Abstract
Copyright © 2016 American Association for Aerosol Research
EDITOR:
Strongly light-absorbing aerosols emitted from natural and anthropogenic sources, such as refractory black carbon (rBC) and iron oxides, exhibit positive climate forcing by directly heating the atmosphere and indirectly reducing the snow albedo. The absorption cross-section of these light-absorbing particles strongly depends on complex refractive indices (i.e., chemical composition), size, and mixing state. The single-particle soot photometer (SP2; Droplet Measurement Technologies, Boulder, CO, USA), a commercial instrument with laser-induced incandescence using an intracavity Nd:YAG laser (λ = 1064 nm) (Stephens et al. Citation2003), is an established technique for fast real-time measurements of mass and mixing state of rBC (Moteki and Kondo Citation2010; Schwarz et al. Citation2015). Using the SP2, the traceable rBC datasets acquired in various field campaigns (Schwarz et al. 2010b) have been used to evaluate global models (Wang et al. Citation2014). In contrast, fast real-time methods for measuring light-absorbing particulate iron oxides have not been available because of the technical difficulties in automatically identifying the various iron oxides (e.g., goethite, hematite, and magnetite) of natural and anthropogenic origins. It is of fundamental importance to identify the iron oxides in aerosol measurements because the complex refractive indices of the different iron oxides are markedly different over the visible-to-near IR wavelength (Zhang et al. Citation2015 and references therein). The particulate iron oxides in bulk aerosol samples collected on a filter can be identified by X-ray absorption (XAS; e.g., Formenti et al. Citation2014 and references therein). To add to the off-line chemical speciation techniques, a fast real-time method for measuring size-resolved number (mass) concentration with less detailed chemical information is important in practice, for example, to perform aircraft observations over wide spatial coverage for comparison with global models.
We demonstrate that we can use the SP2 to identify and quantify particulate light-absorbing iron oxides simultaneously with rBC, if the wavelength bands of the two incandescence channels are adequately chosen. All the configurations of SP2 in this study, including the two wavelength bands for incandescence detection, are identical to those described in Moteki and Kondo (Citation2010). The incandescence in the shorter wavelength band (300–550 nm: blue band) is detected by an H6779 photomultiplier tube (PMT, Hamamatsu Inc., Japan) without any optical filters, and in the longer wavelength band (580–710 nm: red band) with an H6779–02 PMT (Hamamatsu Inc., Japan) equipped with a bandpass optical filter PB0640–140 (Asahi Spectra Inc., Japan). The blue band–red band signal ratio (color ratio) is an indicator of the blackbody temperature of incandescent particles; thus, we use it to discriminate the incandescent iron oxides from rBC. The power flux density of the intracavity laser beam at the center of the Gaussian beam was ∼1.5 × 1010 Wm−2, as estimated from the peak height of the scattering waveform of polystyrene latex sphere with known scattering cross-section (see Schwarz et al. 2010a for this procedure). We used fullerene soot (Alfa Aesar Inc., Stock#40971, Lot#FS12S011) as standard material for rBC (Moteki and Kondo Citation2010). For iron oxides, we used wüstite, magnetite, hematite, goethite (Kojundo Chemical Laboratory Co., Ltd., Japan), and ferrihydrite (provided by Y. Takahashi). The finely powdered reference materials were individually aerosolized from a tube-assembled glass flask dipped in an ultrasonic bath with 1 L min−1 particle-free air flow. Each of the aerosolized reference materials was mass sorted by passing through an aerosol particle mass analyzer (APM model 3601, Kanomax, Japan; Tajima et al. Citation2011) operating at λ = 0.75 and then introduced to the SP2. In addition to these reference materials, we measured four field samples. The first two field samples were Icelandic dust: wind-blown dust from Maelifellssandur deposited on a snowpack and a Halslon dust storm sample. The third field sample was Taklamakan desert dust (36°25′N, 81°58′E, 2656 m ASL). These dust samples were individually aerosolized using the same method as that with the standard materials and were directly introduced to the SP2. The fourth field sample was ambient air in Tokyo. The ambient air was sampled from an aerosol inlet installed on the sixth floor (20 m above ground) of Science Bldg. 1 in the Hongo campus of the University of Tokyo (N35°42′, E139°45′) and was continuously introduced to the SP2 for 8 h (11–19 local time) on 7 September 2015.
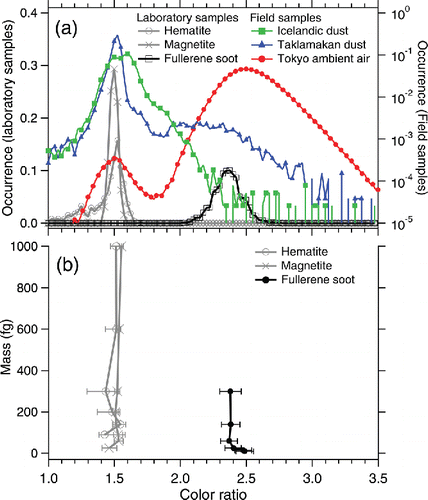
The color ratios of fullerene soot and incandescent iron oxides (wüstite, magnetite, and hematite) were measured at ∼2.4 and ∼1.5, respectively (a). The SP2 signals for wüstite were omitted from the figures because they were very similar to those for magnetite. Goethite and ferrihydrite did not incandesce in the laser beam of SP2. Assuming the incandescent temperature of fullerene soot to be T = 4230 K (Moteki and Kondo, Citation2010), the observed color ratio of ∼1.5 for iron oxides corresponds to T ∼3300 K. This estimated incandescent temperature of iron oxides is not very different from the boiling point of iron (3134 K; Lide Citation2000). The color ratios for rBC and incandescent iron oxides did not strongly depend on particle mass (b). Thus, we can discriminate rBC from incandescent iron oxides for any mass according to the color ratio assuming a threshold value of ∼1.8. a also shows the color ratio for the field samples. The Tokyo ambient air shows two distinct modes: a tiny mode at ∼1.5 and a huge mode at ∼2.5 that can be attributed to iron oxides and rBC, respectively. Taklamakan dust shows the dominant mode of iron oxides at ∼1.5 and a tiny broad hump over >1.8 that is interpreted as rBC deposited from the atmosphere. Icelandic dust shows a single but broad mode peak around 1.5–1.6. In this study, any SP2 responses for the two Icelandic dust samples were very similar and we only present the results for the Halslon sample. For Icelandic dust, we interpreted the enhanced relative occurrence of color ratio around 1.7–2.0 as an indication of the presence of other strongly light-absorbing minerals (e.g., titano-magnetite) that could be of volcanic origin. Thus, the field samples need to be checked for the occurrence distributions of the observed color ratio before automatically classifying the incandescent species using a threshold value of color ratio.
Figure 1. (a) Normalized color ratio (blue band/red band peak height) for laboratory and field samples. The vertical axis in logarithmic scale is for the field samples. (b) The median and 25–75th percentile of the measured color ratio for laboratory samples of known particle mass.
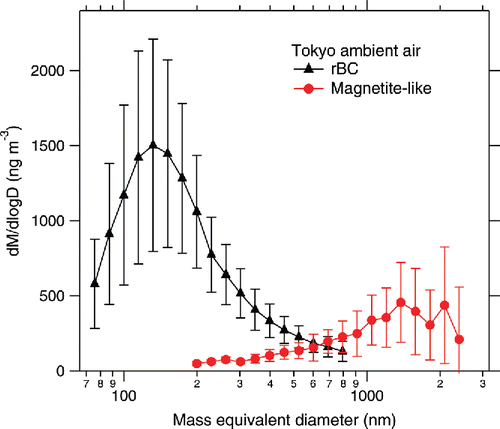
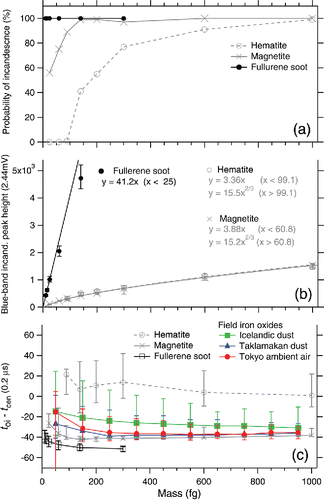
summarizes the observed SP2 signals related to incandescence as a function of particle mass. a shows the probability of incandescence (only for laboratory samples) defined as the fraction of incandescent particles in all particles triggered by the scattering signal. The probability of incandescence of iron oxides decreased with decreasing mass, likely owing to the lesser absorption efficiency of the smaller particles. The relation between the peak height of the blue-band incandescence signal and particle mass is shown in b. The intensity of incandescence for fullerene soot is proportional to the particle mass in the small-mass domain of m < 25 fg, as it is theoretically expected when the particle size is comparable to or smaller than the wavelength of incandescence detection (Moteki and Kondo Citation2010). A linear function fitted to the data points in the m < 25 fg domain is shown in b. The experimental data for magnetite (hematite) in the small-mass domain m < ∼100 fg were successfully fitted by a linear function. The remaining data points of magnetite (hematite) in the mass domain of 100 fg < m < 1000 fg were successfully fitted using a power function of m2/3 dependence. For masses m > 1000 fg beyond the sortable limit in our APM, we extrapolated the power function of m2/3 dependence to this domain based on the following. First, it is theoretically predicted that the emission cross-section of a particle is proportional to the surface area when the particle size is much larger than the wavelength (i.e., geometric optics limit). Second, it is expected that iron oxides melt and transform to spherical droplets (surface area ∝ m2/3) prior to the onset of incandescence in the laser beam. c shows the onset of incandescence (toi) relative to the timing of the particle when it reaches the center of the laser beam (tcen). Smaller toi − tcen suggests earlier onset of incandescence, that is, the particle heats up more rapidly in the laser beam. The standard deviation of the Gaussian beam profile was ∼27 (0.2 μs). It was observed that fullerene soot (rBC) and magnetite begin to incandesce markedly earlier than hematite, and the toi − tcen values did not strongly depend on particle mass (c). The slower heating of hematite compared to magnetite in the SP2 laser beam is attributed to its less imaginary part of the complex refractive index at near-IR wavelengths (see figure 1 of Zhang et al. Citation2015). Thus, we use the toi − tcen as an indicator of the different incandescent iron oxides. The incandescent iron oxide particles (color ratio <1.8) in the Taklamakan dust and Tokyo ambient air showed toi − tcen values close to that of magnetite and substantially less than that of hematite (c). Hence, the incandescent iron oxide particles in these field samples were considered magnetite-like, that is, similar to magnetite but substantially different from hematite. The result of Icelandic dust in c was different from those of the Taklamakan and Tokyo samples. The toi − tcen values for the incandescent iron oxide particles (color ratio < 1.8) were broadly distributed between the values for magnetite and hematite. The identification of magnetite-like particles in Taklamakan dust and Tokyo ambient air is consistent with previous reports. For instance, Torii et al. (Citation2001) identified magnetite particles in a Taklamakan desert dust sample based on the Curie temperature and Verwey transition. Abdul-Razzaq and Gautam (Citation2001) identified magnetite particles in diesel exhausts; diesel emissions were estimated to contribute to ∼40% of the total particulate matter emissions in Tokyo (Yamamoto et al. Citation2007). We estimated the mass of each magnetite-like particle in Taklamakan and Tokyo samples from the peak height of the blue-band incandescence signal using the calibration curve for laboratory magnetite (b). Regarding data on Icelandic dust in c, the procedure used for estimating the mass of a magnetite-like particle was applied for estimating the mass of each incandescent iron oxide particle. The mass-equivalent diameter Dme for rBC and magnetite-like particles was calculated from the particle mass by assuming bulk densities of 1.80 g cm−3 and 5.17 g cm−3, respectively. The mass-size distributions of rBC and magnetite-like particles in the Tokyo ambient air are shown in . In our SP2, the detectable size (mass) range of magnetite-like particle is ∼200 nm (22 fg) < Dme (m) < ∼2400 nm (40,000 fg). For Dme < 200 nm, the low signal-to-noise ratio does not allow unambiguous discrimination of iron oxide from rBC. The data acquisition system of the incandescence channels saturates at the signal intensity corresponding to Dme ∼2400 nm, according to the calibration curve for laboratory magnetite. The possible decrease of the incandescence probability of magnetite-like particles in m < ∼100 fg (Dme < ∼330 nm), as observed in laboratory magnetite, was not considered. The average mass (number) concentrations of rBC and magnetite-like particles in this period were 780 ng m−3 (410 cm−3) and 250 ng m−3 (0.72 cm−3), respectively. Further research is necessary for elucidating in detail the chemical properties and emission sources (formation mechanisms) of the magnetite-like particles in Tokyo. Field observations of these strongly light-absorbing magnetite-like particles using a modified SP2 will improve the presently limited understanding of the role of light-absorbing iron oxides in climate.
Figure 2. Observed signals related to the incandescence of laboratory samples as a function of particle mass: (a) probability of incandescence in the laser beam of SP2, (b) peak height of the blue-band incandescence signal and associated fitting curves, and (c) timing of the onset of incandescence (toi) relative to the timing at the center of the Gaussian laser beam (tcen). Marker with error bars in panels (a) and (c) denotes the median and 5–95th percentile.
Acknowledgments
The authors thank Y. Takahashi for providing the ferrihydrite sample.
Funding
This study was supported by the GRENE Arctic Climate Change Research Project, the global environment research fund of the Japanese Ministry of the Environment (2–1403), and JSPS KAKENHI Grant No. 15H05465.
References
- Abdul-Razzaq, W., and Gautam, M. (2001). Discovery of Magnetite in the Exhausted Material from a Diesel Engine. Appl. Phys. Lett., 78:2018–2019.
- Formenti, P., Caquineau, S., Chevaillier, S., Klaver, A., Desbeoufs, K., Rajot, J. L., Belin, S., and Briois, V. (2014). Dominance of Goethite Over Hematite in Iron Oxides of Mineral Dust from Western Africa: Quantitative Partitioning by X-ray Absorption Spectroscopy. J. Geophys. Res. Atmos., 119(22):12,740–12,754.
- Lide, D. R. (2000). CRC Handbook of Chemistry and Physics, 81st ed., CRC Press, Boca Raton, FL, pp. (4) 124–125, (6) 105–108.
- Moteki, N. and Kondo, Y. (2010). Dependence of Laser-Induced Incandescence on Physical Properties of Black Carbon Aerosols: Measurements and Theoretical Interpretation. Aerosol Sci. Technol., 44(8):663–675.
- Schwarz, J. P., Spackman, J. R., Gao, R. S., Perring, A. E., Cross, E., Onasch, T. B., Ahern, A., Wrobel, W., Davidovits, P., Olfert, J., Dubey, M. K., Mazzoleni, C., and Fahey, D. W.. (2010a). The Detection Efficiency of the Single Particle Soot Photometer. Aerosol Sci. Technol., 44(8):612–628.
- Schwarz, J. P., Spackman, J. R., Gao, R. S., Watts, L. A., Stier, P., Schulz, M., Davis, S. M., Wofsy, S. C., and Fahey, D. W. (2010b). Global-Scale Black Carbon Profiles Observed in the Remote Atmosphere and Compared to Models. Geophys. Res. Lett., 37:18.
- Schwarz, J. P., Perring, A. E., Markovic, M. Z., Gao, R. S., Ohata, S., Langridge, J., Law, D., McLaughlin, R., and Fahey, D. W. (2015). Technique and Theoretical Approach for Quantifying the Hygroscopicity of Black-Carbon-Containing Aerosol Using a Single Particle Soot Photometer. J. Aerosol Sci., 81:110–126.
- Stephens, M., Turner, N., and Sandberg, J. (2003). Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity. Appl. Opt., 42(19):3726–3736.
- Tajima, N., Fukushima, N., Ehara, K., and Sakurai H. (2011). Mass Range and Optimized Operation of the Aerosol Particle Mass Analyzer. Aerosol Sci. Technol., 45(2):196–214.
- Torii, M., Lee, T.-Q., Fukuma, K., Mishima, T., Yamazai, T., Oda H., and Ishikawa, N. (2001). Mineral Magnetic Study of the Taklamakan Desert Sands and Its Relevance to the Chinese Loess. J. Geophys., 146(2):416–424.
- Wang, Q., Jacob, D. J., Spackman, J. R., Perring, A. E., Schwarz, J. P., Moteki, N., Marais, E. A., Ge, C., Wang, J., and Barrett, S. R. H. (2014). Global Budget and Radiative Forcing of Black Carbon Aerosol: Constraints from Pole-to-Pole (HIPPO) Observations Across the Pacific. J. Geophys. Res.: Atmos., 119(1):195–206.
- Yamamoto, N., Muramoto, A., Yoshinaga, J., Shibata, K., Endo, M., Endo, O., Hirabayashi, M., Tanabe, K., Goto, S., Yoneda, M., and Shibata, Y. (2007). Comparison of Carbonaceous Aerosols in Tokyo Before and After Implementation of Diesel Exhaust Restrictions. Environ. Sci. Technol., 41(18):6357–6362.
- Zhang, X. L., Wu, G. J., Zhang, C. L., Xu, T. L., and Zhou, Q. Q. (2015). What's the Real Role of Iron-Oxides in the Optical Properties of Dust Aerosols? Atmos. Chem. Phys. Discuss., 15(4):5619–5662.