ABSTRACT
We developed an improved technique for measuring the size distribution of black carbon (BC) particles suspended in liquid water to facilitate quantitative studies of the wet deposition of BC. The measurement system, which consists of a nebulizer and a single-particle soot photometer, incorporates two improvements into the system that we developed earlier. First, we extended the upper limit of the detectable BC size from 0.9 μm to about 4.0 μm by modifying the photo-detector for measuring the laser-induced incandescence signal. Second, we introduced a pneumatic nebulizer (Marin-5) with a high extraction efficiency (∼50.0%) that was independent of particle diameter up to 2.0 μm. For BC mass concentrations less than 70 μg L−1, we experimentally showed that the diameters of BC particles did not appreciably change during the Marin-5 extraction process, consistent with theoretical calculations. Finally, we demonstrated by laboratory experiments that the size distributions of ambient BC particles changed little during their growth into cloud droplets under supersaturation of water vapor. Using our improved system, we measured the size distributions of BC particles simultaneously in air and rainwater in Tokyo during summer 2014. We observed that the size distributions of BC particles in rainwater shifted to larger sizes compared with those observed in ambient air, indicating that larger BC particles in air were removed more efficiently by precipitation.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Black carbon (BC) particles are emitted by incomplete combustion of fossil fuels and biomass. BC particles heat the atmosphere by absorbing visible solar radiation (Ramanathan and Carmichael Citation2008; Bond et al. Citation2013; Kondo Citation2015), and when deposited on snow and ice, they reduce albedo and accelerate ice melting (Warren and Wiscombe Citation1980; Bond et al. Citation2013). The temporal and spatial distributions of BC particles are controlled by emission, transport, and deposition. Emitted BC particles are hydrophobic and become hydrophilic by coating with hygroscopic materials during transport. Such coated BC particles are activated as cloud condensation nuclei (CCN), depending on their size and mixing states (Kuwata et al. Citation2007, Citation2009), and eventually are removed by precipitation. Therefore, measuring the size distribution of BC particles suspended in rainwater and snow should improve our quantitative understanding of the wet deposition of BC.
The size distribution and mass concentration of BC particles in liquid water have been measured by a system consisting of an ultrasonic nebulizer (U5000AT: Cetac Technologies Inc., Omaha, NE, USA) and a single-particle soot photometer (SP2) (McConnell et al. Citation2007; Ohata et al. Citation2011, Citation2013; Schwarz et al. Citation2012; Mori et al. Citation2014). The U5000AT nebulizer converts the liquid water into small droplets on the surface of a piezoelectric transducer and dries the droplets to release some particles into the air. The extracted particles are transferred to the SP2, which measures the masses of individual BC particles by the laser-induced incandescence technique (Stephens et al. Citation2003; Moteki and Kondo Citation2010). The combined U5000AT/SP2 system can measure the size distribution and mass concentration of BC particles in rainwater and snow, typically requiring only 5 mL of liquid water sample (Ohata et al. Citation2011). Ohata et al. (Citation2011, Citation2013) and Mori et al. Citation(2014) measured the size distributions of BC particles in rainwater and snow in two urban areas (Tokyo, 35.7°N, 139.8°E, and Sapporo, 43.1°N, 141.4°E) and at a remote site (Cape Hedo, 26.9°N, 128.7°E) in Japan. The extraction efficiency of the U5000AT nebulizer is particle size dependent: Ohata et al. Citation(2013) obtained an extraction efficiency of about 10% in the diameter range 200–500 nm at the liquid and air flow rates used in their experiments, but for particles with diameters greater than 500 nm the extraction efficiency decreased sharply, resulting in large uncertainties in the measurement of BC size distributions.
Using a collision-type nebulizer, Schwarz et al. (Citation2012, Citation2013) observed a substantial fraction of BC particles in the diameter range 0.6–2.0 μm in snow samples, suggesting the importance of measuring BC particles with diameters larger than 600 nm and the necessity of improving extraction efficiency for large particle sizes. The extraction efficiency of the APEX-Q pneumatic nebulizer (Elemental Scientific Inc., Omaha, NE, USA) has been shown to be largely size independent in the particle diameter range 100–1000 nm (Wendl et al. Citation2014). Lim et al. Citation(2014) measured the extraction efficiency of APEX-Q nebulizer to be about 72% in the diameter range 150–600 nm at their flow rates.
In general, a higher extraction efficiency is associated with a higher extraction accuracy. In addition, statistical uncertainties of BC particle detection are generally reduced at higher extraction efficiencies. Thus, improvements in the extraction efficiency can significantly improve the measurement accuracy of BC concentrations in liquid.
However, there are no demonstrated methods for accurately measuring the size distribution of BC particles with diameters up to a few micrometers in liquid water samples, for two main reasons. First, the extraction efficiencies of nebulizers for particles with diameters larger than 1.0 μm have not been reliably evaluated. Second, incandescence signals of the SP2 used in previous studies were saturated for large BC particles (>1.0 μm) (Moteki and Kondo Citation2010). To overcome these difficulties, we extended the upper limit of the size range of BC particles detectable by our SP2 and combined the SP2 with a pneumatic nebulizer for accurate measurements of BC particles with diameters up to about 2.0 μm.
To better understand the wet deposition of BC, possible changes in BC particle size during the nebulizer extraction process must be assessed. It is also important to investigate possible changes in the shapes of the size distributions of airborne BC particles during their growth into cloud droplets. We conducted laboratory experiments to investigate these points, and we also interpreted the results based on theoretical calculations.
We used our technique to measure the mass concentrations and size distributions of BC particles in ambient air and rainwater in Tokyo during summer 2014 to demonstrate reliability of the technique for field observations.
2. Methods
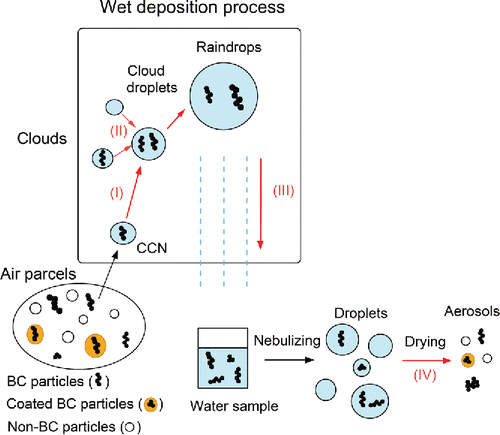
illustrates the wet deposition processes of BC and its extraction from liquid water by a nebulizer. There are four important processes through which the sizes of BC particles might change: (I) Growth of BC-containing particles into cloud droplets, (II) collisional growth of cloud droplets, (III) formation of raindrops by coalescence, and (IV) extraction of BC particles from collected rainwater by the nebulizer. The effects of these processes are examined quantitatively in this article. We first describe the improved system for measuring the size distributions of BC particles in liquid water.
Figure 1. Schematic diagram of the wet deposition processes of BC and its extraction into air by a nebulizer: (I) growth of BC-containing particles into cloud droplets; (II) collision of cloud droplets; (III) formation of raindrops by coalescence of cloud droplets; (IV) extraction of BC from liquid water by the nebulizer. The size distributions and chemical composition of non-BC particles extracted by process (IV) are different from those in the air parcels.
2.1. Nebulizer/wide-range SP2 setup
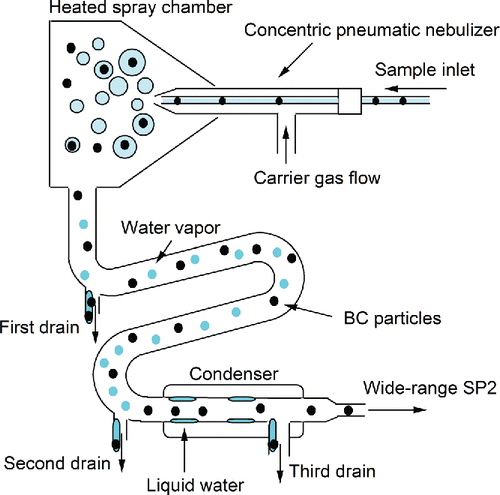
shows the experimental setup for measuring the size distribution of BC particles in liquid water. The structure of the Marin-5 pneumatic nebulizer (Cetac Technologies Inc.) is shown in .
Figure 2. Experimental setup for measuring BC particles in rainwater and snow. Air flow rates are given in units of standard temperature and pressure (STP; 273 K and 1013 hPa) cm3 s−1.
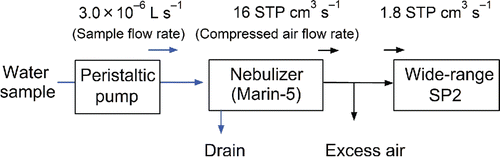
As shown in , the water sample is fed into the Marin-5 nebulizer by a peristaltic pump (REGRO Analog; ISMATEC SA, Feldeggstrasse, Glattbrugg, Switzerland) at a constant flow rate (Vpump: 3.0 × 10−6 L s−1). A portion of the introduced water is converted into droplets by the concentric pneumatic nebulizer, using dry air as the driving force at a constant flow rate (Fneb: 16 cm3 s−1 at standard temperature and pressure [STP]), and the diameters of the droplets are estimated (as described in Section 3.2). Immediately after nebulization, the droplets are evaporated in a heated spray chamber at 140°C, generating a mixture of BC particles, non-BC particles, and water vapor. A fraction of the non-aerosolized water sample is removed through the first drain. The particles and water vapor are transferred to a condenser at 3°C. The water vapor condenses and is removed through the second and third drains. The extracted BC particles are introduced into a modified SP2 (a wide-range SP2; WR-SP2) at a constant flow rate of 1.8 STP cm3 s−1. The WR-SP2 measures the masses of the individual BC particles with mass-equivalent diameters between 70 and 4170 nm (Section 2.2).
2.2. Wide-range SP2
The SP2 is an instrument for measuring the size-resolved BC mass concentration by the laser-induced incandescence technique (Stephens et al. Citation2003; Moteki and Kondo Citation2010). In our SP2 (called the standard SP2 in this article), two of the four detection channels are utilized for detecting incandescence in two different wavelength bands (a blue band at λ = 300–550 nm, and a red band at λ = 580–710 nm). Incandescence in the 300–550 nm band is detected by an H6779 photomultiplier tube (PMT; Hamamatsu Inc., Hamamatsu, Shizuoka, Japan) equipped with a KG5 band-pass filter (Schott Inc., Elmsford, NY, USA). Incandescence in the 580–710 nm band is detected by a H6779–02 PMT (Hamamatsu Inc.) equipped with a narrow band-pass filter (PB0640–140, Asahi Spectra Inc., Tokyo, Japan). The other detection channels are utilized for detecting scattered light at 1064 nm, which is detected by a Si-avalanche photodiode (APD; C30916E, Perkin–Elmer Inc., Waltham, MA, USA) and a position-sensitive Si-avalanche photodiode (PS-APD; C30927E-01, Perkin–Elmer Inc.). Each detection channel has high-gain and low-gain detectors. The sensitivity of the high-gain detectors is ten times that of the low-gain detectors. The detectable mass-equivalent diameter ranged from 70 to 920 nm.
To expand the upper limit of the BC particle mass-equivalent diameter (DBC) detectable by the standard SP2, we replaced the PS-APD with the H6779 PMT equipped with the KG5 filter and then lowered the sensitivity of the PMT by a factor of about 140. Thus, the WR-SP2 had three incandescence detection channels and one scattering detection channel. As a result, the detection limit was extended to 4170 nm, assuming a density of BC particles of 1.8 g cm−3 (Moteki and Kondo Citation2010). shows the relationship between the peak incandescence signal (S) and the BC particle mass (MBC) up to 30.0 fg (DBC = 317 nm) for fullerene soot (FS; Alpha Aeser Inc., Wardhill, MA, USA). We linearly fitted the data for masses smaller than 10.0 fg (DBC = 220 nm) to derive EquationEquation (1)[1] :
Figure 4. (a) Calibration curves of the WR-SP2 for fullerene soot and for extremely non-compact and compact shapes of BC particles. (b) Mass size distributions of BC particles in Tokyo air, determined by using the three different calibration curves. For the size distribution of BC particles with extremely compact shapes, the dM/dlogDBC value for the diameter of 4780 nm (8.74 μg m−3) is not shown. Bars indicate 1σ of a Poisson distribution.
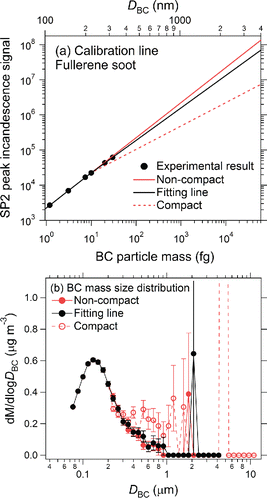
The relationship was extrapolated to diameters up to 4170 nm, in the form of S = a(MBC)b. The values of a and b were determined by least-squares fitting of the experimental data with MBC < 30.0 fg:[2] The value of b derived in this study is similar to that derived in our previous study (Moteki and Kondo Citation2010).
For masses larger than 10.0 fg (DBC = 220 nm), the calibration curve depends on the shapes of the BC particles, as demonstrated by Moteki and Kondo Citation(2010) who also showed that the shapes can be represented by their effective densities (ρeff). Here, we call BC particles with ρeff of about 0.30 non-compact (highly branched aggregates), and we call those with ρeff of about 2.0 compact (spherical). To estimate the uncertainties arising from the different shapes of the BC particles, we also determined calibration curves for BC particles with extremely non-compact (b = 1.0; EquationEquation (3)[3] ) and compact (b = 0.667; EquationEquation (4)
[4] ) shapes for masses larger than 10.0 fg (DBC = 220 nm) ():
[3]
[4] Snon-compact and Scompact are the peak incandescence signals derived from the MBC of particles with extremely non-compact and compact shapes, respectively. For BC particles with extremely non-compact and compact shapes, the parameter b approaches 1.0 and 2/3 ( = 0.667), respectively, because each volume element and the surface area of the BC particles interact with the incident radiation (Moteki and Kondo Citation2010). Parameter a in EquationEquation (4)
[4] was determined so that the Snon-compact value would equal the Scompact value at MBC = 10.0 fg. As an example, shows the dependence of the mass size distributions of BC particles derived from the SP2 data for ambient air in Tokyo on the calibration curves (). The derived size distribution strongly depends on b in the extrapolated BC size range. In this study, we assumed that there were very few BC particles with extremely compact shapes in the air and rainwater of Tokyo.
The evaluation of the overall performance of the WR-SP2 is described in the online supplemental information.
2.3. Number concentration of polystyrene latex spheres in water
To determine the extraction efficiency of the Marin-5 nebulizer, we prepared polystyrene latex (PSL) suspensions of known number concentration. The mean diameters of the PSL spheres (DPSL) were 202 ± 5.0, 254 ± 8.0, 309 ± 9.0, 402 ± 6.0, 603 ± 14, and 814 ± 19 nm (JSR Inc., Kamitu, Ibaraki, Japan); 1025 ± 30 and 1537 ± 77 nm (Polyscience Inc., Warrington, PA, USA); and 2.0 ± 0.02, 3.0 ± 0.03, 4.0 ± 0.03, and 5.0 ± 0.04 μm (Duke Scientific Corp., Palo Alto, CA, USA). We determined the size-resolved number concentrations of the PSL suspensions (N(DPSL)) by measuring the attenuation of a solid-state 532 nm laser (GSHG-3020F; KTG Co. Ltd., Kochi, Japan) (Ohata et al. Citation2013). The values of N(DPSL) ranged from 7.04 × 104 to 2.53 × 109 particles cm−3. The PSL suspensions were diluted further to determine the extraction efficiency of the Marin-5 nebulizer.
2.4. Extraction efficiency with PSL suspensions
We determined the extraction efficiencies (ϵ) of the Marin-5 by measuring the number concentrations of PSL suspensions with the 12 diameters mentioned above. Here, the ϵ values of PSL and BC were assumed to be identical (Ohata et al. Citation2013).
shows the measured ϵ in the diameter range 200–5000 nm. The ϵ values of the U5000AT in the diameter range 100–1025 nm (Ohata et al. Citation2013) are also plotted for comparison. The ϵ values of the Marin-5 were 50.0 ± 4.4% on average in the diameter range 200–2000 nm, and they linearly decreased at larger sizes, as fitted with linear EquationEquations (5)[5] and Equation(6)
[6] :
Figure 5. Measured extraction efficiency of the Marin-5, obtained by using polystyrene latex (PSL) spheres and AquaBlack 162 in water. Error bars show standard deviations for repeated measurements. For comparison, the extraction efficiency of an ultrasonic nebulizer (U5000AT) is also shown (Ohata et al. Citation2013).
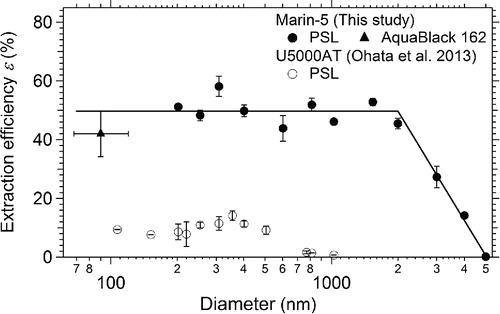
These values may depend on the liquid and air flow rates. The ϵ values of the Marin-5 were much higher than those of the U5000AT. The size-independent and high ϵ values greatly improved the measurement accuracy of the size distributions of BC in water in the diameter range 200–2000 nm using a water sample of only a few milliliters. For diameters larger than 2000 nm, the ϵ values linearly decreased, possibly owing to particle losses by impaction on the walls of various parts of the system and gravity settling of droplets.
To determine the ϵ value of the Marin-5 for diameters smaller than 200 nm, we prepared a known mass concentration (35 μg L−1) of AquaBlack 162 (AB-162; Tokai Carbon Co. Ltd., Tokyo, Japan), which is a laboratory standard of BC particles suspended in water (Ohata et al. Citation2011, Citation2013). The measured mass median diameter of the mass size distribution was 90 nm, and the geometric standard deviation was 1.33. The mass fraction of BC particles with diameters smaller than 70 nm undetectable by the SP2 was estimated to be 7.1%. The ϵ value for AB-162 was estimated to be 42 ± 7.9%, indicating that the estimated ϵ value agreed to within 20% of the ϵ value (50%) for the diameter range 200–2000 nm determined with the PSL suspension (). Therefore, in this study, BC mass concentration in rainwater for diameters smaller than 200 nm was calculated using the ϵ value of 50%.
2.5. Reproducibility
We assessed the reproducibility of the measured BC size distributions in two rainwater samples collected at Cape Hedo, Okinawa Island, Japan. The BC mass concentrations in these samples were 50 and 106 μg L−1, respectively. The size distributions of BC in the two rainwater samples were measured on two different days, 22 days apart (Days 1 and 23). shows the number and mass size distributions of BC for one of the samples on Days 1 and 23, together with the Day 23:Day 1 ratios of the respective distributions up to 300 nm. For diameters larger than 300 nm, the ratios are not shown, because the BC particle concentrations were very low. These BC number and mass size distributions agreed to within 12%. The count median diameter (CMD) and geometric standard deviation (σgc) of the number size distributions of the two samples were reproducible to within 1.0%. This result showed that the BC size distribution in liquid water changed little during about three weeks, consistent with the previous study (Ohata et al. Citation2013).
Figure 6. (a) Number and (b) mass size distributions of BC in Cape Hedo rainwater samples (bottom panels). The ratios of the number and mass concentrations of BC in rainwater on two different days are also shown (top). Bars indicate 1σ values.
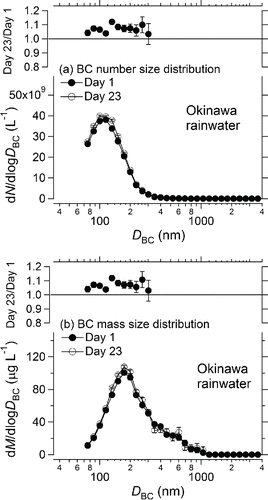
The overall uncertainty of the measurements of BC in water by the Marin-5/WR-SP2 system was estimated to be about 16%. This value comprises the uncertainties of ϵ (±4.4%), the size-resolved BC mass concentration in air measured by the WR-SP2 (±15%), Fneb (±5%), and Vpump (±5%). The deviation of the Day 23/Day 1 ratio from 1 is within this estimated uncertainty of the BC size distribution measurement.
3. Independent estimates of the extraction efficiency of the Marin-5 and the droplet size distribution
Ammonium sulfate (AS) solutions of known mass concentration were used to independently estimate the ϵ of the Marin-5. In addition, we estimated the size distribution of the droplets generated by the concentric pneumatic nebulizer. These data were used to estimate possible changes in the size of BC particles during the extraction process by the Marin-5 (Section 4).
3.1. Extraction efficiency
To estimate ϵ of the Marin-5 and the droplet size distribution, we first prepared AS solutions of six different mass concentrations (). These AS solutions were fed to the Marin-5 by a peristaltic pump to generate droplets of AS solution, and then the AS particles were extracted (). The generated AS particles were further dried to a relative humidity of about 12% by passing them through a Nafion tube to minimize the effect of hygroscopic growth of the particles. The dried AS number size distributions (15–900 nm) were measured with an accuracy of about 10% by a scanning mobility particle sizer spectrometer (SMPS 3936; TSI Inc., Shoreview, MN, USA) consisting of a differential mobility analyzer (Model 3081, TSI Inc.) and a condensation particle counter (Model 3010, TSI Inc.). The contribution of non-AS particles to the total volume of the extracted particles was negligibly small.
Figure 7. Experimental setup for estimating the extraction efficiency of the pneumatic nebulizer (Marin-5) and the size distribution of the generated droplets of ammonium sulfate (AS) solution. SMPS, scanning mobility particle sizer spectrometer; RH, relative humidity.
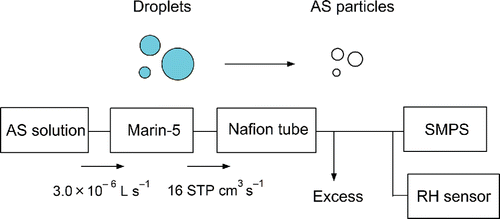
Table 1. Mass concentrations of ammonium sulfate (AS) solutions used to estimate the extraction efficiency of the Marin-5 nebulizer and corresponding count median diameter (CMD) and geometric standard deviation (σgc) values of the AS particles and droplets generated by the nebulizer.
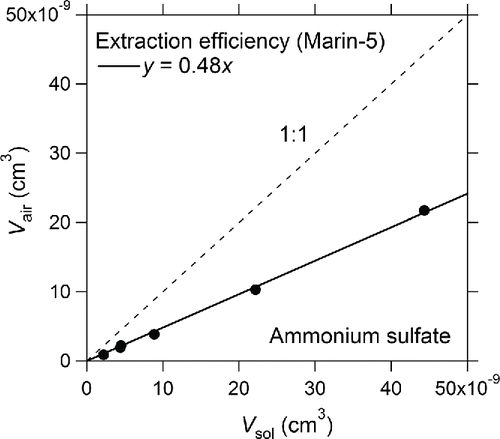
The ϵ of the Marin-5 was determined as the ratio of the volume of AS particles extracted per second (Vair (cm3)) to the volume of AS in the solution supplied per second (Vsol (cm3)). shows the correlation between Vair and Vsol. The value of ϵ, derived as the slope of the correlation line, was 48 ± 6.3% (1σ). This value is consistent with that determined by using the PSL suspensions (Section 2.4).
3.2. Droplet size distribution
We also estimated the size distribution of droplets generated from the nebulizer, so that possible changes in the size of BC particles occurring during the extraction process by the Marin-5 could be estimated (Section 4). The known mass concentration of AS in solution (X) is expressed as the ratio of the mass of solute (AS) to that of the solution (deionized water + AS):[7] Da and Dd are the diameters of AS particles and droplets, respectively, and ρa and ρd are the densities of the AS particles (1.77 g cm−3) and droplets, respectively. We approximated ρd as water density (ρl: 1.00 g cm−3), because the mass mixing ratios of AS were less than 30 parts per million (ppm). Therefore, using ρl, Dd is derived by EquationEquation (8)
[8] :
[8]
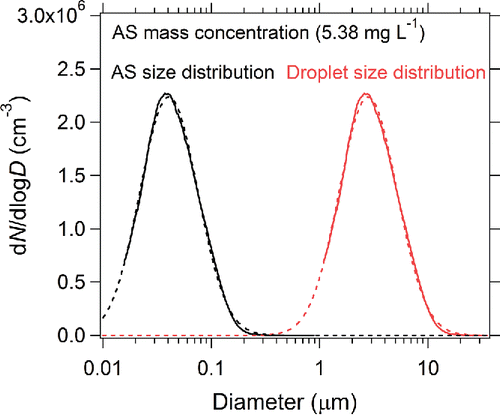
shows the measured size distribution of AS particles generated using solution 4 (). The AS mass concentration of 5.38 mg L−1 is similar to the typical value for rainwater in East Asia (Fujita et al. Citation2000). also shows the droplet size distribution estimated from the AS size distribution (EquationEquation (8)[8] ). The CMD and σgc values of the AS and droplet number size distributions of each sample are summarized in . The average CMD of the droplet number size distribution was 2.8 ± 0.4 μm, and the average σgc was 1.82 ± 0.04.
4. Changes in BC size in the nebulizer
4.1. Experimental evaluation
BC particle size can change because of coagulation of BC particles during the extraction process. First, we evaluated this effect by laboratory experiments. We prepared two kinds of BC samples with known mass concentrations: AB-162 and FS. The mass median diameter and average geometric standard deviation of FS were 174 nm and 1.54, respectively. The number concentrations of AB-162 particles were about ten times higher than those of FS particles with the same mass concentrations because of the smaller diameters of AB-162.
The mass concentrations of BC particles in the AB-162 and FS samples were 70 and 137 μg L−1, respectively. We diluted the samples with deionized water and prepared six different BC mass concentrations in water. The ranges of the mass concentrations of BC in the AB-162 and FS samples were 7.3–70 and 6.9–137 μg L−1, respectively. These BC mass concentrations are typical for rainwater collected in Tokyo and at Cape Hedo (Ohata et al. Citation2011; Mori et al. Citation2014).
We measured the number size distributions of BC particles in the AB-162 and FS samples with the Marin-5/WR-SP2 system. The measured number concentrations of BC particles in the AB-162 and FS samples ranged from 20 × 109 to 166 × 109 particles L−1 and from 1.2 × 109 to 19 × 109 particles L−1, respectively, indicating that the number concentrations of BC particles in the AB-162 samples were about ten times those in the FS samples. shows the normalized number size distributions of BC particles in the AB-162 and FS samples for different BC mass concentrations. In this figure, we show only the data for BC concentrations sufficiently high for reliable statistical analysis. To evaluate the effect of coagulation of BC particles during the extraction process, we used AB-162 and FS samples with BC mass concentrations of 8.0 and 18 μg L−1 as reference samples. also shows ratios of the normalized number concentrations of BC particles in the AB-162 and FS samples (dNsamp) to those of the references (dNref) at different sizes. The AB-162 and FS ratios were normalized by the diameters of 76 and 115 nm, respectively. These results show that the effect of coagulation of BC particles during the extraction process was negligibly small for BC mass concentrations of less than about 70 μg L−1 for the FS samples and less than about 30 μg L−1 for the AB-162 samples.
Figure 10. (a, b) Normalized number size distributions of BC particles in AquaBlack 162 (AB-162) samples. (c, d) Same as (a) and (b) but for fullerene soot (FS) samples. Ratios of normalized number concentrations of BC particles in AB-162 and FS samples to reference concentrations at different sizes are also shown, which were normalized by 76 and 115 nm for AB-162 and FS, respectively. The mass concentrations of the reference samples of AB-162 and FS were 8.0 and 18 μg L−1.
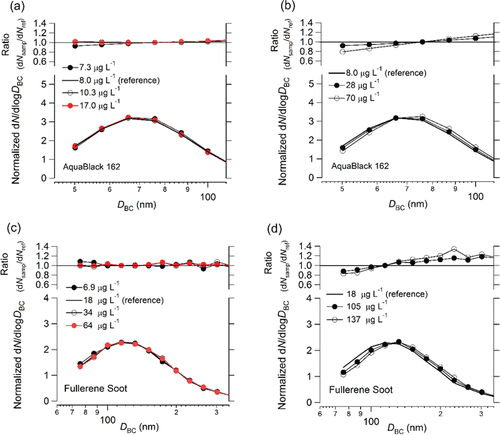
The BC number size distributions shifted to larger sizes for BC mass concentrations substantially higher than these threshold mass concentrations (). We attribute this shift to the coagulation of BC particles during the extraction process. The threshold mass concentration of AB-162 was lower than that of FS, reflecting the higher number concentration for a given mass concentration, as discussed above.
4.2. Theoretical evaluation
We theoretically calculated the effect of coagulation of BC particles during the extraction process and compared the result with the experimental results. By using the theoretical model developed by Moteki and Mori Citation(2015), the number size distribution of BC particles extracted by the nebulizer can be predicted from the droplet number size distribution and the BC number size distribution in the water sample. The calculated probability of the number of BC particles in an individual droplet is a measure of the effect of coagulation of BC particles. This probability is described by a Poisson distribution because the number of BC particles and droplets produced by a nebulizer is a discrete random variable. More details of the model are given by Moteki and Mori Citation(2015).
The effect of coagulation is represented by the change of the calculated size distribution relative to the BC mass concentration in water. The calculated CMD of the extracted FS size distribution changed from 118 to 120 nm when the mass concentration in water changed from 6.9 to 64 μg L−1. The predicted change in the standard deviation was about 1%. Similar changes in these parameters were predicted for AB-162. These small changes in the predicted size distributions provide a mathematical basis for the interpretation of the results of the laboratory experiments.
5. Changes in BC particle size in cloud droplets
The BC particle size might change during the condensation, coalescence, and precipitation processes shown in . To investigate possible changes of the BC size distribution through these processes, we carried out an experiment that included all of these processes, although the conditions were different from those in the natural atmosphere. In this experiment, we measured size distributions of BC particles in ambient Tokyo air by the standard SP2 and compared them with those of BC particles collected in water by a particle-into-liquid sampler (PILS) (). The PILS consists of a particle growth chamber and an impactor component (Weber et al. Citation2001; Orsini et al. Citation2003; Miyazaki et al. Citation2006). Orsini et al. Citation(2003) showed that almost all olive oil particles in the diameter range 0.03–6.0 μm were captured in the growth chamber under a high supersaturation of water vapor.
Figure 11. Experimental setup for measuring changes in the size distribution of BC particles during their collection by a particle-into-liquid sampler (PILS).
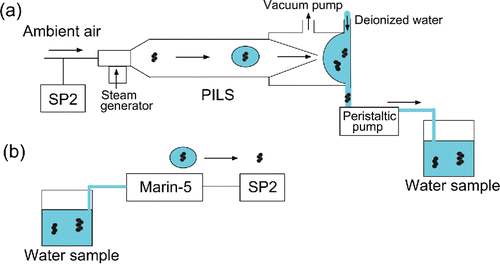
In this particle growth chamber, ambient particles containing BC are mixed with supersaturated water vapor to produce droplets through condensational growth. The droplets formed are collected by a polycarbonate impaction plate. The collected liquid sample is continually transported with a stream of ultrapure deionized water to a glass bottle by a peristaltic pump (IPC/IPC-N; ISMATEC, Wertheim, Germany) at a constant flow rate of 3.0 × 10−6 L s−1 (). The air sample flow rate for the PILS was maintained at 266 cm3 s−1. The water sample was transferred to the Marin-5 and converted into aerosols (), and the BC particle size distributions were measured by the standard SP2.
Measurements were made on 25 December 2014 and 26 January 2015, and BC particles were collected for half an hour; summarizes relevant values for the BC particles in air and the collected water samples. Average mass concentrations of BC in air on the first and second days were 0.21 ± 0.04 and 0.55 ± 0.02 μg m−3, respectively. Mass concentrations of BC in the collected water samples on the first and second days were 0.76 and 2.27 μg L−1, respectively. The effect of coagulation of BC particles during the extraction process was negligibly small (Section 4). shows the normalized number size distributions of BC in air and the collected water samples for the diameter range 100–400 nm on the second day. This figure also shows the ratios of the normalized number concentration of BC in the collected water sample (dNPILS) to that in air (dNair) for the same diameter range. The average dNPILS/dNair ratio was 0.95 ± 0.09 (1σ), and the CMD and σgc values for air and the collected water sample agreed to within 5.1% and 4.7%, respectively. The ratio was 1.0 ± 0.2 (1σ) for the diameter range 100–350 nm. The large errors of the ratios () were caused by low BC particle counts. For lower BC mass concentrations on the first day, the average dNPILS/dNair ratio was very similar (0.94 ± 0.18 (1σ)), and the CMD and σgc values for air and the collected water sample also agreed to within 7.1% and 6.3%, respectively.
Figure 12. Normalized number size distributions of BC particles in ambient air and those collected by a particle-into-liquid sampler (PILS) in the diameter range 100–400 nm (bottom). The ratio of the normalized number concentration in water (dNPILS) to that in air (dNair) at different sizes is also shown (top). Bars indicate 1σ values.
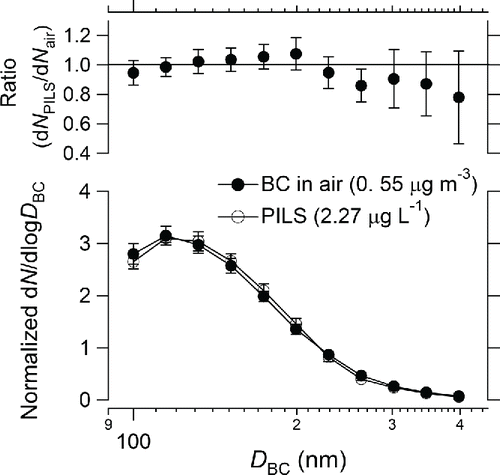
Table 2. Mass concentration, count median diameter (CMD), and geometric standard deviation (σgc) values of BC particles in Tokyo ambient air and the collected water samples on the first and second days.
In this experiment, all ambient BC particles detectable by the SP2 should be captured in the particle growth chamber under high supersaturation, considering the results of Orsini et al. Citation(2003). We showed that the size distribution of BC particles activated as CCN was the same as the size distribution of BC in water condensed from water vapor. More specifically, we can conclude that the sizes of BC particles do not change during condensational growth.
6. Size distribution of BC particles in rainwater
We carried out overall tests of the Marin-5/WR-SP2 system by measuring the size distributions of BC in air and rainwater simultaneously in Tokyo during summer 2014. We developed an automated rain sampling system that enabled the rain samples to be analyzed within about 3 min after the start of rainfall. First, we collected rainwater with a plastic funnel (30 cm maximum diameter). The rainwater was transferred to a glass bottle through a Tygon tube and then directly introduced into the Marin-5/WR-SP2 system by a peristaltic pump (). To estimate the losses of BC particles due to adhesion to the plastic funnel and the Tygon tube, we compared the mass concentration and size distribution of BC in rain samples collected at Cape Hedo with and without transferring them through the plastic funnel and Tygon tube. The BC mass losses during the transfer were less than 7%, with negligible changes in the shape of the size distribution. In addition, we estimated the effect of dry deposition of BC particles on the funnel. The BC mass concentration deposited on the funnel was measured by pouring deionized water into the funnel. The effect of dry deposition of BC particles on the funnel was estimated to be negligibly small for measurement of BC in rainwater in Tokyo.
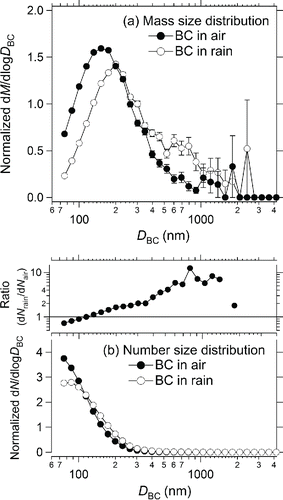
shows the normalized mass and number size distributions of BC in air 1 h before a precipitation event, which lasted for about 2 h, on 10 August 2014. Rainwater was collected about 50 min after the initiation of the precipitation. The average mass concentration of BC in air was 0.07 ± 0.02 μg m−3, and the average mass concentration of BC in rainwater was 5.5 ± 1.3 μg L−1. also shows the ratio of the normalized number concentration in rainwater (dNrain) to that in air (dNair) for different particle sizes. The ratios are normalized to a diameter of 130 nm. The size of BC in rainwater was larger than that in air, indicating that large BC particles were more efficiently removed by precipitation. The difference between the size distribution in air and that in rainwater was much larger than that caused through processes I–IV (). Because of the thin coating thickness of BC particles in Tokyo air (Kondo et al. Citation2011), larger BC particles can be more active as CCN. The existence of BC particles with diameters larger than 1 μm also indicates that further studies of wet deposition of BC will require the use of the WR-SP2.
Figure 13. (a) Normalized mass size distributions of BC in air and rainwater collected in Tokyo on 10 August 2014. (b) Same as (a) but for normalized number size distributions. Ratios of the normalized size distribution in rainwater to that in air are also shown. The ratios are normalized by a diameter of 130 nm. Bars indicate 1σ of a Poisson distribution.
7. Summary
We improved the technique for measuring the size distribution of BC in rainwater and snow by combining a pneumatic nebulizer (Marin-5) with a modified SP2 (WR-SP2). First, we extended the upper limit of the size of BC detectable by the SP2 from 0.9 μm to about 4.0 μm by modifying the photo-detector for measuring the laser-induced incandescence signal. Second, we demonstrated that the extraction efficiency of the Marin-5, measured by using PSL, was as high as 50.0 ± 4.4% and was size independent in the diameter range 200–2000 nm. The efficiency determined by using AB-162 samples agreed with this value to within 20% for diameters smaller than 200 nm. The average efficiency determined with AS solutions also agreed to within 5%. The combined Marin-5/WR-SP2 system enabled accurate measurement of the size distribution of BC particles in liquid water up to a particle diameter of 4170 nm. The reproducibility of the measured mass size distribution of BC in rainwater was about 12% in the diameter range 70–300 nm.
In the laboratory, we investigated possible changes in the size distribution of BC particles during the extraction process by the nebulizer and condensational growth of BC. First, we showed that the effect of coagulation of BC particles during the extraction process by the Marin-5 was negligibly small for BC mass concentrations in liquid water of less than 70 μg L−1, consistent with theoretical calculations. Second, we showed that BC diameter did not change during condensational growth in the particle growth chamber of a PILS.
To evaluate the overall performance of the Marin-5/WR-SP2 system, we measured the size distribution of BC in air and rainwater simultaneously in Tokyo during summer 2014. The size distribution of BC in rainwater was larger than that in air, indicating that larger BC particles were removed more efficiently by precipitation.
These results, based on laboratory experiments, theoretical calculations, and field observations strongly support the application of the Marin-5/WR-SP2 system to quantitative studies of wet deposition of BC.
UAST_1147644_Supplemental_File.zip
Download Zip (115 KB)Funding
This study was supported by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, the GRENE Arctic Climate Change Research Project, the Arctic Challenge for Sustainability (ArCS) project, the Global Environment Research Fund of the Japanese Ministry of the Environment (2–1403), and Project Research KP-15 of the National Institute of Polar Research.
References
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., Ghan, S., Kärcher, B., Koch, D., Kinne, S., Kondo, Y., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S. (2013). Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res., 118:1–173, doi:10.1002/jgrd.50171.
- Fujita, S., Takahashi, A., Weng, J. H., Huang, L. F., Kim, H. K., Li, C. K., Huang, F. T. C., and Jeng, F. T. (2000). Precipitation Chemistry in East Asia. Atmos. Environ., 34:525–537.
- Kondo, Y. (2015). Effects of Black Carbon on Climate: Advances in Measurement and Modeling. Monogr. Environ. Earth Planets., 3:1–85, doi:10.5047/meep.2015.00301.0001.
- Kondo, Y., Sahu, L., Moteki, N., Khan, F., Takegawa, N., Liu, X., Koike, M., and Miyakawa, T. (2011). Consistency and Traceability of Black Carbon Measurements Made by Laser-Induced Incandescence, Thermal–Optical Transmittance, and Filter-Based Photo-Absorption Techniques. Aerosol Sci. Technol., 45:295–312, doi:10.1080/02786826.2010.533215.
- Kuwata, M., Kondo, Y., Mochida, M., Takegawa, N., and Kawamura, K. (2007). Dependence of CCN Activity of Less Volatile Particles on the Amount of Coating Observed in Tokyo. J. Geophys. Res., 112:D11207, doi:10.1029/2006JD007758.
- Kuwata, M., Kondo, Y., and Takegawa, N. (2009). Critical Condensed Mass for Activation of Black Carbon as Cloud Condensation Nuclei in Tokyo. J. Geophys. Res., 114:D20202, doi:10.1029/2009JD012086.
- Lim, S., Faïn, X., Zanatta, M., Cozic, J., Jaffrezo, J. L., Ginot, P., and Laj, P. (2014). Refractory Black Carbon Mass Concentrations in Snow and Ice: Method Evaluation and Inter-Comparison with Elemental Carbon Measurement. Atmos. Meas. Tech., 7:3307–3324, doi:10.5194/amt-7–3307–2014.
- McConnell, J. R., Edwards, R., Kok, G. L., Flanner, M. G., Zender, C. S., Saltzman, E. S., Banta, J. R., Pasteris, D. R., Carter, M. M., and Kahl, J. D. W. (2007). 20th-Century Industrial Black Carbon Emissions Altered Arctic Climate Forcing. Science, 317:1381–1384, doi:10.1126/science.1144856.
- Miyazaki, Y., Kondo, Y., Takegawa, N., Komazaki, Y., Fukuda, M., Kawamura, K., Mochida, M., Okuzawa, K., and Weber, R. J. (2006). Time-Resolved Measurements of Water-Soluble Organic Carbon in Tokyo. J. Geophys. Res., 111: D23206, doi:10.1029/2006JD007125.
- Mori, T., Kondo, Y., Ohata, S., Moteki, N., Matsui, H., Oshima, N., and Iwasaki, A. (2014). Wet Deposition of Black Carbon at a Remote Site in the East China Sea. J. Geophys. Res. Atmos., 119:10485–10498, doi:10.1002/2014JD022103.
- Moteki, N., and Kondo, Y. (2010). Dependence of Laser-Induced Incandescence on Physical Properties of Black Carbon Aerosols: Measurements and Theoretical Interpretation. Aerosol Sci. Technol., 44:663–675, doi:10.1080/02786826.2010.484450.
- Moteki, N., and Mori, T. (2015). Theoretical Analysis of a Method to Measure Size Distributions of Solid Particles in Water by Aerosolization. J. Aerosol Sci., 83:25–31, doi:10.1016/j.jaerosci.2015.02.002.
- Ohata, S., Moteki, N., and Kondo, Y. (2011). Evaluation of a Method for Measurement of the Concentration and Size Distribution of Black Carbon Particles Suspended in Rainwater. Aerosol Sci. Technol., 45:1326–1336, doi:10.1080/02786826.2011.593590.
- Ohata, S., Moteki, N., Schwarz, J., Fahey, D., and Kondo, Y. (2013). Evaluation of a Method to Measure Black Carbon Particles Suspended in Rainwater and Snow Samples. Aerosol Sci. Technol., 47:1073–1082, doi:10.1080/02786826.2013.824067.
- Orsini, D., Ma, Y., Sullivan, A., Sierau, B., Baumann, K., and Weber, R. (2003). Refinements to the Particle-Into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition. Atmos. Environ., 37:1243–1259, doi:10.1016/S1352–2310(02)01015–4.
- Ramanathan, V., and Carmichael, G. (2008). Global and Regional Climate Changes due to Black Carbon. Nature Geosci. 1:221–227, doi:10.1038/ngeo156.
- Schwarz, J. P., Doherty, S. J., Li, F., Ruggiero, S. T., Tanner, C. E., Perring, A. E., Gao, R. S., and Fahey, D. W. (2012). Assessing Single Particle Soot Photometer and Integrating Sphere/Integrating Sandwich Spectrophotometer Measurement Techniques for Quantifying Black Carbon Concentration in Snow. Atmos. Meas. Tech., 5:2581–2592, doi:10.5194/amt-5–2581–2012.
- Schwarz, J. P., Gao, R. S., Perring, A. E., Spackman, J. R., and Fahey, D. W. (2013). Black Carbon Aerosol Size in Snow. Nature Sci. Rep., 3:1356, doi:10.1038/srep01356.
- Stephens, M., Turner, N., and Sandberg, J. (2003). Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity. Appl. Opt., 42:3726–3736.
- Warren, S. G., and Wiscombe, W. J. (1980). A Model for the Spectral Albedo of Snow, II, Snow Containing Atmospheric Aerosols. J. Atmos. Sci., 37:2734–2745.
- Weber, R. J., Orsini, D., Daun, Y., Lee, Y.-N., Klotz, P. J., and Brechtel, F. (2001). A Particle-Into-Liquid Collector for Rapid Measurement of Aerosol Bulk Chemical Composition. Aerosol Sci. Technol., 35:718–727.
- Wendl, I. A., Menking, J. A., Färber, R., Gysel, M., Kaspari, S. D., Laborde, M. J. G., and Schwikowski, M. (2014). Optimized Method for Black Carbon Analysis in Ice and Snow Using the Single Particle Soot Photometer. Atmos. Meas. Tech., 7:2667–2681, doi:10.5194/amt-7–2667–2014.