ABSTRACT
Recently, Pathak et al. Citation(2013) conducted a series of non-isothermal D2O nanodroplet growth studies in the free molecular regime. They found that under highly non-equilibrium conditions, the condensation (qc) and evaporation coefficients (qe) can differ from each other and from the expected value of 1. Here, we confirm these observations by analyzing comparable experiments using n-propanol. We show that the best agreement with the non-isothermal Hertz–Knudsen growth law corresponds to setting (qc, qe) = (1, 0.6) or (qc, qe) = (1.3, 1). The approach of retarded evaporation yields values close to those observed by Pathak et al. for D2O, but is difficult to justify theoretically. Enhancing the condensation coefficient is consistent with long-range attractive interactions between the vapor molecules and droplets in the nanometer size range.
© 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Almost all vapor-to-liquid phase transitions initiated by homogenous nucleation involve droplet growth in the free molecular regime. Understanding this phenomenon is, therefore, critical to a broad range of natural and industrial applications. In the atmosphere, for example, new particle formation yields clusters with diameters on order of 1–1.5 nm, but most instruments can only detect particles after they have grown to be 3–10 nm in size. Obtaining accurate nucleation rates, based on the appearance of the larger particles can be quite sensitive to assumptions made regarding the free molecular growth of the initial nuclei to detectable size (Korhonen et al. Citation2014). In an industrial setting, improving the efficiency of steam turbines requires droplet growth laws that are accurate from the free molecular to continuum regime under conditions that are far from equilibrium (Hughes et al. Citation2015).
Recently, Pathak et al. Citation(2013) investigated the growth rates of nonane and D2O nanodroplets formed via homogenous nucleation in a supersonic nozzle. In their experiments, the Knudsen number was always greater than 10, where λ is the mean free path of the carrier gas and
is the average droplet radius. Thus, droplet growth was always in the free molecular regime and the rate of droplet growth by condensation should be governed by the Hertz–Knudsen equation,
[1] where
is the molecular volume of the condensate,
is the mass of the condensate monomer,
is the Boltzmann constant,
is the partial pressure of the vapor, T is the gas temperature, and
is the droplet temperature. The vapor pressure above a droplet of radius
and temperature
is denoted as
and is given by
[2] where
is the equilibrium vapor pressure above a flat surface,
is the surface tension of the liquid, and
is determined by solving the coupled heat and mass fluxes to the growing droplets as summarized in Section 3 of this article and described in more detail in Pathak et al. Citation(2013). Finally, the condensation and evaporation coefficients qc and qe, are most often assumed to both equal 1, but there are arguments, based on experimental results, that these values do not need to be the same when the system is not at equilibrium (Young Citation1982; Marek and Straub Citation2001; Pathak et al. Citation2013). Furthermore, recent molecular dynamic (MD) simulations also concluded that the condensation and the evaporation coefficients can differ from each other and from 1 under non-equilibrium condensation or evaporation (Meland et al. Citation2004).
In the nonane nanodroplet growth experiments, Pathak et al. Citation(2013) found that coagulation was negligible on the timescale of the experiment because the aerosol number density was low enough. Furthermore, the condensational growth rates predicted by the Hertz–Knudsen equation (Equation Equation(1)[1] ) agreed very well with the experimentally determined values when qc = qe = 1. In these experiments, the high degree of supersaturation (
) of the alkane ensured that the condensational growth rates were essentially independent of the temperature of the growing droplets. Growth was only limited by the rate of arrival of monomer to the droplet surface and not by the re-evaporation of molecules from the rapidly growing droplet, and, thus, it was not possible to test whether qe was <1.
In contrast, in D2O nanodroplet growth experiments, rapid condensational growth gave way to coagulation-driven growth as the condensable was depleted from the vapor phase. More importantly, the non-isothermal droplet growth rates calculated using Equation Equation(1)[1] and qc = qe = 1, underestimated the experimentally measured values even though the droplet temperatures predicted by theory were lower than those estimated directly from the experimental data. The differences between the experimental and theoretical growth rates and droplet temperatures were greatly reduced by setting qe = ∼0.5 while maintaining qc = 1. Pathak et al.'s Citation(2013) approach was motivated by the work of Young Citation(1982) who found that models of low pressure steam condensation in supersonic nozzles only matched experimental results when the ratio qe/qc was less than 1. Evaluating Young's Equation (31), using the parameters suggested by author (qc = 1.0, α = 9) under Pathak et al.'s conditions gives qe/qc ∼ 0.65. The value qe = 0.5 determined by Pathak et al. was also comparable to the value (qe = 0.57 ± 0.06) reported by Drisdell et al. Citation(2008) who examined the evaporation of liquid D2O jets into vacuum.
One concern with Pathak et al.'s Citation(2013) D2O experiments is that the droplets were highly supercooled. With droplet temperatures in the range of 220 K < Td < 260 K, uncertainty in the analysis could arise from the physical properties for D2O being extrapolated by as much as 50 K. To examine whether evaporation coefficients that differ from 1 are reasonable or not requires commensurate experiments using a material whose growth also occurs under moderate supersaturation but where the liquid is not in a supercooled state. In addition, comparable number densities and droplet sizes would ensure equivalent influence from coagulation on the experimental results.
The first goal of this work is, therefore, to analyze nanodroplet growth experiments conducted with n-propanol to confirm the enhanced growth rate effect. Alcohols are a good choice because they condense in the supersonic nozzle at reasonable supersaturations and at temperatures well above their melting points (Ghosh et al. Citation2008; Mullick et al. Citation2015). Mullick et al. Citation(2015) recently published a set of position resolved measurements for n-propanol droplet growth that used the same set of matched nozzles (Nozzle C3/C2) as Pathak et al.'s Citation(2013) D2O experiments. Furthermore, the aerosol droplet sizes (D2O: ∼5.2 nm, n-propanol: ∼6.2 nm) and number densities (D2O: ∼3.2 × 1019 kg−1, n-propanol: ∼6.8 × 1019 kg−1) matched quite closely, and Kn was always greater than 100.
A second goal of this work is to better understand the origin of the enhanced growth rates. Pathak et al. Citation(2013) only considered the option of reducing qe to ∼0.5 while maintaining qc = 1. Here, we explore the alternate choice, i.e., allowing qc > 1 while maintaining qe = 1. Although the condensation rate expression generally only considers the velocity and density of vapor molecules and the surface area of a droplet, Vasil'ev and Reiss (Citation1996a,b) pointed out that the attractive potential of the liquid droplet should also play a role. In particular, the presence of such a potential could increase the number of vapor molecules impinging onto the droplet surface and, as a result, increase the condensation rate. Vasil'ev and Reiss defined the condensation enhancement factor as
[3] where β and β(c) are the condensation rates of vapor molecules onto a droplet in the presence and absence of an attractive potential. For small enough droplets under dilute vapor conditions, they found that by considering van der Waals interactions η could be as high as 2 or 3. They also showed that when the droplet size is large enough, or the carrier gas is dense enough, η again approaches 1 (Vasil'ev and Reiss Citation1996a).
Similar considerations also govern enhanced coagulation of nanoparticles in the free molecular regime. Here, experimental data (Fuchs and Sutugin Citation1965; Graham and Homer Citation1972; Okuyama et al. Citation1984; Mullick et al. Citation2015) and simulations (Marlow Citation1980, Citation1982; Kennedy Citation1987; Harris and Kennedy Citation1988; Gopalakrishnan and Hogan Citation2011; Ouyang et al. Citation2012) both show enhanced coagulation rates that depend on the strength of the long-range van der Waals interactions between particles and are a strong function of particle size and gas pressure. In a sense, condensation can be thought of as coagulation between a vapor molecule and the growing droplet. Indeed, the MD simulations by Gopalakrishnan and Hogan Citation(2011) showed that the dimensionless collision rates for both condensation and coagulation are essentially identical over the whole range of Kn. Thus, it would seem reasonable that if long-range interactions are important in coagulation, they should also be observable in nanodroplet growth studies.
2. Experiments
A detailed description of the flow system and the experimental methods used to conduct the n-propanol experiments are included in earlier papers (Ghosh et al. Citation2008; Mullick et al. Citation2015). To summarize briefly, expansions started from a stagnation temperature T0 of 49.80°C and stagnation pressure p0 of 30.18 ± 0.03 kPa. The carrier gas was N2 and the partial pressure of n-propanol was 0.43 kPa. In the Pressure Trace Measurements (PTM), the static pressure was measured as a function of position along the centerline of the flow with (wet trace) and without (dry trace) the condensate. Small Angle X-ray Scattering (SAXS) measurements were conducted using the 12-ID_C beam line at the Advanced Photon Source, Argonne National Labs, Argonne, IL to determine the mean radius of the droplets , the width of the size distribution σ, the aerosol number density N, and, thus, the mass fraction of condensate, g. The other properties of the flow, i.e., temperature T, density ρ, and velocity u, are then derived in by solving the equations that describe supersonic flow in the nozzle using p and g as the measured inputs. Further details of this integrated data analysis approach are available in Pathak et al. Citation(2013). The physical properties of n-propanol and N2 used in the calculations are those presented by Ghosh et al. Citation(2008).
3. Growth models
The two main processes that affect droplet growth in supersonic nozzle experiments are condensation and coagulation. We assume that these growth mechanisms are independent and, thus, the total growth rate is given by[4]
The expression for condensational growth is given in Equation Equation(1)[1] , where the droplet temperature is determined by balancing the mass
and heat
fluxes from the droplets to the surrounding gas, i.e.,
[5] and
is the latent heat of condensation. Equation Equation(5)
[5] neglects the small contribution from
that corresponds to the change in temperature of condensable vapor from T to Td, where cp,v is the specific isobaric heat capacity of the condensable vapor. In the free molecular regime,
is given by
[6] and
by
[7] where
is the specific isobaric heat capacity of nitrogen. To calculate the theoretical droplet temperature, Td,exact, we solve Equations Equation(5)–(7) using the experimental values for
, T,
, and
. To calculate the experimental droplet temperature, Td,exp, we equate
in Equation Equation(7)
[7] with the experimentally derived heat flux (Tanimura et al. Citation2010)
[8] where
is the number of particles per kg of flow and
is the experimentally determined mass flux.
For coagulation, we use the equation derived by Pathak et al. Citation(2013) based on the experimental values for and
:
[9]
4. Condensation enhancement factor
In a series of two papers, Vasil'ev and Reiss explored the effect long-range interactions could have on the growth of small clusters (Vasil'ev and Reiss Citation1996a,b). Their motivation was to examine the effect of increased capture cross sections on vapor-liquid nucleation rates. Although the droplets in our growth studies are well past the critical cluster size characteristic of supersonic nozzle nucleation experiments, they are reasonably close in size to critical clusters at lower saturations (Manka et al. Citation2012; Viisanen and Strey Citation1994). Thus, Vasil'ev and Reiss' reasoning should apply equally well to our situation.
To evaluate the condensation enhancement factor η, Vasil'ev and Reiss considered both the simple potential (Vasil'ev and Reiss Citation1996a) and the more realistic potential (Vasil'ev and Reiss Citation1996b)
[10] derived by integrating the simple potential over a sphere of radius R0 where
,
is the droplet radius, and a is the dimension of the hard repulsive molecular core. The values of
and
are related to the characteristic Lennard–Jones energy
and length
by
and
. Finally,
is the distance between the particles and
is the molecular number density in the condensed phase.
The increase in over
is found by integrating
[11] where
is the molecular velocity, ρ is the density of vapor, and
corresponds to the largest initial velocity a molecule with impact parameter R can have and still be captured by the drop. For the given potential, they found that the enhancement factor η could be written in terms of the two independent parameters μ
[12] and ν
[13] as
[14] where
[15]
Here, we use the approach in the second paper of Vasil'ev and Reiss Citation(1996b) to calculate η for n-propanol, D2O, and nonane where .
Vasil'ev and Reiss Citation(1996a) also noted that if the carrier gas density is high enough or the particle size is large enough because the attractive potential from other molecules also affects the condensing vapor molecules, and as a result, cancels out the enhancement. The screening effect becomes important when the intermolecular distance of carrier gas is much smaller than droplet size, i.e.,
, where
[16] and the subscript 1 indicates the properties of the carrier gas.
5. Result and discussion
The n-propanol static pressure (p/p0) and SAXS () measurements we will analyze are presented in along with the derived quantities (T, g, S and scaled nucleation rate J/Jmax). All the data are shown with respect to the flow time where the latter is calculated from the position and flow velocity in the nozzle via
. The time origin, t = 0, corresponds to the point in the flow where the supersaturation S reaches its maximum value.
Figure 1. The n-propanol data set comprises both PTM and SAXS experiments. Directly measured quantities (pressure ratios,) are shown in black. The remaining quantities, derived from the integrated analysis, are shown in gray (blue). (a) The static pressure ratio and estimated temperatures. (b) The mean droplet size
and the spread in the size distribution
. (c) The mass fraction condensate g and the n-propanol supersaturation S. The upper dashed-dot line corresponds to the mass fraction of n-propanol entering the nozzle, ginf. The lower long gray dashed line indicates
whereas the short dashed line (red) indicates
, i.e., the supersaturation corresponding to that of the droplets at Td,exp. (d) The aerosol specific number,
, and normalized nucleation rate (J/JMAX). The number density is normalized by the gas density in order to account for the continued expansion of the flowing mixture. In the absence of coagulation
is constant. Size and number density data were previously published in Mullick et al. Citation(2015). The error bars indicate the ± 5% uncertainties in the absolute calibration for the SAXS measurements.
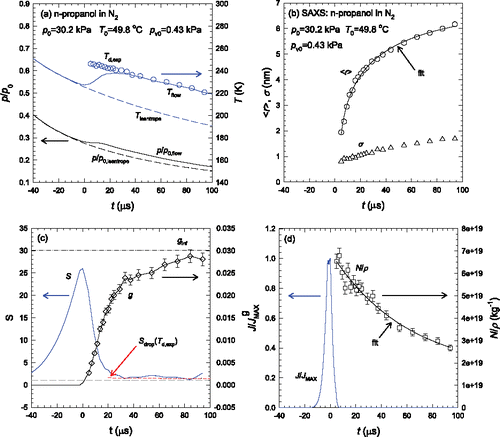
As illustrated in , both the temperature and pressure profiles of the condensing flow follow those of the non-condensing flow until the curves deviate as heat is released to the flow due to droplet formation and growth. The onset of sudden particle growth is also reflected in the values of , and g, . In the first ∼30 μs after onset, particles grow from
∼2 nm to
∼4.8 nm and g increases to ∼80% of the incoming value. Particle growth slows considerably after this point, and by t ∼ 80 μs, particle size reaches ∼6.2 nm and ∼93% of the available n-propanol has condensed. The width of the size distribution, σ also increases gradually as the aerosol evolves. shows the supersaturation S peaks sharply near the onset point before approaching the supersaturation
consistent with the droplet size and temperature when t ∼30 μs. The behavior of S and g suggests that condensational droplet growth should be less important for t > 30 μs and that the continued increase of
is largely due to a competing mechanism. The rapid decrease of
in suggests that droplet coagulation is the dominant mechanism for continued droplet growth when t > 30 μs. The normalized nucleation rate J/JMAX in , calculated using the Becker–Döring version of Classical Nucleation Theory and the values of S and T derived from the experiments (Becker and Döring Citation1935), suggests that particle formation is essentially complete when we are first able to detect particles.
Figure 2. (a) The n-propanol droplet temperatures and the gas temperature, and (b) the temperature difference between droplets and gas. (c) The experimental growth rates (circles) are calculated from a fit to the measured values of . The lines correspond to the growth rate predicted using the Hertz Knudsen growth model, Equation Equation(1)
[1] , using qc = qe = 1 and the indicated droplet temperatures.
![Figure 2. (a) The n-propanol droplet temperatures and the gas temperature, and (b) the temperature difference between droplets and gas. (c) The experimental growth rates (circles) are calculated from a fit to the measured values of . The lines correspond to the growth rate predicted using the Hertz Knudsen growth model, Equation Equation(1)[1] , using qc = qe = 1 and the indicated droplet temperatures.](/cms/asset/5a181fb1-bf79-4843-9618-be7175d6f017/uast_a_1185084_f0002_oc.gif)
show the droplet temperatures and the difference in temperature between the droplets and the gas mixture. The experimental droplet temperature (Td,exp) was determined by setting the heat flux, arising from the collisions of the nitrogen gas with the growing n-propanol droplets (Equation Equation(7))[7] , equal to the heat released from the condensation of n-propanol (Equation Equation(8)
[8] ). The theoretical droplet temperature (Td,exact) was determined by the implicit solution of Equations Equation(5)–(7) assuming that qc = qe = 1. During the early stages of condensation, droplet temperatures are 10–15 K higher than the gas temperature but this temperature difference (ΔT) approaches zero by t ∼30 μs. After this time point, ΔT is almost zero because the condensation rate has decreased and the heat release due to coagulation is negligible. Since we use the raw dg/dt values rather than fitting and smoothing the data, ΔTexp fluctuates even when we expect it to be zero. Since ΔTexp is always less than 1.3 K in this region, we consider 1.3 K to be a measure of the experimental uncertainty in this quantity. Thus, at earlier times the difference between Td,exp and Td,exact is too large to be explained by experimental uncertainty alone.
compares the droplet growth rates determined from the experiments to those calculated assuming condensation is the only growth mechanism. As expected, the experimental growth rates decrease rapidly until ∼30 μs, and more gradually thereafter. The growth rates calculated using Equation Equation(1)[1] and Td,exp or Td,exact both lie below the measured droplet growth rate especially when t is less than ∼30 μs. Comparing leads to an interesting dilemma when we try to explain the discrepancy between the theoretical and experimental growth rates given the error in Td,exact. suggests Td,exact is too low, but the low growth rates in suggest that Td,exact is still too high.
The results for n-propanol illustrated in , i.e., that ΔTd,exp > ΔTd,exact at the same time that (dr/dt)exp > (dr/dt)exact, mirror the results of Pathak et al. Citation(2013) for D2O nanodroplet growth. Thus, the current experiments—experiments in which the liquid condensate is never supercooled—confirm and strengthen Pathak et al.'s observations.
To improve agreement between theory and experiments, we first adopt the approach used by Pathak et al. Citation(2013), to match the theoretical and experimental growth rate of D2O nanodroplets. As illustrated in , if we reduce qe to 0.6 while maintaining qc = 1, the agreement between ΔTd,exp and ΔTd,exact for the current n-propanol experiments improves significantly. Since the improvement under our experimental conditions reflects the change in the ratio qe/qc, rather than the particular value of either parameter, one can equally set qe = 1 and increase qc to a value larger than 1. shows that setting qc = 1.3 and qe = 1 also leads to good agreement between ΔTd,exp and ΔTd,exact. Furthermore, the growth rates at the shortest times are also predicted more accurately. Although not shown here, Pathak et al.'s D2O data can also be well fit using qc = 1.3 and qe = 1.
Figure 3. (a) The experimental temperature differences between the droplets and surrounding gas are compared to theoretical droplet temperatures determined using the following two assumptions: i: (qc, qe) = (1, 0.6), and ii: (qc, qe) = (1.3, 1.0). (b) The theoretical growth rates calculated with Td,exact for case ii agree quite well with the values measured during rapid particle growth. Including the effect of coagulation improves the agreement when t > ∼30 μs. The growth rates for case i lie almost on top of those for case ii and are not shown to maintain clarity.
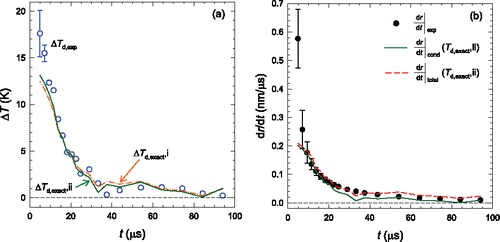
While the refined values of qe and qc provide improved droplet temperatures and growth rate, the theoretical growth rates, , again starts to diverge from the experimental growth rate for t > ∼30 μs. As discussed earlier with respect to , for t > ∼30 μs droplet growth is dominated by coagulation. Thus, as illustrated in , adding the coagulation growth rate to the condensational growth rate removes this difference. Once again, the results for n-propanol are very similar to those observed by Pathak et al. Citation(2013) for D2O.
Although it is not a standard approach in the droplet growth literature, the possibility of qc > 1 was suggested by Vasil'ev and Reiss (Citation1996a,Citationb). Their interest was in exploring the effect a droplet's attractive potential could have on enhancing the droplet's capture cross section for vapor molecules and thereby change the nucleation rate. Using the equations in Section 4 of this article, we calculated the condensation enhancement factors η for n-propanol, D2O, and nonane as a function of position in the nozzle and averaged the values. summarizes the Lennard–Jones parameters used in the calculations and the average values of η.
Table 1. The Lennard–Jones parameters for n-propanol, D2O, and nonane (Poling et al. Citation2001), and the average values for η calculated using Equations Equation(10)–(13).
In all cases, the values of η are greater than 1. Although not shown here, the values are not very sensitive to the particular set of Lennard–Jones parameters chosen for the calculation. For n-propanol and D2O, the enhancement factors are in line with the experimentally determined values of qc required to achieve good agreement with the experimental growth rates. The value for η for nonane initially seems to be inconsistent with the result of Pathak et al.'s Citation(2013) experiments that showed good agreement between experimental and theoretical growth rates when qc = qe = 1. This discrepancy can, however, be explained by considering the screening effect proposed by Vasil'ev and Reiss Citation(1996a), i.e., that η ≈ 1 when where the ∼
is the average carrier gas intermolecular distance and
is the droplet size.
When we checked the importance of screening in the experiments, we found that for n-propanol and D2O, is 3∼4 times larger than
during the early stages of condensation and only becomes comparable to
in the region where coagulation dominates growth. In contrast, for nonane the condition
holds for all but the first few droplet measurements. Thus, even though we calculate an average value of η equal to 1.26, the actual value of η should be very close to 1 because of the dense carrier gas “screening effect.” This is consistent with the experimental growth rate of nonane being in good agreement with the theoretical growth rate with qc = 1. Although Pathak et al. Citation(2013) assumed qe = 1, the experiments cannot constrain qe because the theoretical results are not sensitive to this parameter.
Fundamentally, the (qc, qe) values determined here for n-propanol (or D2O) are not unique. Rather they represent appropriate values corresponding to the assumptions of qc = 1 or qe = 1, and other choices are possible. For n-propanol (D2O), however, our experiments support the observation of others that qe/qc < 1 (Young Citation1982). Furthermore, the analysis of Vasil'ev and Reiss Citation(1996a,Citationb) and results from the coagulation literature (Fuchs and Sutugin Citation1965; Graham and Homer Citation1972; Marlow Citation1980, Citation1982; Okuyama et al. Citation1984; Kennedy Citation1987; Harris and Kennedy Citation1988; Gopalakrishnan and Hogan Citation2011; Ouyang et al. Citation2012; Mullick et al. Citation2015) suggest that qc > 1 is reasonable. Clearly, under equilibrium conditions above a flat surface, qc must equal qe, but under non-equilibrium conditions this is not a given.
In the Hertz–Knudsen equation (Equation Equation(1)[1] ), the evaporation rate of condensable species is derived based on the fact that the evaporation and condensation rates are equal under metastable equilibrium conditions, i.e.,
, and Td = T. The evaporation coefficient qe in Equation Equation(1)
[1] should be unity when the following conditions are satisfied; (1) the condensation rate is equal to the collision rate on a hard sphere of radius
in the absence of an attractive force at Sdrop = 1, and (2) the evaporation rate at any Sdrop is equal to that at Sdrop = 1. Thus, the coefficient qe indicates deviations from these two conditions and is more complicated to analyze than the coefficient qc. We therefore avoid any further discussion regarding qe in this article.
6. Summary and conclusion
We analyzed the growth rates of n-propanol nanodroplets produced in a supersonic nozzle using the results of PTM and SAXS experiments. Working with alcohols has the advantage, relative to experiments with H2O or D2O, that liquid droplets are not supercooled with respect to the solid phase. The key result of our analysis of the n-propanol growth rates is very much in line with the D2O results of Pathak et al. Citation(2013). In particular, the theoretical droplet temperatures and growth rates are inconsistent with the experimentally derived values if we assume qc = qe = 1. Good agreement is possible if we assumed either that (i) (qc, qe) = (1, 0.6) or (ii) (qc, qe) = (1.3, 1.0). Approach (i) is consistent with the observations of Young Citation(1982) but is more difficult to justify from a theoretical standpoint. Approach (ii) can be justified by considering the attractive potential between the condensing vapor molecules and the growing droplets. Since long-range interactions are known to affect the coagulation rate of nanoparticles (Fuchs and Sutugin Citation1965; Graham and Homer Citation1972; Marlow Citation1980, Citation1982; Okuyama et al. Citation1984; Kennedy Citation1987; Harris and Kennedy Citation1988; Gopalakrishnan and Hogan Citation2011; Ouyang et al. Citation2012; Mullick et al. Citation2015), and condensation is, in a sense, coagulation between a vapor molecule and the droplet, it should not be surprising that long-range interactions can play a role in nanodroplet growth.
Acknowledgments
The authors would like to thank S. Seifert and R. Winans for help with the SAXS experiments.
Funding
Support was provided by NSF grants CHE-1213959 and CHE-1464924. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357.
References
- Becker, R., and Döring, W. (1935). Kinetische Behandlung der Keimbildung in Übersättigten Dämpfen. Ann. Phys., 24:719–752.
- Drisdell, W. S., Cappa, C. D., Smith, J. D., Saykally, R. J., and Cohen, R. C. (2008). Determination of the Evaporation Coefficient of D2O. Atmos. Chem. Phys., 8:6699–6706.
- Fuchs, N. A. and Sutugin, A. G. (1965). Coagulation Rate of Highly Dispersed Aerosol. J. Colloid. Sci., 20:492–500.
- Ghosh, D., Manka, A., Strey, R., Seifert, S., Winans, R. E., and Wyslouzil, B. E. (2008). Using Small Angle X-Ray Scattering to Measure the Homogeneous Nucleation Rates of n-Propanol, n-Butanol, and n-Pentanol in Supersonic Nozzle Expansions. J. Chem. Phys., 129:124302-1–124302-14.
- Gopalakrishnan, R., and Hogan, C. J. (2011). Determination of the Transition Regime Collision Kernel from Mean First Passage Times. Aerosol Sci. Technol., 45:1499–1509.
- Graham, S. C., and Homer, J. B. (1972). Coagulation of Molten Lead Aerosol. Faraday Symp. Chem. Soc., 7:85–96.
- Harris, S. J., and Kennedy, I. M. (1988). The Coagulation of Soot Particles with Van der Waals Forces. Combust. Sci. Tech., 59:443–454.
- Hughes, F. R., Starzmann, J., White, A. J., and Young, J. B. (2015). A Comparison of Modeling Techniques for Polydispersed Droplet Spectra in Steam Turbines. J. Eng. Gas Turbines Power, 138:042603-1–042603-9.
- Kennedy, I. M. (1987). The Evolution of a Soot Aerosol in a Counterflow Diffusion Flame. Combust. Flame, 68:1–16.
- Korhonen, H., Kerminen, V., Kokkola, H., and Lehtinen, K. E. J. (2014). Estimating Atmospheric Nucleation Rates from Size Distribution Measurements: Analytical Equations for the Case of Size Dependent Growth Rates. J. Aerosol Sci., 69:13–20.
- Manka, A. A., Wedekind, J., Ghosh, D., Hohler, K., Wolk, J., and Strey, R. (2012). Nucleation of Ethanol, Propanol, Butanol, and Pentanol: A Systematic Experimental Study along the Homologous Series. J. Chem. Phys., 137:054316.
- Marek, R., and Straub, J. (2001). Analysis of the Evaporation Coefficient and the Condensation Coefficient of Water. Int. J. Heat and Mass Trans., 44:39–53.
- Marlow, W. H. (1980). Lifshitz-van der Waals Forces in Aerosol Particle Collisions. 1. Introduction: Water Droplets. J. Chem. Phys., 73:6288–6295.
- Marlow, W. H. (1982). Lead Aerosol Brownian Collision Rates at Normal and Elevated Temperature: Theory. J. Colloid Interface Sci., 87:209–215.
- Meland, R., Frezzotti, A., Ytrehus, T., and Hafskjold, B. (2004). Nonequilibrium Molecular-Dynamics Simulation of Net Evaporation and Net Condensation, and Evaluation of the Gas-Kinetic Boundary Condition at the Interphase. Phys. Fluids, 16:223–243.
- Mullick, K., Bhabhe, A., Manka, A., Wölk, J., Strey, R., and Wyslouzil, B. E. (2015). Isothermal Nucleation Rates of n-Propanol, n-Butanol, and n-Pentanol in Supersonic Nozzles: Critical Cluster Sizes and the Role of Coagulation. J. Phys. Chem. B, 119:9009–9019.
- Okuyama, K., Kousaka, Y., and Hayashi, K. (1984). Change in Size Distribution of Ultrafine Aerosol Particles Undergoing Brownian Coagulation. J. Colloid Interface Sci., 101:98–109.
- Ouyang, H., Gopalakrishnan, R., and Hogan, C. J. (2012). Nanoparticle Collisions in the Gas Phase in the Presence of Singular Contact Potentials. J. Chem. Phys., 137:064316-1–064316-9.
- Pathak, H., Mullick, K., Tanimura, S., and Wyslouzil, B. E. (2013). Nonisothermal Droplet Growth in the Free Molecular Regime. Aerosol Sci. Technol., 47:1310–1324.
- Poling, B. E., Prausnitz, J. M., and O'Connell, J. P. (2001). The Properties of Gases and Liquids. 5th ed. McGraw-Hill Professional, New York.
- Tanimura, S., Wyslouzil, B. E., and Wilemski, G. (2010). CH3CH2OD/D2O Binary Condensation in a Supersonic Laval Nozzle: Presence of Small Clusters Inferred from a Macroscopic Energy Balance. J. Chem. Phys., 132:144301.
- Vasil'ev, O. V., and Reiss, H. (1996a). Effect of the Attractive Potential of a Drop in Vapor Phase Nucleation. Phys. Rev. E, 54:3950–3954.
- Vasil'ev, O. V., and Reiss, H. (1996b). Capture of Vapor Molecules by a Realistic Attraction Potential of a Drop. J. Chem. Phys., 105:2946–2947.
- Viisanen, Y., and Strey, R. (1994). Homogeneous Nucleation Rates for n-Butanol. J. Chem. Phys., 101:7835–7843.
- Young, J. B. (1982). The Spontaneous Condensation of Steam in Supersonic Nozzle. PCH Physcio Chem. Hydrodynam., 3:57–82.
