ABSTRACT
Refractory black carbon (rBC) is an aerosol that has important impacts on climate and human health. rBC is often mixed with other species, making it difficult to isolate and quantify its important effects on physical and optical properties of ambient aerosol. To solve this measurement challenge, a new method to remove rBC was developed using laser-induced incandescence (LII) by Levin et al. in 2014. Application of the method with the Single Particle Soot Photometer (SP2) is used to determine the effects of rBC on ice nucleating particles (INP). Here, we quantify the efficacy of the method in the laboratory using the rBC surrogate Aquadag. Polydisperse and mobility-selected samples (100–500 nm diameter, 0.44–36.05 fg), are quantified by a second SP2. Removal rates are reported by mass and number. For the mobility-selected samples, the average percentages removed by mass and number of the original size are 88.9 ± 18.6% and 87.3 ± 21.9%, respectively. Removal of Aquadag is efficient for particles >100 nm mass-equivalent diameter (dme), enabling application for microphysical studies. However, the removal of particles ≤100 nm dme is less efficient. Absorption and scattering measurements are reported to assess its use to isolate brown carbon (BrC) absorption. Scattering removal rates for the mobility-selected samples are >90% on average, yet absorption rates are 53% on average across all wavelengths. Therefore, application to isolate effects of microphysical properties determined by larger sizes is promising, but will be challenging for optical properties. The results reported also have implications for other instruments employing internal LII, e.g., the Soot Particle Aerosol Mass Spectrometer (SP-AMS).
© 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
1.1. Ambient refractory black carbon
Refractory black carbon (rBC), commonly referred to as soot, is directly emitted into the atmosphere from combustion sources, e.g., vehicles, coal-fired power plants, biomass burning, wood stoves, etc. These particles, while a small fraction of the total ambient aerosol mass loading, play a large role in climate forcing due to their strong direct absorption of light and resultant atmospheric heating (Jacobson Citation2000; Ramanathan and Carmichael Citation2008; Cappa et al. Citation2012; Bond et al. Citation2013). In addition to the direct effect, rBC also has numerous indirect effects on climate. Indirect effects include cloud interactions, e.g., the ability to function as cloud condensation nuclei (CCN; Petzold et al. Citation2005; Dusek et al. Citation2006; Koehler et al. Citation2009; Petters et al. Citation2009) and ice nucleating particles (INP; Cozic et al. Citation2008; Twohy et al. Citation2010). Together, the combination of direct and indirect effects has resulted in rBC being identified as potentially the second most important global warming contributor, behind carbon dioxide (Bond et al. Citation2013). In addition to climate, rBC is also linked to adverse health effects in humans (Pope and Dockery Citation2006; Janssen et al. Citation2011).
Traditional measurement of ambient rBC concentrations has involved numerous techniques over the years. Most are filter-based optical methods that have well-known biases associated with them (Subramanian et al. Citation2007; Lack et al. Citation2008, Citation2014) and also assume a mass absorption cross-section (MAC). As a result, the advent of continuous wave laser-induced incandescence (LII) application to single particle measurement of rBC (Petzold et al. Citation2013, Citation2014) resulted in a new de facto standard (Stephens et al. Citation2003; Schwarz et al. Citation2006), which is most equivalent to elemental carbon measured by thermal-optical transmittance (Kondo et al. Citation2011).
Until recently most applications of the LII technique have focused on the interpretation of the emitted incandescence and light scattering signals, linking them to properties of rBC-containing particles. Two applications using LII coupled with mass spectrometry for aerosol chemical composition are the Soot Particle Aerosol Mass Spectrometer (SP-AMS, Onasch et al. Citation2012) and the Laser Induced Incandescence-Mass Spectrometric Analyzer (LII-MS, Miyakawa et al. Citation2014). Here we explore a new application using LII as a selective rBC-removal technique to isolate the effects of rBC on ambient mixed aerosol properties. Levin et al. Citation(2014) first applied the approach to INP measurements and also measured the number and size of residual particles following LII using a scanning mobility particle sizer. We now investigate the application in more detail and discuss other potential applications. Since minimal modification of the Single Particle Soot Photometer (SP2) is required, the method is accessible and has a high potential impact. The presence of SP2s around the globe and ease of the modifications needed to use the technique could allow for universal application as a pretreatment for analysis of mixed aerosols containing rBC, most notably for ambient aerosol sampling.
1.2. Measurement of rBC using laser-induced vaporization and incandescence
LII involves irradiating sample particles in a high-intensity laser beam. LII of rBC occurs at temperatures between 2500 K and 4500 K, at which point the particles vaporize and incandesce. If particles absorb light sufficiently they heat up and emit radiation, which can be detected and used to determine sample properties. Laser powers above a threshold of 0.2 J/cm2 are required to vaporize soot via laser induced vaporization (LIV) using a 1064 nm Nd:YAG laser (Dasch Citation1984). In pulsed LII approaches an ensemble of particles is heated briefly by a pulsed laser and the resulting decay in emitted light intensity is used to determine various particle properties. In single particle approaches, including the SP2, sample particles are drawn through a continuous, high-intensity laser beam and if sufficiently absorbing are heated to their vaporization temperature before exiting the laser profile. The peak incandescence signal is converted to single particle rBC mass using a calibrated response curve.
Previous studies have shown that nanoparticles can nucleate, either homogeneously or heterogeneously, from vaporized rBC for laser powers >0.22 J/cm2 (Michelsen et al. Citation2007), specifically 0.5 J/cm2 at 1064 nm (Vander Wal et al. Citation1998; Yoder et al. Citation2005). These laser powers are required for rBC to reach its equilibrium vaporization temperature of ∼4000 K (Leider et al. Citation1973). The exponential decay of both light scattering and incandescence signals in early experiments with increasing laser powers indicated that rBC particles shrink, as opposed to fragment, indicating vaporization of rBC for laser powers above the aforementioned LII threshold (Dasch Citation1984). While both fragmentation and vaporization have been reported to occur when using different wavelengths and laser fluencies as summarized by Michelsen et al. Citation(2007), vaporized particles are thought to dominate over fragmentation or disaggregation of rBC undergoing LII. LIV has been demonstrated for rBC with transmission electron microscopy (TEM) micrographs (Vander Wal et al. Citation1998). Modeled loss of particle volume (Michelsen et al. Citation2007) also confirms LIV to be the dominant source of these small particles for higher laser fluencies.
The SP2 uses powers well above the range in which rBC is vaporized (∼5 J/cm2) using calculations based on an average measured laser power of 1.7 × 105 W/m2 and the Gaussian time profile of the laser to which each particle is exposed (Schwarz et al. Citation2006). LIV temperatures in the SP2 when run within the standard operating procedures were determined to be in the range of 3700 to 4300 K (Schwarz et al. Citation2006, Citation2010).
1.3. Selective removal of rBC using LII and the SP2
Since LII occurs as rBC is vaporized in the SP2, an SP2 can in principle be used as a pretreatment to selectively remove rBC-containing particles from mixed aerosol samples. When placed upstream of another aerosol instrument, the SP2 can be cycled between laser on and laser off modes as in other LII applications (Onasch et al. Citation2012; Miyakawa et al. Citation2014). By quickly cycling between the two modes, direct comparison can be made between theoretically rBC-removed aerosol and unperturbed aerosol by instruments sampling in series behind the SP2. Levin et al. Citation(2014) exploited this phenomenon to remove rBC from an aerosol sample prior to measuring its ice nucleation activity. They compared INP measured using a diffusion chamber downstream of an SP2 while cycling between laser on (theoretically rBC-free samples) and laser off (unperturbed samples). Here we probe the efficacy of this method in detail, including characterization of the particles present after exposure to LII in the SP2, and evaluate its suitability for investigating additional applications. This rBC removal technique could allow interrogation of the effects of rBC mixing on aerosol optical (e.g., isolate brown carbon (BrC) light absorption) and microphysical properties (e.g., cloud and ice nucleation) directly by difference studies (SP2 laser cycling on and off), similar to thermal denuding, and thereby reduce uncertainties in their parameterizations used in climate models.
One current frequently used approach to study the properties of rBC-containing particles is by the difference method of selectively removing volatile species with a thermal denuder (TD; Huffman et al. Citation2008; Jimenez et al. Citation2009; Cross et al. Citation2010) and then quantifying the differences in physical and optical properties of the remaining nonvolatile material, assumed to be dominated by rBC (Cappa et al. Citation2012; Liu et al. Citation2015). This method allows for the direct measurement and comparison of rBC-containing particles versus the total aerosol. The rBC removal method represents a complementary approach by providing a way to isolate externally mixed non-rBC from rBC-containing particles. It would allow for the direct measurement of non-rBC-containing aerosols versus the total aerosol for ambient and other mixed aerosols. Sampling without interference from rBC can be useful for a variety of applications, including the study of light absorption by non-rBC components like BrC and mineral dust.
We report a feasibility study for the specific removal of rBC from direct aerosol measurements using an rBC surrogate and common calibrant, Aquadag (Baumgardner et al. Citation2012). Both mobility diameter-selected and polydisperse samples of Aquadag were sampled with a downstream SP2 and three wavelength photoacoustic soot spectrometer (PASS-3) after exposure to LII in a primary SP2. Incandescent size distributions and optical properties are reported for the initial Aquadag particles and those present after exposure to LII measured from a secondary SP2 operated inline and downstream of the primary SP2 under standard conditions.
2. Experimental
2.1. Aerosol measurements
In the SP2, a 1064 nm Nd:YAG laser irradiates the particles and induces incandescence in the particles containing absorbing species, primarily rBC. The incandescence signal is used to determine the total mass of rBC on a single particle basis. A scattering channel is also used to detect nonabsorbing particles and determine coating thicknesses on rBC-containing particles (Schwarz et al. Citation2006).
Two SP2s were run using standard operation conditions to optimize rBC detection and coating measurements (Schwarz et al. Citation2010). Calibrations were made with “Classic” Aquadag from 100 nm to 500 nm mobility diameters (dm), equivalent to 0.40–31.6 fg (Moteki et al. Citation2010; Gysel et al. Citation2011) for the incandescent channel, and polystyrene latex spheres (PSLs; Thermo Scientific, Waltham, MA, USA) from 150 nm to 300 nm dme for the scattering channel. Lasers were aligned prior to the start of all experiments, and scattering and incandescent signals were optimized. Laser power was stable for both SP2s (SP2 #1 7.39 ± 0.22 V, SP2 #2 7.40 ± 0.045 V) during the experiments. Data analysis was performed with the Paul Scherrer Institut Toolkit (PSI, Martin Gysel, Villigen, Switzerland) developed for SP2 analysis within Igor Pro (Wavemetrics, Inc.). Incandescent rBC data are reported within the detection range of the SP2 (Schwarz et al. Citation2006).
The Three-Wavelength Photoacoustic Soot Spectrometer (PASS-3; Droplet Measurement Technologies, Boulder, CO, USA) is an online photoacoustic spectrometer that measures absorption and scattering coefficients on the same air mass at three wavelengths: 405 nm, 532 nm, and 781 nm (Arnott et al. Citation1999; Cross et al. Citation2010; Flowers et al. Citation2010; Nakayama et al. Citation2015). A resonant acoustic cavity and microphone measure the pressure wave created from the thermal energy absorbed by the particles within the sample cell in real time. Scattering coefficients are collected by a reciprocal nephelometer with an integrating sphere (Varma et al. Citation2003; Abu-Rahmah et al. Citation2006). Direct online measurement avoids the potential biases that are common with traditional filter-based methods (Lack et al. Citation2008; Lack et al. Citation2009). The PASS-3 was calibrated initially with NO2(g) at 532 nm (Arnott et al. Citation2000; Gyawali et al. Citation2012), and confirmed with absorbing and nonabsorbing particles at all wavelengths for optical closure as is detailed in the instrument manual (Droplet Measurement Technologies). Calibrations are consistent for all wavelengths since the microphone and the nephelometer are independent of wavelength.
Optical properties of the original Aquadag and the incandescent particles remaining after LII were measured with a PASS-3 to assess if the method can be used to directly measure light absorption by BrC. The optical properties of the particles remaining after LII are different from the original incandescent particles due to the reduction in size distributions and are also indicative of potential changes in chemical composition. No size-dependent optical properties are presented due to the presence of multiply charged particles in this study. A discussion of the measured Aquadag optical properties, including MAC, absorption angstrom exponent (AAE), single-scatter albedo (SSA), and their calculations, are included in the online supplementary information (SI).
2.2. Experimental design
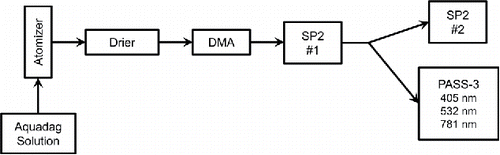
A schematic of the experimental configuration is shown in . rBC was atomized from aqueous solution and dried with a diffusion drier (TSI Model 3062, TSI Inc., Shoreview, MN, USA) filled with silica gel spheres to remove excess water before the particles were selected based on mobility diameter (dm) with a differential mobility analyzer (DMA; TSI Model 3081). Polydisperse samples were also analyzed. Aquadag® (Aqueous Deflocculated Acheson Graphite; Acheson Industries, Inc., Port Huron, MI, USA, production lot #9054, known as “Classic Aquadag” within the SP2 community) is the recommended rBC surrogate used for calibration of rBC instruments due to its known density, solubility in water, and similarities to ambient rBC (Baumgardner et al. Citation2012). It is a water-based colloid of ultra-fine graphite composed of thin graphitic crystalline plates as can be seen in the transmission electron microscope (TEM) images in Moteki et al. Citation(2009).
Mobility-selected and polydisperse aerosol samples were sent to the primary or upstream SP2 (SP2 #1), which was configured to selectively remove rBC via LII by cycling between laser on and laser off modes. The exhaust flow pump was bypassed using conductive tubing connected directly to the exhaust port on SP2 #1 and the two instruments operating downstream. After passing through SP2 #1, the sample was split using a y-splitter and sent to a secondary SP2 (SP2 #2) and a PASS-3. Initial concentrations are reduced by a factor of ten when they reach SP2 #2 and PASS-3 because the (aerosol-free) sheath and optics purge flows used in SP2 #1 to collimate the particles all exit through the same exhaust as the aerosol sample flow. The dilution factor depends on the sample, sheath, and purge flow rates, which can be reduced for this application in future studies to maintain higher sample concentrations. The only difference in the two modes of operation is whether the SP2 #1 laser is on causing rBC particles in the sample to incandesce or off allowing all particles to pass through the cavity unperturbed. Sheath and sample flows in the DMA were kept as close to a 10:1 ratio as possible for an optimized DMA transfer function and narrow size distributions. Multiple charging occurring in the DMA was not filtered out with an impactor or charger (Romay-Novas and Pui Citation1988; Gupta and Mcmurry Citation1989) due to issues of signal and particle loss since in this preliminary study aerosol concentrations were already being reduced by a factor of ten due to the sheath flow in SP2 #1. Multiple charging is addressed in this analysis by determining removal rates in two ways. First we calculate removal rates for the original mobility-selected diameter only, via Gaussian fitting of the original peak, which is defined as the singly charged mobility-selected diameter. Then we calculate the total removal rate for all incandescent particles detectable by SP2 #2, which includes the multiply charged mobility diameters from the original (unperturbed) sample. The second approach is comparable to the optical measurements, where the singly charged particles cannot be isolated from multiply charged particles.
Total mass concentrations measured by the detecting SP2 #2 range from 75 ng m−3 to 5.0 µg m−3 and incandescent number concentrations of 100 to 750 cm3, both within the limits of detection and counting statistics for the SP2 (Schwarz et al. Citation2010). Mobility-selected Aquadag is reported from the SP2 within the size range detected, from 80 nm to 500 nm dme, (0.27–65 fg). No data is reported for purely scattering particles as none were detected either in the original samples or after LII. Levin et al. Citation(2014) also established that purely scattering particles are unaffected by the SP2 laser. PASS-3 absorption and scattering data are reported for signals >6 Mm−1 to eliminate any data that might be limited by signal-to-noise.
2.3. Removal rate calculations
Atomized and dried Aquadag samples were exposed to LII in SP2 #1 and then characterized downstream by SP2 #2 and the PASS-3 (see . All SP2 data were analyzed from the downstream or detecting SP2 (SP2 #2). By using the data from the downstream SP2, we eliminate the need for any nontrivial cross-calibration of the two instruments to eliminate biases (Schwarz et al. Citation2015). The original mass and number concentrations are compared when the laser is on for rBC removal versus when the laser is off for the unperturbed sample in the pretreatment SP2 (SP2 #1). Two types of removal rates are calculated for the mobility-selected diameter data and are shown below (Equations (1) and (2)). The first removal rate (R(dm)) sums only the signal within ±2 Sigma of the original mobility-selected diameter, while the second (Rtot(dm)) sums over the total detectable incandescent signal range of SP2 #2. The two different methods allow for the exclusion and incorporation of the multiply charged peaks in the removal rate calculations as they are excluded in the first method and included in the second.[1]
[2]
SP2 #2 signal is defined as either number or mass, resulting in a total of four removal rates calculated for each mobility-selected sample. Removal rates are reported as percentages except where noted. Sigma is determined by fitting a Gaussian to the singly charged particle peak detected in SP2 #2 and reported by mass-equivalent diameter (dme), using the effective density for Aquadag published by Gysel et al. Citation(2011). The signal beneath the curve ±2 Sigma (σ, defined as the square root of two times the standard deviation of the mean or the Gaussian root mean squared width) is integrated to determine the original, unperturbed mass and number concentrations. The Gaussian fits can be seen in blue (or gray) for the data presented in . Gaussian σ values ranged from 8.9 nm to 45.3 nm dme by mass and 9.4 to 37.3 nm dme by number, with the larger σ values corresponding to the larger dm due to the transfer function of the DMA. The mass and number concentrations remaining after exposure to LII with the laser on was determined by integrating the area under the same diameter range as was determined from the original dm. Since the effective density for the incandescent particles remaining after LII is unknown, unit effective density (1 g cm−3) was used for simplicity. The second calculation of removal rates from the mobility-selected samples is done by integrating across the total mass and number detected by SP2 #2. Just as for the first dm calculation, laser on versus laser off modes are used to determine removal rates.
3. Results and discussion
3.1. Reduction in the incandescent particle signal after LII
DMA-selected dm include multiply charged species as can been seen in . Multiply charged particles are not shown in the 500 nm dm data because their mass exceeded the upper detection limit of SP2 #2. After exposure to LII in SP2 #1, the multiply charged particles with higher masses than the singly charged particles are also removed and can no longer be seen in the size distributions. The removal of the multiply charged particles is not emphasized here as it does not appear to be significantly different from the singly charged data.
Figure 2. Mass-weighted size distributions of Aquadag before (solid black) and after (dashed) undergoing LII. Initial size distributions for DMA-selected mobility diameters of (a) 100 nm, (b) 150 nm, (c) 300 nm, (d) 350 nm, (e) 500 nm, and (f) polydisperse samples as detected by incandescence in the SP2. Plotted diameter is dm with residuals (ρeff = 1, dme = dm for spherical particles). Gaussian fits (gray [blue]) are used to determine the reported Sigma (σ) values.
![Figure 2. Mass-weighted size distributions of Aquadag before (solid black) and after (dashed) undergoing LII. Initial size distributions for DMA-selected mobility diameters of (a) 100 nm, (b) 150 nm, (c) 300 nm, (d) 350 nm, (e) 500 nm, and (f) polydisperse samples as detected by incandescence in the SP2. Plotted diameter is dm with residuals (ρeff = 1, dme = dm for spherical particles). Gaussian fits (gray [blue]) are used to determine the reported Sigma (σ) values.](/cms/asset/98266174-18a1-46a1-813e-ba0ad06f5f77/uast_a_1173647_f0002_oc.gif)
3.1.1. Mass
After Aquadag undergoes LII, on average 88.9 ± 18.6% of the original mass from the initial mobility-selected samples that is fit under the Gaussian curve is removed when the laser is on versus off. summarizes the results and variability of all the dm sampled. For the particles of 300 nm dm and larger, the mass remaining is no more than 1.2 ± 0.003%. The mass fraction removed for the smallest Aquadag particles, 100 nm dm, is 56.3%. This removal rate is significantly less efficient than that of 90.0–99.9% for the larger particles. The lower removal rate of the 100 nm Aquadag is related to the incomplete vaporization and limits of detection within the SP2 (Schwarz et al. Citation2010). The polydisperse sample is similar to the mobility-selected samples with a 93.3 ± 0.2% total mass removal rate, indicating that the mobility-selected samples overall are representative of the polydisperse sample.
Table 1. Removal rates for Aquadag exposed to LII by mass concentration, number concentration, percent scattering, and absorption. Both the mobility-selected particle dm range as determined by ±2σ from the Gaussian fits to the original size distributions and the total particles measured by the SP2 are reported for the mass and number concentrations. Reported averages are from the mobility-selected samples, and uncertainties are the standard deviations.
If the whole size range of the original Aquadag sample including multiply charged particles is analyzed as opposed to only the Gaussian fit to the singly charged mobility-selected sample, the removal rates are similar except for the 100 nm data. The overall average mass percentage removed is 90.5 ± 3.2% from all sampled sizes. The smaller particles have a higher mass percentage remaining likely due to incomplete vaporization of the smaller particles in the laser beam, a limitation of the SP2, and/or a higher fractional contribution from new particle nucleation after LII.
Overall, these removal rates are relevant within the SP2 size range for detection, but could be upper limits for rBC-containing particles outside of this range (80–500 nm dme). However, the mass of particles below 80 nm is small in terms of the total mass due to its small size, and ambient rBC particles are mostly above this range except when sampling direct or near-source emissions. rBC particles larger than the detection range of the SP2 are less common in the atmosphere and should not bias these reported removal rates.
3.1.2. Number
On average, 87.3 ± 21.9% of the number of rBC particles are removed from the original size range of the mobility-selected Aquadag samples (). The results are size-dependent as were those from the mass concentration data, with less efficient removal for the smaller diameters. For the 150 nm sample, 88.7 ± 1.82% of the particles are removed, but for the samples from 300 nm and above, the average number removed is ≥99.4%. These removal rates are similar to the original mobility-selected diameter results for mass concentration removal discussed above.
When the whole size distribution is analyzed by number, the number of particles present after LII exhibits the opposite trend to that found for mass removal. The number remaining over the total size range measured by SP2 (i.e., including sizes below the original dm selected) increases with particle diameter. On average from all mobility-selected data, the ratio of the total incandescent number concentration after and before LII (laser on/laser off) is 1.45 ± 1.03. There are more particles present across the whole size distribution than were present in the original unperturbed sample. These new particles centered at diameters <200 nm dme () indicate that larger particles that undergo LII are potentially undergoing LIV and then recondensing and/or are fragmenting.
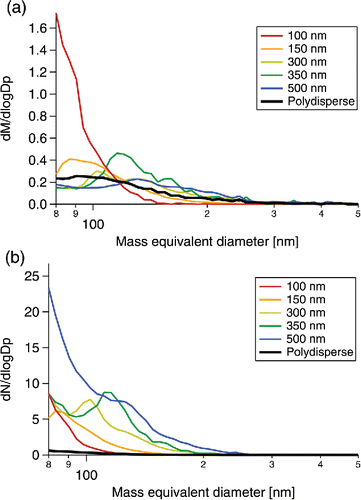
Figure 4. (a) Mass and (b) number size distributions of the incandescent particles remaining after LII normalized by the original Aquadag mass and number concentrations, respectively.
For the 100 and 150 nm dm data and the polydisperse sample, the total number of particles remaining after LII within the SP2 detection limit is less than that of the original Aquadag sample, but the removal rate is not as high as it was by mass. The 100 nm dm has a significant particle number concentration remaining after the sample undergoes LII in the SP2, with only 49.0% of the original number removed. This number could be overestimating removal rates if particles below the SP2 detection limit were somehow measured and included. This hypothesis is supported by , which indicates that the number size distributions peak below 80 nm dme for the 100 nm and polydisperse samples. For this reason, we calculate the number fraction for the 100 nm data using a Gaussian to fit data below the SP2 detection limit. Since the number size distribution remaining after LII for 150 nm initial particles peaks above the lower detection limit of the SP2, at 83.2 nm, if the particle size distribution is fit to a Gaussian, the ratio of particles remaining after LII to the original sample is 1.77, accounting for the particles below SP2 detection limit assuming a mono-modal size distribution. This interpretation could underestimate the total number if more than one mode is present below the detection range of the SP2, but this is unlikely based on the data presented in Levin et al. Citation(2014).
Figure 3. Number size distributions of Aquadag before (solid black) and after (dashed) undergoing LII. Initial size distributions for DMA-selected mobility diameters of (a) 100 nm, (b) 150 nm, (c) 300 nm, (d) 350 nm, (e) 500 nm, and (f) polydisperse samples as detected by incandescence in the SP2. Plotted diameter is dm with residuals (ρ = 1, dme = dm for spherical particles). Gaussian fits (gray [blue]) are used to determine the reported Sigma (σ) values.
![Figure 3. Number size distributions of Aquadag before (solid black) and after (dashed) undergoing LII. Initial size distributions for DMA-selected mobility diameters of (a) 100 nm, (b) 150 nm, (c) 300 nm, (d) 350 nm, (e) 500 nm, and (f) polydisperse samples as detected by incandescence in the SP2. Plotted diameter is dm with residuals (ρ = 1, dme = dm for spherical particles). Gaussian fits (gray [blue]) are used to determine the reported Sigma (σ) values.](/cms/asset/76239ea1-b963-4a26-98fc-187cb54ffea0/uast_a_1173647_f0003_oc.gif)
Two modes are observed in for the 300 nm and 350 nm samples after exposure to LII, but are not discernible for the other samples. One limitation in understanding this is the size range of the SP2. However, it is possible that the larger diameter samples exhibit a larger fraction of fragmented particles in addition to the freshly nucleated rBC-containing particles that form after vaporization that are thought to be represented by the small diameter rBC observed after LII. This could explain the second mode of particles remaining after LII in the 300 nm and 350 nm samples. Further support for this is the fact that the 150 nm sample exhibits a peak that appears to be clearly above the 80 nm limit and the shoulder in the 500 nm sample. Both of these indicate that it is likely that there are two (or more) modes of rBC-containing particles after LII that could be explained by the presence of both freshly nucleated particles and fragments of the original samples (.
Detection of the nucleated and/or fragmented particles remaining after LII is limited by the size range detected by the SP2. The particles remaining would only represent a small fraction of the mass due to their very small diameters. In addition, the second mode appears to increase in diameter as a function of the original mobility diameter sampled yet remain independent of the total mass concentration sampled, as shown in . Hence, the second mode of particles remaining after LII could be the result of fragmentation in the laser beam.
3.1.3. Scattering and absorption
includes the removal rates for the optical signals after Aquadag aerosol is exposed to LII. The average removal rates for the scattering coefficient are similar to the rBC mass removal rates, as expected, at 91 ± 10% and 99 ± 0.01% for 405 nm and 532 nm. The polydisperse sample is also similar to the average of the mobility-selected data with 93.5% and 97.0% of the scattering coefficient removed at 405 nm and 532 nm, respectively. These removal rates are consistent with the significant reduction in size of the particles removed by the first SP2 and the smaller size of the incandescent particles remaining after LII. Data from 781 nm are not reported due to low signals and signal-to-noise ratios.
The percentage of the absorption coefficients removed from the polydisperse sample is 92.6 ± 1.7% across all wavelengths, similar to all the other measurements. However, the mobility-selected removal rates are significantly less: 30 ± 32% at 405 nm, 62 ± 27% at 532 nm, 67 ± 13% at 781 nm. The nonuniform reduction in optical coefficients implies a change in the size distributions and/or the chemical composition of the incandescent particles remaining after LII in comparison to the original Aquadag sample. Therefore, the limited removal of aerosol absorption coefficients could pose a challenge for future measurements seeking to probe the optical properties of BrC independently from rBC by employing this removal technique.
3.2. Incandescent particles after LII
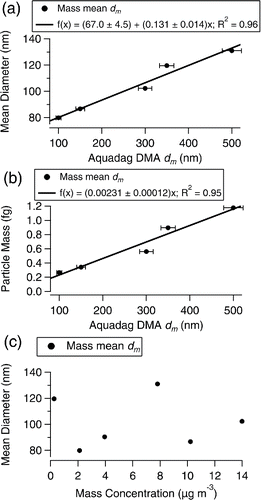
The average incandescent mass per particle after LII is 0.58 ± 0.38 fg and 0.35 ± 0.18 fg, respectively, for mean dme weighted by mass and number size distributions. The mass mean diameter of the particles after LII is on average 99.9 ± 21.6 nm for a density of 1 g cm−3. Number mean diameters are limited by the detection range in the SP2, and are estimated to be <85.6 nm dm from all mobility-selected samples.
shows the normalized size distributions from all samples after LII. Most of the particle mass and number after LII is below 200 nm dme (4.2 fg) at 91.0 ± 0.1% and 99.0 ± 0.01% on average with the dominant mode or modes below 150 nm dme (1.8 fg). The size distribution varies in both number and mass for the different original diameters sampled as shown in . Measured number size distributions are limited by the detection range of the SP2, but the mass size distributions indicate that the mean diameter of the dominant particle mode above 80 nm dme increases with the original diameter sampled ().
The total mass-weighted mean dm increases by 13.1 ± 1.4% relative to the original diameter selected with an offset of 67.0 ± 4.5 nm. Applying this relationship to the number-weighted mean dm of the polydisperse sample, 225.3 nm, predicts a mean dm of 96.0 ± 7.6 nm, encompassing the measured value of 90.4 nm dm. It is possible that there are smaller modes below the detection limit of the SP2, but for the particles above 80 nm dm the mean dm has a positive correlation with the diameter of the original sample. No trend was observed for the relationship between the mass mean dm and the number mean dm with the total mass concentrations sampled ().
The optical properties of the particles present after LII differ from the original sample (see the SI), thus indicating different chemical and/or physical properties. The average AAE from all data after LII is 1.38 ± 0.16, higher than nascent rBC and the original Aquadag sampled here, which was spectrally neutral, indicating wavelength-independent absorption. Measured AAE > 1 is indicative of particles with increased absorption at shorter wavelengths, e.g., absorbing organic carbon species such as BrC (Saleh et al. Citation2014; Liu et al. Citation2015) and/or small size distributions (Bond Citation2001) dependent upon the refractive index of the sampled particles. For these reasons, further inquiries and analysis must be made before trying to differentiate rBC from BrC within the remaining particles after LII by using AAE (Lack and Langridge Citation2013).
3.3. Efficacy and limitations of using LII as an rBC removal technique for online aerosol sampling
Numerous laboratory studies have characterized the physical and optical properties of laboratory-generated aerosols of single components (e.g., ammonium sulfate) or major aerosol classes such as secondary organic aerosol, mineral dust, or combustion emissions. The ability to separate complex mixtures into different classes allows us to compare measurements of ambient aerosol with single component laboratory analyses. This rBC removal technique is unique in that it could be used to separate rBC-containing aerosol from the total mixed ambient aerosol to isolate rBC-dominated microphysical properties and processes in the atmosphere. Chemical sub-selection based on thermal properties is routine for removing volatile organics via thermal denuding; however, removal based on optical properties is a significant challenge. rBC removal would have different applications from thermal denuding since thermal removal separates the volatile species from the refractory, which includes rBC, dust, and sea salt, while this technique would only remove rBC-containing particles.
In general, for the experimental configuration used here, less than ∼10% of the original rBC mass remains after undergoing LII in the SP2 for incandescent particles >80 nm dme. However, significant mass and number fractions remain of particles <100 nm, and small particle formation is strongly evident in the number size distribution. Therefore, the SP2 can be used as a removal technique for the majority of the incandescent mass, e.g., rBC or soot, in order to conduct direct online measurements downstream. For example, this rBC removal technique can be used to directly discriminate the role of rBC from within large ambient external mixtures containing rBC particles (>150 nm) on the microphysics of ice and water nucleation (Levin et al. Citation2014). As these microphysical properties are often dominated by the larger particle sizes, these studies can be done with minimal effect from the small sizes (<100 nm) that are present after exposure to LII in the SP2 with this technique.
However, the removal of rBC by SP2 results in the formation of small particles by fragmentation and recondensation of the volatilized material that, while small in mass, have a significant effect on the residual absorption and incandescent particle number remaining. Our results show conclusively that incandescent particles are present after LII in the SP2 for typical SP2 operating conditions, and that care should be taken for interpretation of results when using a SP2 in-line with other instrumentation. The results shown here are representative for standard SP2 operational settings, but we do not expect significant changes in the particles being formed without decreasing the laser power to levels that would not normally be used by the SP2 in order to get optimal rBC and rBC-coating information. While only Aquadag was tested here, since the rBC is effectively pyrolized by the laser within the SP2, the particles present after LII are expected to be similar in structure even when formed from different precursor chemical compositions. Previous work supports this in finding that new particles formed in similar conditions from different carbonaceous compounds are graphitic in nature, also known as pyrolytic graphite (Borghesi and Guizzetti Citation1991; Bengtsson and Alden Citation1995; Wagner et al. Citation2003).
3.4. Future work and application
Future work should include the study of removal rates in the absence of multiply charged particles. Either a mass-selective instrument such as an aerosol particle mass analyzer (AMP) or using one of the removal techniques that can be used with a DMA should be incorporated. One way to reduce the concentration of multiply charged particles would be the use of a single-stage impactor (commercial or micro-orifice impactor depending on the desired size to be selected) in series, located downstream of the DMA as is described by Romay-Novas and Pui Citation(1988). The DMA-impactor method, while able to reduce the concentration of multiply charged particles to as low as 6%, is limited by the reduction of total particles by a factor of 2 to 10 due to the flow rate through the impactor stage. Another option would be the use of a custom charger in addition to the DMA as described by Gupta and McMurry Citation(1989), known as the low-ion-concentration-charger-DMA technique. The charging method only allows neutral particles to be selected and charged singly to enter the DMA. A third approach is possible with the recent development of a classification instrument that does not require particle charging called the Aerodynamic Aerosol Classifier (Tavakoli et al. Citation2014). This technique allows the selection of a true monodisperse particle distribution based on aerodynamic diameter and will be commercially available within the next year. These studies will allow for a more fundamental interpretation and quantification of the small particles and their formation processes, in addition to the size-resolved study of optical properties.
Different soot surrogates, ambient rBC, and internally mixed rBC should be investigated to determine variability in removal rates for other materials. Offline filter collections and analysis by TEM could help to determine the morphology and shape of the particles remaining after LIV and should be examined in follow-up studies. Future studies could also investigate the effect of varying laser power on the amount and size of particles produced by the SP2. These experiments were beyond the scope of this work as this experiment was designed to show the results under standard operating conditions as a proof of concept for future work.
Since the results here apply to the removal of external mixtures, future work should be conducted not only on other rBC surrogates and rBC, but also on internal mixtures to determine those removal rates. This work is advised for most ambient measurement applications to ensure no confounding issues occur when internally mixed particles undergo LIV. However, bulk thermal–optical analysis of dried Aquadag indicates that ∼83% of the total mass is carbon with ∼76% from elemental carbon (EC) and ∼7% from organic carbon (OC) (Gysel et al. Citation2011). Therefore, this work does demonstrate that an rBC surrogate with a small amount of non-rBC material did not produce any detectable scattering (non-rBC-containing) particles after undergoing LIV. Thickly coated particles could potentially not fully vaporize and/or non-rBC material can detach, as has been shown for internally mixed rBC particles with very thick coatings (>250 nm; Moteki and Kondo Citation2007; Miyakawa et al. Citation2014). While thickly coated rBC cores should not have much effect on surface-dependent measurements such as INP, they may be important for other applications, e.g., optical and absorption measurements. As such, further work should look at the direct application to ambient sampling in order to discern any complications from both internal mixtures and also ambient rBC size distributions.
After fundamental studies involving improved sampling methods and characterization of the removal efficacies for different rBC samples, new applications can be explored in addition to INP. Application to CCN studies could be explored since many types of rBC have been studied, and it has been shown that graphitic types are especially hydrophobic and do not activate below the supersaturation of the Kelvin line (Koehler et al. Citation2009). Since the remaining small particles are expected to be hydrophobic, even if they had a Kappa of 0.01 (Petters and Kreidenweis Citation2007), it is expected that they would have critical supersaturations above 1% for all sizes below 100 nm diameters. For this reason, the application to CCN may have minimal complications due to the remaining small rBC.
However, removal of the small particles remains a high priority for improving this new technique for future applications. One application where the removal of the small rBC particles remaining after LIV would be required is for the study of BrC. One approach would be to introduce a size classifier between the SP2 (or other LIV process) and an optical instrument. The classifier would select a larger size where rBC would be fully removed by LIV and pass the distribution to the optical instrument for measurement of the absorption and scattering properties of the rBC-free particles. Any measured absorption would arise only from non-rBC materials, such as BrC. Normally, the relatively low transmission and charging efficiencies, coupled with complications arising from multiply charged particles, would make interpretation of the DMA size-selected optical measurements difficult. This obstacle could be overcome by using an AAC (Tavakoli et al. Citation2014) instead of a DMA as the size classifier, which has higher transmission efficiencies and no charging effects, allowing for measurement of the optical properties of size-selected, rBC-free particles. Measuring wavelength-dependent light absorption by BrC, together with INP effects, represent two dominant uncertainties in anthropogenic climate forcing. Therefore, the application to BrC is one that should be prioritized.
Additionally, applications using other LII instrumentation could also be explored. One such example is within the SP-AMS, which also uses the high temperatures achieved through LII to measure the composition of the rBC-containing particles (Onasch et al. Citation2012). However, the SP-AMS is different from the SP2 in terms of how the rBC is measured. The SP-AMS detects the rBC signal using a mass spectrometer, which is calibrated based on the total rBC mass in the sample (Onasch et al. Citation2012). Taking into account differences in detection between the SP2 and the SP-AMS, size-dependent studies should be pursued for LII in the SP-AMS (Onasch et al. Citation2012) to determine if incomplete vaporization at smaller sizes and/or any size-dependent recondensation or fragmentation is occurring as was observed in this study. Since LII within the SP-AMS occurs under vacuum, this is expected to limit these issues. However, if significant, this would warrant a size-dependent calibration that is not currently employed in the calibration method used to determine total rBC mass (Onasch et al. Citation2012).
4. Conclusions
There is a need and opportunity to isolate rBC in ambient samples to understand the role of particle chemistry on physical processes in the atmosphere. An initial feasibility and characterization study for using LII within an SP2 operating under standard conditions to remove rBC from an aerosol sample is described using both mobility-selected and polydisperse samples of a common rBC surrogate used in the laboratory, Aquadag. On average, over 90% of the original incandescent mass is removed after undergoing LII in the SP2, indicating that the technique could be a viable method for rBC removal from ambient and/or laboratory aerosol sampling. Results are also relevant to other LII instrumentation, such as the SP-AMS (Onasch et al. Citation2012) and pulsed LII systems.
Aquadag particles of 300 nm and larger are removed almost entirely, with <1.2% mass remaining, while at 100 nm 43.7% of the mass is still present after LII. Using the SP2 for removal of Aquadag is efficient for particles greater than 300 nm in diameter, but the efficiency decreases with diameter. In this dataset, the decrease in both mass and number for the original particle size range is a factor of ∼2 for 100 nm dm, and a factor of ∼10 for 150 nm dm.
Complications such as incomplete vaporization can occur for smaller rBC particles, less than approximately 100 nm mass equivalent diameter (dme), in the SP2 (Schwarz et al. Citation2010). Another issue is the recondensation and/or nucleation of vaporized carbon. Both of these processes can confound this removal technique because a fraction of the rBC is not completely removed in the SP2 laser and could affect measurements made downstream. Our optical property data clearly indicate such an effect. In summary, any small incandescent particles present after LII in the SP2 could be from one of three sources: (1) freshly nucleated particles from the vaporized rBC; (2) fragments of the larger rBC agglomerates; and (3) particles below the detection limit of the SP2 (<70 nm dme) that do not vaporize. Since the third type is not detectable with the SP2, this article focuses on the first two sources of particles that can remain after rBC is vaporized in the SP2. More information on the rBC below the range of detection in the SP2 is included in the previous work by Levin et al. Citation(2014).
This new rBC removal technique has been proven to directly interrogate the role of rBC within mixed aerosol sampling for ice nucleation (Levin et al. Citation2014). In that case, the remnant smaller particles do not influence ice nucleation, most likely because smaller particles are less efficient as INP and the surface properties important to ice nucleation are likely to be significantly altered from the original rBC even if the particles are not fully removed. It is expected that similar application for other microphysical studies, such as liquid-phase cloud formation, is feasible. Potential applications for rBC removal related to studying light absorption by organic aerosol components will prove more challenging since a significant percentage of absorption remains despite the scattering signal being reduced to <10%.
Supplemental_Files_1173647.docx
Download MS Word (40.4 KB)Acknowledgments
We thank J. Taylor (University of Manchester) for his initial thoughts and discussion on the application of the SP2 in this manner, Brad Flowers (URS) for preliminary laboratory work on Aquadag with the PASS-3, and John Walker for his support of the PASS-3 at DMT. We also thank the FLAME-IV campaign and subsequent funding agencies for enabling the co-location of instruments that allowed us to conduct this novel research.
Funding
This work was supported by LANL LDRD Director's Postdoctoral Fellowship (Allison C. Aiken), DOE ASR grant F265 (Manvendra K. Dubey), and NASA Earth Science Division award NNX12AH17G (Paul J. DeMott; CSU). Although this work was funded by the DOE, it has not been subject to formal review and thus no official endorsement should be inferred.
References
- Abu-Rahmah, A., Arnott, W. P., and Moosmuller, H. (2006). Integrating Nephelometer with a Low Truncation Angle and an Extended Calibration Scheme. Meas. Sci. Technol., 17(7):1723–1732.
- Arnott, W. P., Moosmuller, H., Rogers, C. F., Jin, T. F., and Bruch, R. (1999). Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description. Atmos. Environ., 33(17):2845–2852.
- Arnott, W. P., Moosmuller, H., and Walker, J. W. (2000). Nitrogen Dioxide and Kerosene-Flame Soot Calibration of Photoacoustic Instruments for Measurement of Light Absorption by Aerosols. Rev. Sci. Instrum., 71(12):4545–4552.
- Baumgardner, D., Popovicheva, O., Allan, J., Bernardoni, V., Cao, J., Cavalli, F., Cozic, J., Diapouli, E., Eleftheriadis, K., Genberg, P. J., Gonzalez, C., Gysel, M., John, A., Kirchstetter, T. W., Kuhlbusch, T. A. J., Laborde, M., Lack, D., Muller, T., Niessner, R., Petzold, A., Piazzalunga, A., Putaud, J. P., Schwarz, J., Sheridan, P., Subramanian, R., Swietlicki, E., Valli, G., Vecchi, R., and Viana, M. (2012). Soot Reference Materials for Instrument Calibration and Intercomparisons: A Workshop Summary with Recommendations. Atmos. Meas. Techn., 5(8):1869–1887.
- Bengtsson, P. E., and Alden, M. (1995). Soot-Visualization Strategies Using Laser Techniques—Laser-Induced Fluorescence in C2 from Laser-Vaporized Soot and Laser-Induced Soot Incandescence. Appl. Phys. B-Laser. Opt., 60(1):51–59.
- Bond, T. C. (2001). Spectral Dependence of Visible Light Absorption by Carbonaceous Particles Emitted from Coal Combustion. Geophys. Res. Lett., 28(21):4075–4078.
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., and Ghan, S. (2013). Bounding the Rold of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res.-Atmos., 118:5380–5552.
- Borghesi, A., and Guizzetti, G. (1991). Graphite (C), in Handbook of Optical Constants of Solids II, E. D. Palik, ed., Academic Press, New York, pp. 449–460.
- Cappa, C. D., Onasch, T. B., Massoli, P., Worsnop, D. R., Bates, T. S., Cross, E. S., Davidovits, P., Hakala, J., Hayden, K. L., Jobson, B. T., Kolesar, K. R., Lack, D. A., Lerner, B. M., Li, S. M., Mellon, D., Nuaaman, I., Olfert, J. S., Petaja, T., Quinn, P. K., Song, C., Subramanian, R., Williams, E. J., and Zaveri, R. A. (2012). Radiative Absorption Enhancements Due to the Mixing State of Atmospheric Black Carbon. Science., 337(6098):1078–1081.
- Cozic, J., Mertes, S., Verheggen, B., Cziczo, D. J., Gallavardin, S. J., Walter, S., Baltensperger, U., and Weingartner, E. (2008). Black Carbon Enrichment in Atmospheric Ice Particle Residuals Observed in Lower Tropospheric Mixed Phase Clouds. J. Geophys. Res. Atmos., 113(D15). DOI: 10.1029/2007JD009266.
- Cross, E. S., Onasch, T. B., Ahern, A., Wrobel, W., Slowik, J. G., Olfert, J., Lack, D. A., Massoli, P., Cappa, C. D., Schwarz, J. P., Spackman, J. R., Fahey, D. W., Sedlacek, A., Trimborn, A., Jayne, J. T., Freedman, A., Williams, L. R., Ng, N. L., Mazzoleni, C., Dubey, M., Brem, B., Kok, G., Subramanian, R., Freitag, S., Clarke, A., Thornhill, D., Marr, L. C., Kolb, C. E., Worsnop, D. R., and Davidovits, P. (2010). Soot Particle Studies Instrument Inter-Comparison Project Overview. Aerosol Sci. Technol., 44(8):592–611.
- Dasch, C. J. (1984). Continuous-Wave Probe Laser Investigation of Laser Vaporization of Small Soot Particles in a Flame. Appl. Opt., 23(13):2209–2215.
- Dusek, U., Reischl, G. P., and Hitzenberger, R. (2006). CCN Activation of Pure and Coated Carbon Black Particles. Environ. Sci. Technol., 40(4):1223–1230.
- Flowers, B. A., Dubey, M. K., Mazzoleni, C., Stone, E. A., Schauer, J. J., Kim, S. W., and Yoon, S. C. (2010). Optical-Chemical-Microphysical Relationships and Closure Studies for Mixed Carbonaceous Aerosols Observed at Jeju Island; 3-Laser Photoacoustic Spectrometer, Particle Sizing, and Filter Analysis. Atmos. Chem. Phys., 10(21):10387–10398.
- Gupta, A., and Mcmurry, P. H. (1989). A Device for Generating Singly Charged-Particles in the 0.1–1.0-Mum Diameter Range. Aerosol Sci. Technol., 10(3):451–462.
- Gyawali, M., Arnott, W. P., Zaveri, R. A., Song, C., Moosmuller, H., Liu, L., Mishchenko, M. I., Chen, L. W. A., Green, M. C., Watson, J. G., and Chow, J. C. (2012). Photoacoustic Optical Properties at UV, VIS, and Near IR Wavelengths for Laboratory Generated and Winter Time Ambient Urban Aerosols. Atmos. Chem. Phys., 12(5):2587–2601.
- Gysel, M., Laborde, M., Olfert, J. S., Subramanian, R., and Grohn, A. J. (2011). Effective Density of Aquadag and Fullerene Soot Black Carbon Reference Materials Used for SP2 Calibration. Atmos. Meas. Techn., 4(12):2851–2858.
- Huffman, J. A., Ziemann, P. J., Jayne, J. T., Worsnop, D. R., and Jimenez, J. L. (2008). Development and Characterization of a Fast-Stepping/Scanning Thermodenuder for Chemically-Resolved Aerosol Volatility Measurements. Aerosol Sci. Technol., 42(5):395–407.
- Jacobson, M. Z. (2000). A Physically-Based Treatment of Elemental Carbon Optics: Implications for Global Direct Forcing of Aerosols. Geophys. Res. Lett., 27(2):217–220.
- Janssen, N. A. H., Hoek, G., Simic-Lawson, M., Fischer, P., van Bree, L., ten Brink, H., Keuken, M., Atkinson, R. W., Anderson, H. R., Brunekreef, B., and Cassee, F. R. (2011). Black Carbon as an Additional Indicator of the Adverse Health Effects of Airborne Particles Compared with PM10 and PM2.5. Environ. Health Perspect., 119(12):1691–1699.
- Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S. H., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., Ng, N. L., Aiken, A. C., Docherty, K. S., Ulbrich, I. M., Grieshop, A. P., Robinson, A. L., Duplissy, J., Smith, J. D., Wilson, K. R., Lanz, V. A., Hueglin, C., Sun, Y. L., Tian, J., Laaksonen, A., Raatikainen, T., Rautiainen, J., Vaattovaara, P., Ehn, M., Kulmala, M., Tomlinson, J. M., Collins, D. R., Cubison, M. J., Dunlea, E. J., Huffman, J. A., Onasch, T. B., Alfarra, M. R., Williams, P. I., Bower, K., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Salcedo, D., Cottrell, L., Griffin, R., Takami, A., Miyoshi, T., Hatakeyama, S., Shimono, A., Sun, J. Y., Zhang, Y. M., Dzepina, K., Kimmel, J. R., Sueper, D., Jayne, J. T., Herndon, S. C., Trimborn, A. M., Williams, L. R., Wood, E. C., Middlebrook, A. M., Kolb, C. E., Baltensperger, U., and Worsnop, D. R. (2009). Evolution of Organic Aerosols in the Atmosphere. Science., 326(5959):1525–1529.
- Koehler, K. A., DeMott, P. J., Kreidenweis, S. M., Popovicheva, O. B., Petters, M. D., Carrico, C. M., Kireeva, E. D., Khokhlova, T. D., and Shonija, N. K. (2009). Cloud Condensation Nuclei and Ice Nucleation Activity of Hydrophobic and Hydrophilic Soot Particles. Phys. Chem. Chem. Phys., 11(36):7906–7920.
- Kondo, Y., Sahu, L., Moteki, N., Kahn, F., Takegawa, N., Liu, X., Koike, M., and Miyakawa, T. (2011). Consistency and Traceability of Black Carbon Measurements Made by Laser-Induced Incandescence, Thermal-Optical Transmittance, and Filter-Based Photo-Absorption Techniques. Aerosol Sci. Technol., 45(2):295–312.
- Lack, D. A., Cappa, C. D., Covert, D. S., Baynard, T., Massoli, P., Sierau, B., Bates, T. S., Quinn, P. K., Lovejoy, E. R., and Ravishankara, A. R. (2008). Bias in Filter-Based Aerosol Light Absorption Measurements Due to Organic Aerosol Loading: Evidence from Ambient Measurements. Aerosol Sci. Technol., 42(12):1033–1041.
- Lack, D. A., Cappa, C. D., Cross, E. S., Massoli, P., Ahern, A. T., Davidovits, P., and Onasch, T. B. (2009). Absorption Enhancement of Coated Absorbing Aerosols: Validation of the Photo-Acoustic Technique for Measuring the Enhancement. Aerosol Sci. Technol., 43(10):1006–1012.
- Lack, D. A., and Langridge, J. M. (2013). On the Attribution of Black and Brown Carbon Light Absorption Using the Angstrom Exponent. Atmos. Chem. Phys., 13(20):10535–10543.
- Lack, D. A., Moosmuller, H., McMeeking, G. R., Chakrabarty, R. K., and Baumgardner, D. (2014). Characterizing Elemental, Equivalent Black, and Refractory Black Carbon Aerosol Particles: A Review of Techniques, Their Limitations and Uncertainties. Anal. Bioanalyt. Chem., 406(1):99–122.
- Leider, H. R., Krikoria, O. H., and Young, D. A. (1973). Thermodynamic Properties of Carbon up to Critical-Point. Carbon., 11(5):555–563.
- Levin, E. J. T., McMeeking, G. R., DeMott, P. J., McCluskey, C. S., Stockwell, C. E., Yokelson, R. J., and Kreidenweis, S. M. (2014). A New Method to Determine the Number Concentrations of Refractory Black Carbon Ice Nucleating Particles. Aerosol Sci. Technol., 48(12):1264–1275.
- Liu, S., Aiken, A. C., Gorkowski, K., Dubey, M. K., Cappa, C. D., Williams, L. R., Herndon, S., Massoli, P., Fortner, E., Chhabra, P. S., Brooks, W. A., Onasch, T. B., Jayne, J. T., Worsnop, D. R., China, S., Sharma, N., Mazzoleni, C., Xu, L., Ng, N. L., Liu, D., Allan, J. D., Lee, J. D., Fleming, Z. L., Mohr, C., Zotter, P., Szidat, S., and Prevot, A. S. H. (2015). Enhanced Light Absorption by Mixed Source Black and Brown Carbon Particles in UK Winter. Nat. Commun., 6:8435.
- Michelsen, H. A., Tivanski, A. V., Gilles, M. K., van Poppel, L. H., Dansson, M. A., and Buseck, P. R. (2007). Particle Formation from Pulsed Laser Irradiation of Soot Aggregates Studied with a Scanning Mobility Particle Sizer, a Transmission Electron Microscope, and a Scanning Transmission X-Ray Microscope. Appl. Opt., 46(6):959–977.
- Miyakawa, T., Takeda, N., Koizumi, K., Tabaru, M., Ozawa, Y., Hirayama, N., and Takegawa, N. (2014). A New Laser Induced Incandescence-Mass Spectrometric Analyzer (LII-MS) for Online Measurement of Aerosol Composition Classified by Black Carbon Mixing State. Aerosol Sci. Technol., 48(8):853–863.
- Moteki, N., and Kondo, Y. (2007). Effects of Mixing State on Black Carbon Measurements by Laser-Induced Incandescence. Aerosol Sci. Technol., 41(4):398–417.
- Moteki, N., Kondo, Y., and Nakamura, S. (2010). Method to Measure Refractive Indices of Small Nonspherical Particles: Application to Black Carbon Particles. J. Aerosol Sci., 41(5):513–521.
- Moteki, N., Kondo, Y., Takegawa, N., and Nakamura, S. (2009). Directional Dependence of Thermal Emission from Nonspherical Carbon Particles. J. Aerosol Sci., 40(9):790–801.
- Nakayama, T., Hiroyuki, S., Kagamitani, S., Ikeda, Y., Uchiyama, A., and Yutaka, M. (2015). Characterization of a Three Wavelength Photoacoustic Soot Spectrometer (PASS-3) and a Photoacoustic Extinctiometer (PAX). J. Meteorol. Soc. Japan. Ser. II, 93:285–308.
- Onasch, T. B., Trimborn, A., Fortner, E. C., Jayne, J. T., Kok, G. L., Williams, L. R., Davidovits, P., and Worsnop, D. R. (2012). Soot Particle Aerosol Mass Spectrometer: Development, Validation, and Initial Application. Aerosol Sci. Technol., 46(7):804–817.
- Petters, M. D., Carrico, C. M., Kreidenweis, S. M., Prenni, A. J., DeMott, P. J., Collett, J. L., and Moosmuller, H. (2009). Cloud Condensation Nucleation Activity of Biomass Burning Aerosol. J. Geophys. Res. Atmos., 114. DOI: 10.1029/2009JD012353.
- Petters, M. D., and Kreidenweis, S. M. (2007). A Single Parameter Representation of Hygroscopic Growth and Cloud Condensation Nucleus Activity. Atmos. Chem. Phys., 7(8):1961–1971.
- Petzold, A., Gysel, M., Vancassel, X., Hitzenberger, R., Puxbaum, H., Vrochticky, S., Weingartner, E., Baltensperger, U., and Mirabel, P. (2005). On the Effects of Organic Matter and Sulphur-Containing Compounds on the CCN Activation of Combustion Particles. Atmos. Chem. Phys., 5:3187–3203.
- Petzold, A., Ogren, J. A., Fiebig, M., Laj, P., Li, S. M., Baltensperger, U., Holzer-Popp, T., Kinne, S., Pappalardo, G., Sugimoto, N., Wehrli, C., Wiedensohler, A., and Zhang, X. Y. (2013). Recommendations for Reporting “Black Carbon” Measurements. Atmos. Chem. Phys., 13(16):8365–8379.
- Pope, C. A., and Dockery, D. W. (2006). Health Effects of Fine Particulate Air Pollution: Lines That Connect. J. Air Waste Manag. Assoc., 56(6):709–742.
- Ramanathan, V., and Carmichael, G. (2008). Global and Regional Climate Changes Due to Black Carbon. Nat. Geosci., 1(4):221–227.
- Romay-Novas, F. J., and Pui, D. Y. H. (1988). Generation of Monodisperse Aerosols in the 0.1–1.0-Mu-M Diameter Range Using a Mobility Classification Inertial Impaction Technique. Aerosol Sci. Technol., 9(2):123–131.
- Saleh, R., Robinson, E. S., Tkacik, D. S., Ahern, A. T., Liu, S., Aiken, A. C., Sullivan, R. C., Presto, A. A., Dubey, M. K., Yokelson, R. J., Donahue, N. M., and Robinson, A. L. (2014). Brownness of Organics in Aerosols from Biomass Burning Linked to Their Black Carbon Content. Nat. Geosci., 7(9):647–650.
- Schwarz, J. P., Gao, R. S., Fahey, D. W., Thomson, D. S., Watts, L. A., Wilson, J. C., Reeves, J. M., Darbeheshti, M., Baumgardner, D. G., Kok, G. L., Chung, S. H., Schulz, M., Hendricks, J., Lauer, A., Karcher, B., Slowik, J. G., Rosenlof, K. H., Thompson, T. L., Langford, A. O., Loewenstein, M., and Aikin, K. C. (2006). Single-Particle Measurements of Midlatitude Black Carbon and Light-Scattering Aerosols from the Boundary Layer to the Lower Stratosphere. J. Geophys. Res. Atmos., 111(D16). DOI: 10.1029/2006JD007076.
- Schwarz, J. P., Perring, A. E., Markovic, M. Z., Gao, R. S., Ohata, S., Langridge, J., Law, D., McLaughlin, R. J., and Fahey, D. W. (2015). Technique and Theoretical Approach for Quantifying the Hygroscopicity of Black-Carbon-Containing Aerosol Using a Single Particle Soot Photometer. J. Aerosol Sci., 81:110–126.
- Schwarz, J. P., Spackman, J. R., Gao, R. S., Perring, A. E., Cross, E., Onasch, T. B., Ahern, A., Wrobel, W., Davidovits, P., Olfert, J., Dubey, M. K., Mazzoleni, C., and Fahey, D. W. (2010). The Detection Efficiency of the Single Particle Soot Photometer. Aerosol Sci. Technol., 44(8):612–628.
- Stephens, M., Turner, N., and Sandberg, J. (2003). Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity. Appl. Opt., 42(19):3726–3736.
- Subramanian, R., Roden, C. A., Boparai, P., and Bond, T. C. (2007). Yellow Beads and Missing Particles: Trouble Ahead for Filter-Based Absorption Measurements. Aerosol Sci. Technol., 41(6):630–637.
- Tavakoli, F., Symonds, J. P. R., and Olfert, J. S. (2014). Generation of a Monodisperse Size-Classified Aerosol Independent of Particle Charge. Aerosol Sci. Technol., 48(3):i–iv.
- Twohy, C. H., DeMott, P. J., Pratt, K. A., Subramanian, R., Kok, G. L., Murphy, S. M., Lersch, T., Heymsfield, A. J., Wang, Z. E., Prather, K. A., and Seinfeld, J. H. (2010). Relationships of Biomass-Burning Aerosols to Ice in Orographic Wave Clouds. J. Atmos. Sci., 67(8):2437–2450.
- Vander Wal, R. L., Ticich, T. M., and Stephens, A. B. (1998). Optical and Microscopy Investigations of Soot Structure Alterations by Laser-Induced Incandescence. Appl. Phys.; B-Laser. Opt., 67(1):115–123.
- Varma, R., Moosmuller, H., and Arnott, W. P. (2003). Toward an Ideal Integrating Nephelometer. Opt. Lett., 28(12):1007–1009.
- Wagner, H. G., Emelianov, A. V., Eremin, A. V., and Jander, H. (2003). Comparison of Properties of Carbon Particles Formed by Pyrolysis of C3O2 and C2H2 Behind Shock Waves. Kinet. Catal., 44(4):463–470.
- Yoder, G. D., Diwakar, P. K., and Hahn, D. W. (2005). Assessment of Soot Particle Vaporization Effects during Laser-Induced Incandescence with Time-Resolved Light Scattering. Appl. Opt., 44(20):4211–4219.