ABSTRACT
Wood pellets have been used in domestic heating appliances for three decades. However, because the share of renewable energy for heating will likely rise over the next several years, alternative biomass fuels, such as short-rotation coppice or energy crops, will be utilized. We tested particulate emissions from the combustion of standard softwood pellets and three alternative pellets (poplar, Miscanthus sp., and wheat straw) for their ability to induce inflammatory, cytotoxic, and genotoxic responses in a mouse macrophage cell line. Our results showed clear differences in the chemical composition of the emissions, which was reflected in the toxicological effects. Standard softwood and straw pellet combustion resulted in the lowest PM1 mass emissions. Miscanthus sp. and poplar combustion emissions were approximately three times higher. Emissions from the herbaceous biomass pellets contained higher amounts of chloride and organic carbon than the emissions from standard softwood pellet combustion. Additionally, the emissions of the poplar pellet combustion contained the highest concentration of metals. The emissions from the biomass alternatives caused significantly higher genotoxicity than the emissions from the standard softwood pellets. Moreover, straw pellet emissions caused higher inflammation than the other samples. Regarding cytotoxicity, the differences between the samples were smaller. Relative toxicity was generally highest for the poplar and Miscanthus sp. samples, as their emission factors were much higher. Thus, in addition to possible technical problems, alternative pellet materials may cause higher emissions and toxicity. The long-term use of alternative fuels in residential-scale appliances will require technological developments in both burners and filtration.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
A number of recent decrees aimed at lowering carbon dioxide and other greenhouse gas emissions from energy production have inevitably also increased the amount of renewable fuels for energy production (EU 2030 framework; Executive Office of the President, June 2013). It is thus likely that the use of wood-based biomass for energy production will rise even further in the next few years (Alakangas et al. Citation2012; CitationEuropean Commission, SWD/2014/259).
Legislative measures in Europe (Directive Citation2009/125/EC) limit the particulate and gaseous emissions from biomass combustion in small-scale domestic heating appliances, but appliances that use outdated technology are still widely in use. These old-technology appliances generally produce higher amounts of particulate emissions than modern-technology pellet boilers (Lamberg et al. Citation2011), and additionally, their emissions have elicited significant negative effects in vitro (Jalava et al. Citation2012, Tapanainen et al. Citation2012). In contrast, it has been shown that nearly complete combustion of high-quality wood pellets in domestic appliances results in low particulate matter (PM) emissions (Schmidl et al. Citation2011; Lamberg et al. Citation2013) and that these emissions cause typically mild to moderate toxic effects in vitro and in vivo (Jalava et al. Citation2012; Happo et al. Citation2013). A switch from old-technology domestic appliances to new-technology appliances would thus lead to an overall reduction of harmful PM emissions.
However, factors limiting the increasing use of wood-based biomass fuels include the high costs of sustainable forestry and competition for the raw material by other industries (Alakangas et al. Citation2012). Hence, alternative biomass fuels, which grow faster and are more economically profitable, will be needed to fulfill the renewable energy targets set by authorities (EU 2030 framework; The President's Climate Action Plan, June 2013).
Possible alternatives to traditional forestry products are short-rotation coppices (SRC), such as poplar and willow, which demand little of the soil and can be grown with good efficiency on fallow land (Porsö and Hanssen, Citation2014). However, due to the high ash content of SRC, it is not possible to use them to produce pellets of the highest quality standards (European Pellet Council Citation2013).
Another alternative to wood-based biomass fuels are herbaceous energy crops, such as reed canary grass, Miscanthus sp., and straw from various grains such as rice, wheat, barley, rye, and oat. These crops can yield high amounts of biomass per acre of cultivated land in a short time (Brosse et al. Citation2012), but most combustion studies on these fuels have come to similar conclusions: slagging and fouling increase due to the high ash content of the fuels and low ash melting temperatures. Additionally, ash deposits can cause significant corrosion in the burners and boilers (Brunner et al. Citation2013; Lamberg et al. Citation2013; Kortelainen et al. Citation2015). Another downside to these alternative biomass fuels is that we know very little about the toxicological properties of their emissions and the associated adverse health effects.
In the present study, we burned pellet fuels from different raw materials, Populus sp. (poplar), Miscanthus sp., and straw from Triticum sp. (wheat), as well as standard softwood pellets from spruce (Picea sp.) in two domestic pellet boilers and a combined woodchip/pellet boiler. We collected the combustion emissions and determined their chemical composition as well as the chemical composition of the pellet fuels. A mouse macrophage cell line was exposed to increasing concentrations of the PM emissions. After 24 h, we determined parameters of cell viability, inflammation, and DNA damage, and evaluated how the effects of the alternative pellets' emissions differed from standard softwood pellets' emissions.
2. Materials and methods
2.1. Appliances, fuels, and online measurements
After careful evaluation of different pellet boiler systems that are available, a BioWin 210 (Windhager, Austria, nominal boiler capacity: 21 kWth) was selected for standard softwood pellet combustion, a Powerchip 30 (Guntamatic, Austria, nominal boiler capacity: 30 kWth) for Miscanthus sp. and straw pellet combustion, and a Vitoligno 300-P (Viessmann, Germany, nominal boiler capacity: 20 kWth) for poplar pellet combustion. The boilers were chosen based on their ability to utilize biomass with high ash contents and low ash melting temperatures, as well as the possibility to influence air-staging to achieve optimized burnout conditions in the secondary combustion zone.
All boilers had staged combustion and were equipped with automated ignition, boiler cleaning, and de-ashing systems, and a water-cooled secondary combustion zone. The BioWin and the Vitoligno are overfed burners with fixed grates, while the Powerchip has an inclined moving grate. The combustion conditions were controlled through furnace temperature measurements in the BioWin and via a λ-probe in the PowerChip and Vitoligno. The furnaces were operated for 10 h according to a daily load cycle derived from typical field data for average winter days (Kelz et al. Citation2010). The cycle included stable full and partial load operation phases as well as a considerable number of startup, load change, and shutdown procedures.
Gaseous emissions were analyzed with nondispersive infrared sensors (ND-IR; CO, CO2 —Rosemount NGA 2000, Emerson, USA), flame ionization detectors (FID; SmartFID, ErsaTec, Germany), and paramagnetic sensors (O2—Rosemount NGA 2000, Emerson, USA). PM emissions were characterized using an electrical low-pressure impactor (ELPI™; Dekati®, Finland), a Berner-type low-pressure impactor (BLPI; LPI 30/0, Hauke, Austria), a Dekati® gravimetric impactor (DGI; Dekati®, Finland), and filter methods.
2.2. Sample collection and sample preparation for the toxicological analyses
PM sampling for the chemical and toxicological analyses was performed using a DGI with an air flow of 70 L per min. A detailed description of the DGI sampling setup can be found in Ruusunen et al. Citation(2011). The flue gas was diluted with porous tube and ejector diluters and DGI sampling was conducted at a flue gas temperature of +40°C. The sampling covered almost the entire operation cycle. In this study, PTFE filters (Fluoropore™, Merck Millipore, Germany) were used as DGI substrates. The filters were weighed before and after the sample collection to determine the mass of the collected PM. The collected PM with an aerodynamic diameter smaller than 1 µm (PM1) mass was then extracted from the PTFE filters as described previously by Tapanainen et al. Citation(2011). Briefly, PTFE filters were quartered aseptically and transferred to 50 mL glass tubes before adding 30 mL HPLC-grade methanol. The tubes were then sonicated in an ultrasonic water bath at RT for 30 min. The particle suspension was collected in a 1000 mL round bottom flask and the sonication procedure was repeated with fresh methanol. Thereafter, the methanol was evaporated in a rotary evaporator at 150 mbar and +35°C until approximately 10–20 mL of the particulate suspension was left. This suspension was divided into 10 mL glass tubes before the rest of the methanol was evaporated completely using N2-gas and the PM1 mass was determined. The tubes were stored at −20°C until the chemical analysis or the toxicological testing.
2.3. Chemical analysis
Moisture and ash content of the pellet raw materials were determined according to standards EN 14774-1:2009 and EN 14775:2010. The chemical elements C, H, and N were analyzed according to standard EN 15104:2011, and Si, Ca, Mn, K, Na, and Zn according to standard EN 15290:2011. The chloride content of the pellets was detected by ion chromatography (IC, standard EN 15289:2011). All analyses were performed as described previously by Kelz et al. Citation(2010) and Jalava et al. Citation(2012).
Chemical analysis of the PM1 samples was conducted as described previously in detail by Kelz et al. Citation(2010) and Jalava et al. Citation(2012). Briefly, organic carbon (OC), elemental carbon (EC), and inorganic carbon (IC) contents were determined using a carbon/hydrogen analyzer (LECO, RC-612), and chemical elements (Ca, Cd, Cl, K, Mg, Mn, P, Pb, S, Zn) were analyzed with either inductively coupled plasma optical emission spectrometry (ICP-OES) or inductively coupled plasma mass spectrometry (ICP-MS).
For the analysis of polycyclic aromatic hydrocarbons (PAH), the samples were extracted with dichloromethane and total of 30 PAH were analyzed using a gas chromatograph and a mass selective detector (6890N GC-5973 INERT MSD, Agilent Technologies, CA, USA) (Lamberg et al. Citation2011).
2.4. Cell culture and study design
RAW264.7 (ATCC, USA) cells were routinely cultured in RPMI-1640 medium with 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum and 100 U/mL penicillin/streptomycin (all Gibco®, Life Technologies, USA) in a humidified atmosphere at +37°C and 5% CO2. For the exposure experiments, we seeded 1,000,000 cells/well in 6-well plates (Corning, USA) and left them to attach and grow for 24 h. After this growth period, the culture medium was replaced and the cells were left to acclimatize for 1 h. Meanwhile, the dried PM1 samples were dispersed at 5 mg/mL in a 10% DMSO solution in pyrogen-free water (Sigma Aldrich Corp., USA). The complete dispersion of the particulate samples was verified by sonication for 30 min (FinnSonic M03, FinnSonic Ltd., Finland). The cells were then exposed to increasing sample concentrations (15, 50, 150, and 300 µg/mL, corresponding to 3.1 µg/cm2, 10.4 µg/cm2, 31.3 µg/cm2, and 62.5 µg/cm2), as well as 0.3% DMSO as a vehicle control and a number of positive and negative methodological controls. All experiments were conducted in duplicate. The exposure period was 24 h. After the exposure period, the culture medium of both duplicates was frozen at −80°C for enzyme-linked immunosorbent analysis (ELISA) of the pro-inflammatory mediators tumor necrosis factor α (TNFα) and macrophage inflammatory protein 2 (MIP-2). One milliliter of phosphate-buffered saline was added to the wells of one experimental duplicate and the cells were then detached by scraping and suspended by pipetting. Two 100-µL aliquots of the cells were transferred to 96-well plates for the analysis of their cellular metabolic activity (CMA) by MTT assay. The remaining 800-µL cell suspension was divided in half and 400 µL were used for the flow cytometric determination of membrane integrity by PI exclusion assay. The remaining 400 µL were fixed in 70% ethanol for cell cycle analysis. The cells of the other duplicate wells were used for the analysis of DNA damage by single-cell gel electrophoresis (SCGE) assay.
2.5. Toxicological endpoints
The toxicological endpoints were assessed as described previously (Jalava et al. Citation2010, Kasurinen et al. Citation2015). Briefly, CMA was assessed by MTT assay in which a yellow-colored compound (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is converted to purple formazan by metabolically active cells. The cells' membrane integrity and cell cycle analysis were determined after staining of the cells with propidium iodide (PI) by flow cytometry (CyAn™ ADP Analyzer, Beckman Coulter, USA). Cellular secretion of the pro-inflammatory markers TNFα and MIP-2 was assayed by ELISA (R&D-Systems, Minneapolis, MN, USA) using 3,3′,5,5′-tetramethylbenzidine (TMB) as substrate for the horseradish peroxidase. DNA damage was assessed by alkaline single-cell gel electrophoresis (SCGE) with olive tail moment (OTM: [(tail mean − head mean) × tail%DNA/100]) as a parameter for statistical analyses.
2.6. Statistical analysis and relative toxicity
The statistical analysis of the data was conducted as described previously by Jalava et al. Citation(2012) and Uski et al. Citation(2015). Correlation analysis was carried out as described by Happo et al. Citation(2013). IBM SPSS statistical software (version 19) was used for all statistical analyses.
To illustrate the relative toxicity of the samples, the PM1 dose in µg/mL was divided by the amount of PM1 emitted per MJ of obtained energy, thus obtaining a dose of kJ/mL. The number of PI-positive cells, the number of cells in the subG1-phase, the concentration of MIP-2, or the OTM value was then plotted against the dose for each sample.
3. Results
3.1. Chemical composition of the pellets and PM emissions
The ash contents of the straw, Miscanthus sp., and poplar pellets were all considerably higher than the ash content of the standard softwood pellets (in the online supplemental information [SI]). Additionally, we detected markedly higher contents of chloride (10–66 times) and potassium (7–13 times) in these fuels compared to the standard softwood pellets (see the SI).
Representative long-term operations under good gas phase burnout conditions were achieved in all cases. The average CO emissions amounted to 45.4 mg/MJ for standard softwood pellets, 45.0 mg/MJ for poplar pellets, 40.0 mg/MJ for Miscanthus sp. pellets, and 67.5 mg/MJ for straw pellets (. The average PM1 emissions ranged from 12.0 mg/MJ (straw) to 26.3 mg/MJ (Miscanthus sp.) and 30.2 mg/MJ (poplar), which were all higher than the emissions from the standard softwood pellet combustion (10.9 mg/MJ) ().
Table 1. Overview of the different pellet fuels used in this study and their emissions.
The PM1 emissions from Miscanthus sp. and straw pellet combustion contained OC levels of 18% and 26% and EC concentrations under 2.8% (). The OC and EC concentrations from the poplar pellet combustion were comparable to the OC and EC concentrations from standard softwood pellet combustion (<5%) (). The inorganic aerosol fractions of the samples were dominated by potassium (K)-salts, and we detected high chloride (Cl−) concentrations in the PM emissions of poplar, Miscanthus sp., and straw combustion, as well as high sulfur (S) concentrations in the PM emissions from poplar and standard softwood pellet combustion (). Moreover, the contents of the easily volatile metals zinc (Zn), lead (Pb), and cadmium (Cd) in the PM emissions increased from Miscanthus sp. and straw to wood fuels (standard softwood pellets, poplar) (). All samples contained rather low concentrations of total and genotoxic PAH compounds ( and the SI).
Table 2. Carbonaceous and chemical compositions [in ng/mg PM mass] of the PM emission samples from different biomass fuels.
3.2. Inflammatory markers
Exposure to the PM1 emissions from standard softwood pellet combustion caused a very mild and dose-dependent increase of the TNF-α secretion by RAW264.7 cells (, while no dose dependency was detected in the secretion of MIP-2 (see the SI).
Figure 1. Production of the pro-inflammatory marker TNFα by RAW264.7 macrophages after a 24-h exposure of the cells to four doses (15, 50, 150, and 300 µg/mL) of PM1 samples from the combustion of different pellet fuels. Bars represent the average concentration of TNFα in pg/mL and whiskers represent the standard error of the mean (SEM). Asterisks indicate statistical significance relative to the negative control; “a” indicates a statistically significant difference relative to standard softwood pellet combustion (ANOVA/Dunnett's; n = 6, p ≤ 0.05).
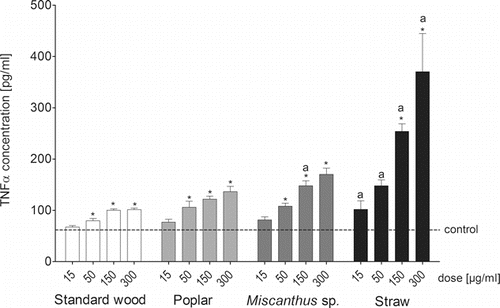
Exposure to the poplar PM1 emissions induced a significant and dose-dependent increase of the TNFα secretion (); however, MIP-2 secretion reached a plateau at sample doses of 150 µg/mL and 300 µg/mL (see the SI). The overall inflammatory response of the cells to this sample was only slightly more pronounced than to the standard softwood pellets PM1 emissions ().
The Miscanthus sp. pellets induced similar inflammatory responses in RAW264.7 cells as the poplar pellets () with a significant and dose-dependent increase of the cellular production of TNFα and a plateau in MIP-2 production (see the SI).
The highest inflammatory responses were seen after exposure of the cells to the straw pellet PM1 emissions. The secretion of both inflammatory markers in response to the straw pellet PM1 emissions was significantly higher at all doses compared to standard softwood PM1 emissions (; see the SI).
3.3. Cell viability
We found a strong, dose-dependent, and significant decrease of the CMA at all concentrations after exposure to the PM1 emissions from standard softwood pellet combustion (a), but the number of PI-positive cells was far less increased (b). At the highest sample concentration, these emissions caused a significant increase of the subG1-population to almost 20% (c). The amount of cells in the subG1-population was lower than after exposure to the alternative biomass pellet combustion at all doses except for 300 µg/mL (c).
Figure 2. Cell viability of RAW264.7 macrophages assessed with (a) the MTT test and (b) the PI exclusion assay and (c) by flow cytometric analysis of the cell cycle/SubG1-cells after a 24-h exposure of the cells to four doses (15, 50, 150, and 300 µg/mL) of particulate samples from the combustion of different pellet fuels. Each bar represents the experimental mean + standard error of the mean (SEM). Asterisks indicate statistical significance relative to the negative control; “a” indicates statistical significance relative to the standard softwood pellet sample (PI exclusion and SubG1-cells: Kruskal-Wallis: n = 3, p ≤ 0.05, MTT: ANOVA/ Dunnett's; n = 6, p ≤ 0.05).
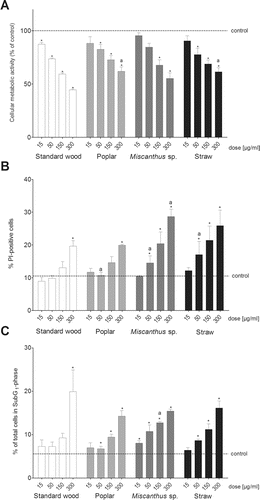
The PM1 emissions from the poplar pellets caused a significant and dose-dependent decrease of the CMA (a). The overall decrease of the CMA was not as pronounced as after the exposure to the emissions from the standard softwood pellets; however, significance between these two samples was observed only at 300 µg/mL. Poplar PM1 emissions slightly increased the number of PI-positive cells compared to the standard softwood pellet emissions with significance at 50 µg/mL (b). Additionally, the poplar PM1 emissions caused a significant and dose-dependent increase of the subG1-population (c).
The PM1 emissions from the Miscanthus sp. and straw pellets elicited similar responses in the three cell viability endpoints. Both samples induced a dose-dependent decrease of the CMA (a), a dose-dependent increase of the number of PI-positive cells (b), and a dose-dependent increase of the number of cells in the subG1-population (c). For both samples, the decrease of CMA was less than for the standard softwood pellet emission (a); however, the number of PI-positive cells (b) and the number of cells in the subG1-population (c) was higher than after the standard softwood pellet exposure.
3.4. Genotoxicity/DNA damage
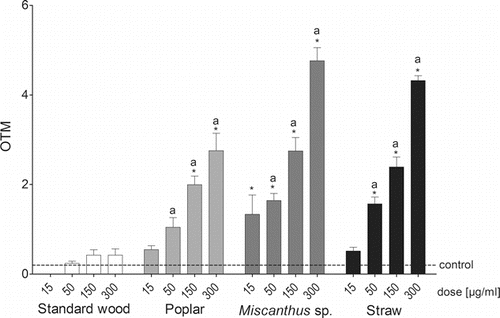
We saw large differences in the ability of the samples to induce DNA damage in RAW264.7 cells (). The PM1 emissions from standard softwood pellets did not cause significant DNA damage at any sample concentration tested. However, the other three samples induced significant, dose-dependent increases in the OTM of the cells (). All three PM1 samples from the combustion of the alternative pellet materials caused significantly larger DNA damage compared to the PM1 emissions from standard softwood pellets at sample concentrations of 50 µg/mL, 150 µg/mL, and 300 µg/mL ().
Figure 3. DNA damage evaluation by the SCGE assay in RAW264.7 cells after a 24-h exposure to three (sample standard softwood pellets, 50, 150, and 300 µg/mL) or four different concentrations (15, 50, 150, and 300 µg/mL) of particulate samples from the combustion of different pellet fuels expressed as the olive tail moment (OTM: (tail.mean-head.mean) x (tail%DNA/100)). Each bar represents the experimental mean + standard error of the mean (SEM). Asterisks indicate statistical significance relative to the negative control; a indicates statistical significance relative to the standard softwood pellet sample (Kruskal–Wallis n = 3, p ≤ 0.05).
3.5. Relative toxicity calculated with emission factor
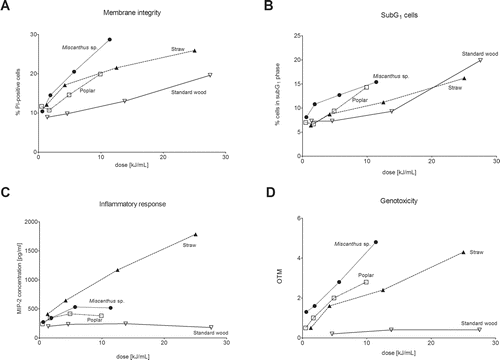
To represent more realistic exposure scenarios, the toxicological responses were divided by the amount of PM1 emitted per MJ of produced energy of the fuel (). For CMA, the relative toxicity cannot be calculated, as the result is already a ratio between unexposed and exposed cells and the results can thus only range between 0 and 100%. We saw clear differences in the relative toxicity of the samples. The PM1 emissions from the standard softwood pellets had the lowest relative toxicity across all investigated endpoints in this study () due to two factors: PM1 emissions (mg/MJ) were the lowest of the four pellet fuels tested in this study, and the overall inflammatory and genotoxic responses were generally less pronounced than those of the emissions of the other samples. In contrast, we found that the highest relative toxicity for PI positive cells and genotoxicity was elicited by the poplar and Miscanthus sp. PM1 emissions (). For straw, the relative toxicity was second lowest, except for inflammation (). This was due to low mass emissions, which could not diminish the highest inflammatory effect of the straw pellet combustion sample.
Figure 4. Relative toxicity of four selected endpoints: (a) membrane integrity as assayed by PI exclusion assay, (b) cells in the SubG1-phase as assayed by cell cycle analysis, (c) inflammation assayed by measurement of MIP-2, and (d) genotoxicity assayed by the SCGE assay. The scale on the X-axes is the PM1 dose in µg/mL divided by the amount of PM1 emitted per MJ of obtained energy.
3.6. Correlation between the samples' chemical composition and the toxicological effects
We found that several chemical components of the emission samples were linked to the toxicological endpoints. A high OC and Cl− content of the sample correlated well with elevated responses in all measured toxicological endpoints (). The Pb content of the samples was positively correlated with an increased production of inflammatory mediators and increased DNA damage, while high EC, Zn, magnesium (Mg), and calcium (Ca) contents of the emission samples were linked to increased cell death (MTT-assay, PI-exclusion assay, increase in subG1-population). The analyzed factors are presented in detail in .
Table 3. Spearman correlation coefficients (ρ) between the chemical constituents of the particulate samples from the combustion of different pellet fuels and the toxicological responses of RAW264.7 cells.
4. Discussion
The aim of this study was to investigate the influence of inorganic aerosol composition on health effects of PM1 emissions from the combustion of different pellet fuels. To achieve this goal, boilers providing the best possible burnout conditions for each fuel had to be selected. Thus, we could compare the effects of the PM1 emissions obtained under optimal conditions rather than the effects of the combustion quality.
4.1. Standard softwood pellets
Emissions from the combustion of standard softwood pellets generally contain only minimal amounts of carcinogenic PAH compared to emissions from fossil fuel combustion. However, they can contain considerable amounts of transition metals, namely zinc (Tapanainen et al. Citation2011; Jalava et al. Citation2012; Uski et al. Citation2014). In cases of almost complete combustion, zinc has been found to be the cause of most of the cytotoxic effects seen in in vitro and in vivo studies (Uski et al. Citation2012, 2015; Leskinen et al. Citation2014). These previous results are supported by our present findings, as the PM1 sample from the standard softwood combustion contained the highest amount of Zn and also induced the largest cytotoxic responses. Additionally, we found a very strong correlation between the Zn content of the samples and the reduction of CMA and the increase in the number of cells in the subG1-population. We saw similar correlations for Mg, but the overall Mg content of the samples was comparatively low. The sulfur content of the samples also correlated positively with the decrease of CMA; however, we previously determined that sulfur is mostly conjugated with potassium to form potassium sulfate (K2SO4), and sulfur therefore probably functions more as a surrogate substance than a cause of toxicity itself (Torvela et al. Citation2014). The emissions from the standard softwood pellet combustion induced only minimal inflammatory responses.
Adjustment of the toxicological responses with the emission factor showed that the cytotoxic responses induced by the emissions from the standard softwood pellets were not as distinct. In fact, the emissions from the Miscanthus sp. and poplar pellets induced considerably larger relative effects. This further underlines the importance of minimizing PM1 emissions during domestic pellet combustion.
4.2. Poplar pellets
Although the mechanical and physicochemical properties of poplar pellets have been investigated previously (Díaz-Ramírez et al. Citation2012; Monedero et al. Citation2015), the adverse health effects of emissions from poplar pellet combustion remain elusive to this date.
We found interesting differences between the chemical composition of the emissions from poplar pellet and standard softwood pellet combustion: the poplar emissions contained much more Pb, which was not fully explained by the fuel analysis. Thus, the Pb content of the emission sample must be interpreted with caution, especially because the poplar pellet emissions also contained the highest amount of Cd. Additionally, the chloride content of the poplar pellet emissions was almost three times higher than that of the standard softwood pellet emissions. Our correlation analysis showed that the Cl−-content of the samples was connected to all measured toxicologic endpoints; however, the reason for this remains unclear.
After adjusting the toxicological responses with the emission factors, we detected clear differences between the emissions from the standard softwood pellets and the poplar pellets. Because poplar pellet combustion yielded three times more PM1 emissions per MJ of fuel energy, the toxicological effects were seen at much lower relative PM1 doses. One explanation for the higher mass emissions from the poplar pellet combustion may be the different ratio between wood and bark compared to standard softwood pellets. Moreover, poplar trees generally take up metals more efficiently than most other trees (Ruttens et al. Citation2011). This might cause the evaporation of excess metals into the emissions. The markedly higher particulate emissions of SRC must be considered when promoting their widespread use as biomass fuels for residential heating, in which filters are not applied for PM emission control. They should preferably be used in larger-scale biomass combustion plants, which apply electrostatic precipitators or baghouse filters for particle removal.
4.3. Miscanthus sp. pellets
Previous studies on the combustion of herbaceous plant material have focused on the physicochemical composition of the emissions and their effects on the combustion appliances (Demirbas Citation2004; Brunner et al. Citation2013; Forbes et al. Citation2014) and have not investigated their possible negative health effects. Thus, to the best of our knowledge, the results presented here are the first to consider the toxic effects of PM1 from Miscanthus sp. fuel.
In addition to the well-known technical disadvantages over standard softwood pellets, we found that the genotoxic responses induced in RAW264.7 cells by the PM1 emissions from the Miscanthus sp. pellets were significantly higher than the effects induced by the emissions from the standard softwood pellets. None of the measured chemical compounds was the highest in the emissions from the Miscanthus sp. pellets, but some of the compounds were higher than average. Namely, OC, Mg, and phorsphorus (P) concentrations were above average, and we found positive associations with those compounds in correlation analysis. The addition of the emission factor into the results increased the difference between the emissions from standard softwood pellets and Miscanthus sp. pellet combustion, especially for genotoxicity but also for cytotoxicity and inflammation.
4.4. Wheat straw pellets
Previous studies have reported high particulate emissions and increased slagging tendencies of straw pellets, even with improved air staging in domestic appliances (Örberg et al. Citation2014; Kortelainen et al. Citation2015), which has limited their use to mainly medium- and large-scale combustion systems (Nordgren et al. Citation2013). When the toxicity of the emissions was evaluated, we found that straw pellet combustion induced the most pronounced inflammatory effects of the pellet fuels, but otherwise the responses were on a similar level to the emissions from the Miscanthus sp. pellets. The inflammatory effect of straw combustion emission is also higher than that observed with various wood combustion emissions (Jalava et al. Citation2012), but is still generally low to moderate. Correlation analyses showed that both the Cl− and the P content of the emissions correlated strongly with the induction of inflammatory responses and DNA damage. Both of these elements were found in high concentrations in the PM1 emissions from the wheat straw pellets. We can thus conclude that the high chloride content of the emissions is not only linked to possible damage to the combustion appliances (Bankiewicz et al. Citation2011; Tissari et al. Citation2015) but may also be responsible for toxic effects in vitro or at least function as a surrogate substance for the induced effects. However, the exact reason why Cl− induces toxic effects in vitro cannot be determined by the means of this study and remains to be investigated further. Additionally, the OC content of the straw pellet emissions was the highest of the samples tested in this study, and we also detected a strong positive correlation of the OC content with all toxic responses. Due to the good air staging setup, the straw pellet combustion showed PM1 emissions comparable to the PM1 emissions from the standard softwood pellets. Thus, after adjusting the toxicological effects with the emission factors, the cytotoxic and genotoxic effects induced by straw pellet emissions were less pronounced than the effects induced by the emissions from Miscanthus sp. or poplar pellet combustion, which both had higher mass emissions.
5. Conclusions
The PM1 emissions from the combustion of standard softwood pellets elicited the least toxic effects. This was even more evident when the emission factor was taken into consideration. PM1 emissions from poplar pellet combustion induced similar responses as the PM1 emissions from standard softwood pellet combustion; however, the genotoxic responses were higher. The considerably higher mass emissions from poplar pellet combustion caused more prominent relative toxicity than standard softwood pellets. Thus, they may contribute to the negative health effects induced by the emission PM more strongly. In some cases, poplar combustion emissions may cause a greater release of heavy metals (e.g., Pb, Cd) than other wood material, as they can efficiently extract metals from soil.
It is well established that the combustion of herbaceous biomass causes numerous problems in small-scale appliances. These technical challenges, in addition to the pronounced toxic effects of the emissions, could prevent the widespread use of these energy crops in a domestic setting at the moment. The emissions from Miscanthus sp. pellets were much higher than the emissions from standard softwood and straw pellet combustion. These high emissions combined with the most severe genotoxic responses and high cytotoxic responses caused the most prominent relative toxicity, which may lead to adverse health effects. The emissions from straw pellet combustion caused the highest inflammatory response, which may lead to short-term health consequences and irritation. However, with proper appliances and settings, the PM mass emissions were very low and comparable to wood pellet combustion. Thus, to promote the future use of alternative biomass for pellet production, further improvements are needed in the prevalent combustion technology of domestic appliances as well as filtration systems should be developed and applied.
UAST_1121198_Supplementary_File.zip
Download Zip (43.2 KB)Acknowledgments
The authors wish to thank Mrs. Miia Koistinen of the University of Eastern Finland for her excellent technical assistance in the toxicological studies. The PAH analysis of particulate samples by MSc Annika Virén of the University of Eastern Finland is also much appreciated.
Funding
This study was funded by the Finnish Funding Agency for Innovation (Tekes) as part of the ERA-NET program of the EU, the Austrian Research Promotion Agency (FFG) and the Research Program on Sustainable Energy of the Academy of Finland. This project belongs to the strategic funding of the University of Eastern Finland (Sustainable Bioenergy, Climate Change and Health).
References
- Alakangas, E., Junginger, M., van Dam, J., Hinge, J., Keränen, J., Olsson, O., Porsö, C., Martikainen, A., Rathbauer, J., Sulzbacher, L., Vesterinen, P., and Vinterbäck, J. (2012). EUBIONET III—Solutions to Biomass Trade and Market Barriers. Renew. Sust. Energy Rev., 16:4277–4290.
- Bankiewicz, D., Alonso-Herranz, E., Yrjas, P., Laurén, T., Spliethoff, H., and Hupa, M. (2011). Role of ZnCl2 in High-Temperature Corrosion in a Bench-Scale Fluidized Bed Firing Simulated Waste Wood Pellets. Energy Fuels, 25:3476–34843.
- Brosse, N., Dufour, A., Meng, X., Sun, Q., and Ragauskas, A. (2012). Miscanthus: A Fast-Growing Crop for Biofuels and Chemicals Production. Biofuels. Bioprod. Biorefin., 6:580–598.
- Brunner, T., Biedermann, F., Kanzian, W., Evic, N., Obernberger, I. (2013). Advanced Biomass Fuel Characterization Based on Tests with a Specially Designed Lab-Scale Reactor. Energy Fuels, 27:5691–5698.
- Carroll, J.P., Finnan, J. (2012). Physical and Chemical Properties of Pellets From Energy Crops and Cereal Straws. Biosys. Eng., 112:151–159.
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; A policy framework for climate and energy in the period from 2020 to 2030, COM(2014) 15 final. 22.1.2014
- Demirbas, A. (2004). Combustion Characteristics of Different Biomass Fuels. Prog. Energ. Combust. Sci., 30:219–230.
- Díaz-Ramírez, M., Boman, C., Sebastián, F., Royo, J., Xiong, S., and Boström, D. (2012). Ash Characterization and Transformation Behaviour of the Fixed-Bed Combustion of Novel Crops: Poplar, Brassica, and Cassava Fuels. Energy Fuels, 26:3218–3229.
- Directive 2009 / 125 / EC of the European Parliament and of the Council of 21 October 2009 establishing a framework setting of eco-design for energy related products
- European Commission. (2014). Commission Staff Working Document SWD 259 final, 28.7.2014.
- European Pellet Council. (2013). Handbook for the Certification of Wood Pellets for Heating Purposes, Version 2.0, April 2013.
- Executive Office of the President. June 2013. The President's Climate Action Plan. whitehouse.gov, Washington, D. C., June 25, 2013.
- Forbes, E.G.A., Easson, D.L., Lyons, G.A., and McRoberts, W.C. (2014). Physico-Chemical Characteristics of Eight Different Biomass Fuels and Comparison of Combustion and Emission Results in a Small Scale Multi-Fuel Boiler. Energ. Conver. Manag., 87:1162–1169.
- Happo, M.S., Uski, O., Jalava, P.I., Kelz, J., Brunner, T., Hakulinen, P., Mäki-Paakkanen, J., Kosma, V.-M., Jokiniemi, J., Obernberger, I., and Hirvonen, M.-R. (2013). Pulmonary Inflammation and Tissue Damage in the Mouse Lung After Exposure to PM Samples From Biomass Heating Appliances of Old and Modern Technologies. Sci. Total Environ., 443:256–266.
- Jalava, P.I., Salonen, R.O., Nuutinen, K., Pennanen, A.S., Happo, M.S., Tissari, J., Frey, A., Hillamo, R., Jokiniemi, J., and Hirvonen, M.-R. (2010). Effect of Combustion Condition on Cytotoxic and Inflammatory Activity of Residential Wood Combustion Particles. Atmos. Environ., 44:1691–1698.
- Jalava, P.I., Happo, M.S., Kelz, J., Brunner, T., Hakulinen, P., Mäki-Paakkanen, J., Hukkanen, A., Jokiniemi, J., Obernberger, I., and Hirvonen, M.-R. (2012). Invitro Toxicological Characterization of Particulate Emissions From Residential Biomass Heating Systems Based on Old and New Technologies. Atmos. Environ., 50:24–35.
- Kasurinen, S., Jalava, P.I., Tapanainen, M., Uski, O., Happo, M.S., Mäki-Paakkanen, J., Lamberg, H., Koponen, H., Nuutinen, I., Kortelainen, M., Jokiniemi, J., and Hirvonen, M.-R. (2015). Toxicological Effects of Particulate Emissions – A Comparison of Oil and Wood Fuels in Small- and Medium-Scale Heating Systems. Atmos. Environ., 103:321–330.
- Kelz, J., Brunner, T., Obernberger, I., Jalava, P.I., and Hirvonen, M.-R. (2010). Untersuchung Des Gesundheitsgefährdungspotentials von Feinstaubemissionen aus Biomasse-Kleinfeuerungsanlagen. BIOENERGY 2020 +GmbH Endbericht Teil 1, Bericht Nr. TR I-2-13 01. (In German).
- Kortelainen, M., Jokiniemi, J., Nuutinen, I., Torvela, T., Lamberg, H., Karhunen, T., Tissari, J., and Sippula, O. (2015). Ash Behaviour and Emission Formation in a Small-Scale Reciprocating-Grate Combustion Reactor Operated with Wood Chips, Reed Canary Grass and Barley Straw. Fuel, 143:80–88.
- Lamberg, H., Nuutinen, K., Tissari, J., Ruusunen, J., Yli-Pirilä, P., Sippula, O., Tapanainen, M., Jalava, P., Makkonen, U., Teinilä, K., Saarnio, K., Hillamo, R., Hirvonen, M.-R., and Jokiniemi, J. (2011). Physicochemical Characterization of Fine Particles From Small-Scale Wood Combustion. Atmos. Environ., 45:7635–7643.
- Lamberg, H., Tissari, J., Jokiniemi, J., and Sippula, O. (2013). Fine Particle and Gaseous Emissions from a Small-Scale Boiler Fueled by Pellets of Various Raw Materials. Energy Fuels, 27:7044–7053.
- Leskinen J., Tissari, J., Uski, O., Virén, A., Torvela, T., Kaivosoja, T., Lamberg, H., Nuutinen, I., Kettunen, T., Joutsensaari, J., Jalava, P.I., Sippula, O., Hirvonen, M.-R., and Jokiniemi, J. (2014). Fine Particle Emissions in Three Different Combustion Conditions of a Wood Chip-Fired Appliance – Particulate Physico-Chemical Properties and Induced Cell Death. Atmos. Environ., 86:129–139.
- Monedero, E., Portero, H., Lapuerta, M. (2015). Pellet Blends of Poplar and Pine Sawdust: Effects of Material Composition, Additive, Moisture Content and Compression Die on Pellet Quality. Fuel Proc. Technol., 132:15–23.
- Nordgren D., Hedman, H., Padban, N., Boström, D., and Öhmann, M. (2013). Ash Transformations in Pulverised Fuel Co-Combustion of Straw and Woody Biomass. Fuel Proc. Technol., 105:52–58.
- Örberg, H., Jansson, S., Kalén, G., Thyrel, M., and Xiong, S. (2014). Combustion and Slagging Behavior of Biomass Pellets using a Burner Cup Developed for Ash-Rich Fuels. Energy Fuels, 28:1103–1110.
- Porsö, C., Hansson, P.-A. (2014). Time-Dependent Climate Impact of Heat Production from Swedish Willow and Poplar Pellets—In a Life Cycle Perspective. Biomass Bioenerg., 70:287–301.
- Ruttens, A., Boulet, J., Weyens, N., Smeets, K., Adriaensen, K., Meers, E., van Slycken, S., Tack, F., Meiresonne, L., Thewys, T., Witters, N., Carleer, R., Dupae, J., Vangronsveld, J. (2011). Short Rotation Coppice Culture of Willows and Poplars as Energy Crops on Metal Contaminated Agricultural Soils. Int. J. Phytoremediat., 13:194–207.
- Ruusunen, J., Tapanainen, M., Sippula, O., Jalava, P.I., Lamberg, H., Nuutinen, K., Tissari, J., Ihalainen, M., Kuuspalo, K., Mäki-Paakkanen, J., Hakulinen, P., Pennanen, A., Teinilä, K., Makkonen, U., Salonen, R.O., Hillamo, R., Hirvonen, M.-R., and Jokiniemi, J. (2011). A Novel Particle Sampling System for Physico-Chemical and Toxicological Characterization of Emissions. Anal. Bioanalyt. Chem., 401:3183–3195.
- Schmidl, C., Luisser, M., Padouvas, E., Lasselsperger, L., Rzaca, M., Cruz, C.R.-S., Hendler, M., Peng, G., Bauer, H., and Puxbaum, H. (2011). Particulate and Gaseous Emissions from Manually and Automatically Fired Small Scale Combustion Systems. Atmos. Environ., 45:7443–7454.
- Tapanainen, M., Jalava, P.I., Mäki-Paakkanen, J., Hakulinen, P., Happo, M.S., Lamberg, H., Ruusunen, J., Tissari, J., Nuutinen, K., Yli-Pirilä, P., Hillamo, R., Salonen, R.O., Jokiniemi, J., and Hirvonen, M.-R. (2011). In vitro Immunotoxic and Genotoxic Activities of Particles Emitted from Two Different Small-Scale Wood Combustion Appliances. Atmos. Environ., 40:7546–7554.
- Tapanainen, M., Jalava, P.I., Mäki-Paakkanen, J., Hakulinen, P., Lamberg, H., Ruusunen, J., Tissari, J., Jokiniemi, J., and Hirvonen, M.-R. (2012). Efficiency of Log Wood Combustion Affects the Toxicological and Chemical Properties of Emission Particles. Inhalat. Toxicol., 24:343–355.
- Tissari, J., Sippula, O., Torvela, T., Lamberg, H., Leskinen, J., Karhunen, T., Paukkunen, S., Hirvonen, M.-R., Jokiniemi, J. (2015). Zinc Nanoparticle Formation and Physicochemical Properties in Wood Combustion—Experiments with Zinc-Doped Pellets in a Small-Scale Boiler. Fuel, 143:404–413.
- Torvela T., Uski, O., Karhunen, T., Lähde, A., Jalava, P.I., Sippula, O., Tissari, J., Hirvonen, M.-R., and Jokiniemi, J. (2014). Reference Particles for Toxicological Studies of Wood Combustion: Formation, Characteristics, and Toxicity Compared to Those of Real Wood Combustion Particulate Mass. Chem. Res. Toxicol., 27:1516–1527.
- Uski, O.J., Happo, M.S., Jalava, P.I., Brunner, T., Kelz, J., Obernberger, I., Jokiniemi, J., and Hirvonen, M.-R. (2012). Acute Systemic and Lung Inflammation in C57Bl/6J Mice After Intratracheal Aspiration of Particulate Matter from Small-Scale Biomass Combustion Appliances Based on Old and Modern Technologies. Inhalat. Toxicol., 24:952–965.
- Uski, O., Jalava, P.I., Happo, M.S., Leskinen, J., Sippula, O., Tissari, J., Mäki-Paakkanen, J., Jokiniemi, J., and Hirvonen, M.-R. (2014). Different Toxic Mechanisms are Activated by Emission PM Depending on Combustion Efficiency. Atmos. Environ., 89:623–632.
- Uski O., Jalava, P.I., Happo, M.S., Torvela, T., Leskinen, J., Mäki-Paakkanen, J., Tissari, J., Sippula, O., Lamberg, H., Jokiniemi, J., and Hirvonen, M.-R. (2015). Effect of Fuel Zinc Content on Toxicological Responses of Particulate Matter from Pellet Combustion in vitro. Sci. Total Environ., 511:331–340.
- World Health Organization (WHO) (1998). Selected Non-heterocyclic Polycyclic Aromatic Hydrocarbons. Environmental Health Criteria 202. WHO International Program of Chemical Safety (IPCS), Geneva, Switzerland.
