ABSTRACT
Surgical face masks are commonly used by the general public in indoor environments. However, masks could be contaminated, resulting in secondary microbial infections when they act as touchable fomites. Therefore, we evaluated the ability and durability of a covalently bound antimicrobial surfactant coated onto mask surfaces before use to reduce the bacterial burden upon exposure to aerosols. With regard to bacteria that settled onto the mask surface, this antimicrobial product provided >99.3% efficiency for all three tested bacterial species. In addition, the antimicrobial ability of the coated mask maintained efficacy at least one week after coating. For bioaerosols that came into contact with the mask (103 CFU/m3), the antimicrobial agent reduced the average colony rates by 91.8%, but the rates decreased with increased bioaerosol concentrations. Moreover, regardless of whether the coated mask was processed with the bioaerosol penetration test or the National Institute for Occupational Safety and Health-certified sodium chloride aerosol test, the filtration performance of the surgical mask was not significantly altered. These results demonstrate that this antimicrobial product has a durable inhibitory activity for the reduction of bacterial burdens on masks.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Surgical masks are widely used but are often confused with filtering face piece respirators (FFRs). FFRs are commonly used to protect people from respiratory infections. However, surgical masks are used to prevent airborne droplets produced by the wearer from contaminating the surrounding environment (Rengasamy et al. Citation2009). Both surgical masks and FFRs may be contaminated through continuous bioaerosol exposure, resulting in secondary microbial infections when the devices act as touchable fomites (Heimbuch et al. Citation2014; Williams et al. Citation2014).
Relative to respirators, surgical masks are used more often by the public in indoor environments. This fact is especially true for a pandemic disease outbreak, in which supply shortages of FFRs may occur (Viscusi et al. Citation2011; Heimbuch et al. Citation2014). People may use surgical masks instead of FFRs because of economic reasons or non-availability (Rengasamy et al. Citation2009). Although surgical masks may not be used to reduce the wearer's exposure to small airborne particles (which is the case for FFRs), it is nonetheless possible for bacteria to be deposited on the mask. Luksamijarulkul et al. Citation(2014) indicated that both bacterial and fungal contamination were significantly greater on the exterior layer of used masks than on the interior layer. In addition, the contaminating bacterial load on the exterior layer exhibited a significantly positive correlation with the bacterial concentration in the air. Culturable Mycobacterium tuberculosis has also been recovered from patient surgical masks (Williams et al. Citation2014). These findings suggest a potential infection risk for health care workers who handle used masks.
Recently, the antimicrobial agent Goldshield 5 (GS5; 5% active ingredient; AP Goldshield, LLC, Locust Valley, NY, USA) demonstrated highly reduced bacterial recovery on fabric over a period of 14 days (Baxa et al. Citation2011). A recent study also demonstrated that hospital-acquired infections could be prevented by as much as 5–10% by the application of Goldshield 75 (0.83% active ingredient) (Perez et al. Citation2015). The major component of GS5 is quaternary ammonium salt (3-trihydroxysilyl propyldimethyl-octadecyl ammonium chloride; Si-QAC). Based on these antimicrobial properties, Si-QAC has been applied in the food and medical fields (Windler et al. Citation2013). Material surfaces treated with Si-QAC are coated with octadecyldimethylammonium ions by covalent binding, providing a durable antimicrobial effect that damages microbes that are exposed to the surface. The antimicrobial properties are related to Si-QAC's structure and size, but they primarily depend on the length of long alkyl chains (12–18 carbon atoms) (Ferreira et al. Citation2011; Windler et al. Citation2013). These long alkyl chains can penetrate the cytoplasm membrane of microbes. Consequently, Si-QAC can attract negatively charged microorganisms and irreversibly bind to the bacterial membrane compounds, such as phospholipids and proteins, resulting in the impairment of membrane permeability by long alkyl chains (Maris Citation1995).
For other chemical or physical techniques, they are mostly applied for decontamination after microbial contamination has already occurred. In contrast, GS5 can provide a “pre-decontamination” effect. When GS5 is applied on a clean surgical mask before use, it can protect the material from bacterial contamination. More importantly, this pre-decontamination effect could last for several days after GS5 coating. Currently, GS5 is registered with the US Environmental Protection Agency (USEPA) and has demonstrated effective inhibition toward bacteria, mold, and algae (Baxa et al. Citation2011; Perez et al. Citation2015). Although GS5 has been evaluated on fabric (Baxa et al. Citation2011) and hospital surfaces (Perez et al. Citation2015), it has not been applied to prevent microbial contamination on a mask surface. To date, some decontamination agents, such as benzalkonium, chloride hypochlorite, ethylene oxide, and hydrogen peroxide, have been demonstrated to exhibit promising effects in killing microbes on different filter surfaces. Nonetheless, these techniques may also damage the filter's structural integrity and decrease filtration efficiency (Viscusi et al. Citation2007, Citation2009, Citation2011; Heimbuch et al. Citation2014). In addition, the potential for residual chemicals on the filter surface to threaten human health after decontamination is a concern. Therefore, the evaluation of the decontamination effects of GS5 on surgical face masks is necessary.
The purpose of this study was to evaluate the pre-decontamination effects of GS5 on commonly used surgical face masks in the chamber study. The challenged bacterial aerosols in this study were Acinetobacter baumannii, Enterococcus faecalis, and Staphylococcus aureus. These bacterial species are highly related to nosocomial infection and can cause airborne dispersal and transmission (Hsiao et al. Citation2012; Hsiao et al. Citation2014; Tseng et al. Citation2015). The pre-decontamination effects of GS5 were studied and divided into two aspects. One was to investigate the survival of bacteria deposited as droplet nuclei on the surface of GS5-coated mask layers and the durability of the pre-decontamination effect after GS5 coating. Another was to evaluate the survival of bacterial aerosols that were collected by the GS5-coated mask layers. In addition, the filtration efficiency of surgical face masks against bioaerosols and a sodium chloride (NaCl) aerosol was also evaluated when GS5 was coated on the mask. This test was to determine whether the structural integrity and filtration performance of a surgical mask could be affected by GS5 coating.
2. Materials and methods
2.1. Phase one: Pre-decontamination of settled bacterial particles
2.1.1. Test microorganisms
The antibiotic-sensitive A. baumannii (ATCC 17978), E. faecalis (ATCC 29212), and S. aureus (ATCC 29213) reference strains were purchased from the Bioresource Collection and Research Center (Hsin-Chu, Taiwan). All three active bacterial cultures were inoculated in lysogeny broth (LB) and incubated for 24 h at 37°C. After cultivation, the obtained microbe pellets were aseptically washed with sterile phosphate-buffered saline (PBS) and transferred to a sterile 15-mL conical centrifuge tube, which was capped and centrifuged (500 × g, 5 min). Finally, the supernatant was discarded, and the pellets were resuspended in sterile distilled water for the preparation of spray suspensions.
2.1.2. Surgical mask selection and GS5 coating
The single model of surgical mask (AERO PRO, Shuenn Bao Shing Co., Chang Hua, Taiwan) was used in this study. This surgical mask is commercially available and commonly used by health care workers. It has also been cleared by the United States Food and Drug Administration (FDA) as a medical device. The surgical mask comprised three layers: an inner hydrophilic layer, a middle filter layer with electrostatic charge, and an outer hydrophobic layer. In the decontamination test for deposited bacteria, GS5 was diluted in sterile water for application at a concentration of 1% active ingredient. Subsequently, the three layers of the mask were individually investigated and coated with diluted GS5 using a common spray bottle. Briefly, we cut the individual mask layer into 10-cm2 squares and applied four sprays of GS5 on its surface. The distance between the spray bottle and mask layer was 10 cm to ensure that GS5 suspensions were directly sprayed onto the mask layer. The applied volume of GS5 was approximately 1 mL on each mask layer, which was confirmed by weighing the spray bottle before and after applying four sprays. Therefore, the total amount of 1% Goldshield product on the mask layer should be approximately 0.1 mL/cm2.
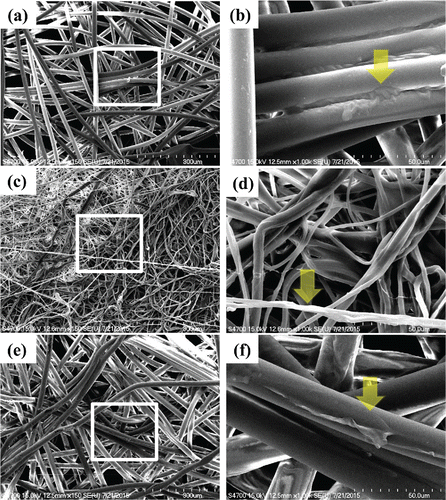
After GS5 coating, one sample of the three coated layers was processed for scanning electron microscopy (SEM) observation. Since it is unlikely that people will wear a wet surgical mask, the GS5-coated mask layer was allowed to completely dry for 24 h. The SEM picture from demonstrates that there was a white and opaque GS5 layer adhered to the filter layers of the mask. Next, durability tests were conducted in which the masks were subjected to the decontamination test at various periods (1, 2, 4, or 8 days) after the application of GS5. To obtain the survival rate of the deposited bacteria, the sterile water-coated masks served as controls and were treated under the same decontamination tests within the same test periods.
Figure 1. The exterior (a, b), internal (c, d), and interior (e, f) layers of the surgical mask were individually coated with GS5 using a common spray bottle and then processed for SEM observation. Representative SEM images are shown of the filter structure of GS5-coated layers, given four sprays (approximately 1 mL) of 1% GS5 on the surface. The left figures (a, c, and e) were taken under a magnification of 150×. The white boxes in (a), (c), and (e) were then magnified by 1000× (b, d, and f), and arrows indicate that there was a white and opaque GS5 layer adhered to the filter layers of the mask.
2.1.3. Aerosol preparation and test system
The aerosol test system in this study was modified from the American Society for Testing and Materials (ASTM) method 2721-10 to simulate aerosol deposition of bacteria onto surfaces (). The test chamber was constructed of acrylic plastic and had a volume of 24.3 L (27 × 30 × 30 cm). A Collison three-jet nebulizer (BGI Collison Nebulizer, BGI Inc., Waltham, MA) was used to nebulize the A. baumannii, E. faecalis, and S. aureus stock in deionized water at 3 L/min with dry, filtered, and compressed laboratory air. The three bacterial suspensions in the nebulizer were approximately 108 colony forming units (CFU)/mL (coefficient of variation (CV) = 4.0%) in each experiment. The stream of aerosolized bacterial particles toward the test chamber inlet was combined with a downward injection of entrance air (flow rate = 50 L/min) comprising a mixture of dry and humid air. This combination resulted in an aerosol residence time of 28 s. In addition, GS5 is positively charged, and the antimicrobial effect is mostly based on ionic bonds and electrostatic interaction. Therefore, non-neutralized bioaerosols were generated to evaluate the antimicrobial effect of GS5 for inactivating Gram-positive and Gram-negative bacteria with different levels of negative charge.
Figure 2. Schematic diagram of the bioaerosol test system for (a) decontamination of settled bacterial particles (phase one study), and (b) decontamination of bacterial aerosols that remained on the mask surface, and the filtration performance test (phase two study). The distance from the surface of the test mask to the airflow inlet of the chamber is 60 cm in the phase one study and 30 cm in the phase two study.
The generated bacterial aerosols were monitored by an AGI-30 impinger in the chamber to ensure that the three bacterial aerosols were maintained at 108 CFU/m3 and would be stable for at least 90 min (CV = 16%). In reality, it is nearly impossible to obtain such an extremely high bioaerosol concentration in an indoor environment. However, this high concentration was appropriate to challenge GS5 against settled bacteria. Decontamination was evaluated under a relative humidity (RH) of 55%. To maintain RH in the test chamber, a humidified gas stream was generated by passing pure compressed air through a humidity saturator. The water vapor content in the gas stream was adjusted by changing the flow rate ratio of the humidified gas stream to the dry gas stream, and the actual RH in the chamber was monitored by a hygrometer (; Rotronic AG, Bassersdorf, Switzerland). In addition, a 6-STG impactor (Andersen Samplers Inc., Atlanta, GA, USA) was used to determine the size distributions of culturable bacteria with size classes of 0.65–1.1, 1.1–2.1, 2.1–3.3, 3.3–4.7, 4.7–7.0, and >7.0 μm at a flow rate of 28.3 L/min (Andersen Samplers Inc.).
2.1.4. Pre-decontamination tests for settled bacteria
Based on the aerosol test system, the airflow inside the chamber permitted the three bacterial aerosols to settle directly onto the GS5-coated mask and the control mask. Therefore, the GS5-coated masks and control masks were directly exposed to the three settled aerosols for 60 min. After aerosol exposure, the individual mask layer was quickly eluted by rinsing with 5 mL of sterile deionized water (within 5 min) that contained 0.07% (wt/vol) Lecithin (Sigma, St. Louis, MO, USA) and then inoculated separately onto LB agar. Lecithin was used to neutralize quaternary ammonia compounds. Finally, the culturable cell counts from the mask layer were calculated based on the dilution ratio and plated volume. Finally, the log reduction in colony counts was calculated as the log10 of Nt/N0, where N0 is the number of bacterial colonies recovered on the control mask, and Nt is the number of colonies on the GS5-coated mask.
2.2. Phase two: Pre-decontamination of bacterial aerosols that remained on the mask surface
2.2.1. Test microorganisms, surgical mask selection, and GS5 coating
In this phase of study, the target microorganisms, surgical mask type, and GS5 coating method were the same as the decontamination test for settled bacterial particles. However, the evaluated area of the individual mask layer was 81 cm2 (9 × 9 cm), and the masks were not tested at various periods after the application of GS5 (durability test).
2.2.2. Aerosol preparation and test system
The aerosol test system was basically the same as the phase one study (). However, the three bacterial suspensions in the nebulizer were adjusted to approximately 103 and 104 CFU/mL (CV = 3.0%). Consequently, three bacterial aerosols could be generated at different concentrations of 103 and 104 CFU/m3 in each experiment to simulate high bioaerosol concentration in indoor environments (CV = 5.0%). According to filter test regulations, filter certification for particles is typically conducted at a rate of 85 or 95 L/min, which corresponds to a high physical workload (Janssen et al. Citation2005). This is based on the air flow rate of 95 L/min and the commercial surgical mask area of approximately 167 cm2, which corresponds to a filtration velocity of 9.5 cm/s. Therefore, we chose a facial velocity of 9.5 cm/s to conduct decontamination and penetration tests. This facial velocity could be reached by conducting an air flow rate of 46 L/min in our chamber with a mask filtration area of 81 cm2. The bacterial aerosol concentrations of 103 and 104 CFU/m3 in the chamber with a total air flow rate of 46 L/min were monitored by an AGI-30 impinger to ensure that three bacterial aerosols were stable for at least 90 min (CV = 15%). For both decontamination and bioaerosol filtration tests, the three individual mask layers were challenged with the three non-neutralized bioaerosols at an RH of 55%.
2.2.3. Pre-decontamination test for bacterial aerosols that contact the mask surface
For the decontamination test, the GS5-coated masks and control masks were directly exposed to the three aerosolized bacteria for 60 min. Therefore, those bacteria that did not penetrate the mask layer might be exposed to 1% GS5 when they were collected by the filter media. The mask surface sampling was conducted with replicate organism detection and counting (RODAC) plates (Becton and Dickinson, Lincoln Park, NJ, USA), which contain an area of approximately 23.7 cm2. In a phase two study, the test concentrations of the bacterial aerosols were 103 and 104 CFU/m3, which were much lower than the concentration used in the phase one study (108 CFU/m3). If the mask layer (81 cm2) was cut into small pieces to recover the bacteria as in the phase one study process, the colony recovery would be low. Therefore, the RODAC plate method was used instead of eluting bacteria from the filter layer with water. The RODAC plates were used to sample the side of the mask layer that was on the side of the chamber air, which was treated as the front of the mask layer (the same side as the coated GS5). While sampling, we held the RODAC plate to press plate bottom firmly against the surface of the mask layer. We attempted to use the same amount of pressure for every mask sample and attempted to keep the plate laterally immobile. After sampling, the plates were removed from the layer surface and incubated for 24 h at 37°C. Their survival rates were determined by colony counts on the GS5-coated mask (Nt) against colony counts on the control mask (N0) by agar-contact surface sampling. Based on the limitations of the RODAC plate method, only bacteria deposited on the mask surface were evaluated. The colony reduction efficiency of bacteria that remained on the mask surface was defined as follows:
2.2.4. Determination of filtration performance for bioaerosols
The penetration rate of the bioaerosols was calculated as the ratio of Cf/Cin, where Cf (CFU/m3) is the concentration with a mask filter operation, and Cin (CFU/m3) is the concentration without a mask filter operation. Finally, the filtration efficiency of the mask was defined as follows:
The test mask layer was coated by GS5, and the untreated or sterilized water coating layer served as controls for efficiency comparison. All concentration measurements were sampled downstream of the mask filter by the Andersen 1-STG impactor (Andersen Samplers Inc., Atlanta, GA, USA).
2.2.5. Determination of filtration performance for NaCl aerosol
In this test, the mask was no longer separated into three individual layers. We evaluated the filtration performance of whole masks and the masks with GS5 coated only on the most exterior side to simulate normal wear conditions. The GS5 coating method was the same as the previous tests, and the mask was allowed to completely dry for 24 h. After drying (the first day after coating), a Model 8130 Automated Filter Tester (AFT) (TSI Inc., St. Paul, MN, USA) was used to measure the initial percentage filter aerosol penetration for all of the GS5-coated and control masks. The experimental system for the mask penetration test was the same as in a previous study (Huang et al. Citation2005). The neutralized NaCl aerosol was generated first in a mixing chamber, and passed through the filter holder. AFT delivers a polydispersed NaCl aerosol, and all the tests were conducted at room temperature with two continuous airflows of 30 and 85 L/min, in accordance with National Institute for Occupational Safety and Health (NIOSH) Citation(2007) certification. The median diameter of the neutralized NaCl aerosol was 0.075 μm, the mass mean diameter was 0.26 μm, and the geometric standard deviation was less than 1.86. Under high humidity, some filter materials, such as electret filters, may exhibit low filtration efficiency (Motyl and Lowkis Citation2006). Since GS5 was diluted in sterile water, we used water-coated masks instead of untreated masks as control masks to exclude the possible effects of water coating.
2.3. Statistical analysis
Since the sample sizes in both phase one (N = 108) and phase two studies (N = 240) were larger than 50, we used the Kolmogorov–Smirnov test to determine whether the sample data were normally distributed. After statistical analysis, non-parametric tests were conducted for the data analysis because the probability associated with the Kolmogorov–Smirnov test of normality is <0.05. In the phase one study, differences in the level of colony reduction between Gram-positive and Gram-negative bacteria were determined using the Mann–Whitney–Wilcoxon test. The differences in colony reduction among the three layers of the mask were compared using the Kruskal–Wallis test. The same statistical method was also used for the durability test, followed by Dunn's test of multiple comparisons to evaluate statistically significant differences (p < 0.05). In the phase two study, the Kruskal–Wallis test was used to compare (1) differences in the colony reduction rates among the three bacterial species; (2) colony reduction rates among the three mask layers; (3) the filtration efficiency among the three species; and (4) the filtration efficiency by three different coating processes. Finally, the filtration efficiency between two different bacterial concentrations of 103 and 104 CFU/m3 was assessed by the Mann–Whitney–Wilcoxon test.
3. Results
3.1. Phase one: Pre-decontamination of settled bacterial particles
3.1.1. Characteristics of aerosolized bacteria
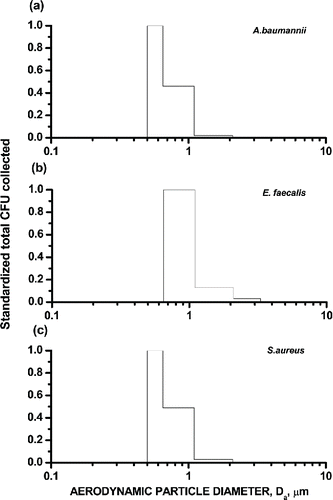
The particle size distributions of culturable aerosols are presented in . The culturable aerosols of A. baumannii, E. faecalis, and S. aureus were primarily collected in Stages 5 and 6 in the 6-STG impactor sampler. More than 95% of the recovered CFU colonies were less than 2.1 μm in diameter.
3.1.2. Pre-decontamination test for settled bacteria
The bacterial colonies that had settled on different layers of control mask ranged from 1.0 × 103 to 3.0 × 105 colonies per layer. Overall, there were no bacterial colonies recovered from the GS5-coated mask (Nt = 0) under all test conditions in the phase one study, no matter which bacterial species was evaluated. However, to quantify detection limit for reduction efficiency (R%), we used the detection limit of our culture assay (5 CFU/cm2) and divided by the average colony counts that were recovered from the three control layers. The detection limit of R varied from 99.3 to 99.9%, depending on the counts recovered from the control layers. Therefore, it can be concluded that the colony reduction was >99.3% for all tested microorganisms and for all three mask layers. In addition, the antimicrobial ability of the coated mask maintained efficacy for at least one week after coating.
3.2. Phase two: Pre-decontamination of bacterial aerosols that remained on the mask surface
3.2.1. Pre-decontamination test for bacterial aerosols that contacted mask surface
shows different GS5-coated mask layers that were challenged by three bioaerosols with two concentrations of 103 CFU/m3 and 104 CFU/m3. There was no significant difference in colony reduction efficiencies among A. baumannii (89.1%), E. faecalis (89.8%), and S. aureus (87.8%; p = 0.768). In addition, the colony reduction rates among the three mask layers also demonstrated no significant difference (87.1% vs. 89.9% vs. 89.8%; p = 0.368). However, the colony reduction rates of GS5-coated masks exposed to aerosol concentrations of 103 CFU/m3 (91.8%) were higher than those of 104 CFU/m3 (86.0%; p = 0.006).
Table 1. Phase two testing for the pre-decontamination efficacy of GS5-coated mask layers against the three challenge bioaerosols.
3.2.2. Determination of filtration performance of the exterior layer exposed to bioaerosols
In the phase two study, the filtration efficiency of the exterior layer (furthest from the wearer) of the mask after different coating processes (untreated, water, and GS5 coating) was evaluated for three challenge aerosols (). The exterior hydrophobic layer of the mask is not primarily used for filtration. Therefore, the filtration efficiencies were not satisfactory and ranged from 42.3 to 69.3%. The filtration efficiency of the exterior layer was not significantly different among A. baumannii (52%), E. faecalis (47.3%), and S. aureus (51.4%; p = 0.135). However, there was a significant difference in the filtration efficiency between two different bacterial concentrations of 103 and 104 CFU/m3 (46.9 vs. 53.5%; p = 0.009). Interestingly, the efficiency of the GS5-coated layer (56.6%) was significantly higher than the untreated (47.2%; p = 0.006) and water-coated layers (46.8%; p = 0.005).
Table 2. Phase two testing for the filtration efficiency of the exterior layers of masks by different coating processes.
3.2.3. Determination of filtration performance of the internal layer exposed to bioaerosols
The internal filter layer is the major filtration part of a surgical mask, and the filtration efficiencies (from 96.2 to 99.9%) were much higher than the exterior and interior layers (). Furthermore, for the internal layer of the mask, the filtration efficiencies among A. baumannii (98.4%), E. faecalis (98.9%), and S. aureus (98.5%) were not significantly different (p = 0.247). In addition, no significant difference was found in filtration efficiency between two different bacterial concentrations of 103 and 104 CFU/m3 (98.4 vs. 98.9%; p = 0.128). Nevertheless, although there was a slight increase in the efficiency of the GS5 coating layers (99.5%), the efficiency was not significantly higher than the efficiencies of the untreated (98.1%; p = 0.525) and water-coated layers (98.2%; p = 0.532).
Table 3. Phase two testing for the filtration efficiency of the internal layers of masks with different coating processes.
3.2.4. Determination of filtration performance of the interior layer for bioaerosols
The interior hydrophilic layer of the mask is worn closest to the skin and has been designed to absorb moisture. Therefore, it is not primarily used for filtration, and its filtration efficiency was similar to the exterior layer from 40.1 to 65.4% (). Similar to the previous two layers of the mask, there was no significant difference in the filtration efficiency among A. baumannii (52.9%), E. faecalis (48.9%), and S. aureus (53.1%; p = 0.129). However, when the higher bioaerosol concentration (104 CFU/m3) was conducted, the filtration efficiency (56.2%) was significantly higher than tests with a lower concentration of 103 CFU/m3 (47%; p = 0.011). Using the GS5 coating, the interior layer of the mask (57.8%) was significantly higher than the untreated (47.3%; p = 0.005) and water-coated layers (49.8%; p = 0.02).
Table 4. Phase two testing for the filtration efficiency of the interior layers of masks with different coating processes.
3.2.5. Filtration performance between sterile water- and GS5-coated whole masks for NaCl aerosol exposure
shows the filtration performance of the whole mask for NaCl aerosols when only the exterior layer was coated with sterile water or GS5. Based on the two air flow rates of 30 and 85 L/min, there was no significant difference in mask resistance between water- and GS5-coated surgical masks (p = 0.130). Furthermore, there was also no significant difference in mask filtration between water- and GS5-coated surgical masks (p = 0.442). However, regardless of coating material, there was a significant increase in both mask resistance and mask filtration (p < 0.001) when the air flow rate was increased from 30 to 85 L/min.
Table 5. The filtration performance of whole sterile water- and GS5-coated masks exposed to NaCl aerosol.
4. Discussion
The size distributions of the three test bacterial aerosols generated in our chamber were similar to those of the bacterial aerosols that included vegetative Escherichia coli, Legionella pneumophila, and Bacillus subtilis endospores, with a GM of 0.7–0.9 μm and a GSD of 1.2 (Li Citation1999; Li et al. Citation2003). The size range distribution of the three bacterial aerosols demonstrated that they were small droplet nuclei, and the filtration performance of the surgical masks with or without GS5 coating should be highly related to these size ranges.
The bacterial loads deposited on the masks from the air in the phase one study (108 CFU/m3) represented a worst-case scenario to evaluate the germicidal effect of GS5-coated masks. The results in the phase one study demonstrated that masks with the GS5 coating can inactivate settled bacteria and showed a log reduction from 2.32 to 5.02. These results were close to some physical methods used to inactivate H1N1 influenza virus on FFRs. For example, the least log reduction for inactivating H1N1 virus on N95 FFRs was 3.36 for ultraviolet germicidal irradiation (UVGI), 3.29 for microwave-generated steam, and 2.96 for warm moist heat (Heimbuch et al. Citation2011). In addition to these physical methods, Heimbuch et al. Citation(2014) also used a commercial cleaning wipe containing benzalkonium chloride (BAC) to clean N95 FFRs after the N95 respirator was contaminated by S. aureus. BAC is also a quaternary ammonium compound, and the colony reduction rate caused by GS5 was similar to that caused by wiping with BAC (99.72% to >99.998%). However, it is worth noting that our GS5 was applied onto the mask layer before wearing rather than cleaning it after the bacterial contamination had occurred. This process may be more meaningful in decreasing the infection risk by touching a contaminated mask surface. More importantly, the durability of the GS5 decontamination effect will last for at least one week after GS5 has been coated onto the surface of the mask layer. These findings could help reduce cleaning costs and increase feasibility.
For the aerosol challenge in the phase two study, the colony reduction rates for all of the tested bacteria ranged from 83.5 to 95.8%. The reduction rates were significantly lower than those in the phase one study and were similar to the reduction rate when using a commercial Pampers wipe for cleaning the FFR after contamination by S. aureus deposition (81.56 to 96.53%) (Heimbuch et al. Citation2014). However, the reduction rates were much lower than those using physical methods for cleaning H1N1 viral aerosols by a similar test (3.32 to 6.58 log reduction) (Heimbuch et al. Citation2011). The difference in the reduction rate may be attributed to different microbial species, different cleaning methods, and different cleaning timing. Nevertheless, it seems that the GS5-coated mask layer would kill the surface-deposited bacteria more easily than killing the bacterial aerosols that came into the mask. This phenomenon may be related to our GS5 coating method. We simply used a bottle for spraying GS5 onto the mask layer because GS5 is readily available, and this method can be easily handled by the public. However, it is known that bacteria may become stuck in the crevices of masks after aerosol challenge, and GS5 could not completely penetrate these shadowed areas using this simplified coating method. Therefore, if a higher reduction rate is required for preventing infections, further evaluation of coating methods is necessary based on methods similar to how Si-QAC is applied on textile materials (Windler et al. Citation2013).
When GS5 was applied to inactivate the higher concentrations of bacteria on the mask layer (the phase one study), a greater significant reduction in Gram-positive E. faecalis and S. aureus was observed in comparison with Gram-negative A. baumannii. These results also agree with a previous study that used higher bacterial concentrations (Baxa et al. Citation2011). The quaternary ammonium groups are positively charged polymers that may trap bacteria and disrupt the cell membrane. Gram-positive bacteria have more peptidoglycan than Gram-negative bacteria. Since peptidoglycan is negatively charged, more quaternary ammonium groups of Si-QAC may become trapped by peptidoglycan in Gram-positive bacteria than in Gram-negative bacteria, resulting in membrane damage (Kawahara et al. Citation2000). However, this finding was not observed when lower bacteria concentrations were used (the phase two study).
According to the current standard ASTM F2100-07 (2007), the bacterial filtration efficiency (BFE) is an indicator of the ability of a mask to block large particles expelled by the wearer. For the basic requirement of a medical/surgical mask, a minimum of 95% BFE is required for S. aureus-containing aerosols with a droplet size of 3.0 μm. The levels from moderate to high protection masks are required to achieve a BFE of 98% and more than 99%. Based on the filtration efficiency of our internal layer for culturable S. aureus with 0.78 μm (96.2–99.9%), our evaluated mask was speculated to meet the basic requirements of a medical/surgical mask. If we combined three layers and evaluated the larger droplet size of 3.0 μm, the evaluated mask should also meet the BFE level from moderate to high. This speculation agreed with the mask manufacturer's claim that the surgical mask tested in this study is 99% for BFE.
For the three tested bacteria, there was no significant difference in the filtration performance because their size distributions were very close. The difference in the filtration performance between the two different bioaerosol concentrations (103 and 104 CFU/m3) was only observed in the exterior and interior layers of the mask. In comparison with the internal layer, the exterior and interior layers are not the major filtration parts of the mask. The filtration pore size of these two layers was large and results in varied filtration efficiencies. In addition, the varied filtration efficiency of surgical masks also related to different manufacturers and different test models, such as different simulated workplace tests, and challenges by monodisperse or polydisperse aerosols (Lawrence et al. Citation2006; Rengasamy et al. Citation2009). Interestingly, if the exterior and interior layers were coated with GS5, the filtration efficiency would increase significantly.
Since GS5 is a quaternary ammonium compound, it is likely that its positive charge interacts with the mask layer and helps to collect bacteria with a negative charge. However, this finding was only observed in the exterior and interior layers and was in contrast to another study (Heimbuch et al. Citation2014). Following BAC wipe cleaning, the N95 FFR exceeded 5% penetration. These differences may be related to different filter materials used for N95 FFR and surgical mask production. The nature of filter materials for N95 FFR is an electret filter that differs from surgical masks that rely on mechanical filters. Compared with mechanical filters, electret filters with electrically charged fibers can improve the collection efficiency of sub-micrometer particles (Janssen et al. Citation2003). However, the drawback of electret filters is that they may lose electret charges and the filtration efficiency may degrade under high humidity (Motyl and Lowkis Citation2006) or when exposed to some chemical compounds, such as nonionic detergent and NaCl (Moyer and Bergman Citation2000; Heimbuch et al. Citation2014). Therefore, the application of quaternary ammonium compounds on FFRs may decrease the filtration efficiency, but the effect on surgical masks was not obvious.
Unlike respirators, surgical masks are not necessarily subjected to filter efficiency testing approved by NIOSH. Other than the bioaerosol test, our study also used NIOSH filter certification testing to evaluate whether the surgical mask could result in the physical degradation of mask and affect filtration efficiency after GS5 coating. Clearly, the physical degradation of surgical masks after GS5 coating appeared to be negligible in both resistance and filtration tests. The filtration performance of our test mask at 85 L/min (77.6–79.4%) was also observed to be superior to the average filtration performance of 37.6% from nine different surgical masks in NaCl aerosol tests. Over 78% (7/9) of the masks showed a filtration performance below 77.2% (Oberg and Brosseau Citation2008).
Unlike FFRs, there is no issue regarding reusing a surgical mask. However, there are issues that must be addressed before the application of chemicals on filter materials, including the chemical's efficacy in killing microbes and maintaining filtration performance and safety for human health. Our study proposes use of a quaternary ammonium compound; GS5 can be coated on a commercially available surgical mask before use and might protect the mask from bacterial contamination while maintaining filtration performance. The advantages of the GS5 coating process are that it is convenient, and the problem of physical degradation of the mask is eliminated. From a health perspective, although Si-QAC is a corrosive chemical, the USEPA Citation(2007) does not consider that it could cause severe effects on human health. In addition, GS5 has been tested for cytotoxicity using the International Organization for Standardization's agarose overlay method, and the results demonstrated that the toxicity of GS5 is of grade 1 (slight reactivity) (Baxa et al. Citation2011). However, Paris et al. Citation(2012) suggested that quaternary ammonium compounds may contribute to work-related asthma. In addition, quaternary ammonium compounds may also be associated with dermatitis, which could be an issue if people wear a mask coated with these compounds (Lodde et al. Citation2012). Although these two studies did not report concentration in the working solution or how the workers applied these compounds in detail, these health issues warrant further study.
Our study has some limitations. First, to test each layer of the mask, we only evaluated one type of mask. If GS5 were to be applied to different types of masks or even FFRs, more studies would be needed before such a practice can be recommended. Second, our study did not utilize different nutrient compounds in the nebulizer to simulate bacteria-containing aerosols released from human respiratory secretions. Some compounds, such as protein or mucin released from human breath condensate, could increase shielding and decrease the susceptibility of bacteria to GS5. Finally, the GS5 coating method in this study can be easily applied by the public, but if higher reduction rates are needed, a strong fixation of GS5 to the filter fiber is desirable to improve the efficacy and durability of the antimicrobial agent.
5. Conclusions
People wear surgical face masks as a basic and simple strategy for preventing the spread of infectious aerosols. However, while a surgical mask can capture bioaerosols, it cannot inactivate them. There is an immediate need to develop a proper decontamination method for surgical masks to reduce secondary microbial infections from virulent bacterial strains. Our study demonstrates that a quaternary ammonium compound, GS5, can be coated onto the filter of surgical layers and provide a durable antimicrobial effect when bacterial aerosols settle down or penetrate the mask. Therefore, this compound may have the potential to be applied on surgical masks and used against organisms that can cause infection. Whether GS5 is appropriate to be coated with FFRs is worthy of additional study. In addition, other quaternary ammonium compounds, such as BAC, are different from GS5 and could result in physical damage of the mask. Therefore, not all quaternary ammonium compounds are suggested for use during coating surgical masks or FFR, and it still needs further assessment before application.
Acknowledgments
The authors appreciate the technical assistance received from personnel at the Electron Microscopy Laboratory at Tzu Chi University.
Funding
This work was supported by the MOST 103-2314-B-320-003-MY2 grant from the Ministry of Science and Technology, Republic of China.
References
- Baxa, D., Shetron-Rama, L., Golembieski, M., Golembieski, M., Jain, S., Gordon, M., and Zervos, M. (2011). In Vitro Evaluation of a Novel Process for Reducing Bacterial Contamination of Environmental Surfaces. Am. J. Infect. Control, 39:483–487.
- Ferreira, C., Pereira, A., Pereira, M., Melo, L., and Simões, M. (2011). Physiological Changes Induced by the Quaternary Ammonium Compound Benzyldimethyldodecylammonium Chloride on Pseudomonas Fluorescens. J. Antimicrob. Chemoth., 66:1036–1043.
- Heimbuch, B. K., Kinney, K., Lumley, A. E., Harnish, D. A., Bergman, M., and Wander, J. D. (2014). Cleaning of Filtering Facepiece Respirators Contaminated with Mucin and Staphylococcus Aureus. Am. J. Infect. Control, 42:265–270.
- Heimbuch, B. K., Wallace, W. H., Kinney, K., Lumley, A. E., Wu, C.-Y., Woo, M.-H., and Wander, J. D. (2011). A Pandemic Influenza Preparedness Study: Use of Energetic Methods to Decontaminate Filtering Facepiece Respirators Contaminated with H1N1 Aerosols and Droplets. Am. J. Infect. Control, 39:e1–e9.
- Hsiao, P. K., Chen, W. T., Chang, K. C., Ke, Y. J., Kuo, C. L., and Tseng, C. C. (2012). Performance of CHROMagar Staph Aureus and CHROMagar MRSA for Detection of Airborne Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus. Aerosol Sci. Tech., 46:297–308.
- Hsiao, P. K., Cheng, C. C., Chang, K. C., Yiin, L. M., Hsieh, C. J., and Tseng, C. C. (2014). Performance of CHROMagar VRE Medium for the Detection of Airborne Vancomycin-Resistant/Sensitive Enterococcus Species. Aerosol Sci. Tech., 48:173–183.
- Huang, S. H., Chen, C. W., and Chang, C. P. (2005). Performance of Commercial Functional Masks. J. Occup. Safe Health, 13:70–77.
- Janssen, L., Anderson, N., Cassidy, P., Weber, R., and Nelson, T. (2005). Interpretation of Inhalation Airflow Measurements for Respirator Design and Testing. J. Int. Soc. Respir. Protect., 22:122.
- Janssen, L. L., Bidwell, J. O., Mullins, H. E., and Nelson, T. J. (2003). Efficiency of Degraded Electret Filters: Part I. Laboratory Testing against NaCl and Dop before and After Exposure to Workplace Aerosols. J. Int. Soc. Respir. Protect., 20:71–80.
- Kawahara, K., Tsuruda, K., Morishita, M., and Uchida, M. (2000). Antibacterial Effect of Silver-Zeolite on Oral Bacteria under Anaerobic Conditions. Dent. Mater., 16:452–455.
- Lawrence, R. B., Duling, M. G., Calvert, C. A., and Coffey, C. C. (2006). Comparison of Performance of Three Different Types of Respiratory Protection Devices. J. Occup. Environ. Hyg., 3:465–474.
- Li, C. S. (1999). Evaluation of Microbial Samplers for Bacterial Microorganisms. Aerosol Sci. Tech., 30:100–108.
- Li, C. S., Tseng, C. C., Lai, H. H., and Chang, C. W. (2003). Ultraviolet Germicidal Irradiation and Titanium Dioxide Photocatalyst for Controlling Legionella Pneumophila. Aerosol Sci. Tech., 37:961–966.
- Lodde, B., Paul, M., Roguedas-Contios, A. M., Eniafe-Eveillard, M. O., Misery, L., and Dewitte, J. D. (2012). Occupational Dermatitis in Workers Exposed to Detergents, Disinfectants, and Antiseptics. Skinmed, 10:144–150.
- Luksamijarulkul, P., Aiempradit, N., and Vatanasomboon, P. (2014). Microbial Contamination on Used Surgical Masks among Hospital Personnel and Microbial Air Quality in Their Working Wards: A Hospital in Bangkok. Oman. Med. J., 29:346–350.
- Maris, P. (1995). Modes of Action of Disinfectants. Rev. Sci. Tech. OIE, 14:47–55.
- Motyl, E., and Lowkis, B. (2006). Effect of Air Humidity on Charge Decay and Lifetime of Pp Electret Nonwovens. Fibres Textiles East. Eur., 14:39–42.
- Moyer, E. S., and Bergman, M. (2000). Electrostatic N-95 Respirator Filter Media Efficiency Degradation Resulting from Intermittent Sodium Chloride Aerosol Exposure. Appl. Occup. Environ. Hyg., 15:600–608.
- National Institute for Occupational Safety and Health (NIOSH). (2007). Determination of Particulate Filter Efficiency Level for N95 Series Filters against Solid Particulates for Non-Powered, Air-Purifying Respirators. Procedure Tebapr-Stp-0059. National Personal Protective Technology Laboratory, NIOSH, Pittsburgh, PA.
- Oberg, T., and Brosseau, L. M. (2008). Surgical Mask Filter and Fit Performance. Am. J. Infect. Control, 36:276–282.
- Paris, C., Ngatchou-Wandji, J., Luc, A., McNamee, R., Bensefa-Colas, L., Larabi, L., Telle-Lamberton, M., Herin, F., Bergeret, A., Bonneterre, V., Brochard, P., Choudat, D., Dupas, D., Garnier, R., Pairon, J. C., Agius, R. M., and Ameille, J. (2012). Work-Related Asthma in France: Recent Trends for the Period 2001–2009. Occup. Environ. Med., 69:391–397.
- Perez, V., Mena, K. D., Watson, H. N., Prater, R. B., and McIntyre, J. L. (In press). Evaluation and Quantitative Microbial Risk Assessment of a Unique Antimicrobial Agent for Hospital Surface Treatment. Am. J. Infect. Control. doi:10.1016/j.ajic.2015.1006.1013.
- Rengasamy, S., Miller, A., Eimer, B. C., and Shaffer, R. E. (2009). Filtration Performance of FDA-Cleared Surgical Masks. J. Int. Soc. Respir. Protect., 26:54.
- Tseng, C. C., Hsiao, P. K., Chang, K. C., Cheng, C. C., Yiin, L. M., and Hsieh, C. J. (2015). Detection of Viable Antibiotic‐Resistant/Sensitive Acinetobacter Baumannii in Indoor Air by Propidium Monoazide Quantitative Polymerase Chain Reaction. Indoor Air, 25:475–487.
- US Environmental Protection Agency (USEPA). (2007). Reregistration Eligibility Decision for Trimethoxysilyl Quaternary Ammonium Chloride Compounds. USEPA, Atlanta, GA.
- Viscusi, D. J., Bergman, M. S., Eimer, B. C., and Shaffer, R. E. (2009). Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg., 53:815–827.
- Viscusi, D. J., Bergman, M. S., Novak, D. A., Faulkner, K. A., Palmiero, A., Powell, J., and Shaffer, R. E. (2011). Impact of Three Biological Decontamination Methods on Filtering Facepiece Respirator Fit, Odor, Comfort, and Donning Ease. J. Occup. Environ. Hyg., 8:426–436.
- Viscusi, D., King, W., and Shaffer, R. (2007). Effect of Decontamination on the Filtration Efficiency of Two Filtering Facepiece Respirator Models. Int. Soc. Respir. Protect., 24:93–107.
- Williams, C. M., Cheah, E. S., Malkin, J., Patel, H., Otu, J., Mlaga, K., Sutherland, J. S., Antonio, M., Perera, N., Woltmann, G., Haldar, P., Garton, N. J., and Barer, M. R. (2014). Face Mask Sampling for the Detection of Mycobacterium Tuberculosis in Expelled Aerosols. PLoS One, 9:e104921.
- Windler, L., Height, M., and Nowack, B. (2013). Comparative Evaluation of Antimicrobials for Textile Applications. Environ. Int., 53:62–73.