ABSTRACT
The CIP 10-M personal sampler measures worker exposure to airborne particles by collecting particles in a rotating metal cup containing a few milliliters of a collection fluid. This device is mainly used to sample microorganisms or microbial components to measure bioaerosol concentrations in various occupational environments. Aqueous liquids are generally used, but their rapid evaporation limits the duration of sampling; alternative collection fluids could alleviate this problem. Indeed, the particle-collection efficiency of the rotating cup has not been extensively studied, and the only data available relate to a discontinued model. This study aimed to measure the collection efficiency of the current rotating cup model containing an aqueous (water) or viscous (ViaTrap mineral oil) collection fluid. The kinetics of evaporation confirmed that ViaTrap does not evaporate, making 8-h sampling campaigns in constant volumes feasible. Particles with a wide range of aerodynamic diameters (between around 0.1 and 10 µm) were produced using various test rigs and mono- or polydisperse test aerosols. Both new and older cup models performed similarly, with a collection efficiency of >80% for larger particles (aerodynamic diameters >2.8 µm), progressively decreasing to around 50% for aerodynamic diameters of 2.1 µm; with aerodynamic diameters of <1 µm, the collection efficiency was generally <10%. In physical terms, collection efficiency was unaffected by the type (aqueous or viscous) or volume (between 0 and 3 mL) of collection fluid used. Bias maps indicated that the inhalable fraction may be underestimated in occupational settings, particularly with aerosols mainly composed of particles with aerodynamic diameters of less than around 3 µm.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Various sampling techniques have been developed to quantify personal exposure to airborne biological agents. One of the devices used is the CIP 10-M microbiological pollutants personal sampler (Capteur Individuel de Polluants Microbiologiques in French). The CIP 10-M was developed on the basis of the CIP 10-I, which is an aerosol sampler designed to collect the conventional inhalable mass concentration and equipped with a plastic rotating cup that contains a porous polyurethane foam filter to collect the airborne particles (Görner et al. Citation2009). The CIP 10-M is equipped with the same annular omnidirectional aerosol inlet orifice () than the CIP 10-I. The CIP 10-M collects particles in a specific rotating, sterilizable metallic cup filled with around 2 mL of a collection fluid. Collection into a liquid is considered to cause less stress to airborne microorganisms and to avoid the desiccation of vegetative bacteria that can occurred when samples are collected on filters (Jensen et al. Citation1992; Crook et al. Citation1997; Li et al. Citation1999; Wang et al. Citation2001) or on the foam of the CIP 10-I's rotating cup, for example. The sampling cup is equipped with radial blades on its upper part () and rotates inside its housing at a speed close to 7000 rpm to induce a 10 L/min sampling airflow through the particle-size selector (Görner et al. Citation2006).
Figure 1. Pictures of the CIP 10-M sampler. (1) Particle-size selector/aerosol aspiration via an omnidirectional annular slot; (2) rotating cup containing the collection fluid; (2a) older-model cup studied by Görner et al. Citation(2006); (2b) cup available since 2008; (3) box containing internal rechargeable batteries/motor/electronic regulator circuit; (4) magnetic start and stop system; (5) housing for the rotating cup; (6) air exhaust orifice. The dotted-line circles highlight the three main differences between the old and the new sampling cups (further explanations on A, B, and C in Section 2.1).

The CIP 10-M is easy to use to perform measurements in occupational atmospheres: it is compact, light, and quiet, it does not require an external pump for active sampling, it is battery operated and can offer full work shift autonomy if necessary, and it can be used to collect personal samples in the workers' breathing zone or to collect stationary air samples.
The CIP 10-M is mainly used to sample microorganisms or microbial components to measure bioaerosol concentrations in various occupational environments. Some examples of its use are listed in Table S1 in the online supplemental information (SI). A range of methods has been used to analyze samples, and the CIP 10-M can be used to measure a large number of particulate microbial entities. It has also been used to sample some chemical components in particulate form, such as 4,4-methylene diphenyl diisocyanate (MDI; Puscasu et al. Citation2015).
The major disadvantage of aqueous collection fluids—the liquids most commonly used with the CIP 10-M—is that they evaporate rapidly. This evaporation has led authors who use water as the collection fluid to limit the duration of sampling to 150 min or less (see Table S1 in the SI). For sampling durations of around 8 h, used, for example, when seeking to assess worker exposure, two collection modes must be distinguished: (1) the start of sampling when particles are collected in a continuously diminishing volume of liquid because of evaporation and (2) the end of sampling when aerosols are sampled in a completely dry cup. An alternative to aqueous liquids would be to work with a viscous, non-evaporating liquid to allow particle collection in a known, constant volume of liquid for the whole duration of sampling. To our knowledge, Puscasu et al. Citation(2015) are the only authors to have tested sampling with a CIP 10-M using a viscous liquid. In their work, the aqueous medium normally used with the CIP 10-M was replaced by a non-volatile viscous solvent (tributylphosphate) containing a derivatization agent (1-(2-methoxyphenyl)piperazine). The MDI aerosol was therefore solubilized in the solvent and rapidly stabilized by reaction with the derivatization agent. The stable derivative formed was then analyzed using liquid chromatography techniques.
Very little experimental data relating to the collection efficiency of the CIP 10-M cup are available. We only found one article, Görner et al. Citation(2006), describing this type of results. In this study, 2 mL of apyrogenic sterile ultra-pure water was used as the collection fluid. The results showed a collection efficiency of 95% for particles with an aerodynamic diameter (dae) greater than 2.8 µm. At smaller diameters, a progressively decreasing sigmoid curve descended to reach around 50% and 15% for aerodynamic diameters of 1.8 µm and 0.8 µm, respectively.
However, the model of the cup studied in Görner et al. Citation(2006) was discontinued a number of years ago; a new version was commercialized in 2008 and is now (exclusively) sold with the CIP 10-M. The differences between the two cup models include altered external and internal geometries. The consequences of these differences on the initial volume of collection fluid or on the physical efficiency of collection have yet to be established. The volume and nature of the collection fluid used (aqueous or viscous) should also be investigated to determine how it influences the physical collection efficiency of the CIP 10-M.
The current study aimed to measure the collection efficiency of the new rotating cup model filled with an aqueous liquid (water) or a viscous liquid (ViaTrap mineral oil). The kinetics of evaporation for the two liquids as a function of the duration of use of the CIP 10-M were measured. We hypothesized that a viscous, non-evaporating liquid could allow an increase in sampling durations and alter the physical performance of the cup. We also wished to compare collection efficiency for the new-model cup to that of the older-model cup.
2. Materials and methods
2.1. Description of the version of the CIP 10-M aerosol sampler tested
The version of the CIP 10-M studied (Tecora, Fontenay sous Bois, France) was equipped with the most recent models of the particle selector for the inhalable fraction (high efficiency CIP 10-I; Görner et al. Citation2009) and of the rotating cup. This version is the only one currently commercially available. The aerosol is aspirated through an omnidirectional slot that is partly shielded by a protective cap. The annular aspiration slot consists of two horizontal circular plates connected to a vertically oriented cylindrical tube. The T-shape of the selector minimizes deposits of particles between the aspiration slot and the rotating cup and is fairly compatible with the selection of the inhalable aerosol fraction to monitor for health-related exposure (Görner et al. Citation2009, Citation2010).
The differences in geometry between the previous cup and the cup available since 2008 are as follows (): (A) the blades located on the external face of the upper region of the new cup (radial grooving) are not beveled on their outer edges; (B) the central conical opening of the new cup is perfectly pointed; and (C) the angle formed by the internal face of the upper crown with the vertical outside edge was 90° on the older model, and is around 115° on the new model. The aim of the first two modifications is to facilitate aspiration by a centrifugal ventilation effect (combination of so-called cyclonic and anti-cyclonic flow) toward the cup's internal cylindrical cavity. The third modification improves recovery of the liquid at the end of sampling, avoiding loss of material as the liquid is poured into an analytical tube.
The flow rate for the CIP 10-M was set to 10 L/min (± 2%). It was periodically verified using a test rig where compensation for pressure drop could be applied, in line with the recommendations in the NF-X-43-262 standard (AFNOR Citation2012). The corresponding cup rotation speed was 6750 ± 50 rotations per minute (rpm), as measured with an optical tachymeter (ST-6236B, Tecora, Fontenay sous Bois, France).
2.2. Collection fluids
The two collection fluids studied were an aqueous liquid (old and new cups), type-2 ultra-pure water (Direct Q-5, Merck Chimie SAS, Fontenay sous Bois, France), and a viscous liquid (only with the new cup), ViaTrap mineral oil (SKC, CAS 8042-47-5, Tecora, Fontenay sous Bois, France), which is commercialized as a support for bioaerosol sampling. The cinematic viscosity (66 × 10−6 m2/s at 40°C) and the saturating vapor pressure (<0.13 mbar at 20°C) for ViaTrap are around 100-fold and 180-fold greater than those for water, respectively. Other tests were performed with a phosphate-buffered saline (PBS, 0.05 M phosphate buffer/0.15 M sodium chloride) solution (kinetics of evaporation) and a new dry cup containing no liquid (collection efficiency).
2.3. Kinetics of evaporation of collection fluids
The kinetics of evaporation of the collection fluids in the cup fitted to the CIP 10-M in use were measured by tracking the cup mass over an 8-h sampling period (AE 163, Mettler-Toledo SAS, Viroflay, France, 0.02 mg reproducibility). The maximal usable liquid volume was first determined by filling the cup with 4 mL of liquid and starting rotation at around 6750 rpm. The maximal volume can be deduced based on the mass of the empty cup and based on the mass/volume conversion of the two fluids using the following densities: 1000 kg/m3 for water, 840 kg/m3 for ViaTrap, and 1019 kg/m3 for PBS. The cup and its contents were then weighed at regular intervals during sampling. Experiments were performed in rooms or in a drying oven where the temperature and relative humidity were maintained constant. Three pairs of conditions, which cover most of the occupational and environmental sampling situations, were achieved: a cold and humid atmosphere (T = 10.0 ± 1.0°C; RH = 58 ± 2%), a warm and dry atmosphere (T = 28.5 ± 1.0°C; RH = 23 ± 2%), and a frequently encountered intermediary level (T = 21.0 ± 0.5°C; RH = 40 ± 2%).
2.4. Laboratory equipment used to measure the CIP 10-M's collection efficiency
To take aerodynamic diameters ranging from 0.1 to 10 µm into consideration, measurements using several instruments were performed on various test rigs with a range of complementary test aerosols ().
Table 1. Laboratory equipment used to measure the collection efficiency of the CIP 10-M.
For a given aerodynamic diameter, the collection efficiency, Ec, can be calculated by comparing the number concentrations measured, alternatively or simultaneously, upstream, Cinlet, and downstream, Coutlet, of the rotating cup, based on the following equation:[1]
Except for protocol C, Cinlet corresponded to the particle number concentration entering the rotating cup's housing, which is the one exiting from the T-shape particle-size selector (upstream of the cup). Concerning protocol C, Cinlet was measured upstream the CIP 10-M's selector, assuming that the penetration efficiency (aspiration and transmission) of submicron particles through the CIP 10′s selector was equal to 100%. Coutlet systematically corresponded to the particle number concentration not collected (downstream of the cup).
This measurement protocols required that perfectly stable aerosols be generated for the whole duration of experiments. The collection efficiency was measured in the first ∼15 min from starting the sampling, when the maximal volume of liquid remained in the rotating cup. To investigate the influence of the volume of liquid, the collection efficiency was also determined using an empty new cup.
2.4.1. Horizontal wind tunnel (protocol A)
For aerodynamic diameters ranging from 0.9 µm to 10 µm, the collection efficiency was measured in the same horizontal aeraulic tunnel as the tests performed by Görner et al. Citation(2006), measuring approximately 10 m in length. The polydisperse test aerosol, composed of glass microspheres (Ballotini 3000, Potters Europe, Châteaubernard, France), was produced at the entrance to the tunnel with a “puldoulit”-type fluidized-bed generator (Guichard Citation1976). The particles generated were electrically neutralized using a corona discharge ionizer (SC-67, Elcowa SA, Mulhouse, France). The CIP 10-M was placed in a test area corresponding to a 1×1 m2 cross-sectioned area located around the middle of the tunnel. Flow was stabilized between the site of aerosol generation and the test area using a 3-m long pyramidal duct fitted with a 6-mm honeycomb mesh and a 0.75-mm grid mesh at its outlet. The air speed at the point of measurement was maintained constant, at 0.14 ± 0.02 m/s (anemometer Velocicalc 9565-P, TSI France Inc., Marseille, France). The number concentration in the measurement zone was continuously checked with an optical particle counter (OPC, 1.109, Grimm, Ainring, Germany) to ensure its stability.
The particle number concentrations for the different aerodynamic diameters were measured alternately upstream and downstream of the cup using an aerodynamic particle sizer spectrometer (APS, Model 3321, TSI France Inc., Marseille, France). The APS allowed real-time monitoring of the particle number size distribution as a function of aerodynamic diameter (dae) for particles with diameters from 0.542 to around 20 µm, at a nominal flow rate of 5 L/min. The particle density of glass beads, 2.46 g/cm3, and the Stokes correction algorithm were applied in the Aerosol Instrument Manager Software (version 8.1.0.0, TSI, December 2007, Wang and John Citation1987). The experimental setup also included a vertical conditioning volume for the aerosol to allow sampling at 5 L/min (toward the APS) from the 10 L/min flow produced by the CIP 10-M, as described by Görner et al. Citation(2001). Further details on the aeraulic tunnel and the method used to measure the collection efficiency are available in Fabriès et al. Citation(1984) and Görner et al. (Citation2001, Citation2006).
2.4.2. Test rig to generate polydisperse submicronic aerosols (protocol B)
For aerodynamic diameters of less than 1 µm, the collection efficiency of the CIP 10-M was measured using the experimental setup shown in .
Figure 2. Diagram of the experimental setups used to measure the collection efficiency of the CIP 10-M cup with (a) polydisperse aerosol of DEHS, (b) monodisperse DEHS aerosols, and (c) polydisperse bacterial aerosol of E. coli.
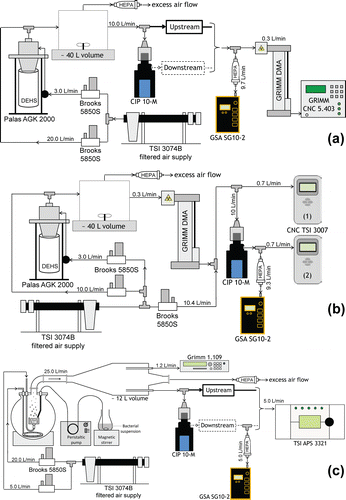
A polydisperse aerosol of Di-Ethyl-Hexyl-Sebacat (DEHS) droplets was generated by nebulization (AGK 2000, Palas, Karlsruhe, Germany) in an approximately 40-L homogenization chamber, inside which air movement was maintained constant using a stirrer. A flow rate of 10 L/min was aspirated from this chamber to feed the CIP 10-M being tested. The particle number size distributions were measured either upstream or downstream of the cup with a scanning mobility particle sizer (SMPS, DMA Vienna Type/CPC 5.403, Grimm, Ainring, Germany). Every concentration measured downstream was associated with an averaged upstream concentration, which stability was verified. Electrical mobility diameters were converted into aerodynamic diameters based on the following implicit relationship (De Carlo et al. Citation2004):[2] where
g/cm3 and
is the Cunningham correction factor in the intermediate and molecular regimes, determined from the following equation:
[3] with
(Kim et al. Citation2005) and
nm at 20°C and atmospheric pressure.
2.4.3. Test rig for the generation of monodisperse submicronic aerosols (protocol C)
For submicron aerodynamic diameters, the collection efficiency was also measured using the experimental setup shown in . A given electrical mobility diameter was selected from a polydisperse aerosol of DEHS droplets (AGK 2000, Palas), using a DMA Vienna Type selector (Grimm, Qsheath = 3 L/min, Qaerosol = 0.3 L/min). Assuming that the proportion of multiply charged particles was negligible (see, e.g., Bau et al. Citation2012), this results in the production of monodisperse aerosols with a given electrical mobility diameter, and a corresponding geometric standard deviation typically smaller than 1.05, as described in previous studies where a model for the DMA transfer function was applied (Bau et al. Citation2014).
Ten aerodynamic diameter values between 0.14 and 0.95 µm were used in this series of experiments. The electrical mobility diameters were converted into aerodynamic diameters using a similar protocol to that described in Section 2.4.2.
The particle number concentrations were simultaneously measured upstream and downstream of the CIP 10-M using two condensation particle counters (CPC 3007, TSI France Inc., Marseille, France). Preliminary tests to compare the two CPCs used in this work confirmed the conformity of the measurements over a range of number concentrations from 4.103 to 9.104 cm−3 and for aerosols with median diameters between 6 and 100 nm (CPC(1) = 1.0563 × CPC(2), R2 = 0.9577, data not shown). The difference in response between the two instruments was small enough so that no correction was necessary when calculating the collection efficiency.
2.4.4. Test rig for bioaerosol generation (protocol D)
A fourth test rig produced particles with aerodynamic diameters between 0.7 and 3 µm. As shown in , this setup included a “Liquid Sparging Aerosolizer”-type bubbling generator and a 12-L homogenization vessel equipped with six sampling probes that have been described elsewhere (Simon et al. Citation2011; Simon and Duquenne Citation2013). The test aerosol consisted of a model microorganism, E. coli (Institut Pasteur CIP 53.126). Liquid cultures of vegetative cells were prepared as described in Simon et al. Citation(2011). A peristaltic pump fed the liquid culture onto the upper surface of a porous stainless steel disk. The liquid culture settled as a liquid film of constant height for the full duration of bioaerosol generation. Bacterial cells were dispersed by bubbling compressed air (5 L/min) through the film of liquid culture; transport of the aerosol produced toward the generator outlet was assisted by injection of upward entraining air (20 L/min).
The stability of the bacterial number concentration generated was continuously monitored with an OPC (1.109, Grimm, size channels in the range 0.3–4 µm). The particle number size distributions were alternately measured upstream and downstream of the CIP 10-M using an APS and a similar method to that described in Section 2.4.1.
2.5. Calculation of CIP 10-M bias based on particle number or mass concentrations
The overall sampling efficiency of the CIP 10-M is the product of the collection efficiency and the penetration efficiency (aspiration and transmission) of the CIP 10′s T-shaped inhalable fraction selector, as measured by Görner et al. (Citation2009, Citation2010).
The collection efficiency curve for the CIP 10-M was modeled by a mathematical function combining three increasing cumulative log-normal functions. The inlet efficiency values for the CIP 10-M's inhalable fraction selector (experimental points at 1 m/s tunnel wind speed) were fitted by a decreasing cumulative log-normal function. The optimized values of the model parameters were determined using least-square optimization procedures. Finally, the product of these two models provided an expression of the overall sampling efficiency as a function of particle aerodynamic diameter between 0.1 and 40 µm. This function can be used to predict the CIP 10-M's sampling behavior with various polydisperse aerosols.
Generally, sampler bias is the percentage difference between the mass concentration measured by the sampler tested with its own sampling efficiency curve, and the mass concentration that would have been measured by an ideal sampler exactly conforming to the target conventional curve (inhalable, thoracic, or respirable). The method for bias calculations has been detailed elsewhere (Lidén and Kenny Citation1992; Görner and Fabriès Citation1996; Fabriès et al. Citation1998; AFNOR Citation2014).
Calculations were performed to simulate the assumed mass concentration sampled in the rotating cup of the CIP 10-M, CM,CIP10-M. This value was compared to the conventional inhalable mass concentration, CM,I, which is the target reference aerosol fraction for any given aerosol mass size distribution, characterized by its mass median aerodynamic diameter (MMAD) and geometric standard deviation (σg). The mass-based bias, BIASM, can be calculated using the following equation:[4]
All the biases represented in the BIASM map (MMAD on the x-axis, σg on the y-axis) were calculated for polydisperse aerosols, in which particles between 0.1 and 40 µm represent more than 95% of the total particle mass, i.e., the diameter range for which the CIP 10-M's sampling efficiency is determined.
Similarly, the number-based bias, BIASN, can be calculated using Equation Equation(5)[5] :
[5] where CN,CIP10-M is the assumed number concentration sampled in the rotating cup of the CIP 10-M; CN,I is the conventional inhalable number concentration strictly conforming to the conventional inhalable curve; and CMAD is the count median aerodynamic diameter for the aerosol considered.
All the biases represented on the BIASN map (CMAD on the x-axis, σg on the y-axis) were calculated for polydisperse aerosols, in which particles between 0.1 and 40 µm represent more than 95% of the total particle number.
Finally, with identical calculation parameters (MMAD = CMAD, same σg), the results for Equations Equation(5)[5] and (6) gave the same bias value, i.e., BIASN = BIASM. Therefore, the mass-based bias, BIASM (MMAD on the x-axis, σg on the y-axis), and the number-based bias, BIASN (CMAD on the x-axis, σg on the y-axis), were presented on the same map.
3. Results
3.1. Kinetics of evaporation of collection fluids
The changes in volume of the collection fluid present in the CIP 10-M cup at different sampling times for two aqueous liquids (water and PBS) and a viscous liquid (ViaTrap) are represented in . To increase the readability, some of the curves were not plotted: T = 10°C/RH = 58% and T = 28°C/RH = 23% for both PBS and ViaTrap.
Figure 3. Kinetics of evaporation for water, PBS, and ViaTrap present in the rotating cup of the CIP 10-M. Error bars represent the 95% confidence interval (IC95).
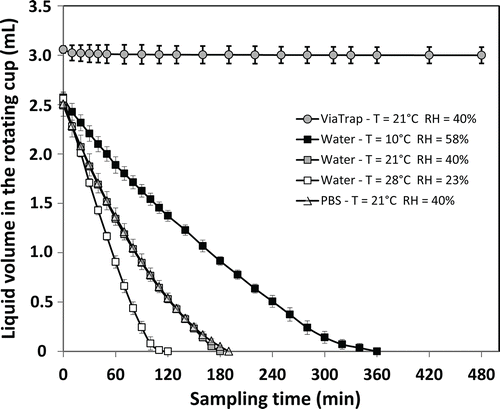
First, we note that the maximal volume of liquid that the cup can hold differed for the two types of collection fluids. When the cup was loaded with 4 mL of fluid, starting rotation led to the immediate expulsion of some of the liquid from the internal cavity due to the centrifugal force. This resulted in a remaining greater volume of ViaTrap (around 3.0 mL) than water and PBS (around 2.5 mL), which can be explained by their differing rheological properties.
Whatever the tested conditions, ViaTrap did not evaporate (data not shown for “10°C/58%” and “28°C/23%”), whereas the curves describing the kinetics of evaporation for water showed a rapid decrease in the initial volume. Curves obtained with PBS are in close agreement with ones for water (data not shown for “10°C/58%” and “28°C/23%”). For aqueous fluids, the kinetics of evaporation at 21°C and 40% of relative humidity decreased by a factor of 2 and 5 within 1 and 2 h, respectively; after 3 h sampling, the cup was completely dry. The evaporation in a colder atmosphere with a greater relative humidity was slower: the initial volume decreased by a factor 2 within around 140 min; the cup was totally dry only after 6 h (). In the warmer and dryer tested atmosphere, the aqueous liquids completely dried out in less than 2 h.
3.2. Collection efficiency of the new CIP 10-M rotating cup
The collection efficiency for the cup is shown in for an extended range of aerodynamic diameters between 0.1 and 10 µm.
Figure 4. Collection efficiency for the CIP 10-M cup filled with different collection fluids (water, ViaTrap) for particles with aerodynamic diameters between 0.1 and 10 µm. Error bars represent the IC95.
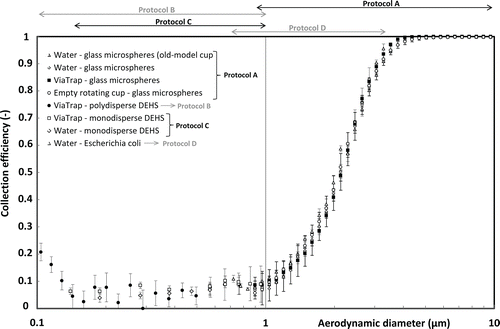
Regardless of the test aerosol or the cup's model, all the experimental points describing the collection efficiency's curve follow the same decreasing sigmoidal trend. Like the older-model cup, the collection efficiency of the new cup was above 80% and 95%, for particles presenting aerodynamic diameters greater than 2.8 µm and 3.5 µm, respectively. For aerodynamic diameters between 0.9 µm and 10 µm, our results also showed similar collection efficiencies for the new- and old-model cups. At smaller diameters, the curve described a progressively decreasing sigmoid reaching around 50% and 10% for aerodynamic diameters of 2.1 µm and 1 µm, respectively. Whatever the collection fluid, shows that the cup's collection efficiency generally remained smaller than 10% for particles' diameters less than 1 µm. The collection efficiency did not depend on the nature of the liquid present in the cup (aqueous or not) nor on its volume (3 mL of ViaTrap, 2.5 mL water, or no liquid).
3.3. Number-based and mass-based bias maps for the CIP 10-M
The overall curve representing the sampling efficiency for the CIP 10-M was composed of two parts presenting a maximum (see Figure S2 in the SI). The increasing part corresponded to the collection efficiency in the rotating cup, as described in . This efficiency was modeled using a combination of three cumulative log-normal functions with the following parameters: CMAD1 = 80.49 µm; σg1 = 23.09; 0.1 ≤ dae < 1.11 µm, CMAD2 = 2.14 µm; σg2 = 1.63; 1.11 ≤ dae < 2.08 µm and CMAD3 = 2.12 µm; σg3 = 1.36; 2.08 ≤ dae ≤ 40 µm.
The decreasing part corresponded to the penetration efficiency for the selector of the inhalable fraction, as measured by Görner et al. (Citation2009, Citation2010, Figure S2). This part was modeled using a decreasing cumulative log-normal function with the following parameters: CMAD = 30 µm; σg = 1.9; 0.1 ≤ dae ≤ 40 µm. The product of the increasing model and the decreasing model provided the expression representing the overall sampling efficiency for the CIP 10-M as a function of aerodynamic diameter between 0.1 and 40 µm and was further used to calculate the bias map, as explained in Section 2.5.
The bias map was plotted as a function of the aerosol size distribution parameters (CMAD or MMAD, σg) and points of equal bias were connected by contour lines (. Such representation highlights the relative discrepancies between the mass concentrations (if MMAD is considered on the x-axis) or the number concentrations (if CMAD is considered on the x-axis) measured by the CIP 10-M and the ones from an ideal sampler of the inhalable fraction. The colored area represents all the parameters of the size distribution for which the bias value is between ±20%. The two gray areas correspond to aerosol distributions for which the bias could not be calculated since, for these regions, less than 95% of the total particle mass (if MMAD is considered on the x-axis) or of the total particle number (if CMAD is considered on the x-axis) was between 0.1 and 40 µm (see Section 2.5).
Figure 5. Bias map for the CIP 10-M for log-normal aerosol size distribution. Contours representing equal mass-based bias (%) relative to the inhalable fraction as a function of MMAD and σg or contours representing equal number-based bias (%) relative to the inhalable fraction as a function of CMAD and σg.
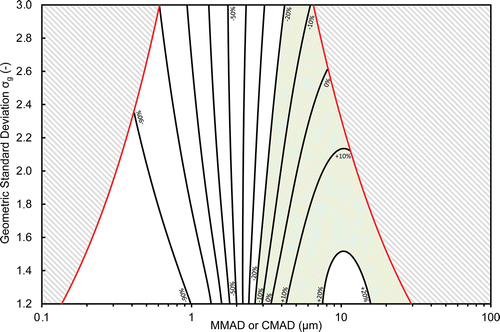
Aerosols containing a majority of fine particles (aerodynamic diameter smaller than about 3 µm) systematically presented negative biases regardless of the geometric standard deviation. For lower aerodynamic diameters, the bias decreased rapidly to −80% or less for the majority of aerosols presenting diameters smaller than 1 µm. The region indicated for aerosols with diameters between approximately 7.5 and 15 µm and σg less than 1.5 presented a bias greater than +20%; sampling of these aerosols with the CIP 10-M will therefore lead to overestimation of the particle concentration if compared to a sampling method exactly representing the inhalable fraction.
4. Discussion
4.1. Kinetics of evaporation of collection fluids and durations of sampling
The kinetics of evaporation of an aqueous liquid, such as water or PBS, resulted in a progressive reduction in the volume of liquid contained in the cup during the sampling time (Section 3.1). Particles are thus collected in a diminishing volume of liquid over the first hours of sampling, then in a dry cup if sampling goes on for longer. Contrary to the ViaTrap behavior, the kinetics of evaporation of aqueous fluids () differed from each other according to the values of temperature and relative humidity. The range of conditions investigated in this article covers most of the sampling situations encountered in occupational areas. However, since the collection efficiency did not depend on the volume of liquid present in the CIP 10-M cup (Section 3.2), the particles will nevertheless be similarly collected for the whole sampling period. Considering only the physical aspects of sampling, the presence of liquid therefore did not appear to be essential to the measurement.
Nevertheless, the presence of liquid in the cup can sometimes be necessary to ensure or improve conservation of the properties of the particles to be measured in the sample. This is the case, for example, with MDI aerosols that must be sampled into a liquid where a solvent and a derivatization agent will rapidly solubilize and stabilize the MDI monomers and oligomers before they polymerize (Lesage et al. Citation2007; Puscasu et al. Citation2015). Sulfuric acid aerosols also need to be stabilized during sampling, for example, by using a liquid that inhibits the chemical reactions between acid droplets and other pollutants present to avoid the modification or alteration of relevant analytes before analysis. Sensitive microorganisms such as vegetative bacterial cells may also undergo stress or injury during sampling, particularly due to desiccation (Jensen et al. Citation1992; Crook et al. Citation1997; Li et al. Citation1999; Wang et al. Citation2001); their collection in a liquid therefore appears less harmful than collection on a dry collecting stage (e.g., filter media). Thus, for some substances, using an aqueous liquid as a collection support in the CIP 10-M cup may be incompatible with the need to perform sampling over several hours in the presence of liquid. The measurement strategy can oblige the sampler to use long-duration sampling methods: measurement of a worker's occupational exposure throughout a full work shift, constraints linked to the performance of subsequent analysis methods (sensitivity, limits of detection, and quantification), etc. The fact that the initial volume of an aqueous liquid is reduced by half after only 1–2.5 h of sampling can thus be a constraint linked to the use of the CIP 10-M for some measurement strategies.
In contrast, the kinetics of evaporation of ViaTrap mineral oil in the CIP 10-M cup showed that this viscous liquid did not evaporate over time. It will therefore be possible to perform sampling over an 8-h period in the presence of a constant volume of liquid. This configuration makes it possible to use the CIP 10-M with a much broader range of sampling durations, thus giving operators more freedom to develop appropriate exposure assessment strategies. However, the compatibility of samples collected in viscous liquids with downstream analytical methods remains to be validated in many cases.
4.2. Complementarity of the test rigs used to measure collection efficiencies
To cover the full range of aerodynamic diameters between 0.1 and 10 µm and perform relevant efficiency measurements, complementary test aerosols had to be used, as illustrated by the results presented in and Figure S1. The complementarities of these setups made it possible to get adequate particle number concentrations for the whole range of diameters studied. The measurements performed with the different submicronic monodisperse and polydisperse aerosols of DEHS thus completed the data obtained with the spherical micronic glass particles, while the E. coli aerosol was used to obtain intermediate experimental points for diameters between 0.7 and 3 µm. It was therefore possible to extend the collection efficiencies measured for the rotating cup on the CIP 10-M to submicronic particles, which constitutes an improvement to the data published by Görner et al. in 2006.
This measurement protocol also required that aerosols be perfectly stable throughout the duration of experiments, in particular when particle number concentrations were measured alternately upstream and downstream of the collection cup.
4.3. Performance and limits of the CIP 10-M
4.3.1. Comparison of the collection efficiency of the new-model and older-model cups
Our results showed that, over the range of aerodynamic diameters between 0.9 and 10 µm, the collection efficiencies of the new cup were identical to those of the older cup (). The modifications to internal and external geometries, made in 2008 and detailed in Section 2.1, therefore did not affect the physical collection efficiency of the CIP 10-M cup; the stream-lines, the collection mechanisms, and the cup's capacity to retain particles were unchanged. The pollutant concentrations measured with a CIP 10-M fitted with an old-model cup can thus be directly compared to those obtained with a CIP 10-M fitted with a new-model cup, provided that all other sampling conditions are identical.
4.3.2. Influence of aerosol size distribution: Usefulness of bias maps
The curve representing the collection efficiency of the new cup showed a gradually decreasing sigmoid at lower aerodynamic diameters, corresponding to a cut-off diameter of 2.1 µm (). The cup presented collection efficiencies systematically smaller than 100% for all particles with an aerodynamic diameter of less than around 3 µm; therefore, using the CIP 10-M to sample the conventional inhalable fraction in occupational atmospheres leads to underestimation of exposure levels for workers exposed to the finest aerosols. The physical mechanisms resulting in the collection of particles in the rotating cup are not yet well determined. According to Görner et al. Citation(2006), particles enter the rotating cup axially, follow a helicoidal trajectory, and are driven by centrifugal force toward the liquid collection surface that is maintained in vertical position in the cup due to centrifugal force. The shape of the collection efficiency curve is quite similar to that of some single-stage microbiological impactors, suggesting that the inertia of particles plays an important role in their collection. Even so, the information provided in this article does not allow to further explain the particle collection mechanisms.
Bias maps were constructed for particle number and mass measurements (Sections 2.5 and 3.3). The mass-based bias map is the most commonly used in the literature; it can be used to estimate the relevance of using a sampling device based on the characteristics of the aerosol encountered when the mass concentration of pollutant (mg/m3) is the indicator sought. In this article, the number-based bias map was also calculated as the CIP 10-M can be used to measure total (cells/m3) number microorganisms concentrations, for example. The two approaches, in mass and number, were therefore necessary to supply complete information covering the various uses of the CIP 10-M. In addition, the bias calculations were performed over a restricted range of size distributions based on the lower (dae = 0.1 µm) and upper limits (dae = 40 µm) for which the sampling efficiency of the CIP 10-M was determined.
Retention of aerosols containing a majority of fine particles (MMAD or CMAD smaller than roughly 3 µm) in the cup is not efficient enough, thus leading to underestimated concentrations with respect to the inhalable fraction ().
The region of the map presenting biases greater than +20% (7.5 ≤ MMAD or CMAD ≤ 15; σg ≤ 1.5) reflected the behavior of the particle selector used in the CIP 10, for which the penetration efficiency overestimates the inhalable fraction for aerodynamic diameters between around 2 and 20 µm (Görner et al. Citation2009, 2010, Figure S2).
Therefore, like all aerosol samplers, the CIP 10-M has limitations that it is wise to be aware of. Our results clearly show that it can be useful for sampling of some aerosols, but may not be relevant in other situations. For example, small particles such as free bacteria (i.e., mostly single cells) or free fungi (i.e., mostly single spores) will be collected less efficiently than larger particles or structures such as clumped microorganisms, mycelium strands, or various agglomerates. This is important as the size distribution of airborne biological entities (microorganisms, endotoxins, allergens, glucans, mycotoxins, etc.) or particulate chemical compounds (sulfuric acid droplets, MDI aerosols, etc.) is highly variable, depending on the environments investigated, the emission sources, the ventilation, and many other determinants. Examples of the variability of aerosol size distribution and consequences on the concentrations measured using the CIP 10-M are discussed further in Section S1 of the SI. In addition to being more variable from one environment or moment in time to another, the size distribution of the aerosol to be sampled is not always known. In this context, a good knowledge of the performance and limitations of the samplers used can help to choose a device that is appropriate for the aerosol to be sampled, and to better interpret the measurement results. The information provided in this article with regard to the CIP 10-M's performance therefore appears to be essential to the proper use of this sampler in the field.
4.3.3. The physical performances of the CIP 10-M compared to those of other bioaerosol samplers
The collection efficiency of the CIP 10-M was similar to that of some single-stage microbiological impactors. The shape of the collection efficiency curve and the value of the 50% efficiency diameter (dae,50 = 2.1 µm) were quite comparable to the collection efficiencies measured for high-flow rate portable microbial samplers such as the Microbiological Air Sampler (MAS-100, 100 L/min), Surface-Air-Sampler (SAS Super 180, 180 L/min), or Millipore Air Tester (140 L/min), as measured by Yao and Mainelis Citation(2006). Like single-stage impactors, the CIP 10-M can also be considered to be an inertial particle separator (with a rotating cup rather than an impaction plate or a Petri dish). For 0.5 µm particles, both types of devices collect 10% or less on the collection stage. Despite similarities in terms of physical collection efficiency, the CIP 10-M and single-stage impactors are used differently to sample bioaerosols for occupational hygiene purposes. Single-stage impactors are more frequently used at stationary positions for short-duration sampling (≤10 min) to measure culturable bacterial or fungal concentrations in environments where contamination tends to be low. The CIP 10-M, in contrast, can be used for personal sampling over longer durations, its use remains possible in particularly contaminated environments and because biological substances can be recovered from the cup, downstream sample treatments are possible (e.g., dilution) along with a larger number of analyses (see Table S1 in the SI).
The performance of the CIP 10-M can also be compared to that of other newly developed personal bioaerosol samplers. For example, the collection efficiency of the CIP 10-M is close to that of the tube in the 2 L/min one-stage cyclone (Chen et al. Citation2004) and of the first tube of the 2 L/min two-stage cyclone (Lindsley et al. Citation2006), developed by the National Institute for Occupational Safety and Health (NIOSH). Other wet-type personal bioaerosol samplers (tested using water) such as the Frit-Bubbler (Agranovski Citation2007) and the PAS-4 or PAS-5 (Tolchinsky et al. Citation2011) have better collection efficiencies than the CIP 10-M for particles smaller than around 3 µm, but show comparable collection efficiencies (∼100%) for larger particles. The Frit-Bubbler has a 4 L/min flow rate and contains 40 mL of collection fluid. Its physical sampling efficiency is equal to ∼95%, ∼98%, and 99.9% for particles with diameters of 0.3, 1, and 3 µm, respectively (Agranovski Citation2007). The PAS-4 and PAS-5 have an ∼8 L/min flow rate and contain 8 mL and 15 mL of collection fluid, respectively. The collection efficiency for particle diameters of 0.76 µm is about 60% and 50% for the PAS-4 and PAS-5, respectively. For particles larger than 1.2 µm, more than 85% of the test aerosol is collected by both samplers. For particles measuring 2 µm in aerodynamic diameter, collection efficiencies of 97% and 99% have been reported for PAS-4 and PAS-5, respectively (Tolchinsky et al. Citation2011). Finally, the estimated cut-off diameter is 0.67 µm for the PAS-4 and 0.75 µm for the PAS-5. Thus, the CIP 10-M collects fewer micronic and sub-micronic particles than these three wet-type samplers. However, we are not aware that these liquid-based samplers are commercially available, and their sampling efficiency for a specific fraction (inhalable, thoracic, respirable, etc.) has yet to be determined.
4.3.4. Discussion about survival/preservation of biological particles during sampling
Because the main use of the CIP 10-M concerns the sampling of biological material, the biological aspect of the sampling efficiency is of great importance. The biological efficiency characterizes the ability of the device to sample the biological particles without altering the biological properties (viability, culturability, or any other biological activity) that are used to analyze or detect these biological particles (Reponen et al. Citation2011). Depending on the stress and damages that microorganisms undergo during the sampling process, their physiological status may be altered and classified in one of the following categories: culturable, viable but not culturable, nonviable but maintaining membrane integrity or cell fragments (Rule et al. Citation2007). Whatever the bioaerosol sampler considered, the microorganisms are unavoidably exposed to stress, but it is crucial that the sampling method maintains as best as possible their initial physiological status to minimize bias in the quantification or identification of microorganisms.
Our results focus on the physical factors affecting the overall sampling performance of the CIP 10-M and biological factors have not been studied. The biological efficiency can be evaluated using two complementary approaches: (1) laboratory assays that are generally based on the generation of controlled experimental bioaerosol and the use of different analytical methods (cultural, molecular, cytological, or fluorescent techniques, for example); (2) field trials where bioaerosol samplers operate side by side in order to compare them in environments with unknown microbial concentrations. To our knowledge, such a work has never been performed to better assess the microbial preservation and recovery efficiency of the CIP 10-M.
The collection into a liquid is a favorable aspect because it is considered to avoid the stress associated with desiccation of sampled microorganisms that occurs when samples are collected on filters (Jensen et al. Citation1992; Crook et al. Citation1997; Li et al. Citation1999; Wang et al. Citation2001). On the other hand, the microorganisms collected in the rotating cup may be subjected to mechanical stresses (high impaction velocity and centrifugal force, e.g.) that can result in metabolic or structural injuries, and in a decrease in the biological efficiency, especially for sensitive species. In parallel, the compatibility of collection fluids with preservation of biological material and downstream analytical methods also need to be addressed. Indeed, collection in fluids with unfavorable ionic or glycerol contents could result in decreased viability of microorganisms, ruptured cell membranes, and ultimately in the degradation of DNA, due to osmotic stress (Chang and Chou Citation2011; King and McFarland Citation2012). As an example, the user's manual advises that ViaTrap mineral oil may not be suitable for polymerase chain reaction (PCR) analysis.
5. Conclusion
The collection efficiency of the new CIP 10-M rotating cup filled with aqueous (water) or viscous (mineral oil) liquid was measured for a wide range of aerodynamic diameters between around 0.1 and 10 µm. First, the kinetics of evaporation of the collection fluid from the rotating cup was measured. Unlike an aqueous liquid such as water or PBS, which completely evaporates after a few hours of sampling, a viscous liquid such as mineral oil can be used to perform longer-duration sampling without significant change to the initial volume. Using a viscous liquid as collection support thus gives users of the CIP 10-M greater freedom in the development of the sampling strategy, in particular with regard to sampling duration.
Regarding the experimental collection efficiency curve, the cut-off aerodynamic diameter for the new-model rotating cup was close to 2.1 µm. Neither the type (aqueous or viscous) nor the volume (between 0 and 3 mL) of the liquid influenced the physical collection efficiency of the CIP 10-M rotating cup. In addition, the collection efficiency of the new cup was similar to that of the older-model cup. Number-based and mass-based biases were calculated to better interpret the concentration results for sampled aerosol of known size distributions. The bias can also be used as a decision-making aid prior to performing measurements with the CIP 10-M. Using the CIP 10-M to sample the inhalable fraction in occupational areas may lead to underestimations of worker exposure, particularly when the aerosols emitted are mainly composed of particles presenting aerodynamic diameters less than around 3 µm. Only if the size distribution of the aerosol to be sampled and the performance of the device are known, will it be possible to ensure that the CIP 10-M is relevant in a given environment and that the concentration results are valid. The results for physical sampling efficiencies should be completed in the future by determining the CIP 10-M's capacity to ensure preservation of the (biological or chemical) properties of the particles present in the sample, as well as the compatibility of collection fluids with downstream analytical methods.
Online_SI_final_version.docx
Download MS Word (362.4 KB)References
- AFNOR. (2012). Qualité de l'Air – Air des Lieux de Travail – Prélèvement d'Aérosols Solides à l'Aide d'une Coupelle Rotative (Fractions Alvéolaire, Thoracique et Inhalable)/Air Quality – Workplace Air –Solid Aerosol Sampling with a Rotating Cup (Respirable, Thoracic and Inhalable Fractions), NF-X-43-262. AFNOR, La Plaine Saint-Denis, pp. 19.
- AFNOR. (2014). Workplace Exposure – Assessment of Sampler Performance for Measurement of Airborne Particle Concentrations – Part 2: Laboratory Performance Test Based on Determination of Sampling Efficiency, NFEN13205. AFNOR, La Plaine Saint-Denis, pp. 39.
- Agranovski, I. E. (2007). Personal Sampler for Viable Airborne Microorganisms: Main Development Stages. Clean, 35:111–117.
- Bau, S., Bémer, D., Grippari, F., Appert-Collin, J.-C., and Thomas, D. (2014). Determining the Effective Density of Airborne Nanoparticles using Multiple Charging Correction in a Tandem DMA/ELPI Setup. J. Nanopart. Res., 16:2629–2642.
- Bau, S., Jacoby, J., and Witschger, O. (2012). Evaluation of the Diffusion Size Classifier (meDiSC) for the Real-Time Measurement of Particle Size and Number Concentration of Nanoaerosols in the Range 20–700 nm. J. Environ. Monit., 14:1014–1023.
- Chang, C.-W., and Chou, F.-C. (2011). Assessment of Bioaerosol Sampling Techniques for Viable Legionella pneumophila by Ethidium Monoazide Quantitative PCR. Aerosol Sci. Tech., 45:343–351.
- Chen, B. T., Feather, G. A., Maynard, A., and Rao, C. Y. (2004). Development of a Personal Sampler for Collecting Fungal Spores. Aerosol Sci. Tech., 38:926–937.
- Crook, B., Kenny, L. C., Stagg, S., Stancliffe, J. D., Futter, S. J., Griffiths, W. D., and Stewart, I. W. (1997). Assessment of the Suitability of Different Substrate Materials for Bioaerosol Sampling. Ann. Occup. Hyg., 41:647–652.
- De Carlo, P. F., Slowik, J. G., Wornsop, D. R., Davidovits, P., and Jimenez, J. L. (2004). Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory. Aerosol Sci. Tech., 38:1185–1205.
- Fabriès, J. F., Carton, B., and Wrobel, R. (1984). Equipment for the Study of Air Sampling Instruments with Real Time Measurement of the Aerosol Concentration. Staub. Reinhalt. Luft., 44:405–409.
- Fabriès, J. F., Görner, P., Kauffer, E., Wrobel, R., and Vigneron, J. C. (1998). Personal Thoracic CIP 10-T Sampler and its Static Version CATHIA-T. Ann. Occup. Hyg., 42:453–465.
- Görner, P., and Fabriès, J. F. (1996). Industrial Aerosol Measurement According to the New Sampling Conventions. Occup. Hyg., 3:361–376.
- Görner, P., Fabriès, J. F., Duquenne, P., Witschger, O., and Wrobel, R. (2006). Bioaerosol Sampling by a Personal Rotating Cup Sampler CIP 10-M. J. Environ. Monit., 8:43–48.
- Görner, P., Simon, X., Wrobel, R., Kauffer, E., and Witschger, O. (2010). Laboratory Study of Selected Personal Inhalable Aerosol Samplers. Ann. Occup. Hyg., 54:165–187.
- Görner, P., Wrobel, R., Micka, V., Skoda, V., Denis, J., and Fabriès, J. F. (2001). Study of Fifteen Respirable Aerosol Samplers Used in Occupational Hygiene. Ann. Occup. Hyg., 45:43–54.
- Görner, P., Wrobel, R., and Simon, X. (2009). High Efficiency CIP 10-I Personal Inhalable Aerosol Sampler. J. Phys. Conf. Ser. 151(012061):1–9.
- Guichard, J. C. (1976). Aerosol Generation Using Fluidized Beds, in Fine Particles – Aerosol Generation, Measurement, Sampling, and Analysis, B. Y. H. Liu, ed., Academic Press, New York, pp. 173–193.
- Jensen, P. A., Todd, W. F., Davis, G. N., and Scarpino, P. V. (1992). Evaluation of Eight Bioaerosol Samplers Challenged with Aerosols of Free Bacteria. Am. Ind. Hyg. Assoc. J., 53:660–667.
- Kim, J. H., Mulholland, G. W., Kukuck, S. R., and Pui, D. Y. H. (2005). Slip Correction Measurements of Certified PSL Nanoparticles using a Nanometer Differential Mobility Analyzer (nano-DMA) for Knudsen Number from 0.5 to 83. J. Res. Natl. Inst. Stan., 110(1):31–54.
- King, M. D., and McFarland, A. R. (2012). Bioaerosol Sampling with a Wetted Wall Cyclone: Cell Culturability and DNA Integrity of Escherichia coli Bacteria. Aerosol Sci. Tech., 46:82–93.
- Lesage, J., Stanley, J., Karoly, W. J., and Lichtenberg, F. W. (2007). Airborne Methylene Diphenyl Diisocyanate (MDI) Concentrations Associated with the Application of Polyurethane Spray Foam in Residential Construction. J. Occup. Environ. Hyg., 4:145–155.
- Li, C. S., Hao, M. L., Lin, W. H., Chang, C. W., and Wang, C. S. (1999). Evaluation of Microbial Samplers for Bacterial Microorganisms. Aerosol Sci. Tech., 30:100–108.
- Lidén, G., and Kenny, L. C. (1992). The Performance of Respirable Dust Samplers: Sampler Bias, Precision and Inaccuracy. Ann. Occup. Hyg., 36:1–22.
- Lindsley, W. G., Schmechel, D., and Chen, B. T. (2006). A Two-Stage Cyclone using Microcentrifuge Tubes for Personal Bioaerosol Sampling. J. Environ. Monit., 8:1136–1142.
- Puscasu, S., Aubin, S., Cloutier, Y., Sarazin, P., Tra, H. V., and Gagné, S. (2015). CIP10 Optimization for 4,4-Methylene Diphenyl Diisocyanate Aerosol Sampling and Field Comparison with Impinger Method. Ann. Occup. Hyg., 59:347–357.
- Reponen, T., Willeke, K., Grinshpun, S., and Nevalainen, A. (2011). Biological Particle Sampling, in Aerosol Measurement: Principles, Techniques and Applications. 3rd ed. P. Kulkarni, P. A. Baron, and K. Willeke, eds., John Wiley & Sons Inc., New Jersey, pp. 549–570.
- Rule, A. M., Kesavan, J., Schwab, K. J., and Buckley, T. J. (2007). Application of Flow Cytometry for the Assessment of Preservation and Recovery Efficiency of Bioaerosol Samplers Spiked with Pantoea agglomerans. Environ. Sci. Tech., 41:2467–2472.
- Simon, X., and Duquenne, P. (2013). Feasibility of Generating Peaks of Bioaerosols for Laboratory Experiments. Aerosol Air Qual. Res., 13:877–886.
- Simon, X., Duquenne, P., Koehler, V., Piernot, C., Coulais, C., and Faure, M. (2011). Aerosolisation of Escherichia coli and Associated Endotoxin Using an Improved Bubbling Bioaerosol Generator. J. Aerosol Sci., 42:517–531.
- TolchinskY, A. D., Sigaev, V. I., Varfolomeev, A. N., Uspenskaya, S. N., Cheng, Y. S., and Su, W. C. (2011). Performance Evaluation of Two Personal Bioaerosol Samplers. J. Environ. Sci. Health, Part A, 46:1690–1698.
- Wang, H. C., and John, W. (1987). Particle Density Correction for the Aerodynamic Particle Sizer. Aerosol Sci. Tech., 6:191–198.
- Wang, Z., Reponen, T., Grinshpun, A. S., Gorny, L. R., and Willeke, K. (2001). Effect of Sampling Time and Air Humidity on the Bioefficiency of Filter Samplers for Bioaerosol Collection. J. Aerosol Sci., 32:661–674.
- Yao, M., and Mainelis, G. (2006). Investigation of Cut-off Sizes and Collection Efficiencies of Portable Microbial Samplers. Aerosol Sci. Tech., 40:595–606.
