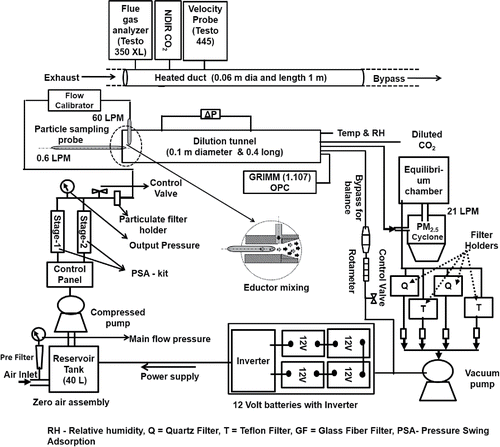
ABSTRACT
This study presents the emission factor of PM2.5, elemental carbon (EC), organic carbon (OC), and water-soluble ions for biomass-fired-induced downdraft gasifier and light duty diesel vehicle (LDDV). A portable dilution system (PDS) developed for on-field measurement of aerosol and their precursors from combustion sources were used for quenching of aerosol at near-atmospheric condition before collection on filters. PDS consists of a heated duct and particle sampling probe, dilution tunnel, zero air assembly, and a power supply unit. PDS was evaluated under controlled conditions in laboratory for gasifier cookstove and LDDV over wide range of dilution ratios to understand the effect of dilution on mixing, particle formation, and loss. The invariability in CO2, recorded along the length and at radial distances of cross-section of dilution tunnel, confirmed the rapid and homogenous mixing inside the dilution tunnel. The particle loss and nucleation inside the dilution tunnel accounted for 6–20% at different dilution ratios (30:1–90:1). PM2.5 emission factors for wood combustion in gasifier cookstove showed mild decrease (13%) with increasing dilution ratio from 75:1 to 108:1. However, a considerable decrease of 37% (221–139 mg km−1) was observed for LDDV with increase in dilution ratio from 39:1 to 144:1. Similar decrease in particulate organic carbon emission rates were observed indicating scarcity of sorptive organics, and insufficient residence time for condensation limited the particle formation from vapor phase organic compounds at high dilution ratios.
© 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Studies (Reddy et al. Citation2004; Verma et al. Citation2006) using global climate models have shown large atmospheric burden of carbonaceous aerosol (organic carbon [OC] and elemental carbon [EC]) and radiative forcing over the Indian subcontinent. Climate effects of carbonaceous aerosol include considerable warming of the top of the atmosphere and a comparably large cooling of the surface of the Indian Ocean (Ramanathan et al. 2001). Global model estimated large radiative forcing due to light absorbing black carbon (BC) was linked with melting of Himalayan snow, perturbation of regional circulation, and precipitation resulting in flood and drought in various part of India (Ramanathan et al. Citation2005). However, a recent (Bond et al. Citation2013) assessment of light absorption by BC over India, China, and other parts of Asia through climate models and satellite retrieved absorbing aerosol optical depth suggested significant underestimation (factor of 3–4) of absorption by climate models. The emission inventories are key input in climate models for such assessment. Incorrect regional emission estimates attributed to very limited measurement of emissions from dominant and less understood sources such as solid fuel (coal, wood, agricultural residue, and dried cattle manure) combustion for residential energy (accounts for 60–80% of BC emissions) (Bond et al. Citation2004; Venkataraman et al. Citation2005), agricultural residue burning in field (Venkataraman et al. Citation2006), coal, wood, and agricultural residue burning in a brick kiln (Weyant et al. Citation2014), diesel and gasoline combustion in automobiles (Pandey and Venkataraman Citation2014), and desert dust (Das et al. Citation2013). Insufficient information on technological advances (e.g., number of advance cookstoves in working condition, number of new and old vehicles operating on road, etc.) and large uncertainty in fuel/energy use in some of the sectors (e.g., open biomass amount, coal use) were also pointed to as important factors for discrepancy (Bond et al. Citation2004; Lu et al. Citation2011; Bond et al. Citation2013).
In the last decade, source characterization for biomass fuel combustion in cooking stoves (Habib et al. Citation2008) and related emission inventory development in India was carried out by few researchers (Parashar et al. Citation2005; Sahu et al. Citation2011; Pandey and Venkataraman Citation2014; Sadavarte and Venkataraman Citation2014), which pointed out the importance of BC emissions from this source. The studies (Ministry of New and Renewable Energy [MNRE] Citation2009; Venkataraman et al. Citation2010; Sutar Citation2015) have emphasized on next-generation cleaner household cookstoves with high efficiency and low emissions but using the same solid biomass fuel readily available in rural area. Since 1980s, many researchers (Mukunda et al. Citation1988, Citation2010; Mukunda Citation2011; Kumar et al. Citation2013) have been engaged in designing and testing advance cookstoves and gasifiers for household use. However, the emission measurement in field has remained a challenge to assess the stove performance. Recent study by Roden et al. Citation(2006) reported large discrepancies between emission factors from on-field measurement and under controlled laboratory conditions, and emphasized more on-field measurement of less understood poor combustion sources.
For vehicular emission, limited measurements (Sharma et al. Citation2005; Dwivedi et al. Citation2006; Garg et al. Citation2006; ARAI Citation2009; CPCB Citation2010) are available for Indian vehicles tested on dynamometer using Indian driving cycle. Importantly, recent studies (Goel et al. Citation2015) have reported considerable differences in on-road operation of vehicles and Indian driving cycle in terms of percentage of time (18–37%) vehicles operate in idling condition, and average vehicle speed (15–25 km/h). Grieshop et al. Citation(2012) have reported that on-road driving speed has a significant effect on emission factors. Several studies (Hansen et al. Citation1995; Sturm et al. Citation1996; Tong et al. Citation2000; Kean et al. Citation2003; Smit et al. Citation2008; Barth and Boriboonsomsin Citation2009) have shown a nonlinear dependence of vehicular speed with emission factors. Emission measurements in tunnel (Rogak et al. Citation1998; Kirchstetter et al. Citation1999; Gertler et al. Citation2002; Geller et al. Citation2005; Ning et al. Citation2008; Roden et al. Citation2009; Ban-Weiss et al. Citation2010; Dallmann et al. Citation2011; Park et al. Citation2011; Dallmann et al. Citation2012) or on-road (Westerdahl et al. Citation2009) indicated that the emission characteristics of vehicles vary significantly from laboratory measurement under controlled conditions. The variabilities were attributed to road conditions, user's habit or on-road driving pattern, type of fuel, and its adulteration. Notably, emissions of aerosol and their precursors from open agricultural residue burning in field after harvest have not been assessed for the Indian region. Therefore, a database of emission factors from on-field measurement of region-specific sources are of utmost importance to improve emission estimates and hence understanding of climate change over India.
For gaseous pollutants and aerosol emission measurement of mobile sources, portable onboard emission measurement systems (PEMS) (Durbin et al. Citation2007; Zhang and Frey Citation2008; Johnson et al. Citation2009) and portable dilution sampling systems (Lipsky and Robinson Citation2005; Wang et al. Citation2012) have been developed. However, some major issues including continuous availability of particle-free air for dilution and uninterrupted power supply have not been addressed for on-field measurements.
Therefore, the aim of this study was to develop a portable dilution sampling system for on-field measurement of stationary and mobile sources. In this article, we present the evaluation of portable dilution system (PDS) designed following Lipsky and Robinson Citation(2005). The PDS is evaluated for PM2.5 and its chemical constituents (carbonaceous aerosols and water-soluble ions) emitted from a gasifier cookstove (detailed in Sutar Citation2015) and a light duty diesel vehicle (LDDV) (INNOVA 2007 model, engine capacity 2400 cc, gross weight 2300 kg) under controlled laboratory conditions. The system is designed to simulate near-ambient conditions in terms of rapid dilution and cooling after the flue gas enters the dilution tunnel along with the zero air, which is supplied continuously throughout the experiment. The portability, continuous zero air, and power supply will allow the use of this system for on-field measurement of various combustion sources. The experiments were performed to determine the effect of dilution ratio on (a) particle loss and particle formation, (b) homogenous and rapid mixing of exhaust and zero air in dilution tunnel and persistence of near-ambient environment inside the dilution tunnel, and (c) PM2.5 and its chemical constituents.
2. Methodology
2.1. Details of dilution system
A PDS () was designed for aerosol sampling at near-atmospheric condition, consisting of five main units, namely a duct (0.06 m diameter and 1.0 m length), a dilution tunnel (0.1 m diameter and 0.4 m length), a particle sampling probe (diameter 2 mm) of stainless steel to avoid contamination, a zero air generation system, and a power supply system (3 KV inverter and four 12 V batteries). The duct and particle sampling probe are heated at temperature above exhaust temperature to avoid the thermophoresis and condensation losses (Baron and Willeke Citation2001; Wang et al. Citation2011). A zero air assembly was comprised of a compressor of flow rate 80 LPM connected to a reservoir of 40 L capacity, a filter holder, an activated carbon cartridge and two molecular sieves. The atmospheric air was stored in a reservoir for continuous generation and supply of zero air. The atmospheric air was passed through a filter, an activated carbon cartridge, and molecular sieves to remove particles, volatiles organic compounds, and the moisture, respectively. Two-stage molecular sieves automatically regenerate by the principle of pressure swing adsorption, where total pressure of assembly “swings” between high pressure in feed and low pressure in regeneration (Ruthven et al. Citation1994; Rezaei and Webley Citation2009). The particle sampling probe consists of a stainless steel nozzle and Teflon tube to allow flexibility during operation. The probe withdraws a fraction of exhaust from the tailpipe, which is co-axially and rapidly mixed with zero air in the dilution tunnel (). Flow rate inside the particle sampling probe can be maintained at 0.2–1.0 LPM by adjusting the distance of the tip of the probe from zero air inlet and varying the negative pressure at the tip of the probe (). In experiments conducted here the sampling flow rate was maintained at 0.6 LPM by inserting the sampling probe to an extent so that the tip of the probe was 0.5–2.0 cm ahead of the zero air inlet to achieve the intended dilution. The flow inside the particle sampling probe was calibrated in separate experiment in the laboratory. The negative pressure at the tip of the probe was varied by varying the zero air flow rate and the distances of the tip from zero air inlet were marked to maintain required flow rate. A stainless steel dilution tunnel of 3L capacity was designed to achieve maximum flow based dilution ratio of 100:1 and complete aerosol quenching in minimum 3 s as reported in literature (Lipsky and Robinson Citation2005). Six ports are provided at the end of dilution tunnel to attach various instruments.
2.2. Experimental procedure
2.2.1. Determination of particle loss
Performance of PDS in terms of particle loss or diffusion loss (Figure S1 in the online supplementary information [SI]) was evaluated using sodium chloride (NaCl) solution. NaCl was fed into the atomizer (Model 3076, TSI Particle Instrumentation Inc., Shoreview, MN, USA) using a syringe pump (Model NE 300 Syringe pump, New Era Pump Systems Inc., Farmingdale, NY, USA). Flow in the particle sampling probe was set at 0.6 LPM and zero air supply rate was maintained to 18, 36, and 54 LPM to achieve dilution ratios 30:1, 60:1, and 90:1 in dilution tunnel. Size distribution of particles were measured by a scanning mobility particle sizer (SMPS; Model 3936, TSI Inc.) consisting of differential mobility analyzer (DMA; Model 3081, TSI Inc.) and condensation particle counter (CPC; Model 3775, TSI Inc.). This experiment was carried out in the Department of Chemical Engineering, Indian Institute of Technology Bombay, India.
Polydisperse NaCl particles, in the size range 20–600 nm, generated from atomization of NaCl solution (5 mg mL−1), were used to determine particle loss in the dilution tunnel. A 20 mL volume of solution was placed in a syringe (Becton Dickinson India Pvt. Ltd., Haryana, India) and passed to the atomizer at a flow rate of 0.3 mL min−1. At the start of each experiment, the system was operated with no particle generation until SMPS indicated zero particle concentration. The DMA used for size distribution varied from 14 nm to 600 nm with a one-scan cycle time of 135 s. The dilution tunnel was attached between the aerosol generation system and the SMPS in a parallel configuration. The experiments were carried out without dilution tunnel, and then with dilution tunnel and providing zero air for 30:1, 60:1, and 90:1 dilution ratio. These size distribution measurements were used to determine particle losses in the dilution tunnel. Three sets of experiments were carried out for each configuration for statistical confidence.
2.2.2. Gasifier exhaust measurement
Experiments with induced downdraft gasifier stove were conducted in the laboratory located in the Department of Mechanical Engineering at Indian Institute of Technology Delhi (Figure S2 in the SI). The fuelwood chips of Jamun (Syzygium cumini) of length 20 mm and diameter 10 mm were burned and induced air was supplied at 16 LPM. The experiment setup was designed by Sutar Citation(2015) following Venkataraman and Rao Citation(2001), consisting of a large hood connected to a duct of 0.16 m diameter. An exhaust fan was fitted at the end of the duct to remove extra exhaust (Figure S2 in the SI).
Smoke from the stove was entrained into the duct (Figure S2 in the SI) with natural dilution from background air having particle mass ∼5–10% of sample collected from wood combustion. A fraction of sample was withdrawn from duct using particle sampling probe and entrained into dilution tunnel for further dilution using zero air to achieve the quenching of aerosol near ambient condition. The dilution due to background air was determined using combustion temperature, temperature inside the duct, and ambient temperature. On the basis of flow rate of zero air and sampled exhaust the dilution ratio inside the dilution tunnel was set to 30:1, 60:1, 90:1, and 120:1. The real dilution ratios were determined (Equation Equation(1)[1] ) by measuring CO2 concentrations inside the duct (CO2)duc, inside the dilution tunnel (CO2)dil, and of ambient air (CO2)amb. A flue gas analyzer (model Testo 350XL, Testo India Pvt. Ltd., Pune, India) based on electrochemical sensors to measure NOx (0–500 ppm), CO2 (0–50%), SO2 (0–5000 ppm), and CO (0–10,000 ppm) was used to record the concentration of gases in the duct at 1-min interval. Additionally, another NDIR-based CO2 analyzer (range 0–20%; Technovation from Polltech Ins. Pvt. Ltd., Mumbai, India) was used to monitor CO2 concentration of exhaust in the duct. CO2 measured from two instruments agreed well (R2 = 0.96, slope = 1.05) and geometaric mean CO2 concentration was used to calculate the dilution ratio at 1-min interval. CO2 inside the dilution tunnel was measured using NDIR-based analyzer (Model 0632–1240; Testo India Pvt. Ltd.). The ambient CO2 concentration was determined before each experiment.
[1]
The temperature, pressure, and relative humidity (RH) (Model Testo 0560–445; Testo India Pvt. Ltd.) inside the dilution tunnel were also recorded each minute to ensure proper dilution and absence of excessive moisture. In order to ensure proper mixing of sampled exhaust with zero air, CO2, temperature, and pressure were measured at four points, 100, 200, 300, and 400 cm, from the upstream end of the dilution tunnel at every 5-min interval during each experiment (Figure S3a in the SI). Also, CO2 concentration in radial direction at seven points (Figure S3b in the SI) was measured for the same purpose. Data from all instruments were recorded using data acquisition system. A LabView (ATOMBERG Pvt. Ltd., Mumbai, India) program was used to visualize the data.
Particles were collected using a multistream aerosol sampler detailed in earlier work (Venkataraman et al. Citation2005; Habib et al. Citation2008) equipped with Air and Industry Hygiene Laboratory (AIHL) cyclone and four filter holders connected at the end of the dilution tunnel (Figure S4 in the SI). The multistream particle sampler was connected at the end of the dilution tunnel. The flow in each stream of the sampler was controlled through a critical orifice provided at the downstream of the filter holders. The sampler was operated at 21.7 LPM to collect particles of aerodynamic diameter 2.5 μm and less (PM2.5). PM2.5 mass was collected on Tissue Quartz (Pall flex 2500 QAT-UP; Pallflex Corp., NY, USA) and Teflon membrane filters (47 mm, 2-μm pore size, Whatmann Corp., PA, USA). The filters were pre- and post-conditioned in controlled environment (temperature 25°C and RH 50%) for 12 h and were stored at −4°C in refrigerator after experiment. The particle mass was determined as a difference of post-weight and pre-weight of the filter measured with Sartorious microbalance (CPA2P-F detection limit 1 μg; Sartorious, Goettingen, Germany). The emission factor was calculated using Equation Equation(2)[2] .
[2] where, C is the aerosol concentration (μg m−3 of sampled air), DR is CO2-based dilution ratio, Vd is STP corrected exhaust velocity in the duct (ms−1), Ad is the duct area (m2), t is sampling time (s), F is fuel burned (kg), and D is the distance traveled (km).
The input flows in the dilution tunnel were 21.7, 36.7, 54.7, and 72.7 LPM including dilution air flow rates 21.1, 36.1, 54.1, and 72.1 LPM, and corresponding exhaust sample flow rate 0.6 LPM, to achieve the dilution ratios 35:1, 60:1, 90:1, and 120:1. Therefore, a bypass at the end of the dilution tunnel was operated at 15.0, 33.0, and 51.0 LPM for experiments with dilution ratios 60:1, 90:1, and 120:1, respectively.
2.2.3. Diesel vehicle emission measurement
The system was evaluated to understand the effect of dilution on particle mass emission from light duty diesel vehicle (INNOVA). Selected vehicle was operated following Indian driving cycle on chassis dynamometer facility available in Manesar, Gurgaon. A limited number of experiments (4) were planned. A duct was connected to vehicle's tail pipe (Figure S5 in the SI) using a heated stainless steel pipe of 6 cm diameter. The duct and connecting pipe were heated at temperature higher than exhaust temperature to avoid particle loss due to thermophoresis. Whole exhaust was allowed to enter into the duct where flue gas/exhaust velocity, concentration of gases, temperature, and RH were monitored and recorded at 1-min interval. Exhaust velocity and temperature in the duct were monitored using vane probe velocity meter (Testo 06356045), which measures velocity in the range 0.6–20 m/s, and temperature up-to 350°C. RH was also monitored using RH sensor (PEM-EM-HFB-0, range 0–100%, Polltech Instrument Pvt Ltd., Mumbai, India). Reynolds number inside the duct was estimated as 24 × 105–40 × 105 indicating turbulent flow. The specifications of various instrument are listed in Table S1 (see the SI).
A fraction of exhaust was taken directly from the tail pipe using heated particle sampling probe and entrained into dilution tunnel. Ambient air free from particles, moisture, and organic vapor, termed as dilution air, was introduced rapidly and co-axially with sampled exhaust at one end of dilution tunnel to accomplish similar dilution process that occurs in the atmosphere. The co-axial introduction of dilution air along with exhaust eliminates the possibility of particle losses on the wall of the dilution tunnel. A needle valve was used to control the dilution air flow in dilution tunnel and a flow calibrator of range 10–100 LPM was installed to monitor the flow in the dilution air supply line. Different dilution ratios were maintained by controlling the flow rate of dilution air and sampled exhaust.
2.3. Chemical characterization of aerosol
The filters were analyzed for ions using an ion chromatograph (Dionex ICS-1000) at Indian Institute of Technology Delhi (IIT-Delhi). Field blanks were also analyzed and used for correction. ECs and OCs were determined using thermal optical reflectance analyzer (TOR, model DRI2100) at Indian Institute of Science Education and Research (IISER), Bhopal. The procedure is described in Sections S1 and S2 in the SI.
3. Result and discussion
3.1. Effect of dilution on aerosol number and volume concentration
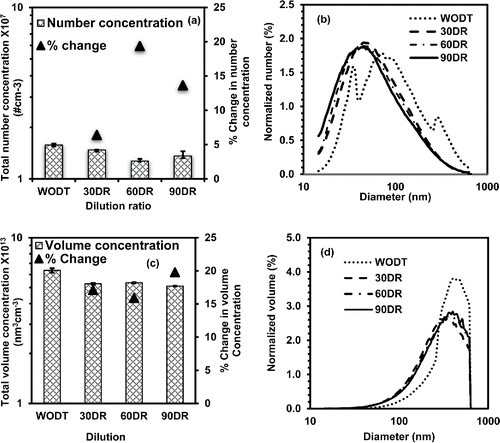
The effect of dilution on aerosol number and volume concentration was determined to evaluate the particle loss and nucleation/coagulation process inside the dilution tunnel. The number distribution was measured for polydisperse NaCl aerosol generated using an atomizer (TSI3061) at IIT Bombay (Figure S1 in the SI). Three experiments for each case including without dilution tunnel and with dilution tunnel at different dilution ratios (30:1, 60:1, and 90:1) were conducted. The average and standard deviation of total number concentration measured in base case (without dilution tunnel WODT) was 1.58 × 107 ± 3.20 × 105. The average of total number concentration measured with dilution tunnel at various dilution ratios 30:1, 60:1, and 90:1 were 1.48 × 107 ± 6.00 × 104, 1.27 × 107 ± 3.93 × 105, and 1.36 × 107 ± 9.01 × 105, respectively (). The variability among the experiments was 0.4–7.0% as depicted by one standard deviation around mean. The change in total number concentration at different dilution ratios 30:1, 60:1, and 90:1 compared to base experiment (WODT) were 6.4, 19.6, and 13.9%, respectively. In experiments with dilution tunnel the shift in number distribution toward ultrafine mode and increase in number concentration of ultrafine particles were observed indicating the nucleation process inside the dilution tunnel. The normalized number concentration () indicated no significant difference at different dilution ratios, also depicted by geomean particle diameters 107.3 ± 4.3 nm, 105.9 ± 3.8 nm, and 103.8 ± 3.9 nm at dilution ratios 30:1, 60:1, and 90:1. The geometric mean of particle diameter at different dilution ratios were significantly (p = 4.16E-07, 4.56E-07, 1.9E-07) lower than the diameter recorded in case of without dilution tunnel (WODT) experiment, also indicating nucleation in presence of dilution tunnel. However, no significant difference in geometric mean diameter at different dilution ratios implies no effect of dilution on nucleation process. The average of total volume concentrations measured were 6.36 × 1013 ± 1.89 × 1012, 5.27 × 1013 ± 4.58 × 1011, 5.35 × 1013 ± 1.80 × 1011, and 5.10 × 1013 ± 1.87 × 1011) for experiment without dilution tunnel, and with dilution tunnel at 30:1, 60:1, and 90:1 dilution ratios, respectively (). Low variability (0.3–3%) depicted by standard deviation indicated consistency in measured concentrations for each case. The percentage change in total volume concentration at different dilution ratios with respect to without dilution tunnel experiment was 17.1, 15.9, and 19.8%, which again indicated low particle loss inside the dilution tunnel (). The volume distribution also indicated the effect of dilution on nucleation process inside the dilution tunnel ().
Figure 2. Total number and volume concentration of NaCl particles and change in concentration at different dilution ratio. The bars in plots (a) and (c) show total number and volume concentrations averaged from three experiments without dilution tunnel (WODT) and with dilution tunnel operated at different dilution ratios (30:1 to 90:1). The triangular legends indicate the change in number and volume concentrations due to presence of dilution tunnel operated at different dilution ratio. The plots (b) and (d) show the normalized number and volume concentrations distribution for without dilution tunnel and with dilution tunnel operated at different dilution ratios.
3.2. Mixing performance of dilution tunnel
Experiments were conducted with a gasifier stove developed by the Department of Mechanical Engineering at Indian Institute of Technology Delhi and described in Sutar Citation(2015) and a light duty diesel vehicle (INNOVA, 2007 Model, 2400 cc) using chassis dynamometer facility at Manesar, Gurgaon.
The experiments were performed at dilution ratios 36:1, 60:1, 90:1 and 120:1. Dilution ratios calculated using CO2 concentration (Equation Equation(1)
[1] ) showed good agreement with flow-based dilution ratios (Figure S6 in the SI). The measured CO2-based dilution ratio showed large variation (35–50%) at high (120:1) dilution ratio depicted by one standard deviation around mean calculated for sampling duration, while the variation at low dilution ratios are within 10–30% (Figure S6 in the SI). In most of the experiments, dilution ratio deviated by less than 20% from 1:1 line except the low dilution ratio (52:1) that deviated by ∼40% from 1:1 line (Figure S6 in the SI).
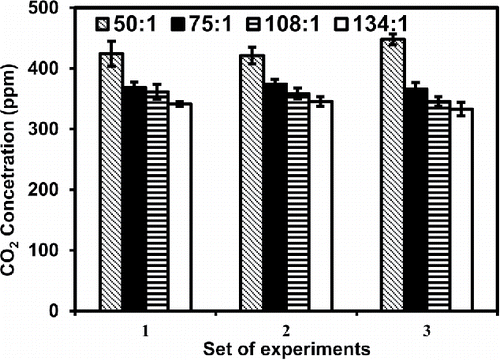
At high dilution ratios, the CO2 concentration showed large variation; therefore, the CO2 was measured both in longitudinal and cross-sectional directions at every 5-min interval to evaluate the mixing of undiluted sampled exhaust and zero air inside the dilution tunnel. CO2 concentrations were measured at four points, 100, 200, 300 and 400 cm, from upstream of the dilution tunnel (Figure S3a in the SI) and at radial distances across the cross-section of the dilution tunnel (Figure S3b in the SI). At a given dilution ratio, the CO2 concentration varied insignificantly at various points along the length of dilution tunnel both for gasifier cookstove (Figure S3c in the SI) and diesel vehicle experiments (Figure S3d in the SI). For gasifier stove the CO2 concentrations averaged over four points showed 0.3–1% variability, and were measured as 415 ± 6 ppm, 360 ± 4 ppm, 338 ± 5 ppm, and 320 ± 2 ppm, respectively, at dilution ratios 44:1, 82:1, 129:1, and 140:1 (Figure S3c in the SI). The concentration of CO2 in the dilution tunnel decreased with increasing dilution ratio; however, the concentration of CO2 in exhaust did not show such decrease and were averaged as 18,260 ± 269, 29,530 ± 290, 43,618 ± 610, and 44,800 ± 286 ppm, respectively, at dilution ratios tested in set of experiments. This indicated the CO2 concentration of diluted mixture decreases with increasing dilution ratio because of introduction of larger volume of zero air at high dilution ratios. In a different set of experiments with gasifier stove, the CO2 concentrations were measured at ports located at radial distances (Figure S3b in the SI). The CO2 concentrations at radial distances also varied insignificantly 1–5% at different dilution ratios (52:1, 75:1, 109:1, and 140:1) ().
Figure 3. Variation of CO2 at different points in radial direction measured in gasifier cookstove experiments conducted at different dilution ratios.
In the set of experiments with diesel vehicle average CO2 concentrations at four points located longitudinally were measured as 1100 ± 60 ppm, 800 ± 40 ppm, 700 ± 30 ppm, and 400 ± 40 ppm at dilution ratios 39:1, 65:1, 96:1, and 144:1, respectively, showing 4–10% variability (Figure S3d in the SI). The corresponding CO2 in exhaust were 43,582 ± 325, 56,062 ± 235, 63,360 ± 753, and 55,500 ± 737 ppm at dilution ratios studied for diesel vehicle. The low variability depicted by standard deviation around mean CO2 concentration of diluted mixture over four points also indicates rapid and homogeneous mixing inside the dilution tunnel regardless of the distance from the inlet point of the exhaust and dilution air. The homogenous mixing is one of the requirement of dilution sampling system (Hildemann et al. Citation1989) to simulate the mixing at near atmospheric condition. Another way to judge the homogenous mixing inside the dilution tunnel is to measure the temperature and differential pressure in longitudinal direction. The temperature inside the dilution tunnel averaged over four points were 31.5 ± 0.8°C and 29.9 ± 1.4°C in experiments with gasifier stove and diesel vehicle, respectively, indicating homogenous and near ambient conditions (Figures S3e and f in the SI). Additionally, the differential pressure was monitored between two points, 100 and 300 cm, and was found consistent in all the experiments (Figure S3e and f in the SI). Excessive moisture should be avoided inside the dilution tunnel to minimize particle loss due to condensation. The RH averaged over four points were low for experiments with gasifier stove (17.1 ± 1.3%) and diesel vehicle (16.1 ± 1.3%) and showed insignificant variation (Figure S3e and f in the SI).
3.3. Effect of dilution ratio on aerosol mass and chemical species
3.3.1 PM2.5 emission factor
Here, we discuss the effect of dilution on PM2.5 emission factors for gasifier cookstove and LDDV. The particles were collected on quartz and Teflon filters intended for different chemical analysis. For gasifier cookstove experiments the difference in PM2.5 emission factors calculated using mass collected on different filters varied from 4% to 8%. PM2.5 were collected from the combustion of Jamun wood fuel (length 20 mm and diameter 10 mm) commonly used in various parts of India (Habib et al. Citation2008). The gasifier used in this study was an induced downdraft type designed in the Mechanical Engineering Department of IIT-Delhi and described elsewhere (Sutar Citation2015), which operated at 16 LPM induced air flow rate. PM2.5 emission factors (gkg−1) at CO2-based dilution ratios 52:1, 75:1, 109:1, and 140:1 were 5.6 ± 0.5, 7.4 ± 0.04, 6.4 ± 0.2, and 6.9 ± 0.6 g kg−1, respectively (). The PM2.5 emission factors for gasifier cookstove are within the range reported in literature () for traditional and advance stoves.
Table 1. Emission factor of PM2.5 and relative contribution of chemical components for wood combustion in gasifier cookstove.
PM2.5 emission factors for gasifier cookstove showed little variation among dilution ratios studied here (). Though the residence time estimated using flow rate inside the dilution tunnel decreased (8.7–2.6 s) with increasing dilution ratio (). Probably because the smoke from stove was pre-diluted with background air before sampling, further dilution inside the dilution tunnel and the extended residence time did not affect much the phase equilibrium of the gas particle partitioning. Decrease in emission factor with increase in dilution ratio was reported by Lipsky and Robinson Citation(2006) for wood smoke sampled at high temperature (130–150°C) and diluted inside the dilution tunnel to achieve near-ambient temperature. Notably no pre-dilution with background air was adopted in Lipsky and Robinson Citation(2006). However, in order to confirm the phase equilibrium and gas to particle partitioning we used OC measured on bare quartz filters and back up quartz filters and investigated against dilution ratio as discussed in next section.
Figure 4. (a) Average PM2.5 emission factors and residence time for wood fuel combustion in gasifier cookstove; (b) average PM2.5 emission factors and residence time from light duty diesel vehicle.
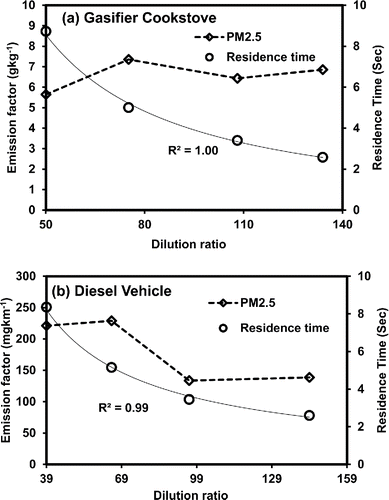
PM2.5 emission factors for diesel vehicle varied considerably from 221 mg km−1 to 139 mg km−1 at 39:1 to 144:1 dilution ratios (). No decrease in emission factor was observed between 39:1 and 65:1 dilution ratios. However, a sharp decrease (41%) in PM2.5 emission factor was observed at 96:1 dilution ratio compared to 65:1 dilution ratio, while further increase in dilution ratio from 96:1 to 144:1 again did not show any such decrease (). Decrease in emission factor with increase in dilution ratio was also reported for low-load diesel generator by Lipsky and Robinson Citation(2006) and medium duty vehicle by May et al. Citation(2013). In the present study, the residence time also showed 39% (8.4–5.1 s) decrease initially from 39:1 to 65:1 dilution ratio; after that the decrease in residence time was 31% (5.1–3.5 s) and 26% (3.5–2.6 s) at higher dilution ratios (). Remember initially the residence times at 39:1 and 65:1 were probably more than the time required for condensation; therefore, no decrease in emission factor was observed between two dilution ratios. However, the further decrease in residence time may have affected the particle formation by limiting homogenous and heterogeneous condensation of organic vapors resulting in low particle emission rate. Additionally the particle free air has been used for dilution, while in real world the dilution occurs with particle laden atmospheric air. This will also reduce the possibility of heterogeneous condensation leading to low emission rate. The effect of dilution on particulate organic carbon and positive artifact has been discussed in following section as a possible explanation of change in PM2.5 emission factor.
Our PM2.5 emission factor (221, 228, 134, and 136 mg km−1) were 1.5–2.5 times higher than average value (92 mg km−1) reported in literature (). While our values are within the range reported in literature. At low dilution ratio (39:1 and 65:1) our emission factors are close to values 250 mg km−1 and 230 ± 120 mg km−1 reported by Parson (2003) and Kim Oanh et al. Citation(2010) for light duty vehicles (). At high dilution ratios (96:1 and 144:1) our PM2.5 emission factors (134 and 139 mg km−1) are within the range (70–150 mg km−1) reported by Paw-armart Citation(2004), Rexeis and Hausberger Citation(2009), and Kerminen et al. Citation(1997). The emission factors reported from this work are 5–15 times higher than the values reported by ARAI Citation(2008) (15 mg km−1) for post-2005 Indian passenger cars (engine capacity 1400–2400 cc) and by Cheung et al. Citation(2010) (27 mg km−1) for diesel passenger cars (Euro-IV-Honda Accord 2200 cc Diesel equipped with exhaust gas recirculation and three-stage catalytic convertor). Overall the emission factors measured in this study encompass the values reported in literature.
Table 2. Emission factor of PM2.5 and relative contribution of chemical components for light duty diesel vehicle.
3.3.2. Effect of dilution on elemental and organic carbon
As expected for gasifier cookstove the elemental carbon a chemically inert component of PM2.5 did not show variation with increasing dilution ratio (). The EC emission factor (g kg−1) regardless of dilution ratios (50:1, 75:1, 108:1, 134:1) were consistent (1.0 ± 0.1, 1.2 ± 0.1, 1.1 ± 0.1 and 1.2 ± 0.2 gkg−1). This also implies that the particle loss in the system was negligible at high dilution ratios. EC emission factors measured in the present study are within the range of values (0.28 g kg−1–7.2 g kg−1) reported for traditional and advance cookstoves (Habib et al. Citation2008; Just et al. Citation2013).
Figure 5. (a) Variation in emission factors of elemental carbon (EC), organic carbon (OC) and total carbon (TC) measured on quartz filters for gasifier cook stove experiments conducted at different dilution ratios. Q is the sum of OC on front quartz (FQ) and back up quartz (QBQ), particulate OC is estimated as Q-QBT (quartz behind Teflon). (b) The variation of EC, OC and TC with dilution ratios for light duty diesel vehicle. The error bars are estimated using maximum (25%) variation in OC, EC measured on three punches of one quartz filter.
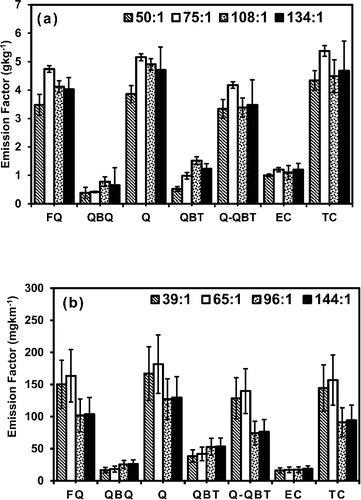
The particulate OC was calculated by adding the OC on front quartz (FQ) and back up quartz behind front quartz filter (QBQ) and subtracting the OC on backup quartz behind teflon filter (QBT). The particulate OC emission factors (FQ+QBQ-QBT) were 3.3 ± 0.3 4.2 ± 0.1, 3.4 ± 0.3, and 3.5 ± 0.9 g kg−1, respectively, at 50:1, 75:1, 108:1, and 134:1 dilution ratios. OC emission factors measured in the present work for gasifier cookstove are within the range of values reported for traditional and advanced cookstove (0.08–8.4 g kg−1) (Fine et al. Citation2001; Schauer et al. Citation2001; Fine et al. Citation2002; Sheesley et al. Citation2003; Fine et al. Citation2004; Habib et al. Citation2008; Just et al. Citation2013; Sen et al. Citation2014). At dilution ratio 108:1 compared to 75:1 the particulate OC emission factor decreased by 19%. This change can be attributed to decrease in OC emission rate in front quartz and increase in artifact OC on QBQ and QBT () indicating the effect of dilution on gas particle partitioning. While further dilution resulted in insignificant change in OC emission rate (2%). Pankow Citation(1994) reported that the formation of particulate organic carbon occurs due to absorption of volatile phase compounds with organic solution and adsorption on preexisting inert components such as elemental carbon and minerals (sorptive material). Therefore, gas to particle partitioning governs by concentration and saturation pressure of vapor phase compounds and the concentration and composition of sportive material. At high dilution ratios the organic aerosol that acts as sorptive material and facilitate conversion of vapor phase organic compounds to particle through absorption was devoid resulting in high concentration of vapor phase organic compounds (QBQ and QBT) and low concentration of particle phase organic aerosol (Q-QBT) (). One shall also note that EC emission rate was consistent regardless of dilution ratio that means sufficient inert EC surface was available for heterogeneous condensation of organic vapor; however, the short residence time at high dilution ratio would have reduced the possible condensation and limited the particle formation. Overall, the results indicated that with increase in dilution ratio the gas particle partitioning plays a significant role in particle formation. However, it shall also be noted that the smoke from gasifier stove was pre-diluted with background air before sampling; the average temperature of smoke entered into dilution tunnel was around 43°C, which was further diluted using particle free air. Therefore, the gas particle partitioning was not prominent as reported by Lipsky and Robinson Citation(2006).
For LDDV, the EC emission factors were consistent as 16.2, 17.0, 17.3, and 18.6 mg km−1 at 39:1, 65:1, 96:1, and 144:1 dilution ratios, respectively (). In previous studies, EC emission factors were reported in the range (2.14–242.2 mg km−1). The OC emission factor decreased by (41%) from 128 mg km−1 to 76 mg km−1 when dilution ratio was increased from 39:1 to 144:1 (). The large decrease in particulate OC emission factor was supported by decrease in OC on front quartz filter and increase in artifact OC on QBQ and QBT. The tail pipe exhaust of temperature around 100–150°C was entered into dilution tunnel and diluted using particle free air of atmospheric temperature; this process has affected the phase equilibrium considerably. As mentioned earlier, such a large decrease in particulate OC emission factor was not observed for gasifier experiments where the smoke was pre-diluted with background air.
Our OC emission factors (74.0; and 76.6 mg km−1) at dilution ratios 96:1 and 144:1, respectively, are within the range (6.3–115.8 mg km−1) reported in literature (Kirchstetter et al. Citation1999; Ban-Weiss et al. Citation2008; Kim Oanh et al. Citation2010), while at dilution ratios 39:1 and 65:1 the present values (128 and 140 mg km−1) are higher than upper range reported in literature.
3.3.3. Water soluble ions
Teflon filters were analyzed for water soluble anions (i.e., Cl−, NO3−, and SO42−) and cations (Na+, NH4+, K+, Ca2+, and Mg2+) using ion-chromatography (Dionex ICS-1000). Relative contribution of ions to PM2.5 emissions from gasifier cookstove at dilution ratios 50:1, 75:1, 108:1, and 134:1 was estimated as 4.1 ± 0.74, 4.9 ± 1.1, 7.6 ± 1.4, and 9.0 ± 1.8%, respectively (). Among ions highest contribution from potassium, a marker of biomass combustion, was observed in all experiments. Other major contributors were sodium, magnesium, ammonium, nitrate, chloride, and sulfate across the experiments conducted at different dilution ratios. Calcium was found in insignificant amount in all the experiments.
Previous studies (Fine et al. Citation2001; Schauer et al. Citation2001; Fine et al. Citation2002; Sheesley et al. Citation2003; Fine et al. Citation2004; Habib et al. Citation2008; Schmidl et al. Citation2008; Zhang et al. Citation2012; Just et al. Citation2013; Sen et al. Citation2014; Shahid et al. Citation2015) have reported the dilution ratio 30–60; therefore, it is appropriate to compare our result at dilution ratio 50:1 with values reported in literature. In the present study, the relative contributions () of all the ions except Mg2+ were within the range reported in literature (Schmidl et al. Citation2008; Zhang et al. Citation2012; Sen et al. Citation2014; Shahid et al. Citation2015). The contribution of Mg2+ was higher than values reported in literature. Total relative contribution of EC, OC, and ions to PM2.5 varied from 73.0 ± 4.1% to 82.1 ± 4.6% at different dilution ratio for gasifier stove.
For diesel vehicles, among cations NH4+, K+, and Na+ were major contributors (3.85–5.12%) to PM2.5 with insignificant contribution from Ca2+ and Mg2+ (). Cations observed in the present study were higher than the range reported in literature for diesel vehicles (Kirchstetter et al. Citation1999; Schauer et al. Citation1999; Geller et al. Citation2005; Ban-Weiss et al. Citation2008; Cheng et al. Citation2010; Cheung et al. Citation2010; Kim Oanh et al. Citation2010; Chiang et al. Citation2012; Kam et al. Citation2012). Relative contribution of anions (0.46–2.22%) for the dilution ratios studied here were within the range reported in literature. Among anions, major contributor is NO3− followed by SO42−(). NOx formation governed by high temperature combustion and sulfate emissions results from sulfur content of diesel fuel (Chiang et al. Citation2012). Notably, total contribution of EC, OC, and ions to PM2.5 varied from 70.5% to 74.4% for LDDV.
The findings from this study have implications in refining emission inventories, as contribution of ECs and OCs from diesel vehicles may be underestimated using EC and OC emission factors (2.8 mg km−1, 7.5 mg km−1) reported by ARAI Citation(2008) for 1400–2400 cc diesel vehicle. Aerosol chemical constituents (i.e., OC and EC) and precursors (i.e., sulfate, nitrate, along with ammonium ion) participate in nucleation (Shi and Harrison Citation1999) and hence in cloud formation, and interact with solar radiation; therefore, their emissions from regionally dominated and less understood sources can play an important role in climate change. The aerosol measurement and characterization at field is an urgent need for improving our understanding of regional climate change and health risk.
4. Conclusions
Large uncertainty in BC emissions from South Asian sources have been questioned recently because of limited measurements. In South Asia, including India, the energy use pattern and technology vary considerably in space and time. Limited efforts have been made to assess the emissions from various combustion sources in India; however, improving air quality associated health risk and understanding climate change have been emerged as regionally important and urgent issues. Without understanding the source strength and its distribution, the above issues cannot be addressed properly. Therefore, aerosol measurement and characterization for climate and health assessment are urgently needed. This study presents the emissions factor of PM2.5, EC, OC, and ions for gasifier cookstove and LDDV using a PDS developed for measurement of aerosol and its precursors. The study showed homogenous and rapid mixing inside the dilution tunnel at near atmospheric temperature. The emission factors of PM2.5, EC, and OC for gasifier cookstove and LDDV were close to values reported in literature. A mild decrease in PM2.5 and OC emission factor was observed in gasifier experiments as smoke diluted with background air was further diluted and sampled; therefore, the induced dilution changed the phase equilibrium insignificantly. In contrast, a sharp decrease in PM2.5 and OC emission factors were observed with increasing dilution ratio for diesel vehicle experiments where hot exhaust was diluted and sampled. The decrease in particulate OC emission factor and increase in semivolatile OC emission factor with increasing dilution ratio indicated the shift of phase equilibrium. At high dilution ratio, inadequate sorptive material (organic compounds) and insufficient residence time limited the particle formation. Overall, low dilution may overestimate the aerosol mass and high dilution and low residence time may limit the particle formation and underestimate the aerosol mass. Therefore, the dilution ratio in the range of 30 to 60 will be used in further measurement of on-road vehicles.
15-150.R1_SI_21Mar2016.pdf
Download PDF (452.6 KB)Acknowledgments
The authors are sincerely thankful to Department of Science and Technology for supporting this work under fast track scheme. We also thank Polltech Instruments Pvt Ltd, Mumbai, for valuable input in the development of PDS. We also pay a sincere gratitude to Professor Chandra Venkataraman, Indian Institute of Technology Bombay, Professor S. Kohli, Indian Institute of Technology Delhi, and Professor Ramya Sunder Raman, Indian Institute of Science Education and Research, Bhopal, for their support in experiments and chemical characterization work.
References
- ARAI. (2008). Emission Factor Development for Indian Vehicles. Air Quality Monitoring Project-Indian Clean Air Programme. The Automotive Research Association of India, Pune.
- ARAI. (2009). Source Profiling for Vehicular Emissions. Air Quality Monitoring Project-Indian Clean Air Programme, Report No. ARAI/VSP-III/SP/RD/08-09/60. The Automotive Research Association of India, Pune.
- Ban-Weiss, G. A., Lunden, M. M., Kirchstetter, T. W., and Harley, R. A. (2010). Size-Resolved Particle Number and Volume Emission Factors for On-Road Gasoline and Diesel Motor Vehicles. J. Aerosol Sci., 41:5–12.
- Ban-Weiss, G. A., Mclaughlin, J. P., Harley, R. A., Lunden, M. M., Kirchstetter, T. W., Kean, A. J., Strawa, A. W., Stevenson, E. D., and Kendall, G. R. (2008). Long-Term Changes in Emissions of Nitrogen Oxides and Particulate Matter from On-Road Gasoline and Diesel Vehicles. Atmos. Environ., 42:220–232.
- Baron, P. A., and Willeke, K., 2001. Aerosol Measurement: Principles, Techniques, and Applications, 2nd ed. John Wiley, Hoboken, NJ.
- Barth, M., and Boriboonsomsin, K. (2009). Energy and Emissions Impacts of a Freeway-Based Dynamic Eco-Driving System. Trans. Res. Part D: Trans. Environ., 14(6):400–410.
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., Deangelo, B. J., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S. (2013). Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos., 118:5380–5552.
- Bond, T. C., Streets, D. G., Yarber, K. F., Nelson, S. M., Woo, J.-H., and Klimont, Z. (2004). A Technology-Based Global Inventory of Black and Organic Carbon Emissions from Combustion. J. Geophys. Res., 109:D14203, doi:10.1029/2003JD003697.
- Cheng, Y., Lee, S. C., Ho, K. F., Chow, J. C., Watson, J. G., Louie, P. K. K., Cao, J. J., and Hai, X. (2010). Chemically-Speciated On-Road PM2.5 Motor Vehicle Emission Factors in Hong Kong. Sci. Tot. Environ., 408:1621–1627.
- Cheung, K. L., Ntziachristos, L., Tzamkiozis, T., Schauer, J. J., Samaras, Z., Moore, K. F., and Sioutas, C. (2010). Emissions of Particulate Trace Elements, Metals and Organic Species from Gasoline, Diesel, and Biodiesel Passenger Vehicles and their Relation to Oxidative Potential. Aerosol Sci. Technol., 44:500–513.
- Chiang, H. L., Lai, Y. M., and Chang, S. Y. (2012). Pollutant Constituents of Exhaust Emitted from Light-Duty Diesel Vehicles. Atmos. Environ., 47:399–406.
- CPCB. (2010). Air Quality Monitoring, Emission Inventory and Source Apportionment Study for Indian Cities. Central Pollution Control Board, Government of India, New Delhi.
- Dallmann, T. R., Demartini, S. J., Kirchstetter, T. W., Herndon, S. C., Onasch, T. B., Wood, E. C., and Harley, R. A. (2012). On-Road Measurement of Gas and Particle Phase Pollutant Emission Factors for Individual Heavy-Duty Diesel Trucks. Environ. Sci. Technol., 46:8511–8518.
- Dallmann, T. R., Harley, R. A., and Kirchstetter, T. W. (2011). Effects of Diesel Particle Filter Retrofits and Accelerated Fleet Turnover on Drayage Truck Emissions at the Port of Oakland. Environ. Sci. Technol., 45:10773–10779.
- Das, S., Dey, S., Dash, S. K., and Basil, G., (2013). Examining Mineral Dust Transport over the Indian Subcontinent Using the Regional Climate Model, Regcm4.1. Atmos. Res., 134:64–76.
- Durbin, T. D., Johnson, K., Cocker, D. R., Miller, J. W., Maldonado, H., Shah, A., Ensfield, C., Weaver, C., Akard, M., Harvey, N., Symon, J., Lanni, T., Bachalo, W. D., Payne, G., Smallwood, G., and Linke, M. (2007). Evaluation and Comparison of Portable Emissions Measurement Systems and Federal Reference Methods for Emissions from a Back-Up Generator and a Diesel Truck Operated on a Chassis Dynamometer. Environ. Sci. Technol., 41:6199–6204.
- Dwivedi, D., Agarwal, A. K., and Sharma, M. (2006). Particulate Emission Characterization of a Biodiesel vs Diesel-Fuelled Compression Ignition Transport Engine: A Comparative Study. Atmos. Environ., 40:5586–5595.
- Fine, P. M., Cass, G. R., and Simoneit, B. R. T. (2001). Chemical Characterization of Fine Particle Emissions from the Fireplace Combustion of Woods Grown in the Northeastern United States. Environ. Sci. Technol., 35:2665–2675.
- Fine, P. M., Cass, G. R., and Simoneit, B. R. T. (2002). Chemical Characterization of Fine Particle Emissions from the Fireplace Combustion of Woods Grown in the Southern United States. Environ. Sci. Technol., 36:1442–1451.
- Fine, P. M., Cass, G. R., and Simoneit, B. R. T. (2004). Chemical Characterization of Fine Particle Emissions from the Wood Stove Combustion of Prevalent United States Tree Species. Environ. Eng. Sci., 21:705–721.
- Garg, A., Shukla, P. R., and Kapshe, M. (2006). The Sectoral Trends of Multi-Gas Emissions Inventory of India. Atmos. Environ., 40:4608–4620.
- Geller, M. D., Sardar, S. B., Phuleria, H., Fine, P. M., and Sioutas, C. (2005). Measurements of Particle Number and Mass Concentrations and Size Distributions in a Tunnel Environment. Environ. Sci. Technol., 39:8653–8663.
- Gertler, A. W., Gillies, J. A., Pierson, W. R., Rogers, C. F., Sagebiel, J. C., Abu-Allaban, Coulombe, W. M., Tarney, L., and Cahill, T. A. (2002). Emissions from Diesel and Gasoline Engines Measured from Motor Vehicles in a Highway Tunnel Emissions in Two Highway Tunnels. Research Report. Health Effects Institute, Boston, MA.
- Goel, R., Guttikunda, S. K., Mohan, D., and Tiwari, G. (2015). Benchmarking Vehicle and Passenger Travel Characteristics in Delhi for On-Road Emissions Analysis. Travel Behav. Soc., 2:88–101.
- Grieshop, A. P., Boland, D., Reynolds, C. C. O., Gouge, B., Apte, J., Rogak, S. N., and Kandlikar, M. (2012). Modeling Air Pollutant Emissions from Indian Auto-Rickshaws: Model Development and Implications for Fleet Emission Rate Estimates. Atmos. Environ., 50:148–156.
- Habib, G., Venkataraman, C., Bond, T. C., and Schauer, J. J. (2008). Chemical, Microphysical and Optical Properties of Primary Particles from the Combustion of Biomass Fuels. Environ. Sci. Technol., 42:8829–8834.
- Hansen, J. Q., Winther, M., and Sorenson, S. C. (1995). The Influence of Driving Patterns on Petrol Passenger Car Emissions. Sci. Tot. Environ., 169(1):129–139.
- Hildemann, L. M., Cass, G. R., and Markowski, G. R. (1989). A Dilution Stack Sampler for Collection of Organic Aerosol Emissions: Design, Characterization and Field Tests. Aerosol. Sci. Technol., 10:193–204.
- Johnson, K. C., Durbin, T. D., Cocker, D. R., Miller, W. J., Bishnu, D. K., Maldonado, H., Moynahan, N., Ensfield, C., and Laroo, C. A. (2009). On-Road Comparison of a Portable Emission Measurement System with a Mobile Reference Laboratory for a Heavy-Duty Diesel Vehicle. Atmos. Environ., 43:2877–2883.
- Just, B., Rogak, S., and Kandlikar, M. (2013). Characterization of Ultrafine Particulate Matter from Traditional and Improved Biomass Cookstoves. Environ. Sci. Technol., 47:3506–3512.
- Kam, W., Liacos, J. W., Schauer, J. J., Delfino, R. J., and Sioutas, C. (2012). On-Road Emission Factors of PM Pollutants For Light-Duty Vehicles (LDVS) Based on Urban Street Driving Conditions. Atmos. Environ., 61:378–386.
- Kean, A. J., Harley, R. A., and Kendall, G. R. (2003). Effects of Vehicle Speed and Engine Load on Motor Vehicle Emissions. Environ. Sci. Technol., 37(17):3739–3746.
- Kerminen, V. M., Makela, T. E., Ojanen, C. H., Hillamo, R. E., Vilhunen, J. K., Rantanen, L., Havers, N., VonBohlen, A., and Klockow, D., (1997). Characterization of the Particulate Phase in the Exhaust from a Diesel Car. Environ. Sci. Technol., 31:1883–1889.
- Kim Oanh, N. T., Thiansathit, W., Bond, T. C., Subramanian, R., Winijkul, E., and Paw-Armart, I. (2010). Compositional Characterization of PM2.5 Emitted from In-Use Diesel Vehicles. Atmos. Environ., 44:15–22.
- Kirchstetter, T. W., Harley, R. A., Kreisberg, N. M., Stolzenburg, M. R., and Hering, S. V. (1999). On-Road Measurement of Fine Particle and Nitrogen Oxide Emissions from Light- and Heavy-Duty Motor Vehicles. Atmos. Environ., 33:2955–2968.
- Kumar, M., Kumar, S., and Tyagi, S. K. (2013). Design, Development and Technological Advancement in the Biomass Cookstoves: A Review. Renew Sustain Energy Rev., 26:265–285.
- Lipsky, E. M., and Robinson, A. L. (2005). Design and Evaluation of a Portable Dilution Sampling System for Measuring Fine Particle Emissions. Aerosol Sci. Technol., 39:542–553.
- Lipsky, E. M., and Robinson, A. L. (2006). Effects of Dilution on Fine Particle Mass and Partitioning of Vapor Phase Organic Compounds Organics in Diesel Exhaust and Wood Smoke. Environ. Sci. Technol., 40:155–162.
- Lu, Z., Zhang, Q., and Streets, D. G. (2011). Sulfur Dioxide and Primary Carbonaceous Aerosol Emissions in China and India 1996–2010. Atmos. Chem. Phys., 11:9839–9864.
- May, A. A., Presto, A. A., Hennigan, C. J., Nguyen, N. T., Gordon, T. D., and Robinson, A. L. (2013). Gas-Particle Partitioning of Primary Organic Aerosol Emissions: (2). Diesel Vehicles. Environ. Sci. Technol., 47:8288–8296.
- Ministry of New and Renewable Energy, India (MNRE) (2009). National Biomass Cookstoves Programme (NBCP) MNRE report. Available at http://www.mnre.gov.in/schemes/decentralized-systems/national-biomass-cookstoves-initiative
- Mukunda, H. S. (2011). Understanding Clean Energy and Fuels from Biomass. Wiley India, New Delhi.
- Mukunda, H. S., Dasappa, S., Paul, P. J., Rajan, N. K. S., Yagnaraman M., Ravi, K. D., and Deogaonkar, M. (2010). Gasifier Stoves—Science, Technology and Field Outreach. Curr. Sci., 98:627–638.
- Mukunda, H. S., Shrinivasa, U., and Dasappa, S. (1988). Portable Single Pan Wood Stoves of High Efficiency for Domestic Use. Sadhana, 13:237–270.
- Ning, Z., Polidori, A., Schauer, J. J., and Sioutas, C. (2008). Emission Factors of PM Species Based on Freeway Measurements and Comparison with Tunnel and Dynamometer Studies. Atmos. Environ., 42:3099–3114.
- Pandey, A., and Venkataraman, C. (2014). Estimating Emissions from the Indian Transport Sector with On-Road Fleet Composition and Traffic Volume. Atmos. Environ., 98:123–133.
- Pankow, J. F. (1994). An Absorption Model of Gas/Particle Partitioning of Organic Compounds in the Atmosphere. Atmos. Environ., 28:185–188.
- Parashar, D., Gadi, R., Mandal, T., and Mitra, A. (2005). Carbonaceous Aerosol Emissions from India. Atmos. Environ., 39:7861–7871.
- Park, S. S., Kozawa, K., Fruin, S., Mara, S., Hsu, Y. K., Jakober, C., Winer, A., and Herner, J. (2011). Emission Factors For High-Emitting Vehicles Based On On-Road Measurements of Individual Vehicle Exhaust with a Mobile Measurement Platform. J. Air Waste Manage. Assoc., 61:1046–1056.
- Paw-armart, I. (2004). Development of Mobile Source Emission Factors for Bangkok Using Modeling and Emission Testing Tools. Master's Thesis, Asian Institute of Technology, Pathumthani, Thailand.
- Ramanathan, N., Lukac, M., Ahmed, T., Kar, A., Praveen, P. S., Honles, T., Leong, I., Rehman, I. H., Schauer, J. J., and Ramanathan, V. (2011). A Cellphone Based System for Large-Scale Monitoring of Black Carbon. Atmos. Environ., 45:4481–4487.
- Ramanathan, V., Chung, C., Kim, D., Bettge, T., Buja, L., Kiehl, J. T., Washington, W. M., Fu, Q., Sikka, D. R., and Wild, M. (2005). Atmospheric Brown Clouds: Impacts on South Asian Climate and Hydrological Cycle. Proc. Nat. Acad. Sci., 102(15):5326–5333.
- Ramanathan, V., Crutzen, P. J., Lelieveld, J., Mitra, A. P., Althausen, D., Andersons, J., Andreae, M. O., Cantrell, W., Cass, G. R., Chung, C. E., Clarke, A. D., Coakley, J. A., Collins, W. D., Conant, W. C., Dulac, F., Heintzenberg, T. M. J., Heymsfield, A. J., Holben, B., Howell, S., Hudson, J., Jayaraman, A., Kiehl, J. T., Krishnamurti, T. N., Lubin, D., McFarquhar, G., Novakov, T., Ogren, J. A., Podgorny, I. A., Prather, K., Priestley, K., Prospero, J. M., Quinn, P. K., Rajeev, K., Rasch, P., Rupert, S., Sadourny, R., Satheesh, S. K., Shaw, G. E., Sheridan, P., and Valero, F. P. J. (2001). The Indian Ocean Experiment: An Integrated Assessment of the Climate Forcing and Effects of the Great Indo-Asian Haze. J. Geophys. Res., 106(D 22):28371–28399.
- Reddy, M. S., Boucher, O., Venkataraman, C., Verma, S., Le´on, J. F., Bellouin, N., and Pham, M. (2004). General Circulation Model Estimates of Aerosol Transport and Radiative Forcing during the Indian Ocean Experiment. J. Geophys. Res., 109(D16205), doi:10.1029/2004JD004557.
- Rexeis, M., and Hausberger, S. (2009). Trend of Vehicle Emission Levels until 2020—Prognosis Based on Current Vehicle Measurements and Future Emission Legislation. Atmos. Environ., 43:4689–4698.
- Rezaei, F., and Webley, P., (2009). Optimum Structured Adsorbents for Gas Separation Processes. Chem. Eng. Sci., 64:5182–5191.
- Roden, C. A., Bond, T. C., Conway, S., and Osorto Pinel, A. B. (2006). Emission Factors and Real-Time Optical Properties of Particles Emitted from Traditional Wood Burning Cookstoves. Environ. Sci. Technol., 40:6750–6757.
- Roden, C. A., Bond, T. C., Conway, S., Pinel, A. B. O., MacCarty, N., and Still, D. (2009). Laboratory and Field Investigations of Particulate and Carbon Monoxide Emissions from Traditional and Improved Cookstoves. Atmos. Environ., 43:1170–1181.
- Rogak, S. N., Pott, U., Dann, T., and Wang, D. (1998). Gaseous Emissions from Vehicles in a Traffic Tunnel in Vancouver. J. Air Waste Manage. Assoc., 48:604–615.
- Ruthven, D. M., Farooq, S., and Knaebel, K. S. (1994). Pressure Swing Adsorption. VCH Publishers, New York.
- Sadavarte, P., and Venkataraman, C. (2014). Trends in Multi-Pollutant Emissions from a Technology-Linked Inventory for India: I. Industry and Transport Sectors. Atmos. Environ., 99:353–364.
- Sahu, S. K., Beig, G., and Parkhi, N. S. (2011). Emissions Inventory of Anthropogenic PM2.5 and PM10 in Delhi during Commonwealth Games 2010. Atmos. Environ., 45:6180–6190.
- Schauer, J. J., Kleeman, M. J., Cass, G. R., and Simoneit, B. R. T. (1999). Measurement of Emissions from Air Pollution Sources. 2. C1 through C30 Organic Compounds from Medium Duty Diesel Trucks. Environ. Sci. Technol., 33:1578–1587.
- Schauer, J. J., Kleeman, M. J., Cass, G. R., and Simoneit, B. R. T. (2001). Measurement of Emissions from Air Organic Compounds from Fireplace Combustion of Wood. Environ. Sci. Technol., 35:1716–1728.
- Schmidl, C., Marr, I. L., Caseiro, A., Kotianová, P., Berner, A., Bauer, H., Kasper-Giebl, A., and Puxbaum, H. (2008). Chemical Characterization of Fine Particle Emissions from Wood Stove Combustion of Common Woods Growing in Mid-European Alpine Regions. Atmos. Environ., 42:126–141.
- Sen, A., Mandal, T. K., Sharma, S. K., Saxena, M., Gupta, N. C., Gautam, R., Gupta, A., Gill, T., Rani, S., Saud, T., Singh, D. P., and Gadi, R. (2014). Chemical Properties of Emission from Biomass Fuels Used in the Rural Sector of the Western Region of India. Atmos. Environ., 99:411–424.
- Shahid, I., Kistler, M., Mukhtar, A., Ramirez-Santa Cruz, C., Bauer, H., and Puxbaum, H. (2015). Chemical Composition of Particles from Traditional Burning of Pakistani Wood Species. Atmos. Environ., 121:35–41.
- Sharma, M., Agarwal, A. K., and Bharathi, K. V. L. (2005). Characterization of Exhaust Particulates from Diesel Engine. Atmos. Environ., 39:3023–3028.
- Sheesley, R. J., Schauer, J. J., Chowdhury, Z., Cass, G. R., and Simoneit, B. R. T. (2003). Characterization of Organic Aerosols Emitted from the Combustion of Biomass Indigenous to South Asia. J. Geophys. Res., 108(D9):4285, doi:10.1029/2002JD002981.
- Shi, J. P., and Harrison, R. M. (1999). Investigation of Ultrafine Particle Formation during Diesel Exhaust Dilution. Environ. Sci. Technol., 33:3730–3736.
- Smit, R., Poelman, M., and Schrijver, J. (2008). Improved Road Traffic Emission Inventories by Adding Mean Speed Distributions. Atmos. Environ., 42(5):916–926.
- Sturm, P. J., Pucher, K., Sudy, C., and Almbauer, R. A. (1996). Determination of Traffic Emissions-Intercomparison of Different Calculation Methods. Sci. Tot. Environ., 189:187–196.
- Sutar, K. (2015). Development of Downdraft Gasifier Cook Stove for Domestic Application, Phd Thesis, Indian Institute of Technology Delhi, New Delhi.
- Tong, S., Schirnding, Y. E. V., and Prapamontol, T. (2000). Environmental Lead Exposure: A Public Health Problem of Global Dimensions. Bull. World Health Org., 78(9):1068–1077.
- Venkataraman, C., Habib, G., Eiguren-Fernandez, A., Miguel, A. H., and Friedlander, S. K. (2005). Residential Biofuels in South Asia: Carbonaceous Aerosol Emissions and Climate Impacts. Science, 307:1454–1456.
- Venkataraman, C., Habib, G., Kadamba, D., Shrivastava, M., Leon, J. F., Crouzille, B., Boucher, O., and Streets, D. G. (2006). Emissions from Open Biomass Burning In India: Integrating the Inventory Approach with High-Resolution Moderate Resolution Imaging Spectroradiometer (MODIS) Active-Fire and Land Cover Data. Global Biogeochem. Cyc., 20:GB2013, doi:10.1029/2005GB002547.
- Venkataraman, C., and Rao, G. U. M. (2001). Emission Factors of Carbon Monoxide and Size-Resolved Aerosols from Biofuel Combustion. Environ. Sci. Technol., 35:2100–2107.
- Venkataraman C., Sagar A. D., Habib G., Lam N., and Smith K. R. (2010). The Indian National Initiative for Advanced Biomass Cookstoves: The Benefits of Clean Combustion. Ene. Sustain. Develop. 14:63–72.
- Verma, S., Boucher, O., Venkataraman, C., Reddy, M. S., Müller, D., Chazette, P., and Crouzille, B. (2006). Aerosol Lofting from Sea Breeze during the Indian Ocean Experiment. J. Geophys. Res., 111:D07208, doi:10.1029/2005JD005953.
- Wang, X., Watson, J. G., Chow, J. C., Gronstal, S., and Kohl, S. D. (2012). An Efficient Multipollutant System for Measuring Real-World Emissions from Stationary and Mobile Sources. Aerosol Air Qual. Res., 12:145–160.
- Wang, X., Westerdahl, D., Wu, Y., Pan, X., and Zhang, K. M. (2011). On-Road Emission Factor Distributions of Individual Diesel Vehicles in and around Beijing, China. Atmos. Environ., 45:503–513.
- Westerdahl, D., Wang, X., Pan, X., and Zhang, K. M. (2009). Characterization of On-Road Vehicle Emission Factors and Micro-Environmental Air Quality in Beijing, China. Atmos. Environ., 43:697–705.
- Weyant, C., Vasudev, A., Ragavan, S., Rajarathnam, U., Lalchandani, D., Maithel, S., Baum, E., and Bond, T. C. (2014). Emissions from South Asian Brick Production. Environ. Sci. Technol., 48(11):6477–6483.
- Zhang, H., Wang, S., Hao, J., Wan, L., Jiang, J., Zhang, M., Mestl, H. E. S., Alnes, L. W. H., Aunan, K., and Mellouki, W. A. (2012). Chemical and Size Characterization of Particles Emitted from the Burning of Coal and Wood in Rural Households in Guizhou, China. Atmos. Environ., 51:94–99.
- Zhang, K., and Frey, C. (2008). Evaluation of Response Time of a Portable System for In-Use Vehicle Tailpipe Emissions Measurement. Environ. Sci. Technol., 42:221–227.