ABSTRACT
Wearable ionization air cleaners are compact in size and marketed for personal respiratory protection by removing air pollutants from users' breathing zone. In this study, ozone emission and particle removal rates of four wearable ionization air cleaners (namely, AC1 through AC4) were evaluated inside a 0.46 m3 stainless steel chamber. Continuous measurements were conducted for ozone concentration, PM2.5 concentration, and particle size distribution in the size range of 18.1–289 nm. Two of the four wearable air cleaners (i.e., AC1 and AC2) had detectable ozone emissions. The 10-h average ozone emission rates were quite different (i.e., 0.67 mg·h−1 for AC1 and 3.40 × 10−2 mg·h−1 for AC2); however, the ozone emissions were negligible for AC3 and AC4. The number removal rates for particles within the measured size range were highly variable (i.e., 2.20 h−1, 0.52 h−1, 8.10 h−1, and 27.9 h−1 for AC1 through AC4, respectively). The corresponding mass removal rates of PM2.5 were 1.85 h−1, 0.48 h−1,1.52 h−1, and 5.37 h−1, respectively. Regulatory guidelines are needed to assure these devices can effectively remove particles without ozone emissions to protect public health.
Copyright © 2016 American Association for Aerosol Research
EDITOR :
1. Introduction
Particulate matter (PM) is a major air pollutant in both indoor and outdoor environments. Acute as well as chronic exposure to PM may result in adverse health outcomes. In 2010, indoor air pollution from household solid fuels and ambient PM pollutants were ranked as the 3rd and the 9th leading risk factors that attribute to the global burden of disease for both sexes (Lim et al. Citation2012). In particular, fine (diameter ≤ 2.5 μm, PM2.5) and ultrafine particles (diameter ≤ 0.1 μm, PM0.1) can penetrate deep into human respiratory system and reach the alveoli, and subsequently cause severe health problems. Previous studies reported exacerbation of the asthma (Norris et al. Citation1999), growing incidence of cardiovascular and cardiopulmonary diseases (Donaldson et al. Citation2001; Delfino et al. Citation2005), and increasing mortality because of human exposure to fine and ultrafine particles (Dockery et al. Citation1993; Pope et al. Citation2002, Citation2009).
Wearable ionization air cleaners are designed to mitigate personal exposure to fine and ultrafine particles. Compared to portable air cleaners, wearable air cleaners are small in their size and people can wear them on their collars or around the neck to remove particles from the breathing zone. Wearable ionization air cleaners remove particles by charging them typically with negative ions. The particles carrying negative charges leave people's breathing zone through either repelling each other or being collected by a collecting plate with the opposite charge.
However, the ionization process of air cleaners may produce ozone, either intentionally or unintentionally. As a strong oxidant, ozone is a toxic substance that needs to be controlled for better indoor air quality. Transient and long-term exposure to ozone can cause detrimental impact on human being's health (Lippmann Citation1989; Jerrett et al. Citation2009). Apart from that, reactions between ozone and indoor chemical compounds yield a variety of harmful and irritating secondary pollutants (Weschler et al. Citation1992; Fan et al. Citation2003; Singer et al. Citation2006). Reactions between ozone and unsaturated volatile organic compounds (VOCs) can lead to formation of secondary organic aerosols (SOA), most of which are in the size range of fine and ultrafine particles (Weschler and Shields Citation1999; Wainman et al. Citation2000; Singer et al. Citation2006; Waring et al. Citation2008; Waring and Siegel Citation2011). SOA formation can undermine particle removal effects of wearable ionization air cleaners. When the SOA generation rate exceeds the particle removal rate, it can even turn an air cleaner into a particle emission source rather than a particle removal device.
Previous studies reported the performance, including particle removal, ozone emission, and SOA generation, of household ionization air cleaners in experimental chambers and realistic indoor environments (Grabarczyk Citation2001; Lee et al. Citation2004; Hubbard et al. Citation2005; Alshawa et al. Citation2007; Grinshpun et al. Citation2007). However, the characteristics of wearable air cleaners are quite different from those of household ones. The target volume of wearable air cleaner is the breathing zone of an individual; whereas, that of household air cleaner is likely the entire space of the target room. Additionally, Corsi et al. Citation(2007) confirmed that chemical reactions between ozone and personal care products can result in deleterious chemicals in individual's near-head region. If wearable ionization air cleaners generated a similar level of ozone in the near-head region, it could be more problematic than that from household ones. Grinshpum et al. Citation(2005) tested the fine particle (˜0.3–3 μm) removal efficiency of wearable ionization air cleaners in confined indoor spaces. They reported that wearable ionization air cleaners were effective in removing fine particles in human breathing zone but the ozone emission rate was not evaluated for the wearable ionization air cleaners tested in their study. Phillips et al. Citation(1999) and Britigan et al. Citation(2006) previously found that ozone emissions from “personal air purifiers” (i.e., marketed name for wearable air cleaners) could make ozone concentrations in breathing zone higher than regulatory limits. However, these studies did not evaluate particle removal (or generation) effect. Consequently, an experimental investigation on both ozone emission and particle removal/generation effects is needed to better understand the performance of wearable ionization air cleaners.
In this study, we evaluated the performance of four wearable ionization air cleaners commercially available in the United States inside a 0.46 m3 stainless steel chamber. Ozone emission rate was determined from continuous measurements of ozone concentrations inside the chamber. Concurrently, we also measured PM2.5 mass concentrations and particle size distributions in a size range of 18.1–289 nm by using real-time instruments. Using the data, we estimated number removal rates of particles in the measured size range and mass removal rates of PM2.5.
2. Methodology
2.1. Wearable air cleaners
Four newly purchased wearable ionization air cleaners from different manufacturers were selected for this study (namely, AC1 through AC4). To select the wearable ionization air cleaner, we searched “wearable air cleaner” or “personal air cleaner” or “personal air purifier” on Amazon.com. Among the search results, the first four air cleaners with description contained “ion” or “ionization” or “ionic” and the sizes of the cleaners were designed for people to wear them on their collars or around the neck were selected in this study. According to their user manuals, all the selected air cleaners had the same removal mechanism by emitting negative ions. Note that these air cleaners have only on/off switches without intermediate power control. Detailed information of selected air cleaners can be found in .
Table 1. Characteristics of the four selected wearable air cleaners.
2.2. Experimental chamber
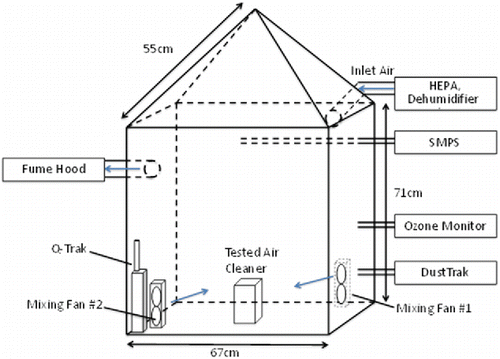
A two-phase investigation was conducted in a 0.46 m3 stainless steel chamber to evaluate the performance of the selected wearable ionization air cleaners. The schematic diagram of the chamber setup is shown in . The chamber was placed in a well-ventilated laboratory. All openings of the chamber were tightly sealed. The inlet air to the chamber was pretreated with HEPA filter, desiccant dehumidifier, and Kr85 neutralizer to remove particles, ozone, water vapor, and organic compounds at the air inlet. The filtered air was supplied into the chamber with a flow rate of 4 l·min−1 and exhausted on the left surface of the chamber. The particle number concentration in the size range of 18.1–289 nm of the inlet air was approximately 50 cm−3 and the ozone concentration was about 0 ppb. Murray and Burmaster Citation(1995) have studied the probabilistic distribution of residential air exchange rates in the United States, the median value of which was approximately 0.5 h−1. Thus, all the experiments were conducted under an air exchange rate of 0.5 h−1 accordingly. To examine the air exchange rate (α) of the chamber, CO2 concentration inside the chamber was continuously measured by Q-Trak (TSI Model 8554, Shoreview, MN, USA). Then, α was determined by fitting the decay curve of the CO2 concentration inside the chamber. The tested air cleaner was located at the center of the chamber floor throughout the experiments. Two mixing fans were placed on the opposite corners of the chamber floor to maintain well-mixed condition in the chamber. The well-mixed condition was verified later through a mixing condition test, as described in the SI (SI 1).
Previous studies and the current testing standard for air cleaners suggested a room-sized chamber to conduct the experiment. GB/T 18801-2008 (GuoBiao/Tuijian by Standardization Administration of the People's Republic of China Citation2008) regulated the volume of a testing chamber to be 30 m3 for household household air cleaners. ANSI/UL 867 (American National Standards Institute/Underwriters Laboratories Citation2000) requires that the volume of the chamber be in the range of 26.9–31.1 m3 to evaluate ozone emissions from electrostatic air cleaners. However, the size and the power of wearable ionization air cleaner are much smaller than a household one. In addition, the target volume of wearable air cleaner is the human breathing zone, much smaller than that of household air cleaner. The ozone emission and particle removal effect of a wearable ionization air cleaner may not be detectable if it is tested in a room-size chamber. Therefore, in this study, ozone emission and particle removal rates of the selected wearable ionization air cleaners were tested in a 0.46 m3 stainless steel chamber.
2.3. Phase 1: Ozone emission test
In the first phase, the ozone emission rate of each wearable ionization air cleaner was quantified by monitoring the ozone concentration inside the chamber. The chamber was preconditioned with the filtered fresh air before each experimental run. An air sampling tube for ozone measurement was placed approximately 0.2 m away from a working wearable air cleaner. Ten-second-averaged ozone concentration was measured by an UV absorbance ozone analyzer (2B Technologies Model 205, Boulder, CO, USA). All the selected wearable ionization air cleaners were fully charged before each sampling run, which lasted for 3 h. Ozone concentration inside the chamber with a working wearable air cleaner was continuously monitored to identify whether there is a significant level of ozone emission. Two selected wearable ionization air cleaners (i.e., AC1 and AC2) were determined to have obvious ozone emission. Thus, these two wearable ionization air cleaners were continuously operated for 10 h to further investigate their ozone emission rates. The ozone mass conservation inside the chamber with a working wearable ionization air cleaner can be expressed as follows:[1] where, Co,in is the ozone concentration inside the chamber (ppb); Co,out is the ozone concentration of the inlet air (ppb); Ko is the ozone deposition rate within the chamber (h−1); Eo is the ozone emission rate from the wearable ionization air cleaner (mg·h−1); t is time (h) and V is the volume of the chamber (m3). Then, Equation (Equation1
[1] ) can be rearranged to determine the ozone emission rate of the working wearable ionization air cleaner by the following equation:
[2]
During the chamber experiments, Co,in was directly monitored by the UV absorbance ozone analyzer; α was determined from the measured CO2 concentration; dCo, in/dt was calculated from the measurements of the ozone analyzer; and Ko was determined from background experiments. The ozone data were smoothed by taking 5-min-averages to estimate dCo, in/dt in Equation (Equation2[2] ).
For the background experiment, the chamber was first re-conditioned with clean air before the experiment (i.e., particle number concentration below 50 cm−3 and ozone concentration at 0 ppb). Ozone was intentionally generated by an UV light ozone generator and supplied into the chamber until its concentration reached up to 200 ppb. Then, the ozone concentration inside the chamber was continuously monitored until it decreased to the background level (i.e., 0 ppb) which can be expressed as the following equation:[3] where, Co, ini is the initial ozone concentration inside the chamber (ppb). The total loss rate of ozone within the chamber (sum of Ko and α) was obtained from the best-fit decay curve of the monitored ozone concentration inside the chamber. Then, Ko was obtained by subtracting α from the total loss rate (i.e., sum of Ko and α). The nonlinear fit was conducted in “Origin 8.5.1” (Microcal Software Inc., Northampton, MA, USA) by the “nonlinear curve fit” function. The adjusted-R2 of the nonlinear fitting of the ozone concentration decay curves were greater than 0.9. The background experiment was repeated three times. The standard deviations from the repeated measurements were utilized to calculate the corresponding error bar of the result.
2.4. Phase 2: Particle removal
In this phase, a burning candle was used as an indoor source for particles (He et al. Citation2004). Number removal rates of particles in the range of 18.1–289 nm and mass removal rates of PM2.5 were determined for each selected wearable ionization air cleaner. Prior to each experiment, the chamber was ventilated by filtered air until the chamber particle number concentration reached the background level (i.e., <50 cm−3). Then, a candle was ignited inside the chamber for 20 s. The particle number concentration inside the chamber reached a level around 1.5 × 106 cm−3 after the ignition. Once the candle was removed, a fully charged wearable ionization air cleaner was placed and operated at the center of the chamber floor. The total and size-resolved number concentrations of particles with diameters of 18.1–289 nm inside the chamber were monitored by a scanning mobility particle spectrometer (SMPS) system (TSI Model 3080 with Model 3085, TSI Inc., Shoreview, MN, USA). The particle number concentrations were scanned across 74 diameter bins within the measured particle size range every 2 min. The sampling point was located at the top center of the left chamber wall, approximately 0.4 m above the tested air cleaner. The mass concentrations of PM2.5 in the chamber were monitored by an aerosol monitor (DustTrak, TSI Model 8520, TSI Inc.), which estimates the PM2.5 mass concentration based on the light scattering signal. The sampling tube for PM2.5 was located at the center of the left chamber wall. The experiment lasted until the number concentration of particles within the measured size range decreased to a relative low level (i.e., <1000 cm−3). The mass conservation of particles inside the chamber can be expressed as follows:[4] where, Cp,in is number concentration of particles (m−3) or PM2.5 mass concentration (μg·m−3) in the chamber; Cp,out is the number concentration of particles (m−3) or PM2.5 mass concentration (μg·m−3) of the filtered air; Kp is the particle deposition rate of the chamber (h−1); R is the number removal rate of particle in the measured size range or PM2.5 mass removal rate of the tested wearable ionization air cleaners (h−1). Cp,out is negligible because the particle concentration of the filtered air was much lower than that in the chamber. Consequently, particle concentration in the chamber can be described with the following by rearranging Equation (Equation4
[4] ), given as:
[5] where, Cp,ini is the initial number concentration of particles in the measured size range (m−3) or initial PM2.5 mass concentration (μg·m−3). Thus, by regressing the decay curve of particle concentration within the chamber, the total particle loss rate of the chamber (sum of α, Kp, and R) can be estimated according to Equation (Equation5
[5] ). R was then obtained by subtracting Kp and α from the total particle loss rate of the chamber. Kp was determined from background experiments similar to those for ozone deposition rate inside the chamber. The nonlinear fit was conducted in “Origin 8.5.1” (Microcal Software Inc., Northampton, MA, USA) by the “nonlinear curve fit” function. The adjusted-R2 of the nonlinear fitting of the particle concentration decay curves were greater than 0.9. The particle concentration decay curve inside the chamber with working wearable ionization air cleaner after the ignition of the candle was measured repeatedly for three times. The standard deviations from the repeated measurements were utilized to calculate the corresponding error bar of the result. It is noteworthy that this method is only valid under an assumption that the effect of particle coagulation is negligible. To validate this assumption, data screening was conducted before fitting the data. The detailed procedure is described in the SI (SI 2).
The particle removal rates of wearable ionization air cleaners at different battery voltage levels were also evaluated to determine the change of particle removal effect as their battery power reduced. AC1 and AC4 were selected for battery testing because they were the most efficient wearable ionization air cleaners tested with and without ozone emissions, respectively. A condensation particle counter (TSI Model 3007, TSI Inc.) was used to monitor the total number concentration of particles in the measured size range inside the experimental chamber. The number removal rates of particles in the measured size range by AC1 and AC4 were evaluated after continuous operations of 0, 12, 24, 36, 48, and 72 h. The experimental setup and calculation formula remained the same as described in the phase 2.
3. Results and discussion
3.1. Ozone emission and ionization
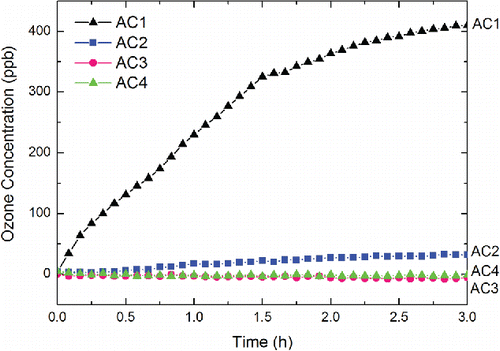
The ozone concentrations in the chamber with the working fully charged wearable ionization air cleaners are shown in . After 3 h of operations, the ozone concentration inside the chamber with AC3 and AC4 remained at the background level of 0 ppb. However, operations of AC1 and AC2 made ozone concentration inside the chamber reach up to 410 ppb and 32 ppb, respectively. This indicates that there were obvious ozone emissions from AC1 and AC2. Ozone concentrations inside the chamber with working AC1 and AC2 were then continuously monitored for 10 h to further evaluate ozone emission rates as a function of time. According to a previous study, ozone emission from a household ionization air cleaner can be obtained from the steady-state ozone concentration (Waring et al. Citation2008). However, for both AC1 and AC2, ozone concentration inside the chamber did not reach a steady state even after 10 h of operation. Thus, the ozone emission rates of working fully charged AC1 and AC2 were estimated by using Equation (Equation2[2] ) in the methodology section.
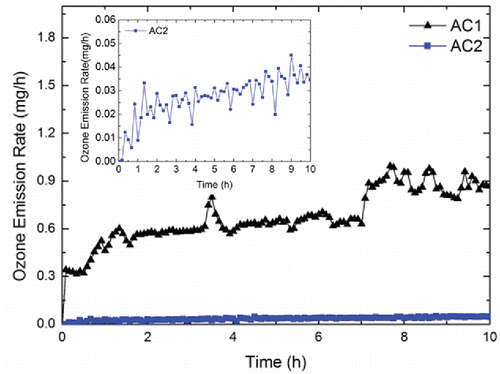
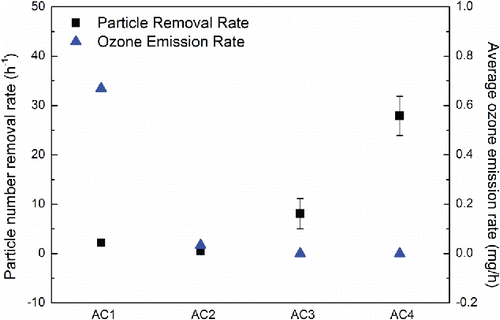
The time-dependent ozone emission rates of AC1 and AC2 are shown in . For AC1, the time-dependent ozone emission rate was fluctuating without an obvious trend, which ranged from 0.315 mg·h−1 to 0.995 mg·h−1. For AC2, the ozone emission rate increased as a function of time. The minimum ozone emission rate of AC2 was 5.36 × 10−4 mg·h−1 and the maximum ozone emission rate of AC2 was 5.07 × 10−2 mg·h−1, which was two orders of magnitude higher than the minimum. shows the time-averaged ozone emission rates of the tested wearable ionization air cleaners. As it shows, the time-averaged ozone emission rates of AC1 and AC2 during the 10-h operation were 0.669 mg·h−1 and 3.40 × 10−2 mg·h−1, respectively. Conversely, the time-averaged ozone emission rates of both AC3 and AC4 remained at 0 mg·h−1.
Figure 4. Removal rates of total particles in the measured size range and time-averaged ozone emission rates of wearable air cleaners.
Comparison of ozone emission rates between wearable ionization air cleaners in this study and other air cleaners from previous studies is shown in . The ozone emission rate of the wearable air purifier measured by Phillips et al. Citation(1999), was approximately 0.102–0.114 mg·h−1, which is less than that of AC1 but greater than AC2. When compared with the household air cleaners, the ozone emission rate of AC1 is approximately 15–30% of those reported in the literature. Nevertheless, the working space of the wearable air cleaner, which is human breathing zone, is much smaller than a typical indoor space for household ones. Thus, the ozone concentration at human breathing zone may reach up to a harmful level if wearable ionization air cleaners are utilized under calm air conditions.
Table 2. Comparison of CADR and ozone emission rate between the selected wearable ionization air cleaner in this study and other air cleaners from references.
Ions are widely adopted for air cleaners. Corona charging is a common method for ion generation, during which process ozone may be generated as a byproduct. The generation of ozone can be simply described by the following two steps (Yagi and Tanaka Citation1979):[6]
[7] where M could be either O2 molecule or N2 molecule. The reaction rate in Equation (Equation6
[6] ) positively correlates with intensity of electron current. The reaction rate in Equation (Equation7
[7] ) decreased as the air temperature increased and the surrounded air is heated by the corona wire surface. Thus, ozone emission rate becomes a function of the intensity of electron current and the corona wire surface temperature. The intensity of ozone generation depends on not only material of the cathode (Boelter and Davidson Citation1997), but also other design parameters of the ion generator (e.g., applied voltage and cathode diameter) (Liu et al. Citation2000). In this study, AC1 and AC2 utilize metallic corona discharge while AC3 and AC4 utilize carbon fiber ionizer for ion generation. Metallic corona discharge mechanism is reported for obvious ozone generation or even utilized for ozone generation (Liu et al. Citation2000; Yanallah et al. Citation2009). This may be the reason why AC1 and AC2 were observed to have obvious ozone emission in this study. For the same level of ionization, a carbon fiber ionizer typically requires lower voltage than metallic cathodes of corona discharge because the diameter of carbon fibers is much smaller (about 5–10 μm) than that of metallic cathodes. Lower voltage applied to carbon fibers is sufficient to trigger ionization at the tip of carbon fiber cathodes; while, the electric field from the applied voltage is not high enough to induce unnecessary ozone reactions (shown in Equations (Equation6
[6] ) and (Equation7
[7] )) (Han et al. Citation2008; Han et al. Citation2009). This may explain the negligible ozone emissions by AC3 and AC4.
3.2. Particle removal and secondary organic aerosol formation
The number removal rates (h−1) of total particles measured in the size range from 18.1 to 289 nm of the four fully charged wearable ionization air cleaners are shown in . Among the four wearable air cleaners tested, the highest number removal rate of total particles in the measured size range was 27.9 h−1 for AC4; whereas, AC2 offered the lowest value as 0.52 h−1.
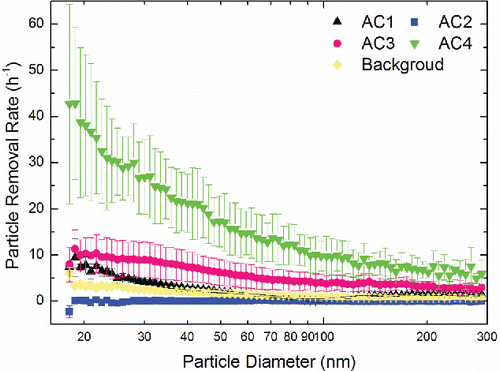
illustrates the size-resolved number removal rates (h−1) of particles in the measured size range by fully charged wearable ionization air cleaners. The x-axis of is particle diameter plotted on a logarithmic scale and the y-axis is number removal rates of size-resolved particles in the measured size range plotted on a linear scale. AC4 provided the highest number removal rate of size-resolved particles in the measured size range, followed by AC3. The number removal rate by AC4 in the measured size range varied from 4.87 h−1 at 278.8 nm to 42.8 h−1 at 18.8 nm. For AC3, the number removal rate within the measured size range varied from 2.37 h−1 at 278.8 nm to 11.3 h−1 at 18.8 nm. In general, the number removal rate of particles in the measured size range decreased as the particle size increased for both AC3 and AC4. As for AC1, the largest number removal rate was 9.45 h−1 at 18.8 nm while the smallest was 0.12 h−1 at 98.2 nm. The number removal rate decreased as the particle diameter increased in the size range of 18.1–98.2 nm and increased as the particle diameter increased in the larger particle size range from 98.2 to 289 nm. AC2 had the lowest number removal rates within the measured range, which varied from –2.30 h−1 at 18.1 nm to 0.26 h−1 at 21.6 nm. There was no significant change of particle number removal rates as diameter increased by AC2. To better understand the difference of particle removal effect between different wearable ionization air cleaners, the time-resolved particle distributions inside the chamber with working AC1 and AC4 are shown in the online supplemental information (SI).
The variable performance of particle number removal rates in the measured size range may be due to the different ion emission densities of the selected wearable ionization air cleaners. Lee et al. Citation(2004) have concluded that high ion emission densities were more efficient in removing fine and ultrafine particles in indoor environments. Unfortunately, ion emission densities of the selected air cleaners were not measured in this experiment. Considering the fact that the surface to volume ratio of the chamber and the ventilation pattern were consistent through the experiments for all the selected air cleaners, it is a reasonable assumption that the higher efficiency of removing particles by AC4 may be due to the higher ion emission density.
For AC2, 43 of the total 78 diameter bins corresponded to negative number removal rates. The negative values indicated that AC2 was rather a source than a sink for these particles. SOA may be generated due to the reactions between ozone and the unsaturated VOCs in this experiment, noting that AC2 was found to emit ozone. Under the scope of this work, the chamber testing was performed without unsaturated VOCs. Nevertheless, the VOCs emitted from the burning candle may react with ozone generated from the wearable ionization air cleaner. Thus, SOA formation would also happen for AC1 since it has significant ozone emissions. Although the SOA formation did not change AC1 from a sink to a source for particles, the SOA formation would weaken its particle removal performance.
In an indoor environment, unsaturated VOCs concentrations can be much higher than our chamber experiments. They can be emitted from various indoor sources: building materials, carpet, consumer products, and so on. Singer et al. Citation(2006) have measured reaction between ozone and VOCs emitted from household cleaning products. According to their results, the SOA formation can increase particle mass concentration by 100 μg·m−3 when 60 ppb ozone reacted with VOCs from realistically applied cleaning products. People spend majority of their time in indoor environments (Klepeis et al. Citation2001); thus, the usage of AC1 and AC2 in indoor environments may become another source of particles due to its ozone emissions and potential indoor VOCs sources.
The measured “particle removal effect” has two possible meanings since SOA formation is a possible scenario due to the reactions between ozone and unsaturated VOCs from burning candle. On one hand, the measured “particle removal rate” represents the true performance of particle removal effect for wearable air cleaners without ozone emission (i.e., AC3 and AC4). On the other hand, for wearable air cleaners with ozone emissions (i.e., AC1 and AC2), “particle removal rate” represents the net effect of particle removal and SOA formation.
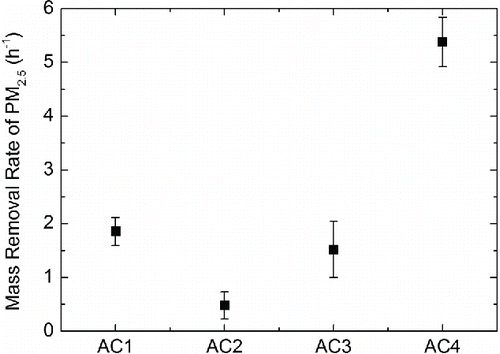
shows the mass removal rates of PM2.5 by the four tested wearable ionization air cleaners. The PM2.5 mass removal rates of AC1, AC2, AC3, and AC4 were 1.85 h−1, 0.48 h−1, 1.52 h−1, and 5.37 h−1, respectively. Similar to particle number removal rates in the measured size range, AC4 still has the highest PM2.5 mass removal rate while AC2 offered the lowest value, close to zero. It can be found that mass removal rate of PM2.5 was less effective than the number removal rates of total particles in the measured size range, especially for AC3 and AC4. For example, the PM2.5 mass removal rate by AC4 was 5.37 h−1 while the corresponding number removal rate of the particles in the measured range was 27.9 h−1. This indicates that the selected wearable ionization air cleaners are more effective to remove submicron particles than micron particles.
3.3. Clean air delivery rate
Clean air delivery rate (CADR) can be directly estimated from the measured particle removal rates for wearable air cleaners without ozone emissions as follows:[8] where V is the volume of the chamber (m3). Thus, the size-resolved CADR of particles in the measured size range varied from 1.09 to 5.20 m3·h−1 for AC3 and 2.24 to 19.7 m3·h−1 for AC4. Regarding total particle number concentration in the measured size range, the CADR was 3.73 m3·h−1 for AC3 and 12.8 m3·h−1 for AC4. As for mass concentration of PM2.5, the CADR was 0.70 m3·h−1 for AC3 and was 2.47 m3·h−1 for AC4. However, CADR for AC1 and AC2 could not be obtained from these experiments because there was potential SOA formation due to the ozone emitted by AC1 and AC2 during the experiments. “Particle removal rate” measured for AC1 and AC2 represents the net effect of particle removal and SOA generation rather than the true performance of removing particles.
compares CADRs between wearable ionization air cleaners in this study and household air cleaners from previous studies. It can be found that CADRs for PM2.5 mass concentration of AC3 and AC4 were much less than those of the household ionization air cleaner measured by Chan and Cheng Citation(2006). When it comes to the CADRs for size-resolved particle number concentrations in the measured range, AC4 showed comparable removal capacity with household ones which was much greater than AC3.
3.4. Particle removal at different battery power levels
AC1 and AC4 were selected for this section because they were demonstrated to be the most effective device of particle removal with and without ozone emissions. The number removal rates of total particles in the measured size range by AC1 and AC4 were evaluated after several hours of operation (i.e., 12, 24, 36, 48, 60, 72 h). presents the number removal rates of total particles in the measured size range and the corresponding voltage of AC1 and AC4. Note that AC1 ran out of battery after 36 h of continuous operation.
Table 3. Total particle number removal rate and voltage by AC1 and AC4 of different battery conditions.
Overall, number removal rates of total particles in the measured size range by both AC1 and AC4 decreased as battery voltage decreased. For AC1, the battery voltage decreased by 17.4% (from 1.93 V to 1.59 V) and the particle number removal rates decreased by 16.5% (from 3.76 h−1 to 3.14 h−1) after running for 36 h. After running for 36 h, AC1 could not operate any more, which may be because the voltage level of 1.59 V is lower than the required voltage level for its operation. As for AC4, the number removal rates of total particles increased as the battery voltage decreased within the first 24 h operation. The voltage decreased by 9.9% (from 4.35 V to 3.92 V) and the particle removal rate increased by 18.4% (from 15.3 h−1 to 18.1 h−1) in the first 24 h operation. Then, the particle number removal rate decreased significantly when the battery voltage decreased in the following operation. The voltage decreased by 11.0% (from 3.92 V to 3.49 V) and the particle removal rate decreased by 65.7% (from 18.1 h−1 to 6.22 h−1) in the next 48 h of operation. Although the particle removal rate of AC4 decreased significantly as the battery voltage decreased, it was still higher than that of any other tested wearable air cleaners even after 72 h of operation (6.22 h−1).
3.5. Limitations
In this study, candle burning was used as the particle emission source to measure particle removal rate of the tested wearable ionization air cleaners. Candle burning was selected since it is one of the major indoor sources for particles (He et al. Citation2004). Using candle emissions would help evaluate the removal rate of particles existing in real daily life by wearable ionization air cleaner. However, the possible VOCs emissions from candle burning would lead to the reaction between VOCs and ozone to generate SOA for ionization air cleaners with ozone emissions (i.e., AC1 and AC2), under which circumstances the real particle removal rates of wearable ionization air cleaners cannot be accurately evaluated.
Additionally, it is noteworthy that the human near-head region is an open space without isolation walls, which is different from the experimental chamber. Particle removal performance of wearable ionization air cleaners within the breathing zone of a human being in a room-size space would be different. A simple experiment was conducted in a small room-size chamber in which particle removal rate of AC4 was determined. The results indicated that AC4 was capacity of removing particles within the breathing zone in a room-sized space. The details of the experimental setup can be found in the SI.
Last but not least, people would wear these wearable air cleaners while they are walking or in outdoor environment. Under such circumstances, the performance of ozone emission as well as particle removal would differ from that in an indoor environment. Thus, future studies would be needed to develop specific testing protocols for wearable air cleaners, which can mimic their operations in the near-head region of users and to set standard for the performance of ozone emission and particle removal.
4. Conclusions
In summary, this study evaluated the ozone emission and particle removal performance of four wearable ionization air cleaners within an experimental chamber. It was found that two of the four air cleaners (i.e., AC1 and AC2) had obvious ozone emissions while the others (i.e., AC3 and AC4) did not. As for particle removal, AC4 was the most effective one while AC2 was the worst. The results illustrated that some wearable ionization air cleaners would lead to elevated ozone concentration within the near-head region with poor particle removal performance while others are capable of removing particles without ozone emissions. Regulatory guidelines for wearable air cleaners are needed to help consumers select useful devices to mitigate their exposures to PM without increasing ozone exposures.
UAST_1139045_Supplemental_File.zip
Download Zip (2.4 MB)Funding
This study was financially supported by the Innovative Research Groups of the National Natural Science Foundation of China (grant number 51521005), Public Scientific Research Project of Ministry of Environmental Protection of China (No. 201409080), and the Shanghai Tongji Gao Tingyao Environmental Science & Technology Development Foundation (STGEF).
References
- Alshawa, A., Russell, A. R., and Nizkorodov, S. A. (2007). Kinetic Analysis of Competition Between Aerosol Particle Removal and Generation by Ionization Air Purifiers. Environ. Sci. Technol., 41:2498–2504.
- ANSI/UL 867 (2000). Standard of Underwriters Laboratories Inc. for Electrostatic Air Cleaners
- Boelter, K. J., and Davidson, J. H. (1997). Ozone Generation by Indoor, Electrostatic Air Cleaners. Aerosol. Sci. Technol., 27:689–708.
- Britigan, N., Alshawa, A., and Nizkorodov, S. A. (2006). Quantification of Ozone Levels in Indoor Environments Generated by Ionization and Ozonolysis Air Purifiers. J. Air. Waste. Manage., 56:601–610.
- Chan, M.-y., and Cheng, B. N. (2006). Performance Evaluation of Domestic Ionizer Type Air Cleaners. Architect. Sci. Rev., 49:357–362.
- Corsi, R. L., Siegel, J., Karamalegos, A., Simon, H., and Morrison, G. C. (2007). Personal Reactive Clouds: Introducing the Concept of Near-Head Chemistry. Atmos. Environ., 41:3161–3165.
- Delfino, R. J., Sioutas, C., and Malik, S. (2005). Potential Role of Ultrafine Particles in Associations Between Airborne Particle Mass and Cardiovascular Health. Environ. Health. Persp., 113:934–946.
- Dockery, D. W., Pope, C. A., Xu, X., Spengler, J. D., Ware, J. H., Fay, M. E., Ferris Jr, B. G., and Speizer, F. E. (1993). An Association Between Air Pollution and Mortality in Six US Cities. New. Engl. J. Med., 329:1753–1759.
- Donaldson, K., Stone, V., Seaton, A., and MacNee, W. (2001). Ambient Particle Inhalation and the Cardiovascular System: Potential Mechanisms. Environ. Health. Persp., 109:523–527.
- Fan, Z. H., Lioy, P., Weschler, C., Fiedler, N., Kipen, H., and Zhang, J. F. (2003). Ozone-Initiated Reactions with Mixtures of Volatile Organic Compounds Under Simulated Indoor Conditions. Environ. Sci. Technol., 37:1811–1821.
- GB/T18801 (2008). Air Cleaner/NationalStandard of People's Republic of China.
- Grabarczyk, Z. (2001). Effectiveness of Indoor Air Cleaning with Corona Ionizers. J. Electrostat., 51:278–283.
- Grinshpun, S. A., Adhikari, A., Honda, T., Kim, K. Y., Toivola, M., Ramchander Rao, K., and Reponen, T. (2007). Control of Aerosol Contaminants in Indoor Air: Combining the Particle Concentration Reduction with Microbial Inactivation. Environ. Sci. Technol., 41:606–612.
- Grinshpun, S. A., Mainelis, G., Trunov, M., Adhikari, A., Reponen, T., and Willeke, K. (2005). Evaluation of Ionic Air Purifiers for Reducing Aerosol Exposure in Confined Indoor Spaces. Indoor Air, 15:235–245.
- Han, B., Hudda, N., Ning, Z., Kim, H.-J., Kim, Y.-J., and Sioutas, C. (2009). A Novel Bipolar Charger for Submicron Aerosol Particles Using Carbon Fiber Ionizers. J. Aerosol. Sci., 40:285–294.
- Han, B., Kim, H.-J., Kim, Y.-J., and Sioutas, C. (2008). Unipolar Charging of Fine and Ultra-Fine Particles Using Carbon Fiber Ionizers. Aerosol. Sci. Technol., 42:793–800.
- He, C., Morawska, L., Hitchins, J., and Gilbert, D. (2004). Contribution from Indoor Sources to Particle Number and Mass Concentrations in Residential Houses. Atmos. Environ., 38:3405–3415.
- Hubbard, H., Coleman, B., Sarwar, G., and Corsi, R. (2005). Effects of an Ozone-Generating Air Purifier on Indoor Secondary Particles in Three Residential Dwellings. Indoor Air, 15:432–444.
- Jerrett, M., Burnett, R. T., Pope III, C. A., Ito, K., Thurston, G., Krewski, D., Shi, Y., Calle, E., and Thun, M. (2009). Long-Term Ozone Exposure and Mortality. New. Engl. J. Med., 360:1085–1095.
- Klepeis, N. E., Nelson, W. C., Ott, W. R., Robinson, J. P., Tsang, A. M., Switzer, P., Behar, J. V., Hern, S. C., and Engelmann, W. H. (2001). The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants. J. Expo. Anal. Env. Epid., 11:231–252.
- Lee, B. U., Yermakov, M., and Grinshpun, S. A. (2004). Removal of Fine and Ultrafine Particles from Indoor Air Environments by the Unipolar Ion Emission. Atmos. Environ., 38:4815–4823.
- Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., and Adair-Rohani, H. (2012). A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet, 380:2224–2260.
- Lippmann, M. (1989). Health Effects of Ozone: A Critical Review. J. Air. Pollu. Control. Assoc., 39:672–695.
- Liu, L., Guo, J., Li, J., and Sheng, L. (2000). The Effect of Wire Heating and Configuration on Ozone Emission in a Negative Ion Generator. J. Electrostat., 48:81–91.
- Murray, D. M., and Burmaster, D. E. (1995). Residential Air Exchange Rates in the United States: Empirical and Estimated Parametric Distributions by Season and Climatic Region. Risk. Anal., 15:459–465.
- Norris, G., YoungPong, S. N., Koenig, J. Q., Larson, T. V., Sheppard, L., and Stout, J. W. (1999). An Association Between Fine Particles and Asthma Emergency Department Visits for Children in Seattle. Environ. Health. Persp., 107:489–493.
- Phillips, T. J., Bloudoff, D. P., Jenkins, P. L., and Stroud, K. R. (1999). Ozone Emissions From a “Personal Air Purifier” 1, 2. J. Expo. Sci. Environ. Epidemiol., 9:594–601.
- Pope, C. A., Burnett, R. T., Thun, M. J., Calle, E. E., Krewski, D., Ito, K., and Thurston, G. D. (2002). Lung Cancer, Cardiopulmonary Mortality, and Long-Term Exposure to Fine Particulate Air Pollution. Jama-j. Am. Med. Assoc., 287:1132–1141.
- Pope, C. A., III, Burnett, R. T., Krewski, D., Jerrett, M., Shi, Y., Calle, E. E., and Thun, M. J. (2009). Cardiovascular Mortality and Exposure to Airborne Fine Particulate Matter and Cigarette Smoke Shape of the Exposure-Response Relationship. Circulation, 120:941–948.
- Singer, B. C., Coleman, B. K., Destaillats, H., Hodgson, A. T., Lunden, M. M., Weschler, C. J., and Nazaroff, W. W. (2006). Indoor Secondary Pollutants from Cleaning Product and Air Freshener Use in the Presence of Ozone. Atmos. Environ., 40:6696–6710.
- Wainman, T., Zhang, J., Weschler, C. J., and Lioy, P. J. (2000). Ozone and Limonene in Indoor Air: A Source of Submicron Particle Exposure. Environ. Health. Persp., 108:1139.
- Waring, M., and Siegel, J. (2011). The Effect of an Ion Generator on Indoor Air Quality in a Residential Room. Indoor Air, 21:267–276.
- Waring, M. S., Siegel, J. A., and Corsi, R. L. (2008). Ultrafine Particle Removal and Generation by Portable Air Cleaners. Atmos. Environ., 42:5003–5014.
- Weschler, C. J., Hodgson, A. T., and Wooley, J. D. (1992). Indoor Chemistry: Ozone, Volatile Organic Compounds, and Carpets. Environ. Sci. Technol., 26:2371–2377.
- Weschler, C. J., and Shields, H. C. (1999). Indoor Ozone/Terpene Reactions as a Source of Indoor Particles. Atmos. Environ., 33:2301–2312.
- Yagi, S., and Tanaka, M. (1979). Mechanism of Ozone Generation in Air-Fed Ozonisers. J. Phys. D. App.l Phys., 12:1509.
- Yanallah, K., Pontiga, F., Fernandez-Rueda, A., and Castellanos, A. (2009). Experimental Investigation and Numerical Modelling of Positive Corona Discharge: Ozone Generation. J. Phys. D. Appl. Phys., 42:065202.