanostructured materials. It allows for the production of high-purity engineered nanoparticles (ENP) and is easily scalable (Dittmeyer et al. Citation2003; Teoh et al. Citation2010). The possibility to kinetically control the particle synthesis facilitates the generation of highly defined and functionalized particles (Binder et al. Citation2009; Huelser et al. Citation2011). After synthesis, ENPs are typically precipitated in filters or cyclones and form a more or less agglomerated powder, which is then processed further (Flörke et al. Citation2008). In many cases, the process chain for the production of nanostructured materials involves the transfer of those particles into a liquid environment. Transfer can also be advisable in order to protect particles against agglomeration and improve handling. As exposition to ENPs can pose serious health risks (Oberdörster et al. Citation2005), handling of ENPs as liquid suspensions is favorable, since the potential risk of release into the air is greatly reduced (Fissan et al. Citation2014).
Suspensions are commonly generated by dispersion of the agglomerated powders into a solvent by ball milling, high shear mixing, or ultrasonication. With increasing energy input, agglomerates will be broken up into ever smaller fragments; ideally until the original particle size distribution (PSD) of the unagglomerated particles is reached. However, even at high specific energy inputs, complete deagglomeration is not always possible, due to the strong interparticular forces. In some cases, high energy inputs even have adverse effects or may lead to particle degradation due to phase transitions (Mandzy et al. Citation2005).
Compared to a powder after precipitation, particles show relatively low degrees of agglomeration shortly after synthesis while they are still in aerosol form. If an aerosol is precipitated directly into a liquid, its particles can be stabilized against agglomeration upon entering the liquid phase. Ideally, this process retains the aerosol’s PSD during the transfer, making further particle dispersion unnecessary.
The direct liquid recovery of ENPs after synthesis has been discussed and tested within the scope of developing safe tools for nanomanufacturing (Sentein et al. Citation2009; Schuster and Lomello Citation2013). However, the efficient collection of gas-borne nanoparticles in liquids remains a problem. Bubble columns typically exhibit a distinct minimum in grade efficiencies around 100 nm, which greatly reduces their effectiveness as a device for the collection of nanoparticles. Even though performance can be improved by increase of residence time or the introduction of submerged granular beds, nanoparticle collection efficiencies typically remain below 95% (Koch and Weber Citation2012; Cadavid-Rodriguez et al. Citation2014). Other common techniques for wet separation such as venturi scrubbers and impingers exhibit even lower removal efficiencies for particles in the nanometer range (Tsai et al. Citation2005; Huang et al. Citation2007; Miljevic et al. Citation2009; Wei et al. Citation2010). Dey and Venkataraman Citation(2012) presented a small-scale wet electrostatic precipitator (WESP) for the direct transfer of nanoparticles into liquid suspensions. Collection efficiencies ranged from 40 to 90% depending on particle size, at residence times of 0.7 s. The mean particle sizes in suspension were comparable to those of the original aerosols.
While nanoparticle removal in the bubble column and venturi scrubber suffers from insufficient collection efficiencies for nanomanufacturing, because it relies on particle diffusivity, the WESP introduces the electrostatic force in order to remove the particles. Once particles are charged in a corona or charging field, their paths can be deflected by electrostatic fields toward a collection electrode. This offers the possibility to precipitate nanoparticles efficiently (Parker Citation1997).
Based on this principle a miniplant scale system was designed, consisting of a flame reactor for nanoparticle synthesis and a WESP for the subsequent direct transfer of particles from the gas into the liquid phase. Due to easy availability and relatively low toxicity of precursor and particles, silica nanoparticles, synthesized from Tetraethylorthosilicate (TEOS), were used as a test substance (Flörke et al. Citation2008). The aim of this investigation is the development of the WESP as an integrated unit operation for the efficient, safe, and continuous liquid recovery of ENPs. A potential industrial application, is the direct transfer of high-value ENPs into stable suspensions in order to preserve these particles from degrading effects, such as phase transitions or loss of surface area due to agglomeration or aggregation. With regard to these prerequisites, the influence of the particle transfer on the PSD was investigated. This is a challenging task, as different techniques need to be applied for aerosol and suspension measurements. In addition to the common methods, use of a novel technique was made, which aerosolizes suspensions in order to measure their PSDs via SMPS. This allows to discuss effects of (de)agglomeration in detail.
EXPERIMENTAL SETUP
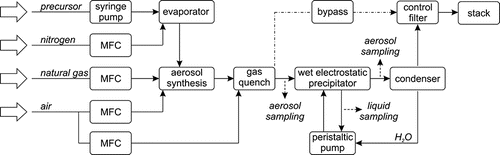
shows the schematic of the experimental setup, which is designed as a closed system on a miniplant like scale. It can essentially be divided into the aerosol section, where the aerosols are generated, and into the precipitation section, where different types of precipitators can be tested.
Aerosol Plant
The aerosol is produced by flame synthesis. By changing the synthesis parameters or precursors, a variety of different aerosols can be generated. The flame is provided by a premixed natural gas burner (type Hegwein ZDAU-240 M), which generates a stable flame over a wide range of fuel gas flows and air ratios. All flows are controlled independently by digital mass flow controllers (MFC) (type Bronkhorst EL-Flow). A nitrogen carrier gas stream introduces the precursor vapor (TEOS 99.9%, Alfa Aesar) into the flame. The precursor is dosed by means of a syringe pump and evaporated into the carrier gas stream inside a specially designed evaporator. It allows very close control of the evaporation temperature and features a low-precursor holdup, allowing fast response times to changes in feed rates. The combustion and reaction zone is shielded inside an Al2O3-ceramic tube within the water-cooled casing of the reactor. After particle synthesis, the hot aerosol enters the gas quench. Here, filtered pressurized air is injected via three jets shifted by 120°. The aerosol is diluted and temperature is rapidly reduced to 150–250°C. This inhibits further particle sintering and reduces agglomeration as well as wall losses due to thermophoresis. The aerosol is then fed to the WESP at gas temperatures of 80–150°C, depending on the operating conditions. Alternatively, the aerosol can be diverted through a bypass into a control filter.
The setup allows the generation of aerosol flows up to 8 m3N/h at SiO2 production rates up to 2 g/h and particle concentrations up to approximately 5 × 108 1/cm3. Naturally, all parameters will influence the characteristics of the produced aerosol. For the presented work, the aerosol flow was kept at 3.7 m3N/h with a fixed SiO2 production rate of 0.5 g/h.
Wet Electrostatic Precipitator (WESP)
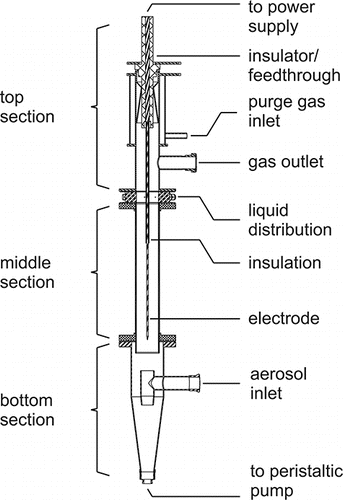
The schematic of the WESP is presented in . It is designed as a single-stage tube-type WESP and consists of three sections. The aerosol enters through the bottom section, which distributes the aerosol into the middle section and collects the suspension. A peristaltic pump continuously circulates the suspensions and generates a liquid film which covers the walls of the middle section and acts as collection electrode. A high voltage is applied between the film on ground potential and the central corona electrode. The particles are charged by the resulting corona discharge and are subsequently precipitated into the suspension while passing the middle section. Afterward, the cleaned gas exits the WESP through an outlet at the top section. A detailed description of the WESP can be found in Anderlohr et al. Citation(2015).
Measurement Setup
As indicated in , aerosol was sampled directly at the WESP’s aerosol inlet and directly after the gas outlet. All aerosol measurements were taken during the actual experiment. Suspension samples were taken from the bulk, after the complete liquid had been drained from the WESP, in order to ensure the samples’ representativity.
Scanning Mobility Particle Sizer (SMPS)
Aerosol PSDs were measured with a scanning mobility particle sizer (SMPS). The SMPS setup used consisted of a Kr85 beta emitter for aerosol charging, a TSI model 3071A Differential Mobility Analyzer with externally regulated sheath air flow, and a TSI model 3775 condensation particle counter operated with n-butanol.
The aerosol was sampled from the center of the aerosol stream by a metal probe. Directly afterward, the aerosol was diluted by a factor of 7–10 with dry particle free air inside a T-connector. The dilution stream was regulated by a MFC (Bronkhorst EL-Flow) and was checked at regular intervals with a Gilian bubble flow meter. After lowering the dew point below ambient temperature and cooling in the first dilution step, the aerosol was fed to a Topas DIL 550 dilution stage offering dilution by a factor of 100. This resulted in a total dilution factor of 700–1000. All presented size distributions are averages of three to five independent measurements.
Liquid Nanoparticles Sizer (LNS)
It is possible to use the SMPS technology for the characterization of particle size distributions of liquid suspensions. Before measurement, the suspension sample has to be transferred into an aerosol, at best without any changes in the particle properties.
The TSI prototype nanoparticle nebulizer 3485 was employed for this purpose. During operation, a sample of the suspension is diluted inline at a defined ratio with ultrapure water (<0.05 µS/cm) and then aerosolized with pressurized air in an atomizer nozzle. The dilution ratio has to be high enough, so no droplet contains more than a single particle. Due to the special design of the nozzle, only very small droplets of sizes around 300 nm can leave the nebulizer. After nebulization, the droplet aerosol is dried by mixing with dry filtered air and heating. The resulting aerosol then can be characterized by SMPS or other techniques. Fissan et al. Citation(2014) give a detailed description of this novel device. The combination of the TSI Nanoparticle Nebulizer and the SMPS will be referred to as liquid nanoparticle sizer (LNS).
Even though the measuring range of the SMPS system used in this work is up to 661.2 nm, the measuring range of the LNS system is much smaller. Due to the generated droplet size distribution, the transfer function becomes less than unity for particles sizes approaching 300 nm, since particles larger than the droplet diameter will not be aerosolized (see the online supplemental information [SI]).
During operation, the nebulizer also generates droplets that do not contain any particles. If nonvolatile substances such as salts or polyelectrolytes are dissolved in these droplets, particles of nonvolatile residues (NVR) will be formed upon drying. The size of this NVR aerosol depends on the initial NVR concentration, the dilution factor, and the droplet size. In the present work, number concentrations of the NVR aerosol were up to two orders of magnitude higher than those of the particles to be characterized. This can cause the NVR aerosol to cover parts of the PSD of the suspended particles. It is possible to partially reconstruct particle size distribution in the region of overlapping PSDs, if a lognormal distribution of the NVR aerosol is assumed. In this method (compare Figure S1) a bimodal lognormal distribution is fitted to the data. The PSD of the smaller NVR aerosol is then subtracted from the measured PSD, removing most of the NVR aerosol’s signal from the data. All LNS size distributions presented in this work have been processed with this method, all of them are averages of at least three measurements.
The number concentration of the generated aerosol is linked via the dilution and conversion factors to the suspension concentration and can be used as a measure of the particle concentration in the suspension. Therefore, the system was calibrated with a defined volume concentration standard of SiO2 particles (mode 30 nm, concentration 5.0 × 1017 nm3/mL) before each set of LNS measurements.
Dynamic Light Scattering (DLS)
Dynamic light scattering (DLS) was used as an additional and established technique to measure the suspensions’ PSDs and zeta potentials. Measurements were carried out on a Malvern Zetasizer Nano ZS. The device has a measurement range from 0.4 to 10,000 nm and also offers the possibility of zeta potential measurements. All presented DLS size measurements are the averages of three to five independent measurements, of 5 min duration each. The zeta potential measurements are averaged from five independent measurements.
Gravimetric Concentration Measurement
The mass concentrations of the suspensions generated in the WESP were determined gravimetrically. The following procedure was used to differentiate between the total precipitated mass and the mass of the stably suspended nanoparticles: The suspension sample was weighed directly after the experiment. Then, it was left to rest for 48 h in order for large and unstably suspended particles to precipitate. Afterward, the supernatant suspension was carefully taken with a pipette and transferred into another container. Both samples were weighed again and dried in a hot-air cabinet at 85 °C for 24 h. After acclimatization, the samples were weighed again and the respective mass concentrations were calculated, taking stabilizer concentrations into account. The electronic balance (type Mettler Toledo PR 2003 Comparator) used for these measurements had an accuracy of 1 mg.
RESULTS
WESP Collection Efficiencies
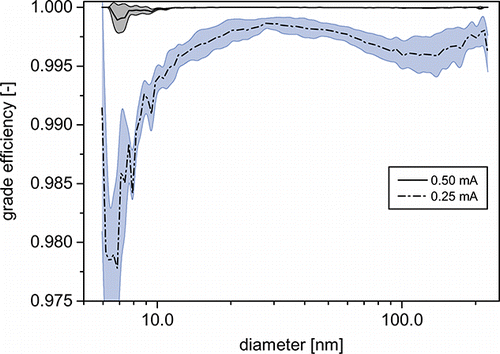
The size distribution of a particle collective can only be kept unchanged during transfer, if all particle sizes are transferred with the same efficiency. Hence, in a first step, the collection efficiency of the WESP was investigated. Since the high-voltage power supply was operated in a constant current mode, collection efficiency and current–voltage characteristic are presented as a function of current in . Residence time inside the WESP was approx. 1.3 s. The efficiencies were calculated from the number concentrations measured at the inlet and outlet of the WESP, according to:
FIG. 3. Overall collection efficiencies, current–voltage characteristic of the WESP at different operating currents.
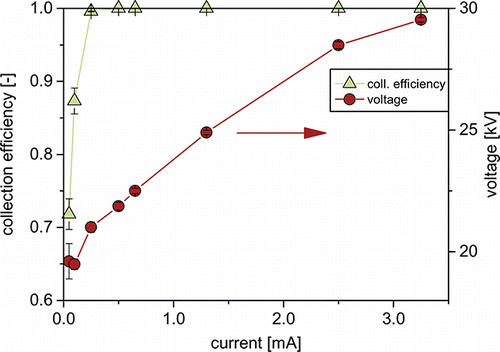
The overall collection efficiency rises steeply with increasing currents. At 0.25 mA, a collection efficiency of 99.5% is reached. shows that the grade efficiencies at this current above 97% for all particle sizes. Two minima in grade efficiency can be distinguished. While the minimum at sizes around 120 nm is the well-known minimum caused by the progression of particle migration velocities (Parker Citation1997), the minimum toward the smallest particle sizes is likely a result of incomplete charging of these very small particles during their residence time in the WESP, due to the reduced charging probability at these sizes (Adachi et al. Citation1985). As the current is increased to 0.50 mA, the minima disappear nearly completely and overall collection efficiency rises to 99.998%.
Even though operation up to 30 kV is possible, lower voltages are preferable. At high field strengths, spraying of the circulating suspension can cause sparkovers and the strong electric wind can lead to partial dewetting of the middle section. During all presented experiments, the WESP was operated at 0.65 mA, since overall collection efficiencies are above 99.999%. Tests with suspensions of up to 10 wt% of colloidal silica showed no influence of the suspension’s concentration on collection efficiencies. During experiments with up to 6 h of continuous operation, no signs of corona quenching or decreasing collection efficiencies were found. Precipitation at higher currents had no measureable influence on the generated suspension size distributions.
Suspension Stability
As per definition, a suspension is considered stable if its PSD remains unchanged over a certain period of time. For the presented investigation, a time span of several days was selected as suitable. Usually, a particle’s zeta potential is used to predict its suspension’s stability. The zeta potential is the electric potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. It is a measure for the degree of electrostatic repulsion between adjacent particles. If the potential is small, attractive forces may exceed this repulsion and cause the suspension to flocculate. If the zeta potential is below −30 mV or above 30 mV, a suspension is expected to be stable, between these boundaries, suspensions are likely to be unstable. A suspension’s pH-value usually has a strong influence on the zeta potential, as it directly influences the particles’ surface charges. Additional effects may come from the adsorption of ions on the particle surface Citation(ISO14887:2000).
In the current setup, the control of the suspension’s pH-value underlies certain limitations. Aqueous solutions will turn acidic due to the absorption of combustion gases inside the WESP. Alkaline pH-value above 9 cannot be maintained, as CO2, abundant from the combustion process, will be absorbed quickly. However, due to the position of the equilibrium, CO2 absorption plays only a minor role at pH-value below 6. The pH-value is further reduced by absorption of SO3 and NO2, forming sulfuric and nitric acid, respectively. It has been shown that SO2 and NOx can be oxidized by active species generated in the nonthermal plasma of the corona discharge (Onda et al. Citation1997; Anderlohr et al. Citation2015). While the oxidation and absorption of SO2 has only a small influence due to the low sulfur content of the fuel gas, NOx is inevitably produced in the combustion. It has been experimentally proven that the HNO3 concentration in the WESP’s liquid is directly dependent on the applied voltage and synthesis conditions. While the absorption of NO2 cannot be prevented, CO2 absorption can be minimized by the use of buffers. This allows for the generation of suspensions with maximum pH-value around 7–8.
Aerosol Synthesis
Two sets of experiments will be discussed in detail. Within each set, an aerosol generated under identical conditions was precipitated into liquids with different concentrations of Na4P2O7. Na4P2O7 is a complex salt commonly used for the stabilization of oxidic nanoparticles in suspension Citation(ISO14887:2000). It also acts as a buffer, increasing the suspension’s pH-value. In each experimental set, four different suspensions A, B, C, and D with the respective stabilizer concentrations 0, 1.0, 2.5, and 5.0 mM Na4P2O7 were generated.
The aerosol was synthesized at a precursor flow rate of 2 mL/h (approx. 0.5 g/h SiO2), a fuel gas flow of 1 LN/min, an air ratio of 1.05, and a quench air flow of 50 LN/min. From set 1 to set 2, residence time between particle synthesis and collection was increased from 0.9 s to 1.9 s by installing an insulated tube between quench and WESP. This increased the aerosol’s geometric mean diameter (GMD) and its geometric standard deviation sigma from 66.0 nm and 1.86 to 81.4 nm and 1.90.
In all experiments, particle synthesis was run until steady state operation in terms of system temperatures and PSD had been achieved. The stability of the aerosol PSD was checked in regular intervals by SMPS measurements. The WESP was then flushed at least three times with deionized water in order to remove all residues of previously generated suspensions. In case of experiments with stabilizers, one additional flushing with the respective stabilizer concentration was performed. During the flushing process, the aerosol was diverted through the bypass directly to the control filter. After flushing was completed, 120 mL of liquid were added to the WESP, the continuous circulation of the liquid was restarted, and the aerosol was fed through the WESP again. After 60 min, the experiment was stopped, the voltage was switched off, and the aerosol was fed through the bypass. Then, liquid circulation was stopped, the suspension was collected in a graded measuring cylinder, and samples for the DLS, LNS, and zeta potential measurements were taken. The remaining suspension was used for gravimetrical measurements. All size distribution measurements presented in this section were done on the day of the experiment.
Measurement Techniques
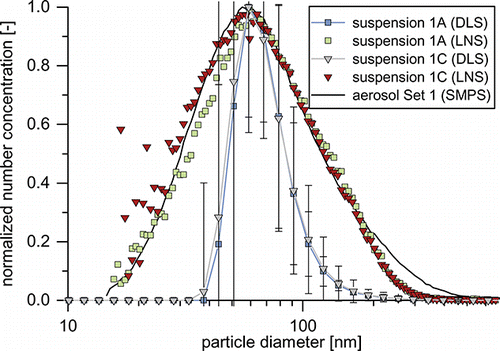
Comparison of the size distributions before and after the transfer from the gas into the liquid phase is a good approach to investigate the WESP’s suitability as a device for the direct particle transfer. Size distributions are commonly measured by SMPS for aerosols and by DLS for suspensions. In order to compare the PSDs to each other, the volume-based PSD from DLS needs to be converted into a number-based PSD as generated by SMPS, or vice versa. presents the normalized number-based PSDs of the aerosol and the corresponding suspensions as measured by SMPS, DLS, and LNS, respectively. In all cases, the DLS PSDs are in the same size range as the aerosol. However, uncertainties are high and size distributions are generally much narrower than those of the original aerosol. This makes definite statements on whether or how much the size distributions have been affected by the transfer virtually impossible. The main cause of this problem is the fact that the two devices are based on fundamentally different measurement principles, the SMPS measuring a number-based mobility distribution while DLS generates an intensity-based size distribution of hydrodynamic diameters.
FIG. 6. Aerosol and suspension particle size distributions for experiments 1A and 1C. For the figure with all error bars, see Figure S3.
The LNS employs the same measurement principle as the SMPS (in fact, the same SMPS was used). Therefore, the generated PSDs are also number-based. This makes this technique highly suitable for the direct comparison to the aerosol PSDs. Since many (partly unknown) parameters (i.e., suspension and aerosol dilution factor inside the LNS, conversion factor from suspension to aerosol, amount of nebulized liquid…) link the number concentration measured by LNS to the actual suspension concentration, particle concentrations of the aerosol and the aerosolized suspension are not directly comparable. In order to compare SMPS and LNS PSDs, both are normalized with their highest concentration. Due to the high size resolution and low uncertainties, even smaller changes in the PSD can now be analyzed, and effects of (de)agglomeration can be studied. On the other hand, the LNS offers only a very limited measuring range (even smaller than the SMPS’). Therefore, it cannot be used as the only method for the characterization of a suspension. While the LNS is good for a close up comparison, the DLS is needed for the bigger picture.
Discussion of Generated Suspensions
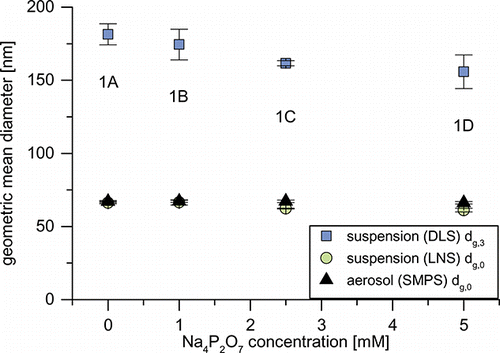
shows the GMDs of the aerosols and their respective suspensions from experimental set 1, measured by SMPS, LNS, and DLS. Since the GMDs from DLS measurements are volume-based, a direct comparison to the aerosol is not sensible. They will be discussed later. Comparison of the number-based GMDs of aerosol (SMPS) and suspension (LNS) indicates a transfer from the gas to the liquid phase with only minor changes in PSDs. The GMDs of suspensions 1A and 1B are nearly identical to those of their respective aerosols. As stabilizer concentration is increased to 2.5 (1C) and 5.0 mM (1D) Na4P2O7, the suspensions’ GMDs are reduced by approx. 5 nm. presents the normalized PSDs of set 1’s aerosol as well as exemplarily the LNS PSDs of suspensions 1A and 1C. At sizes below 180 nm aerosol and suspension PSDs are very similar, but not quite identical. While suspension 1A’s PSD appears to be shifted to larger particle sizes by a few nanometers, suspension 1C’s PSD matches the aerosol quite closely. The increasing scatter of the data points below 30 nm is a result of the stronger NVR background due to the increased stabilizer concentration, which cannot be fully corrected. Looking closer at the particle sizes above 180 nm, a lack of larger particles compared to the aerosol becomes apparent for both suspensions. The reason for the “missing” particles cannot be ultimately stated, since the transfer function of the nanoparticle nebulizer is unknown for these particle sizes. However, data (see the SI) suggests that starting from approx. 180 nm the fraction of aerosolized particles decreases. This would explain the deviating size distributions. If the transfer function is not an issue, deagglomeration of the larger particles in the liquid phase could explain the observed behavior. In this case, a distortion of the size distribution compared to the aerosol would be expected, as partly deagglomerated particles smaller than 180 nm would be added to the distribution. As suspension 1C matches the aerosol PSD very well, this seems unlikely. A third explanation is the agglomeration and growth of these particles to sizes outside the measuring range. This seems unlikely as well, since this collective of growing particles would most likely entail increased sediment formation, which was not observed.
FIG. 5. Geometric mean diameters of the aerosols (number-based) and suspensions (number- and volume-based) of experimental set 1.
The gravimetric measurements of the sediment fractions after 48 h of settling time and the suspensions’ zeta potentials are presented in . About 4–10% of the transferred particle mass were found in the sediment. No clear trend with stabilizer concentration or zeta potential is discernable. Low pressure impactor measurements of the aerosol mass distribution yielded a mass fraction of aerosol particles larger 1 µm of 10 %, which is higher than most sediment fractions. This suggests that the sediment consists mainly of large particles already present in the aerosol stream and is not a result of particle agglomeration during or after the transfer.
FIG. 7. Zeta potentials and sediment fractions of the different suspensions of experimental sets 1 and 2.
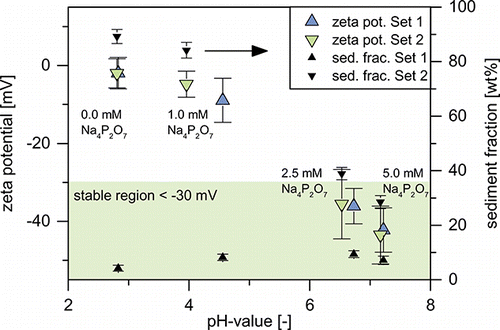
Considering the zeta potentials presented in , suspensions 1A and 1B would be expected to be unstable, as both zeta potentials are above -10 mV. Normally, this should lead to particle agglomeration and sedimentation. The shift of suspension 1A (and 1B) to slightly larger sizes (compare ) is likely a result of this. However, increased sedimentation was not observed. As zeta potential falls below −30 mV for suspensions 1C and 1D, particles are stabilized and possibly even deagglomerated upon transfer into the liquid. This leads to matching PSDs (below 180 nm) of suspension and aerosol.
The difference in PSDs between suspensions 1A and 1C is also reflected in the DLS measurements (compare and Figure S4). The volume-based GMD decreases from approx. 180 nm for suspensions 1A and 1B to approx. 160 nm for 1C and 1D, as particle concentration in the size range from 300 to 700 nm is reduced.
In contrast to the minor influence of the stabilizer concentration on the nanoscopic scale, an effect is macroscopically visible. While the suspension without Na4P2O7 forms a compact precipitate, increasing the Na4P2O7 concentration leads to the formation of large flocs which build up to a voluminous precipitate with the tendency to gel after a few days.
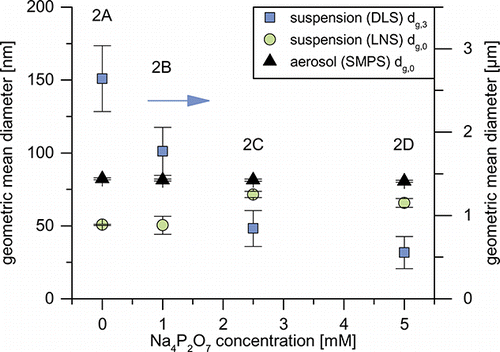
Experimental set 2 was designed in order to investigate the influence of agglomeration on the particle transfer. While the actual degree of agglomeration of the aerosols 1 is unknown, it is clear, that the difference between the aerosol PSDs of set 1 and 2 has to be the result of Brownian coagulation in the gas phase during the increased residence time (from 0.9 to 1.9 s). Unlike the previous set, experimental set 2 behaves “as expected” and exhibits a strong dependency of the sediment fraction on the suspension’s zeta potential (). Suspensions 2A and 2B with zeta potentials of -5 mV and higher consist mainly of precipitate and are instable. As zeta potential drops into the stable region below -30 mV for suspensions 2C and 2D, the sediment fraction decreases to 30–40%. Unlike in experimental set 1, macroscopically no difference in sediment morphology is discernible.
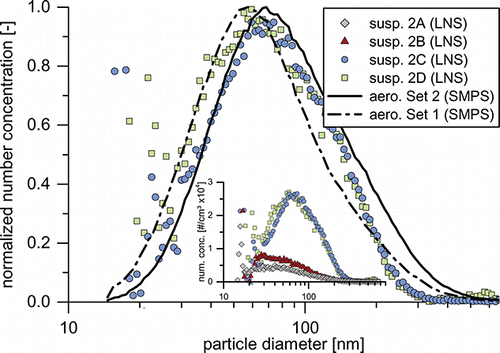
shows the normalized PSDs (LNS) of suspensions 2C and 2D compared to the aerosol PSDs (SMPS) of sets 1 and 2. In the insert, all suspensions of set 2 are compared on a concentration scale. Consistent with the high sediment fractions, suspensions 2A and 2B exhibit much lower number concentrations compared to 2C and 2D, as only a small percentage of the transferred particles is stably suspended. Therefore, the GMDs calculated from these measurements have only limited significance. In accord with lower sediment fractions, suspensions 2C and 2D exhibit much higher number concentrations after aerosolization. Their PSDs show more resemblance to the original aerosol PSD. Closer examination of the size range from 70 to 180 nm shows that the PSDs of suspensions 2C and 2D are within the limits of both aerosol PSDs. This indicates particle deagglomeration, shifting the PSDs toward that of aerosol 1. As discussed before, above 180 nm PSDs are likely to be influenced by the nebulizer. Below 70 nm, the differences between both suspensions become more pronounced. While 2C follows the aerosol PSD of set 2 quite closely, suspension 2D’s PSD is shifted to smaller particles. It is in very good agreement with the aerosol PSD of set 1. The PSD is shifted toward that of the unagglomerated aerosol due to the increased deagglomeration of small particles, as the zeta potential is lowered from −36 mV in 2C to −44 mV in 2D (compare ).
FIG. 8. Aerosol and suspension particle size distributions for experiments 2C and 2D as well as suspension size distributions 2A to 2D on a concentration scale (insert). For the figure with all error bars, see Figure S5.
The strong influence of the stabilizer concentration is also visible in the volume-based DLS measurements ( and Figure S6). The GMD decreases from 2700 nm for suspension 2A to 800 nm for suspension 2C, as the amount of particles larger than 1100 nm is reduced to nearly zero. The further increase in stabilizer concentration mainly lowers particle concentrations between 160 and 1100 nm, reducing the GMD to 500 nm.
FIG. 9. Geometric mean diameters of the aerosols (number-based) and suspensions (number- and volume-based) of experimental set 2.
Comparison of the different experimental sets shows, that residence time after the quench has a strong influence on the particles’ tendency to stabilize themselves in aqueous suspensions. As the suspensions generated in the experimental set 1 are stable regardless of zeta potential, other mechanisms have to be responsible for stabilization. It is possible that, even though temperatures in the reaction zone are far above those required for the pyrolysis of the precursor, organic groups from the precursor remain covalently bonded to the particle surface. These might have stabilizing effects, once the particles are transferred into suspension. The increase in residence time at elevated temperatures from experimental set 1 to 2 might lead to further reaction of these groups, ultimately eliminating their stabilizing effects.
At residence times low enough to avoid excessive agglomeration in the gas phase, particles can be directly transferred from the gas into the liquid with next to no effect on the PSD below 180 nm. If the aerosol consists largely of agglomerates, the PSD cannot be kept unchanged during transfer. Depending on the stabilizer concentration, agglomerates formed in the gas phase will be broken up. Large agglomerates are deagglomerated more easily and at lower stabilizer concentrations than smaller ones.
Direct Transfer vs. Ultrasonication of Powders
In order to investigate the advantages of this new and integrated process for suspension generation over the common route of particle precipitation on a filter and subsequent dispersion in a liquid, both processes were compared.
Particles were synthesized under the same conditions as in experimental set 1; however, the WESP was exchanged with two Gore® ePTFE membrane bag filters in order to precipitate the aerosol particles as powder. The setup was run for several hours until approximately 2.4 g SiO2 particles could be collected. Measurements of the particle concentration after the filter during the experiment confirmed a collection efficiency above 99.99%. After collection, particles were homogenized into a fine powder with a spatula.
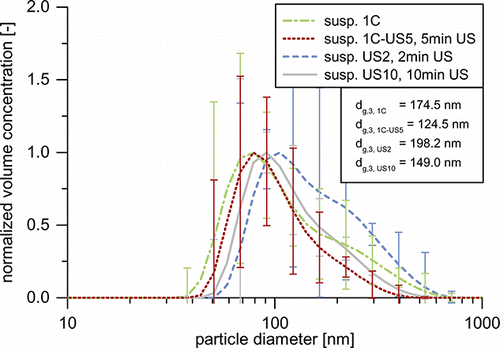
Samples at all investigated Na4P2O7 concentrations were prepared. In order to have comparable stabilization conditions, pH-values were adjusted through addition of HNO3 to match the pH-value of the suspensions from direct transfer. For each suspension, 60 mg of SiO2 powder were weighted with a microbalance (accuracy 0.1 mg) to generate suspension samples of 20 mL volume with mass concentrations (0.3 wt%) matching those of the suspensions from direct transfer (including the sediment). These samples were sonicated with an ultrasonic probe (type Telsonic DG-100) at 16 W for 2 and 10 min. Afterward, particle size distributions were measured with DLS and LNS. In the following paragraph, the results of experiment 1C and the corresponding suspensions US2 and US10 generated via ultrasonication will be compared.
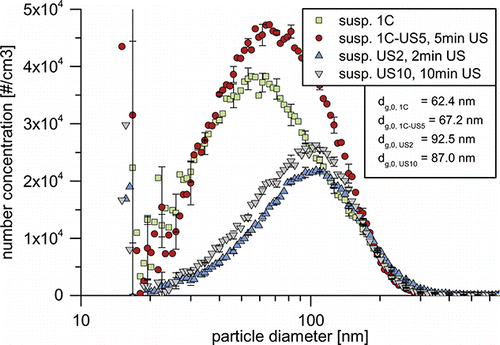
In order to examine the generated suspensions not only in terms of size, but also in terms of particle concentrations, presents the PSD measurements on a concentration scale. Even though it holds no direct information on the mass concentration, it is possible to compare the different suspensions relative to each other, as all measurements were carried out with the same dilution factors and were corrected with the respective calibration measurements. Samples US2 and US10 after 2 and 10 min of ultrasonication show very similar PSDs. While the mode remains at 105 nm, the rise in sonication time increases particle concentrations below 180 nm and reduces the concentrations of larger particles. This leads to an overall rise in particle concentration by a factor of 1.2 and decreases the number-based GMD by approx. 5 nm to 87.0 nm. The DLS measurements of show a somewhat larger influence of sonication time: the volume-based GMD is reduced by nearly 50 nm to 149.0 nm. As discussed above, suspension 1C’s PSD matches that of the original aerosol very closely. It is apparent from that differences between this suspension and the suspensions US2 and US10 are immense. In the size range from 20 to 120 nm, particle concentrations are 2.1–2.7 times higher than those of the samples generated by sonication. In order to rule out possible particle degradation due to the ultrasonication, and study the effect of additional sonication, 20 mL of suspension 1C were sonicated for 5 min at 16 W (1C-US5). The most notable change is the increase in particle concentration between 40 and 180 nm (compare ). At lower sizes, the PSD remains unchanged. The result is a slight increase of the number-based GMD from 62.4 to 67.2 nm. This stands in contrast to the reduction of the volume-based GMD by 50 nm from 174.5 to 124.5 nm (DLS), which is mainly caused by a reduction of particle concentration in the size range from 140 to 600 nm. The additional particles, which can be seen in the LNS measurement, are likely to stem from the deagglomeration of these particles. Comparison of the volume-based GMDs from the DLS measurements () suggest, that both routes generate quite similar suspensions, as the GMDs of samples US2, US10, and 1C are within 50 nm from each other. However, the LNS measurements show that the actual number of suspended particles differs greatly for the different processes. The direct transfer leads to 1.8–2.1 times higher particle number concentrations below 180 nm. Especially, particles smaller than 100 nm are extremely scarce in the suspensions generated from powder. Though larger agglomerates above 100 nm seem to be broken up easily by ultrasonication, complete deagglomeration of the powder to the degree of the original aerosol seems impossible, even at the high specific energy input of 0.45 kJ/mL.
CONCLUSION
It has been shown, that the WESP is a suitable device for the direct transfer of nanoparticles from the gas phase into liquid suspensions. It features high collection efficiencies over the examined size range (5.9–661.2 nm) at low residence times and power consumption. Addition of stabilizing agents into the precipitation liquid, offers the possibility to directly stabilize particles upon entering the liquid phase. This makes particle transfer with nearly complete preservation of the aerosol’s PSD feasible.
In addition to the well-established measurement techniques SMPS and DLS, the novel LNS system for the measurement of suspension PSDs was utilized. Due to its high size resolution, the LNS offers the possibility to closely study effects of (de)agglomeration in the size range from approx. 20 to 180 nm by comparison of the aerosol’s PSD measured by SMPS to the suspension’s PSD measured by LNS.
The direct transfer of two different aerosols of silica nanoparticles showed that suspension stability can differ from the expected. The most likely explanation for this behavior are organic residues from particle synthesis, which influence the stabilization mechanisms. The silica aerosol with a low degree of agglomeration could be transferred into suspension with only minor changes to the PSD. If the aerosol consisted mainly of agglomerated particles, these were (partially) deagglomerated upon transfer. Compared to suspensions produced by the sonication of a particle powder, the direct transfer of the same particles generated much finer suspensions. Even at high energy inputs, it was not possible to deagglomerate the particle powder to the degree of this original aerosol.
UAST_1120857_Supplemental_File.zip
Download Zip (254.7 KB)ACKNOWLEDGMENTS
The authors thank Dr. Axel Zerrath from TSI Inc. for the support providing the nebulizer. The authors also thank the Gas Particle Systems Division of the Institute for Mechanical Process Engineering and Mechanics (KIT) for their support with the aerosol measurements as well as the Division of Combustion Technology at the Engler-Bunte-Institute (KIT) of their support with the suspension measurements. This project is part of the JointLab IP3, a joint initiative of KIT and BASF.
SUPPLEMENTAL MATERIAL
Supplemental data for this article can be accessed on the publisher’s website.
FUNDING
Financial support by the ministry of science, research and the arts of Baden-Württemberg (Az. 33-729.61-3) is gratefully acknowledged.
REFERENCES
- Adachi, M., KousakaY., and OkuyamaK. (1985). Unipolar and Bipolar Diffusion Charging of Ultrafine Aerosol Particles. J. Aerosol. Sci., 16:109–123. 10.1016/0021-8502(85)90079-5
- Anderlohr, C., Brachert, L., Mertens, J., and Schaber, K. (2015). Collection and Generation of Sulfuric Acid Aerosols in a Wet Electrostatic Precipitator. Aerosol. Sci. Technol., 49:144–151. 10.1080/02786826.2015.1008624
- Binder, A., Seipenbusch, M., Muhler, M., and Kasper, G. (2009). Kinetics and Particle Size Effects in Ethene Hydrogenation Over Supported Palladium Catalysts at Atmospheric Pressure. J. Catal. 268:150–155. 10.1016/j.jcat.2009.09.013
- Cadavid-Rodriguez, M. C. C., Charvet, A., Bemer, D., and Thomas, D. (2014). Optimization of Bubble Column Performance for Nanoparticle Collection. J. Hazard Mater. 271:24–32. 10.1016/j.jhazmat.2014.01.040
- Dey, L., and Venkataraman, C. (2012). A Wet Electrostatic Precipitator (WESP) for Soft Nanoparticle Collection. Aerosol. Sci. Technol. 46:750–759. 10.1080/02786826.2012.664295
- Dittmeyer, R., Keim, W., Kreysa, G., and Alfred, O. (2003). Winnacker-Küchler: Chemische Technik: Prozesse und Produkte. Band 2: Neue Technologien. 5th ed. Wiley-VCH, Weinheim, Germany.
- Fissan, H., Ristig, S., Kaminski, H., Asbach, C., and Epple, M. (2014). Comparison of Different Characterization Methods for Nanoparticle Dispersions Before and After Aerosolization. Anal. Methods. 6:7324. 10.1039/C4AY01203H
- Flörke, O. W., Graetsch, H. A., Brunk, F., Benda, L., Paschen, S., Bergna, H. E., Roberts, W. O., Welsh, W. A., Libanati, C., Ettlinger, M., Kerner D, Maier M., Meon W., Schmoll R., Gies H., and Schiffmann D. (2008). Silica. In: Ullmann’s Encycl Ind Chem. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp. 1–89. 10.1002/14356007.a23_583.pub3
- Huang, C.-H., Tsai, C.-J., and Wang, Y.-M. (2007). Control Efficiency of Submicron Particles by an Efficient Venturi Scrubber System. J. Environ. Eng., 133:454–461. 10.1061/(ASCE)0733-9372(2007)133:4(454)
- Huelser, T., Schnurre, S., Wiggers, H., and Schulz, C. (2011). Gas-phase Synthesis of Nanoscale Silicon as an Economical Route Towards Sustainable Energy Technology. Kona. 29:191–207.
- ISO14887:2000. (2000). Sample Preparation - Dispersing Procedures for Powders in Liquids. Distributed through American National Standards Institute, pp. 1–32.
- Koch, D., and Weber, A. P. (2012). Separation of Gas-borne Nanoparticles in Bubble Columns. J. Aerosol. Sci., 53:61–75. 10.1016/j.jaerosci.2012.05.012
- Mandzy, N., Grulke, E., and Druffel, T. (2005). Breakage of TiO Agglomerates in Electrostatically Stabilized Aqueous Dispersions. Powder Technol., 160:121–126. 10.1016/j.powtec.2005.08.020
- Miljevic, B., Modini, R. L., Bottle, S. E., and Ristovski, Z. D. (2009). On the Efficiency of Impingers with Fritted Nozzle Tip for Collection of Ultrafine Particles. Atmos. Environ., 43:1372–1376. 10.1016/j.atmosenv.2008.12.001
- Oberdörster, G., Maynard, A., Donaldson, K., Castranova, V., Fitzpatrick, J., Ausman, K., Carter, J., Karn, B., Kreyling, W., Lai, D., Olin, S., Monteiro-Riviere, N., Warheit, D., and Yang, H. (2005). Principles for Characterizing the Potential Human Health Effects from Exposure to Nanomaterials: Elements of a Screening Strategy. Part Fibre Toxicol., 2:35. 10.1186/1743-8977-2-8
- Onda, K., Kasuga, Y., and Kato, K. (1997). Electric Discharge Removal of SO2 and NOx from Combustion Flue Gas by Pulsed Corona Discharge. Energy Convers. Manag., 38:1377–1387.
- Parker, K. R. (1997). Applied Electrostatic Precipitation. First edit. Parker, K. R., ed. Blackie Academic & Professional, London
- Schuster, F., and Lomello, F. (2013). From Safe Nanomanufacturing to Nanosafe-by-Design Processes. J. Phys. Conf. Ser., 429:012054. 10.1088/1742-6596/429/1/012054
- Sentein, C., Guizard, B., Giraud, S., Yé, C., and Ténégal, F. (2009). Dispersion and Stability of TiO2 Nanoparticles Synthesized by Laser Pyrolysis in Aqueous Suspensions. J. Phys. Conf. Ser., 170:012013. 10.1088/1742-6596/170/1/012013
- Teoh, W. Y., Amal, R., and Mädler, L. (2010). Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale., 2:1324–1347. 10.1039/c0nr00017e
- Tsai, C.-J., Lin, C.-H., Wang, Y.-M., Hunag, C.-H., Li, S.-N., Wu, Z.-X., and Wang, F.-C. (2005). An Efficient Venturi Scrubber System to Remove Submicron Particles in Exhaust Gas. J. Air Waste Manag. Assoc., 55:319–325. 10.1080/10473289.2005.10464622
- Wei, Z., Rosario, R. C., and Montoya, L. D. (2010). Collection Efficiency of a Midget Impinger for Nanoparticles in the Range of 3–100 nm. Atmos. Environ., 44:872–876. 10.1016/j.atmosenv.2009.11.037