ABSTRACT
Aerosol instrument characterization and verification for nanometer-sized particles requires well-established generation and classification instruments. A precise size selection of sub-3-nm charged aerosol particles requires a differential mobility analyzer (DMA), specially designed for the sub-3-nm size range. In this study, a Herrmann-type high-resolution DMA developed at Yale University was characterized in various operation conditions. A relation between sheath flow rate and tetraheptylammonium ion (C28H60N+, THA+, 1.47 nm, mobility equivalent diameter) was established. The maximum particle size that the DMA was able to classify was 2.9 nm with the highest sheath flow rate of 1427 liters per minute (Lpm), and 6.5 nm with the lowest stable sheath flow rate of 215 Lpm, restricted by the maximum and minimum flow rates provided by our blower. Resolution and transmission of DMA are reported for tetrapropylammonium (C12H28N+, TPA+, 1.16 nm), THA+, and THA2Br+ (1.78 nm) ions measured with two different central electrodes and five different sheath flow rates. The transmission varied between 0.01 and 0.22, and the resolution varied between 10.8 and 51.9, depending on the operation conditions.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
Introduction
A differential mobility analyzer (DMA) is an instrument that classifies charged aerosol particles based on their electrical mobility. A typical design of a DMA is based on two flows between two cylindrical electrodes, where the inner one is usually at high potential and the outer electrode is grounded. Of the two flows, the smaller one, containing aerosol particles, is directed close to the outer electrode, ideally parallel to the larger sheath flow that flows between the electrodes. Due to the electric field between the electrodes, charged particles entering the DMA from the inlet with the aerosol flow start to drift toward the central electrode according to their electrical mobility. If the central electrode is at a positive potential, the negatively charged particles drift toward the inner electrode, while the positively charged particles are scavenged to the DMA's outer electrode. A small slit is placed at the end of the inner electrode, into which particles of a certain electrical mobility will hit, exiting the instrument as a monodisperse aerosol flow. The particles of higher electrical mobility will hit the central electrode before the slit, whereas the particles of lower electrical mobility will be carried away by the sheath flow (Hewitt Citation1957; Knutson and Whitby Citation1975).
By varying aerosol and sheath flow rates, their ratio, geometry of the electrodes, or electric field strength, one can design a DMA with parameters suitable for a given experiment (Zhang et al. Citation1995; Reischl et al. Citation1997). A theory of cylindrical DMAs is presented by Stolzenburg and McMurry (Citation2008).
Many conventional commercialized cylindrical DMAs are often operated at an aerosol flow rate of 1–4 L min−1 (Lpm) and with a sheath flow rate of 3–30 (Zhang et al. Citation1995; Birmili et al. Citation1997; Chen et al. Citation1998). Another cylindrical DMA with different design, called Vienna or Hauke DMA, was introduced by Winklmayr et al. (Citation1991). The sheath flow of this DMA can reach up to 60 Lpm without turbulence (Reischl et al. Citation1997). Often in the conventional cylindrical DMAs, the sheath flow becomes turbulent at a flow rate of more than 20 Lpm. The turbulent sheath flow will cause particle transmission through DMA even when no voltage is applied to the central electrode. The shape of the transfer function is also changed, which makes it impossible to use DMA, for example, as part of a differential mobility particle sizer system (Aalto et al. Citation2001; Wiedensohler et al. Citation2012).
Detailed research on the smallest, sub-3-nm nanoparticles requires higher sizing resolution than given by the first DMA designs (Keskinen et al. Citation1995; Rosell-Llompart et al. Citation1996; de Juan and Fernández de la Mora Citation1998). By increasing the ratio of aerosol and sheath flow rate in a DMA, the DMA resolution (V/FWHM) will increase (Flagan Citation1999; Rosser and Fernández de la Mora Citation2005). Simultaneously, in low flow DMAs, the transmission will increase due to lower diffusion losses of particles. The transfer function is defined as “the probability that an aerosol particle which enters the mobility analyzer via the aerosol inlet will leave via the sampling flow, given that its mobility is Zp” (Knutson and Whitby Citation1975).
In recent years, effort has been put for designing DMAs that are optimized for sub-5-nm particle mobility classification (Brunelli et al. Citation2009; Santos et al. Citation2009; Steiner et al. Citation2010; Fernández de la Mora Citation2011; Fernández de la Mora and Kozlowski Citation2013). Of the often used high-resolution DMAs (HDMA), the Herrmann type has not been yet characterized, although it has been used for more than 15 years (Seto et al. Citation1997; Gamero-Castano and Fernández de la Mora Citation2002; Nasibulin et al. Citation2008; Asmi et al. Citation2009; Peineke et al. Citation2009; Sipilä et al. Citation2009; Jiang et al. Citation2011; Kuang et al. Citation2012; Kangasluoma et al. Citation2015). Previous literature has reported, for example, the resolving power of 71.4 (Martinez-Lozano and Fernández de la Mora Citation2005) and the size selection range of up to approximately 12 nm (Saucy et al. Citation2004) for the Herrmann-type DMA. The reader should note that the results could be specific only to the characterized DMA due to variations in the design and dimensions of the manufactured Herrmann DMAs.
In this study, our motivation was to fully characterize our Herrmann-type DMA at various operation conditions. We show the limits for the largest selectable particle size with DMA, pressure drop of DMA, the effect of the aerosol flow rate on the THA+ peak voltage, and list the transmission and resolution at various operation conditions. The experiments also show some of the current challenges in generating molecular mobility standards larger than 2 nm in diameter, and how these challenges can affect the instrument characterization.
Experimental
The dimensions of the Herrmann-type DMA used in our study were R1 = 5 mm, R2 = 9 mm, Lshort bullet = 29 mm, and Llong bullet = 43.5 mm. The Herrmann-type DMA was operated in a closed sheath flow loop arrangement (Jokinen and Mäkelä Citation1997) to keep the sheath flow free of laboratory air contaminants such as water vapor (Kangasluoma et al. Citation2013). When the DMA is operated in a closed loop, the aerosol sample can be either pushed to inlet (pushing mode), or drawn out of outlet (drawing mode). When operating the DMA in a pushing mode, the flow upstream of the inner electrode slit will be at pressure higher than the pressure downstream of the slit (usually ambient pressure), whereas in drawing mode the flow downstream of the slit will be at pressure lower than the pressure upstream of the slit (usually ambient pressure). The pressure drop is due to a very narrow slit of the inner electrode in the Herrmann-type DMA, which constrains the flow rate, and should be considered when operating the DMA in systems sensitive to pressure. We measured the pressure drop corresponding to different operation schemes.
Next we established a relation between the sheath flow rate and the voltage to classify tetraheptylammonium bromide positive monomer ions (THA+) (Ude and Fernández de la Mora Citation2005). This ion is often used to convert DMA classifying voltage to electrical equivalent mobility. The voltage at the DMA inner electrode is inversely proportional to the electrical mobility of the classified particle; therefore, the electrical mobilities corresponding to other voltages can be obtained when one voltage–mobility pair is known.
We added a TSI model 4235 mass flow meter capable of measuring flow rates of up to 1000 Lpm to a closed sheath flow loop setup presented in Kangasluoma et al. (Citation2013). The TSI model 4235 mass flow meter was calibrated against three TSI models 40241 operated in parallel. The maximum measurable flow rate of the 40241 model was 300 Lpm, which gave a flow calibration of up to 900 Lpm for the model 4235. The voltage required to classify the THA+ mobility peak was measured as a function of aerosol and sheath flow rate.
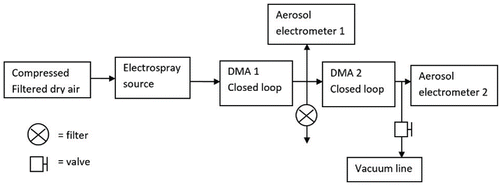
To measure the transmission of DMA, two Herrmann-type DMAs were operated in series, both operated in closed sheath flow loop arrangement (). DMA1 was operated in a pushing mode, and DMA2 in a drawing mode. The DMA2 sheath flow loop was conditioned to 22°C with a water heat exchanger in a sheath flow loop. The DMA1 sheath flow loop was conditioned to room temperature with an air cooler. The sheath flows of both DMAs were filtered with a high efficiency particulate arrestance (HEPA) filter to keep the sheath flow particle free. An aerosol electrometer (TSI 3068B) was placed as close as possible to the inlet and outlet of DMA2. Electrometer (EM1) at the inlet was sampling at 5 Lpm during all experiments. The inlet flow rate of the second aerosol electrometer at the outlet (EM2) varied from 5 to 10 Lpm to vary the aerosol flow rate of DMA2. EM2 was connected to the DMA2 outlet with a T-fitting, of which the extra leg was connected to a vacuum line with 5 Lpm and on/off valve to increase the aerosol flow rate of DMA2 up to 15 Lpm when the inlet flow rate of EM2 was 10 Lpm.
Transmission through DMA2 was measured as a function of particle size, sheath flow rate, and aerosol flow rate, with two different inner electrodes (bullets) in drawing mode. The short bullet allows higher resolution whereas the long bullet allows higher selectable size due to longer size selection length. The transmission was determined by fitting a Gaussian peak to the scan of DMA2, and by calculating the ratio of measured concentrations.
For a DMA resolution characterization, an ideal particle source is DMA, of which resolution is much higher than the resolution of the DMA being characterized. In the case of the Herrmann-type DMA, such a DMA was not available, and therefore we needed to rely on the samples that were sufficiently monomobile while entering the first DMA (Rosell-Llompart et al. Citation1996; Heim et al. Citation2010). An electrospray source ([email protected]) was utilized to generate monomobile ions for experiments. The ions selected for resolution experiments were tetrapropylammonium iodide positive monomer (TPA+), and the five smallest oligomers of tetraheptylammonium bromide (THA+, THA2Br+, THA3Br2+, THA4Br3+, and THA5Br4+), with mobility diameters of 1.16, 1.47, 1.78, 1.97, 2.14, and 2.26 nm, respectively (Ude and Fernández de la Mora Citation2005). The resolution was defined as the ratio of peak voltage and full width at half maximum (FWHM) of peak. The FWHM was obtained from a Gaussian fit to the scan of DMA2. All reported resolutions and transmissions are lower limits, since, for example, impurities that could condense on the ions between DMAs will only make the peaks wider, lowering both resolution and transmission.
As reported by Ude and Fernández de la Mora (Citation2005), spraying of monomobile THABr positive dimer is possible without neutralization of sample, but the trimer and larger n-mers were mixed with the multiply charged droplets. Therefore, to generate ions THA3Br2+, THA4Br3+, and THA5Br4+, the sample flow from the electrospray was lead directly to a 241Am radioactive source before entering the DMA. We showed that generation of monomobile THABr n-mers larger than the dimer was possible in the drawing mode of Herrmann DMA but not in the pushing mode. In the drawing mode, the electrospray was at ambient pressure, while in the pushing mode, the electrospray was at a pressure of 36 kPa above the ambient pressure.
Results
The pressure drop was found to be between 1.6 kPa and 18.4 kPa in pushing mode, and from 2 to 22.9 kPa in drawing mode (). Differences in pressure imply that, for example, when the DMA is operated at the highest sheath flow rate, it should be used in a pushing mode when conducting experiments with condensation particle counters (CPCs) to avoid under pressure at CPC inlet. Similarly, if characterizing a particle generator, the operation of DMA can change the pressure inside the generator if the sample is pushed directly to the DMA without pressure relief.
Table 1. Pressure drop (kPa) of DMA as a function of aerosol flow rate, sheath flow rate, and operation mode.
Operation of DMA at varying aerosol flow rate changed the operation of DMA, as shown in . Pushing the aerosol flow into DMA shifted the position of THA+ ion voltage toward higher voltage, while the opposite behavior was observed in the drawing mode. There are two ways the changed pressure inside the sheath air loop can affect electrical mobility. First, the pressure can change the operation of sheath air loop blower. Second, the pressure affects the mean free path in the size selection region of DMA. The Cunningham slip correction factor for a given particle size in air is governed by the mean free path, which further affects the measured electrical mobility. Physically it can be explained that at lower pressure there will be less collisions with carrier gas molecules, which reduce the drag force. With reduced drag force, the size selection of an ion with fixed electrical mobility can be selected with lower voltage at central electrode.
Figure 2. The THA+ peak voltage as a function of DMA operation mode, and sheath and aerosol flow rate. Lines are to guide the eye.
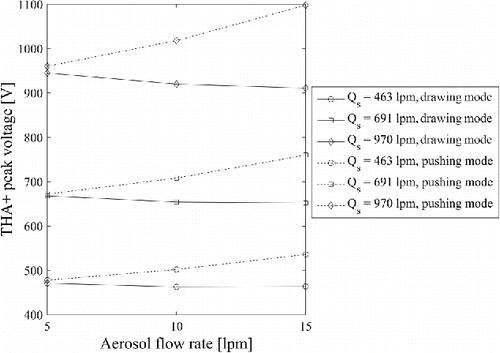
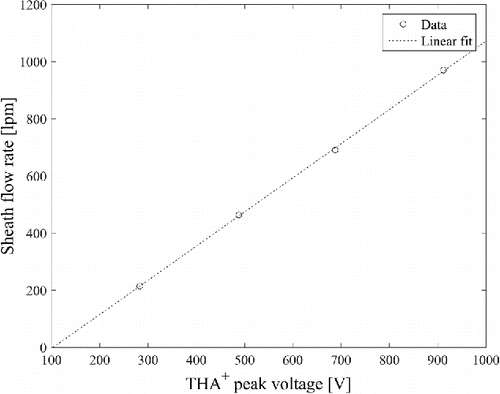
The relation between the THA+ ion peak voltage and the sheath flow rate when the aerosol flow was 15 Lpm in the drawing mode is shown in . The mass flow meter output signal was clearly saturated at the highest sheath air flow rate, for which it was not directly measured. Instead, a linear relation between the THA+ ion peak voltage and the sheath flow rate was assumed (), and the highest sheath air flow rate was estimated from the THA+ ion peak voltage.
Figure 3. DMA sheath flow rate as a function of the THA+ ion peak voltage with 15 Lpm aerosol flow rate in drawing mode.
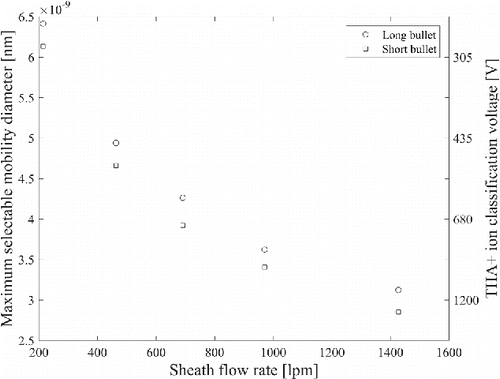
shows the range of different classifying voltages for the THA+ ions used in the study. As observed, the ratio of peak voltages was approximately 0.84 compared with the ratio of the size selection region lengths of 0.66. The difference could be explained by the slightly different geometries of bullets: the short one is conical from the tip to the middle of the bullet, and again conical toward the root of the bullet so that the thickest point is in the middle of the bullet. The long bullet is one cone from the tip to the root.
Figure 4. The THA+ ion scans as a function of sheath flow rate with short and long bullets with an aerosol flow of 15 Lpm in drawing mode.
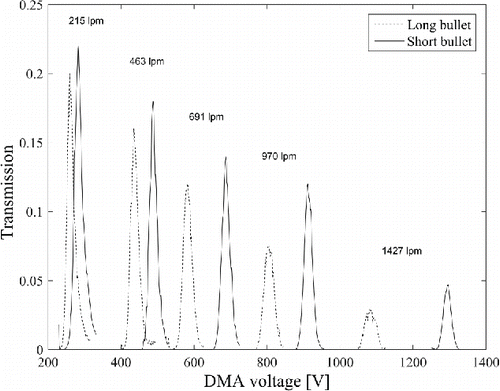
The transmission with the short bullet was higher than with the long one. The lowest sheath flow rate corresponds to the lowest stable sheath flow rate that our blower was able to produce, while the highest was the maximum speed of the blower. The scans were scaled according to the measured transmission (). By assuming a maximum voltage of 5 kV at the central electrode, the maximum selectable mobility equivalent diameter as a function of THA+ peak voltage and sheath flow rate is shown in , which was from 2.9 to 6.1 nm with a short bullet, and from 3.1 to 6.5 nm with a long bullet.
Table 2. Transmissions of TPA+, THA+, and THA2Br+ ions at various sheath and aerosol flow rates.
Figure 5. The maximum selectable size and THA+ ion classification voltage as a function of sheath air flow rate, when 5000 V is assumed as maximum voltage in central electrode.
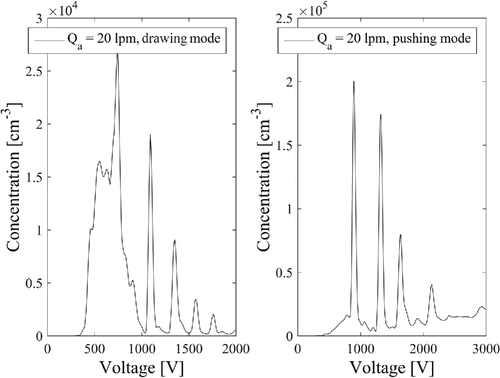
The transmission of the Herrmann DMA was measured only up to THABr dimer because the neutralization of the multiply charged ions was not complete when the DMA1 was operated in a pushing mode. presents two voltage scans of THABr: in pushing and drawing modes with the aerosol and sheath flow rates of 20 Lpm and 691 Lpm, respectively. As seen in , in the drawing mode, the ions from the radioactive source are detected at voltages <1000 V when the pressure upstream of DMA was normal atmospheric pressure. In the pushing mode, background counts, probably from multiply charged droplets, are observed at voltages larger than 1000 V when the pressure upstream of DMA was higher than the ambient pressure. The experiment suggests that higher pressure at electrospray needle deters the evaporation of multiply charged droplets, subsequently making it more difficult to detect monomobile ions larger than 2 nm. The pushing mode spectrum does not present the conditions at which the transmission experiments were conducted, since DMA1 was operated at lower sheath flow rate and without radioactive source. We did not operate two DMAs in series in the drawing mode to keep DMA2 in its “normal” pressure conditions.
Figure 6. Results of scanning of THABr ions in pushing and drawing modes at an aerosol flow rate of 20 Lpm. In the drawing mode, more charger ions are observed at <1000 V, while in the pushing mode, more multiply charged droplets are observed at >1000 V.
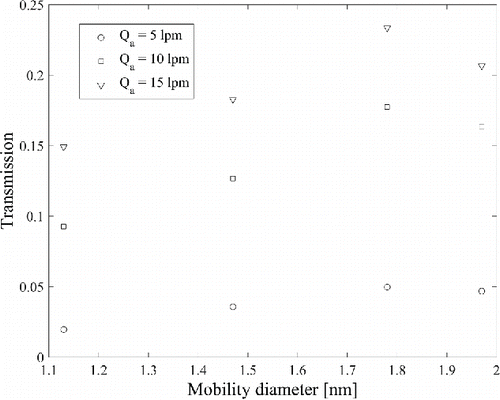
shows an example of DMA transmission curves at a sheath flow rate of 463 Lpm with short bullet. Aerosol flow rate of 5 Lpm is close to the lowest usable aerosol flow rate due to very low transmission of less than 0.05 for particles smaller than 2 nm. By increasing the aerosol flow rate, the DMA reaches a reasonable transmission of >0.1 for all particle sizes. demonstrates the importance of monomobile samples, which is not the case for the THA3Br2+ data points. In the pushing mode generation with DMA1, a small fraction of multiply charged particles was overlapping with trimer. This overlapping resulted in a tail in the scan of DMA2, subsequently lowering the transmission of trimer. The tail can be due to, for example, ion evaporation of multiply charged droplets, as shown, for example, by Hogan and Fernández de la Mora (Citation2010). lists the measured transmissions for the three smallest produced ions, which we believe to be completely monomobile.
Figure 7. Transmission of the Herrmann DMA at a sheath flow rate of 463 Lpm with short bullet at the aerosol flow rates of 5, 10, and 15 Lpm for TPA+, THA+, THA2Br+, and THA3Br2+. The peak of THA3Br2+ was not monomobile.
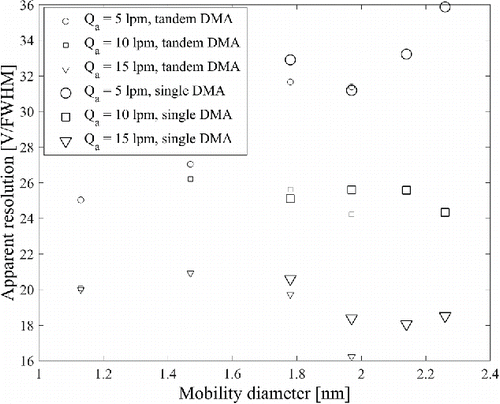
The measurement of resolution was conducted using the DMA2 only in the drawing mode, which allowed generation of THABr positive ions up to pentamer. The pentamer peak was probably monomobile based on the measured resolution of that peak, when the resolution of DMA was 25 or higher. A shoulder peak was observed next to trimer, tetramer, and pentamer, and to clearly separate that from THABr peaks, at least resolution of 25 or more was required. As seen in , resolution with an aerosol flow rate of 15 Lpm becomes lower with increasing size compared with resolution with lower aerosol flow rates, since the DMA was not able to separate the contaminant shoulder peak from the trimer, tetramer, and pentamer peaks. At an aerosol flow rate of 5 Lpm, trimer, tetramer, and pentamer peaks were separated from the shoulder peak due to higher resolution. demonstrates the current challenges in characterizing high resolution DMAs in the super 2-nm size range: generation of monomobile super 2-nm ions, and how to know whether an apparently monomobile sample is monomobile. On the other hand, if the performance of a mobility classifier is known precisely, the resolution of DMA is a good indicator whether a mobility peak is monomobile or not. lists the resolution of the Herrmann DMA at operation conditions for the three smallest ions used in the study, which can be assumed to be completely monomobile.
Table 3. Resolutions (V/FWHM) of TPA+, THA+, and THA2Br+ ions for various sheath and aerosol flow rates.
Figure 8. Apparent resolution of the Herrmann DMA at a sheath flow rate of 463 Lpm with a short bullet, and with the aerosol flow rates of 5, 10, and 15 Lpm for TPA+, THA+, THA2Br+, THA3Br2+, THA4Br3+, and THA5Br4+ ions. With resolution below 25, the DMA was not able to separate the shoulder peak next to the main peak.
Conclusions
In this study, we characterized the Herrmann-type high-resolution DMA. A relation between the DMA sheath flow rate and THA+ ion was established, which is the usual ion to convert voltage axis to mobility axis. The aerosol flow rate affected the voltage at which the THA+ ion was observed: by pushing the flow into the inlet, the THA+ ion appears at higher voltage compared with the case when the sample is drawn from the outlet. Maximum selectable size with DMA was between 2.9 nm and 6.5 nm depending on operation conditions. Finally, the transmission and resolution of DMA were measured under various operation conditions, and we showed that by neutralizing an electrospray sample at ambient pressure, it was possible to generate monomobile samples of even 2.26 nm in mobility diameter.
Acknowledgments
We wish to thank Professor Fernández de la Mora for fruitful discussions.
Funding
This work was partly funded by European Research Council (ATMNUCLE, 227463), Academy of Finland (Center of Excellence Program projects 1118615 and 139656), Nordic Center for Excellence (CRAICC), European Commission's Seventh Framework program (ACTRIS, contract No. 262254; ACTRIS2, contract No. 654109; PEGASOS, contract No. 265148), Office of Science (BER), and US Department of Energy (The Biogenic Aerosols – Effects of Clouds and Climate, BAECC).
References
- Aalto, P., Hämeri, K., Becker, E., Weber, R., Salm, J., Mäkelä, J. M., Hoell, C., O’Dowd, C. D., Karlsson, H., Hansson, H. C., Väkevä, M., Koponen, I. K., Buzorius, G., and Kulmala, M. (2001). Physical Characterization of Aerosol Particles during Nucleation Events. Tellus B, 53:344–358.
- Asmi, E., Sipila, M., Manninen, H. E., Vanhanen, J., Lehtipalo, K., Gagne, S., Neitola, K., Mirme, A., Mirme, S., Tamm, E., Uin, J., Komsaare, K., Attoui, M., and Kulmala, M. (2009). Results of the First Air Ion Spectrometer Calibration and Intercomparison Workshop. Atmos. Chem. Phys., 9:141–154.
- Birmili, W., Stratmann, F., Wiedensohler, A., Covert, D., Russell, L. M., and Berg, O. (1997). Determination of Differential Mobility Analyzer Transfer Functions Using Identical Instruments in Series. Aerosol Sci. Tech., 27:215–223.
- Brunelli, N. A., Flagan, R. C., and Giapis, K. P. (2009). Radial Differential Mobility Analyzer for One Nanometer Particle Classification. Aerosol Sci. Tech., 43:53–59.
- Chen, D. R., Pui, D. Y. H., Hummes, D., Fissan, H., Quant, F. R., and Sem, G. J. (1998). Design and Evaluation of a Nanometer Aerosol Differential Mobility Analyzer (Nano-DMA). J. Aerosol Sci., 29:497–509.
- de Juan, L., and Fernández de la Mora, J. (1998). High Resolution Size Analysis of Nanoparticles and Ions: Running a Vienna DMA of Near Optimal Length at Reynolds Numbers up to 5000. J. Aerosol Sci., 29:617–626.
- Fernández de la Mora, J. (2011). Electrical Classification and Condensation Detection of Sub-3 nm Aerosols, in Aerosol Measurements, P. Baron, P. Kulkarni, and K. Willeke, eds., John Wiley, New York, NY, pp. 697–707.
- Fernández de la Mora, J., and Kozlowski, J. (2013). Hand-Held Differential Mobility Analyzers of High Resolution for 1–30 nm Particles: Design and Fabrication Considerations. J. Aerosol Sci., 57:45–53.
- Flagan, R. C. (1999). On Differential Mobility Analyzer Resolution. Aerosol Sci. Tech., 30:556–570.
- Gamero-Castano, M., and Fernández de la Mora, J. (2002). Ion-induced Nucleation: Measurement of the Effect of Embryo's Size and Charge State on the Critical Supersaturation. J. Chem. Phys., 117:3345–3353.
- Heim, M., Attoui, M., and Kasper, G. (2010). The Efficiency of Diffusional Particle Collection onto Wire Grids in the Mobility Equivalent Size Range of 1.2–8 nm. J. Aerosol Sci., 41:207–222.
- Hewitt, G. W. (1957). The Charging of Small Particles for Electrostatic Precipitation. Am. Inst. Electr. Eng. Trans. 76:300–306.
- Hogan, C. J., and Fernández de la Mora, J. (2010). Ion-Pair Evaporation from Ionic Liquid Clusters. J. Am. Soc. Mass Spectr., 21:1382–1386.
- Jiang, J. K., Attoui, M., Heim, M., Brunelli, N. A., McMurry, P. H., Kasper, G., Flagan, R. C., Giapis, K., and Mouret, G. (2011). Transfer Functions and Penetrations of Five Differential Mobility Analyzers for Sub-2 nm Particle Classification. Aerosol Sci. Tech., 45:480–492.
- Jokinen, V., and Mäkelä, J. M. (1997). Closed-Loop Arrangement with Critical Orifice for DMA Sheath Excess Flow System. J. Aerosol Sci., 28:643–648.
- Kangasluoma, J., Attoui, M., Junninen, H., Lehtipalo, K., Samodurov, A., Korhonen, F., Sarnela, N., Schmidt-Ott, A., Worsnop, D., Kulmala, M., and Petäjä, T. (2015). Sizing of Neutral Sub 3 nm Tungsten Oxide Clusters Using Airmodus Particle Size Magnifier. J. Aerosol Sci., 87:53–62.
- Kangasluoma, J., Junninen, H., Lehtipalo, K., Mikkilä, J., Vanhanen, J., Attoui, M., Sipilä, M., Worsnop, D., Kulmala, M., and Petäjä, T. (2013). Remarks on Ion Generation for CPC Detection Efficiency Studies in Sub-3-nm Size Range. Aerosol Sci. Tech., 47:556–563.
- Keskinen, J., Mattila, T., Ukkonen, A., Jokinen, V., and Mäkelä, J. (1995). Resolution of Vienna-Type DMA in 1-nm Size Range. J. Aerosol Sci., 26(S1):S787–S788.
- Knutson, E., and Whitby, K. (1975). Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications. J. Aerosol Sci., 6:443–451.
- Kuang, C., Chen, M. D., McMurry, P. H., and Wang, J. (2012). Modification of Laminar Flow Ultrafine Condensation Particle Counters for the Enhanced Detection of 1-nm Condensation Nuclei. Aerosol Sci. Tech., 46:309–315.
- Martinez-Lozano, P., and Fernández de la Mora, J. (2005). Effect of Acoustic Radiation on DMA Resolution. Aerosol Sci. Tech., 39:866–870.
- Nasibulin, A. G., Fernández de la Mora, J., and Kauppinen, E. I. (2008). Ion-Induced Nucleation of Dibutyl Phthalate Vapors on Spherical and Nonspherical Singly and Multiply Charged Polyethylene Glycol Ions. J. Phys. Chem. A, 112:1133–1138.
- Peineke, C., Attoui, M., Robles, R., Reber, A. C., Khanna, S. N., and Schmidt-Ott, A. (2009). Production of Equal Sized Atomic Clusters by a Hot Wire. J. Aerosol Sci., 40:423–430.
- Reischl, G. P., Mäkelä, J. M., and Necid, J. (1997). Performance of Vienna-Type Differential Mobility Analyzer at 1.2–20 Nanometer. Aerosol Sci. Tech., 27:651–672.
- Rosell-Llompart, J., Loscertales, I. G., Bingham, D., and Fernández de la Mora, J. (1996). Sizing Nanoparticles and Ions with a Short Differential Mobility Analyzer. J. Aerosol Sci., 27:695–719.
- Rosser, S., and Fernández de la Mora, J. (2005). Vienna-Type DMA of High Resolution and High Flow Rate. Aerosol Sci. Tech., 39:1191–1200.
- Santos, J. P., Hontanon, E., Ramiro, E., and Alonso, M. (2009). Performance Evaluation of a High-Resolution Parallel-Plate Differential Mobility Analyzer. Atmos. Chem. Phys., 9:2419–2429.
- Saucy, D. A., Ude, S., Lenggoro, I. W., and Fernández de la Mora, J. (2004). Mass Analysis of Water-Soluble Polymers by Mobility Measurement of Charge-Reduced Ions Generated by Electrosprays. Anal. Chem., 76:1045–1053.
- Seto, T., Okuyama, K., de Juan, L., and Fernández de la Mora, J. (1997). Condensation of Supersaturated Vapors on Monovalent and Divalent Ions on Varying Size. J. Chem. Phys., 107:1576–1585.
- Sipilä, M., Lehtipalo, K., Attoui, M., Neitola, K., Petäjä, T., Aalto, P. P., O’Dowd, C. D., and Kulmala, M. (2009). Laboratory Verification of PH-CPC's Ability to Monitor Atmospheric Sub-3 nm Clusters. Aerosol Sci. Tech., 43:126–135.
- Steiner, G., Attoui, M., Wimmer, D., and Reischl, G. P. (2010). A Medium Flow, High-Resolution Vienna DMA Running in Recirculating Mode. Aerosol Sci. Tech., 44:308–315.
- Stolzenburg, M. R., and McMurry, P. H. (2008). Equations Governing Single and Tandem DMA Configurations and a New Lognormal Approximation to the Transfer Function. Aerosol Sci. Tech., 42:421–432.
- Ude, S., and Fernández de la Mora, J. (2005). Molecular Monodisperse Mobility and Mass Standards from Electrosprays of Tetra-Alkyl Ammonium Halides. J. Aerosol Sci., 36:1224–1237.
- Wiedensohler, A., Birmili, W., Nowak, A., Sonntag, A., Weinhold, K., Merkel, M., Wehner, B., Tuch, T., Pfeifer, S., Fiebig, M., Fjaraa, A. M., Asmi, E., Sellegri, K., Depuy, R., Venzac, H., Villani, P., Laj, P., Aalto, P., Ogren, J. A., Swietlicki, E., Williams, P., Roldin, P., Quincey, P., Huglin, C., Fierz-Schmidhauser, R., Gysel, M., Weingartner, E., Riccobono, F., Santos, S., Gruning, C., Faloon, K., Beddows, D., Harrison, R. M., Monahan, C., Jennings, S. G., O’Dowd, C. D., Marinoni, A., Horn, H. G., Keck, L., Jiang, J., Scheckman, J., McMurry, P. H., Deng, Z., Zhao, C. S., Moerman, M., Henzing, B., de Leeuw, G., Loschau, G., and Bastian, S. (2012). Mobility Particle Size Spectrometers: Harmonization of Technical Standards and Data Structure to Facilitate High Quality Long-Term Observations of Atmospheric Particle Number Size Distributions. Atmos. Meas. Tech., 5:657–685.
- Winklmayr, W., Reischl, G. P., Lindner, A. O., and Berner, A. (1991). A New Electromobility Spectrometer for the Measurement of Aerosol Size Distributions in the Size Range from 1 to 1000 nm. J. Aerosol Sci., 22:289–296.
- Zhang, S. H., Akutsu, Y., Russell, L. M., Flagan, R. C., and Seinfeld, J. H. (1995). Radial Differential Mobility Analyzer. Aerosol Sci. Tech., 23:357–372.