ABSTRACT
Mixing state refers to the relative proportions of chemical species in an aerosol, and the way these species are combined; either as a population where each particle consists of a single species (‘externally mixed’) or where all particles individually consist of two or more species (‘internally mixed’) or the case where some particles are pure and some particles consist of multiple species. The mixing state affects optical and hygroscopic properties, and quantifying it is therefore important for studying an aerosol's climate impact. In this article, we describe a method to quantify the volatile mixing state of an aerosol using a differential mobility analyzer, centrifugal particle mass analyzer, catalytic denuder, and condensation particle counter by measuring the mass distributions of the volatile and non-volatile components of an aerosol and determining how the material is mixed within and between particles as a function of mobility diameter. The method is demonstrated using two aerosol samples from a miniCAST soot generator, one with a high elemental carbon (EC) content, and one with a high organic carbon (OC) content. The measurements are presented in terms of the mass distribution of the volatile and non-volatile material, as well as measures of diversity and mixing state parameter. It was found that the high-EC soot nearly consisted of only pure particles where 86% of the total mass was non-volatile. The high-OC soot consisted of either pure volatile particles or particles that contained a mixture of volatile and non-volatile material where 8% of the total mass was pure volatile particles and 70% was non-volatile material (with the remaining 22% being volatile material condensed on non-volatile particles).
© 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Aerosols emitted into the atmosphere through natural processes and human activity can adversely impact local air quality and human health (Burnett et al. Citation2014), and the global climate (Myhre et al. Citation2013). Combustion is a significant source of anthropogenic and natural aerosol emissions comprising of black carbon (BC), organic carbon (OC), ash, and inorganic material (e.g., sulfuric acid). At the point of release to the atmosphere, non-volatile BC particles can be mixed with volatile components, where volatile material condenses onto or envelopes BC particles, or volatile or semi-volatile OC and inorganic material formed via nucleation may exist as separate particles (Bond et al. Citation2013). Further atmospheric ageing, oxidation, adsorption, condensation, and coagulation processes generally lead to single particles consisting of multiple species (Zhang et al. Citation2008; Cappa et al. Citation2012). How the different species exist in the aerosol is commonly referred to as the mixing state. Specifically, in an aerosol population comprised of both volatile and non-volatile material, the population is said to be “internally mixed” if all particles contain both species, and “externally mixed” if all particles are either one species or the other. In general, an aerosol population lies between these two extremes.
Optical characteristics of BC particles are significantly affected by the mixing state, with important consequences for the climate impacts of emitted BC. The optical cross-section per unit mass, referred to as the mass absorption cross-section (MAC), of BC particles is significantly affected by the mixing state. In a review of previous studies, Bond et al. (Citation2013) noted that laboratory studies have demonstrated MAC enhancements of up to a factor of 2 for single particles consisting of BC and OC, with OC mass fractions above 80% (Cross et al. Citation2010), while atmospheric aerosol studies show significant variability in MAC enhancement attributed to the relative quantity of non-BC material (Chan et al. Citation2011) and the distribution of non-BC material on the structure of single BC particles (Cappa et al. Citation2012).
The mixing state also affects the hygroscopicity and therefore the interaction between BC particles and clouds—more hygroscopic particles require lower critical supersaturations with respect to water in order to serve as nucleation sites for cloud droplets than less hygroscopic particles (McFiggans et al. Citation2006). Liu et al. Citation(2013) found that the mixing state and composition of the coating material determined the hygroscopic properties of atmospheric BC particles. Furthermore, Kuwata et al. Citation(2009) showed that BC-containing particle hygroscopicity was dependent on the relative mass of non-BC material. Thus, knowing the mixing state of BC particles is of vital importance to providing context to the measured aerosol optical properties and cloud condensation nuclei (CCN) activity.
Several techniques are available to determine the mixing state of BC-containing particles, these can be differentiated into population averaged and single particle techniques. Single particle mixing state techniques include scanning electron microscopy, refractory BCFootnote1 (rBC) incandescence, and particle mass spectrometry. Single particle measurements using these techniques are typically aggregated to give population-level characteristics. Scanning electron microscopy techniques have been used to visually classify the mixing state and quantify morphological characteristics of ambient soot particles (China et al. Citation2013, Citation2015), however these techniques are unable to quantify the mass ratios of BC to non-BC components. The single particle soot photometer (SP2) instrument has been developed to quantify rBC using single particle incandescence signals (Stephens et al. Citation2003). The incandescence signal provides information as to whether an individual particle contains rBC and time difference between incandescence and light scattering signals has been used to classify the mixing state of particles as “thinly coated” or “thickly coated” (Moteki and Kondo Citation2007; McMeeking et al. Citation2011). However, the lower detection limit of the SP2 is approximately 0.3 fg (Schwarz et al. Citation2010; Wang et al. Citation2014), which is approximately 70 nm in diameter for a volume equivalent sphere or approximately 100 nm in diameter for a mobility equivalent sphere from a typical engine combustion source,Footnote2 whereas the count median mobility diameter for many combustion sources (diesel engines, gasoline direct injection engines, gas turbine engines) are below 100 nm. The aerosol mass spectrometer (AMS) was designed to enable quantification and speciation of non-refractory aerosol components (Jayne et al. Citation2000). Recent developments and incorporation of the SP2 principle into the AMS have enabled quantification of rBC in addition to non-refractory aerosol components on population-averaged basis in an instrument called the soot particle aerosol mass spectrometer (SP-AMS; Cross et al. Citation2010; Onasch et al. Citation2012). The addition of a light-scattering module (LS-SP-AMS) allows for the rBC and non-refractory aerosols on a single-particle basis. Recent application of the LS-SP-AMS to urban aerosols by Lee at al. (Citation2015) indicates that measurements of the mixing state of BC-containing particles are currently constrained by a detection limit of around 25 fg of rBC per particle (far larger than the median particle mass for most rBC sources) and that rBC-containing particle number and mass measurements may be underestimated.
Of the aerosol population techniques, tandem differential mobility analyzer (DMA) methods involve classifying an aerosol sample with a first DMA, coating or denuding the resultant monodisperse aerosol to add or remove volatile material, respectively, followed by a second DMA such that the change in mobility diameter, , and mobility-based particle volume can be quantified (Petzold et al. Citation2005). However, this technique is dependent on the particle morphology and for non-spherical particles large amounts of coating can be added without a detectable change in the mobility diameter. For example, Ghazi and Olfert Citation(2013) showed that the mass of a soot particle could be increased up to three times with a coating of dioctyl sebacte without a measurable increase in the mobility diameter. Furthermore, this technique may be susceptible to changes in the structure and mobility of the BC aggregates during coating or denuding (Pagels et al. Citation2009). Thus, the quantification of the amount of volatile material coated on a non-spherical particle is prone to large errors. A derivative of this method is to use a DMA in tandem with a particle mass analyzer (either an aerosol particle mass analyzer [APM; Ehara et al. Citation1996] or centrifugal particle mass analyzer [CPMA; Olfert and Collings Citation2005]). In this technique, a DMA is used to select a monodisperse aerosol that is then denuded or coated, followed by a particle mass analyzer so that the change in particle mass statistics with and without denuding/coating of the aerosol can be quantified. This technique has been used to determine the mass fraction of volatile material on coated particles from flames (Zhang et al. Citation2008; Pagels et al. Citation2009; Ghazi and Olfert Citation2013; Ghazi et al. Citation2013; Schnitzler et al. Citation2014), a Diesel engine (Sakurai et al. Citation2003), gasoline direct injection engines (Momenimovahed and Olfert Citation2015), a natural-gas direct-injection compression-ignition engine (Graves et al. Citation2015), and ambient aerosols (Kuwata et al. Citation2009; Rissler et al. Citation2014).
Recently, Riemer and West Citation(2013) have developed a quantitative measure of aerosol mixing state using measures of “species diversity,” which is a function of the average particle species diversity and the bulk population species diversity. This methodology has been applied to an instrumentation system comprising of a single particle mass spectrometer, aerosol mass spectrometer, and multi-angle absorption photometer to determine particle mixing state of ambient aerosol down to ∼150 nm in aerodynamic equivalent diameter (Healy et al. Citation2014). Also, this methodology has been applied to the spectro-microscopy techniques of scanning transmission X-ray microscopy/near edge X-ray absorption fine structure (STXM/NEXAFS) and computer-controlled scanning electron microscopy/energy dispersive X-ray spectroscopy (CCSEM/EDX; O'Brien et al. Citation2015).
This article demonstrates an experimental methodology for quantifying the mixing state of an aerosol using a DMA, centrifugal particle mass analyzer (CPMA), catalytic denuder (often called a catalytic stripper), and condensation particle counter (CPC). The mixing state information is presented in two ways. First, the data are used to produce a mass distribution (i.e., dM/dlogdm) separated into components of non-volatile material, volatile material condensed onto or enveloping non-volatile material, and volatile material existing as independent particles. This method builds upon DMA-mass analyzer techniques described above to quantify the mobility diameter-dependent mixing state of an aerosol and explicitly includes the independent volatile particle number and mass fractions. While similar techniques have been used before (Sakurai et al. Citation2003), the method described here uses a simpler system to better characterize particle losses, allowing greater accuracy in the volatile number fraction measurements. Second, the data are used to calculate the single particle diversity (Di), average single particle diversity (), bulk population species diversity (
), and mixing state parameter (χ) following the methodology of Riemer and West Citation(2013). In this study, a CPMA has been used to quantify particle mass, but the methods described could also be implemented with an APM. The method is demonstrated on particles generated by a diffusion flame.
2. Method
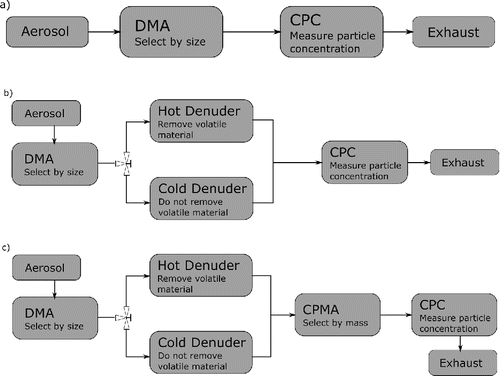
The methodology presented in this article produces a mass distribution that quantifies the non-volatile, surface-condensed volatile (i.e., volatile material condensed on the surface or enveloping a non-volatile particle), and independent volatile components (i.e., particles consisting of only volatile material or “purely volatile particles”) of the aerosol as a function of mobility-equivalent diameter. The different components can also be integrated across the size range to obtain the total mass concentration of each component. This is done with three independent measurements. First, the sample is measured using a DMA and CPC or scanning mobility particle sizer (SMPS; Wang and Flagan Citation1990) to determine the count mobility size distribution by data inversion (Stolzenburg and McMurry Citation2008) as shown in . Second, the volatile number fraction of the particles at each mobility size is determined, measuring the number of particles that are purely volatile using a DMA, catalytic denuder, and CPC as shown in . Third, the volatile mass fraction of the particles containing both volatile and non-volatile material at each size is found, measuring the proportion of the mass that is volatile using a DMA, catalytic denuder, CPMA (Olfert and Collings Citation2005), and CPC as shown in . From these measurements, the mass distribution is calculated. These same measurements and the mass distributions can be used to calculate the species diversities and mixing state parameter.
Figure 1. Experimental setup for measurements of (a) size distribution, (b) volatile particle fraction, and (c) volatile mass fraction.
“Volatile material” is operationally defined as material removed by a catalytic denuder heated to 350°C. This temperature was selected in accordance with the Particle Measurement Programme for automotive particulate emissions who define non-volatile particulate as particles remaining after heating to 300°C to 400°C with sufficient residence time to evaporate, and prevent the re-nucleation of, 30 nm tetracontane particles (UNECE Regulation No. 83). A similar operational definition is proposed for future aircraft certification (SAE Citation2013). Under this definition the majority of OC substances produced in incomplete combustion of hydrocarbons are volatile, as are some inorganic materials (e.g., sulfuric acid, water); while elemental carbon or BC materials are non-volatile.
2.1. Experimental setup
The methodology is demonstrated with measurements on particles produced by a miniCAST soot generator (model 5201C, Jing Ltd., Switzerland) using two different settings. The “High EC” setting is a high elemental carbon (EC) set point with very little volatile material. Conversely, the “High OC” setting is a high organic carbon (OC) set point with a substantial amount of volatile material. shows the flow rate settings for these set points.
Table 1. Flow rates of the miniCAST High EC and High OC set points.
2.2. Size distribution measurement
The size distributions of the samples were measured using a DMA (Model 3081, TSI Inc., Shoreview, MN, USA) and a CPC (Model 3776, TSI Inc.) as shown in . For the size distribution measurement of the high EC setting, a sheath flow of 3 L/min and aerosol flow of 0.3 L/min was used. A sheath flow of 15 L/min and aerosol flow of 1.5 L/min was used for the high OC size distribution measurement, and for the other measurements in this study. The aerosol was classified by electrical mobility in the DMA, which scans through a range of electrical mobility while counting the particles with the CPC to measure their number concentration. The size distribution is calculated from this data using the inversion routine in the TSI AIM software (version 9).
2.3. Volatile particle number fraction
shows the experimental setup for measuring the volatile particle number fraction. The aerosol sample was first size-selected using the DMA that selects the particles of a given range of electrical mobility equivalent diameters. Next, the sample was sent either through a heated catalytic denuder at 350°C (Model CS015; Catalytic Instruments GmbH, Germany), or through a denuder at ambient temperature of the same model. The hot catalytic denuder removed the volatile material from the sample (denuded), while the cold denuder left it unaffected (undenuded). The catalytic denuder has an average gas residence time of 1.1 s at 350°C and 0.5 s at 20°C for flow rates of 1.5 standard L/min. By our operational definition of volatility, the volatile material was precisely the material removed by the denuder at 350°C and flow rate of 1.5 standard L/min. If other denuder temperatures or flow rates were used the amount of volatile material that would evaporate could differ. The particles were then counted in the CPC to measure the number concentration. The difference between the denuded and undenuded concentrations is the number concentration of purely volatile particles removed by the denuder. The lower detection of the CPC used in this study was 2.5 nm, therefore, denuded particles with a diameter less than 2.5 nm would be counted as purely volatile particles. Switching back and forth between the denuded and undenuded lines allowed repeated measurements so as to average out random fluctuations. Repeating this procedure and classifying the sample at different mobility diameters generated the volatile particle number fraction across the size range of the sample.
The measurement of number concentration is sensitive to particle losses, and in particular the ratio between the denuded and undenuded concentrations is sensitive to the ratio of the penetration efficiencies of the hot catalytic denuder and the cold denuder. To minimize this difference, the two lines were made to be the same length and incorporated the same model of denuder, with the cold denuder simply remaining inactive (i.e., unheated). The ratio of penetration efficiencies was measured in order to account for particle deposition due to thermophoresis and diffusion as follows.
2.3.1. Measurement of penetration ratio
Given that the measured number concentration is equal to the actual concentration times the penetration efficiency of the denuder system, the relationship between the actual concentration ratio and the measured concentration ratio is[1] where
[2] where
and
are the denuded and undenuded number concentrations, actual or measured;
and
are the penetration efficiencies of the hot and cold denuders, respectively; and
is the ratio of these penetration efficiencies. In the case of purely non-volatile test aerosols, the actual number concentration should be unaffected by denuding, so that the ratio of the penetration efficiencies of the denuded and undenuded lines is equal to the ratio of the measured number concentrations of the test aerosol. In general, these are functions of particle size. Therefore,
[3] where
is the particle mobility diameter, and
and
are the measured number concentrations of the denuded and undenuded non-volatile test aerosols.
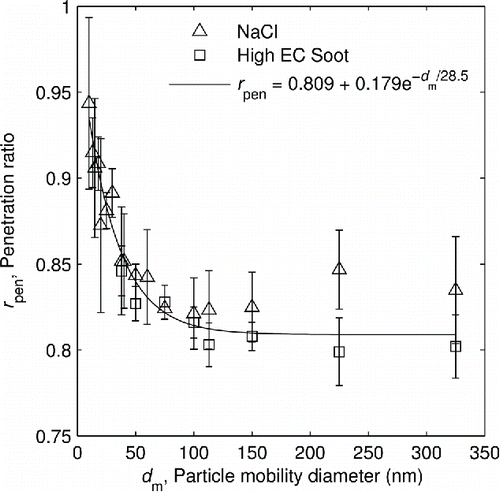
The penetration ratio was measured using two different test aerosols: an atomized salt (NaCl) aerosol and a high EC soot, chosen to be purely non-volatile. The NaCl aerosol was generated using an atomizer (Model 3076, TSI Inc.) with a diffusion dryer. The high EC soot was generated with a miniCAST (Model 4202, Jing Ltd.) operating at the following flow rates: 50 mL/min C3H8, 1.5 L/min oxidation air, 5.5 L/min quench N2, and 30 L/min dilution air.Footnote3 This soot was denuded upstream of the experiment at 350°C with a third catalytic denuder identical to the ones used in rest of the setup (CS015; Catalytic Instruments). Again, by definition, all particles leaving the upstream catalytic denuder are purely non-volatile. After particle generation and conditioning, the penetration ratio measurements used the same experimental setup and method as the volatile particle fraction measurements shown in .
The results of these measurements are shown in , which shows the ratio of the penetration efficiencies of the hot and cold denuders, against the particle mobility diameter. The test aerosols agree within error estimates, with the exception of the point at 225 nm, and both show an initial decrease followed by the penetration ratio being independent of mobility diameter. (This is more pronounced with the salt, for which we were able to take more measurements at small particle sizes.) Furthermore, because rpen becomes constant at higher particle sizes, corrections for multiply charged particles are not required. The larger multiply charged particles are attenuated by the same amount so that no distortion of the penetration ratio occurs. At smaller sizes, the size dependence of the penetration ratio could cause multiply charged particles to distort the measurements, however there the fraction of multiply charged particles is smaller than the uncertainty of the measurement. To capture the behavior of the penetration ratio, a best fit exponential curve was found via error normalized residuals minimization. A similar trend in ratios of penetration efficiencies has been found in other studies (e.g., Graves Citation2015 using a thermodenuder). Penetration efficiencies of the hot and cold denuder can differ due to differences in particle diffusion and thermophoretic losses due to changes in gas viscosity, particle slip correction factor, gas velocity, and temperature gradients. The fact that the penetration ratio is constant above ∼100 nm suggests that inertial losses are negligible as these would increase for larger sizes and would be seen as a difference between the two particle types because they have substantially different densities (i.e., aerodynamic diameters).
2.3.2. Calculation of externally mixed volatile particle fraction
With the penetration ratio characterized, the volatile particle fraction may be calculated from the ratio of the denuded and undenuded number concentrations, correcting for the differences in the losses,[4] where
is the volatile particle fraction; and
and
are the measured number concentrations for the denuded and undenuded soot samples. A suitable best-fit curve may be chosen for the volatile particle fraction, in order to represent it with the simple curve bounded between 0 and 1.
2.4. Volatile mass fraction
shows the experimental setup for measuring the volatile mass fraction. As in the measurement of the volatile particle fraction, the aerosol sample is first classified using a DMA and then it is either denuded or left undenuded. The sample is then sent through a CPMA (Cambustion Ltd., UK) that classifies only the particles of a narrow range of mass-to-charge ratios around a set point. The resolution of the CPMA quantifies the narrowness of the range of classified mass-to-charge ratios; it is equal to the set point divided by the full-width of this range at half-maximum of the transfer function (i.e., the difference between the maximum and minimum values that are classified with at least 50% efficiency at that set point). The CPMA was operated with a flow rate of 1.5 L/min and a resolution of either 3 or 5 depending on the maximum that could be obtained at the given set point. The CPMA is stepped across a range of mass-to-charge set points covering the range of particles exiting the DMA. Finally, the particle number concentration is determined by the CPC from the flow rate and counts of particles.
The DMA selects by electrical mobility after charging the particles with a low charge state, so that most of the particles are either uncharged or singly charged and a small fraction are multiply charged. The electrical mobility is proportional to the product of mobility and charge, so it may allow multiply-charged particles to pass through which are larger than the singly-charged particles. Similarly, the CPMA selects by mass-to-charge ratio, so it may allow particles to pass through with twice the charge and twice the mass expected, for example. However, after classification by the DMA, the relationships between charge, mobility diameter, and mass cause particles in each charge state to have a different mass-to-charge ratio, so that they may in principal be distinguished by the CPMA.
For the particles of a given mobility size, selected by setting the DMA, the CPMA is scanned over a range of particle masses. This procedure is performed using the hot catalytic denuder and repeated using the cold denuder. By doing this a distribution of the number concentration of particles of each mass is generated, for particles of that size and denuding treatment. gives an example of the difference between the mass distributions of undenuded and denuded particles for soot from the high OC setting at three different sizes. The mass distributions are calculated using the inversion routine in the CPMA software (Cambustion CPMA manual). The inversion calculation depends on the resolution of the CPMA, which is assumed to be constant over the range of masses for each scan. For a given flow rate and geometry of the CPMA (length and radii of cylinders), the resolution is a function of voltage and rotational speed of the CPMA and the mobility of the particles (Olfert and Collings Citation2005). Because the particles are classified by mobility in the DMA, for any given CPMA scan the mobility is nearly constant. During a scan the CPMA controls both the rotational speed and voltage, which are selected to keep the resolution constant for a given particle mobility, thus an assumption of constant resolution is reasonable. Furthermore, it is assumed that all particles are singly charged. Because the multiply charged particles classified by the DMA typically have a different mass-to-charge ratio than the expected singly charged particles, the presence of a significant number of multiply charged particles would cause additional modes to appear in the mass distribution. The typical distributions in show a very slight emergence of a second mode corresponding to the +2 charge state (or higher), causing a bump on the right sides of some of the distributions. However, the bump does not distort the location of the peak of the distributions, nor does it significantly affect the mode determined by a lognormal curve fit, so the effect of multiply-charged particles may be neglected. This assumption is also common in DMA measurements (Stolzenburg and McMurry Citation2008). Alternatively, another charge neutralizer can be used before the CPMA to re-neutralize the mobility-classified particles making it easier to distinguish the charge state (Radney and Zangmeister Citation2016).
Figure 3. Mass distributions (distribution of particle concentration over mass) of size selected soot from the high OC setting, showing how the distribution changes when denuded at DMA set points of 38 nm, 100 nm, and 225 nm (left to right). The median mass of each distribution is determined from a lognormal fit. It can be seen that denuding removes mass from the particles from the shift toward lower median masses.
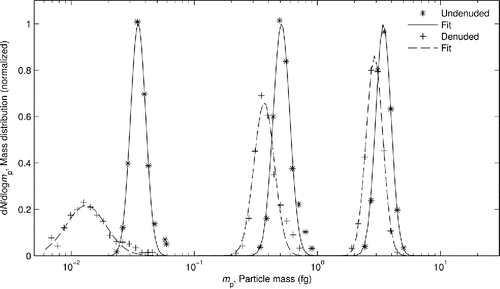
As soot particles have a branched-chain aggregate morphology and are not perfect spheres, there is a range of different masses present at each size. Particles with a more uniform morphology would have a more narrow mass distribution. To simplify calculations to only account for one value of mass at each size, the median mass of the distribution is found using a lognormal approximation, and is taken to represent the mass of particles at that size.Footnote4
A more nuanced calculation could consider the whole distribution of masses at each size, resulting in a two-dimensional distribution (Ruwata et al. 2016). However, this requires many more measurements and is not expected to significantly affect the mass distribution. The difference in the median mass between the undenuded and denuded cases is the volatile mass removed from the mixed particles by the hot catalytic denuder. Repeating this procedure at different sizes gives the volatile mass fraction, across the size range of the sample:
[5] where
is the undenuded particle mobility diameter,
is the median mass of the denuded particles, and
is the median mass of the undenuded particles. A best-fit curve may be selected to match the volatile mass fraction data with a simple function, keeping in mind that the volatile mass fraction is physically constrained to lie between 0 and 1.
Note that if the densities of the volatile and non-volatile material were sufficiently different, and if there was a sufficient proportion of purely volatile particles, the undenuded mass distribution at each size would show two modes. One would originate from the particles with both volatile and non-volatile components, while the other would originate from the purely volatile particles. In our experiment, the proportion of volatile particles was insufficient to produce a noticeable second peak in the mass distribution (), which would allow them to be distinguished. Because of this it was assumed that they do not significantly impact the median mass of the distribution. If the situation had been reversed and the proportion of purely volatile particles was very high, it may instead have been that the mode representing the mass of coated particles was indistinguishable. In that case the mass of the purely volatile particles could be found, but the mass of particles with both species would be difficult to determine.
Note also that if the aerosol population contained significant amounts of both purely non-volatile particles and particles with both species, this could be detected by comparing the undenuded and denuded mass distributions like those shown in . If the distributions could be decomposed into the sum of two distributions, one of which maintained a constant median mass upon denuding (keeping its position on the graph), and one of which reduced its median mass upon denuding (moving left on the graph), this would be evidence of a separation of the non-volatile material into pure particles and mixed particles. The peak that did not move would represent pure non-volatile particles, while the peak that moved left would represent particles of both species. The proportions of these components could be determined by integrating the component distributions. In practice, this would require high CPMA resolution and an effective deconvolution algorithm to determine the component distributions. In the measurements of the miniCAST soot presented here, no such separation of the non-volatile material was observed; either the entire distribution moved (showing all non-volatile material was coated or enveloped) or it stayed in the same position (all non-volatile material was independent).
Finally, note that the median mass of the mobility-selected sample is insensitive to particle losses. Since the particles are all roughly the same mobility size, they are all lost at the same rate, so particle losses merely affect the height of the distribution, not the position of the median. Because of this, this measurement is insensitive to the difference in particle losses between the hot catalytic denuder and the cold denuder, unlike the volatile particle number fraction measurement.
2.5. Mixing state mass distributions
Finally, all these measurements are combined to calculate the mass distributions (distribution of mass over size) of the different material components of the aerosol sample. The component consisting of purely volatile particles has a mass distribution ;
[6] where
is the size distribution (distribution of particle concentration over size), and
is the mass of the independent volatile particles (as a function of size). As noted in Section 2.4, in our case the volatile particles were not prevalent enough to be able to measure their mass. Instead, it has been assumed that the volatile particles are spherical with constant density, so that
. The density of the volatile material,
, is assumed to be equal to unit density of 1000 kg/m3. These assumptions are based on the majority of volatile material being liquid at ambient temperature and pressure, so it can be expected to form into spheres, and furthermore that many volatile organic substances have densities near that of water. Moreover, the mass of the purely volatile component is low and so these assumptions do not significantly impact the calculated mass distribution. If there had been a sufficient proportion of purely volatile particles, this mass could have been measured directly.
The surface-condensed volatile component and the non-volatile component have mass distributions and
, respectively;
[7]
[8] where
is the median mass of undenuded particles at a given mobility size. These equations extract the number of particles of the given component from the size distribution using the volatile particle fraction, then multiplies that by the mass of the component using the undenuded particle mass and the volatile mass fraction.
The size distributions for the coated non-volatile and purely volatile components can also be calculated from the total size distribution using the volatile particle fraction. For the non-volatile component, this is and for the purely volatile component this is
;
[9]
[10]
All of these distributions may be integrated with respect to the logarithm of the particle mobility size to produce the total mass or number concentration of each component, and so the total volatile mass or number fractions can be calculated.
The mixing state can also be quantified using the measures of species diversity and the mixing state parameter, , described in Riemer and West Citation(2013). For an aerosol with volatile and non-volatile material, the species diversity measures
,
, and
range from 1 to 2 and can be thought of as counting the effective number of species present in a particle or in a population, i.e., volatile or non-volatile.Footnote5
The single particle diversity
, average particle diversity
, and bulk population diversity
are calculated using
[11]
[12]
[13] where,
is the mass fraction of species
in particle
,
is the mass of particle
as a fraction of the total mass of the population,
is the mass of species
as a fraction of the total mass of the population,
is the number of species in the population,
is the number of particles in the population,
is the total mass concentration of the population, and
is the total mass fraction of volatile material in the population.
The calculated here as a function of
is not a true single particle diversity but rather is an average of the diversities of the particles at that mobility-equivalent diameter. This is due to the fact that volatile mass fraction is calculated using the median of the CPMA mass distributions. In reality, some particles in the population will have a slightly higher or lower single particle diversity. This
is specifically calculated for the particles containing both volatile and non-volatile material. For the purely volatile particles, the diversity would be equal to 1.Footnote6
The mixing state parameter, , is calculated by
[14] and ranges from 0, indicating the population is externally mixed, to 1, indicating the population is internally mixed.
3. Results and discussion
Using the method and experimental setup outlined in Section 2, the mixing states of the two miniCAST soot samples were determined. As was expected, the high EC sample had a relatively low amount of volatile material, while the high OC sample had a much more significant proportion of volatile material (Mamakos et al. Citation2013).
3.1. Volatile mass fraction
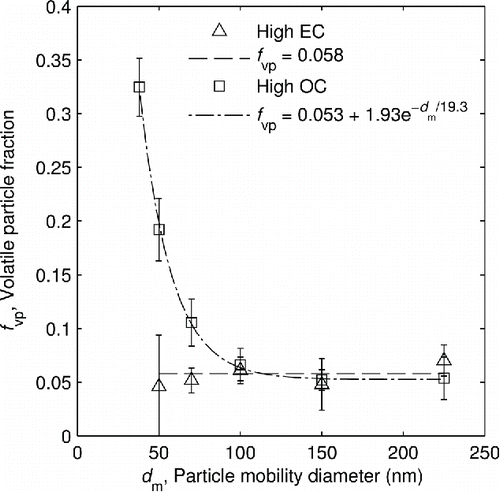
shows the volatile mass fractions of the miniCAST soot at the two different settings. At high EC, the volatile mass fraction remains very close to zero, averaging only 0.3%. It is practically constant, so its best-fit curve was chosen simply to be the mean of the data points. For soot at high OC, the volatile mass fraction begins at over 60% at small sizes and drops to about 15% at large sizes. This soot has a much larger volatile component than the high EC setting, especially for smaller particles. The volatile mass fraction data for high OC was best fit by a power law. A decreasing trend in volatile mass fraction as a function of mobility-equivalent diameter has also been observed for diesel exhaust (Sakurai et al. Citation2003), gasoline direct injection exhaust (Momenimovahed and Olfert Citation2015), natural gas direct-injection compression-ignition engine exhaust (Graves et al. Citation2015), as well as McKenna and inverted burners (Ghazi et al. Citation2013).
Figure 4. Volatile mass fraction and species diversity of the mixed particles in the high OC condition versus undenuded particle mobility diameter.
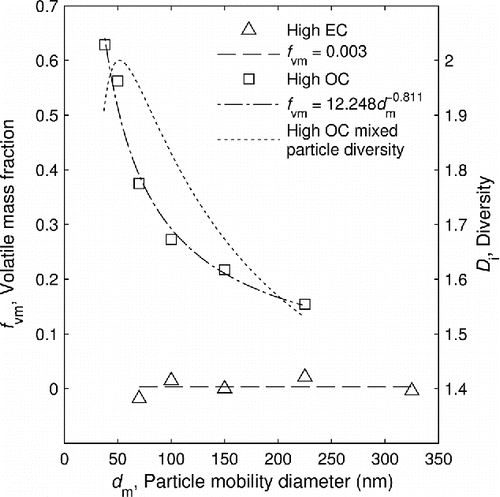
Also shown in is the single particle diversity () of the particles containing both volatile and non-volatile material in the high OC condition, calculated according to Equation (Equation11
[11] ). It achieves a maximum value of 2 when the volatile mass fraction is 0.5, indicating that the particles are composed evenly of two species.
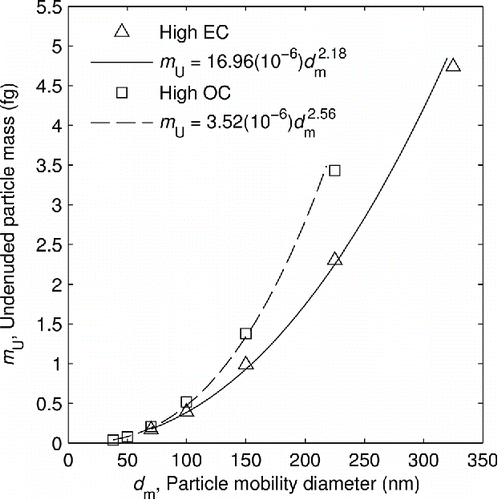
3.2. Size dependence of particle mass
shows the median masses of the undenuded particles at different sizes. Because of the fractal-like aggregate structure of soot particles, the mass increases by less than the cube of the mobility diameter (this exponent is commonly referred to as the mass-mobility exponent; Sorensen Citation2011); this corresponds to larger particles being less dense with respect to their mobility-equivalent volume. Both sets of data are best fit by a power law. The data show that soot from the high OC setting is between 20% and 50% more dense than that from the high EC setting. Presumably, this is due to the volatile material filling voids and producing a particle with a more spherical form.
3.3. Externally mixed volatile particle fraction
shows the volatile particle fractions of the miniCAST soot at the two settings. The error bars in the figure represent 95% confidence intervals on the data points, obtained from between 5 and 10 measurements per data point. The soot generated at the high EC setting has a roughly constant volatile particle fraction around 6%, so it is best fit by its average. This is a small ratio, comparable to the uncertainties, which are also on the order of a few percent for both and
(as shown in ). The high OC soot has a high volatile number fraction of over 30% at small particle sizes (<75 nm), which quickly decreases to roughly the same as the high EC soot. This behavior is best fit by a decaying exponential.
3.4. Mixing state size and mass distribution
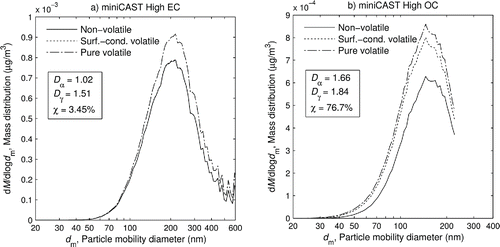
shows the distribution of number concentration, and shows the distribution of mass concentration, over particle mobility diameter and component for the miniCAST soot at (a) the high EC setting and (b) the high OC setting. These were calculated from the smooth best-fit curves from the measurements above. Where data were unavailable, these best-fit curves were used for extrapolation (constraining them to lie within physical limits); this extrapolation only occurred at small sizes where the mass is negligible. Note that the curves in are additive so the uppermost curve represents the total mass distribution, and the distribution of each component alone is represented by the difference between it and the curve below it. It can be seen how the mass distribution is shifted to the right compared to the number distribution; a greater proportion of the mass is in larger particles.
Figure 7. Size distributions of the soot from (a) high EC and (b) high OC showing the number concentration of each component of the sample versus particle mobility diameter. The components are graphed additively; the actual value of each component is given by the difference between its curve and the curve below it.
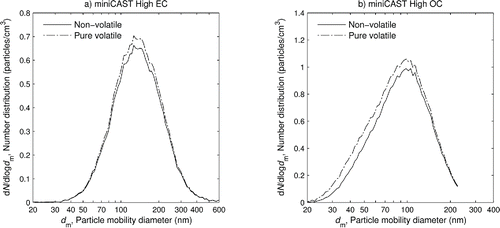
Figure 8. Mixing state of the soot from (a) high EC and (b) high OC. Mass distribution showing the mass concentration of each component of the sample versus particle mobility diameter. As in , the components are graphed additively.
Numerically integrating the above mass distributions with respect to the logarithm of the mobility diameter produces the total mass concentration of each component, and these can be used to find the total mass fraction of each component. The total mass fractions are summarized in .
Table 2. Mass fractions of the different material components of the soot from the different set points.
Uncertainties in these mass concentrations originate from CPMA mass measurement (∼3%, Symonds et al. Citation2013), DMA size measurement (∼3%, Kinney et al. Citation1991), and CPC number concentration (∼10%, TSI CPC specifications). Combined these give an uncertainty on the order of 16% of the total mass concentration (with 95% confidence) neglecting uncertainty due to the SMPS data inversion, as shown by Momenimovahed and Olfert (Citation2015).
The soot from the high EC setting is mostly non-volatile, with virtually no surface-condensed volatile material, and some purely volatile particles (around 5% ). The irregularity at the upper range of the mass distribution comes from the fact that it represents only a very small number of relatively heavy particles. At that end of the SMPS scan, the concentration measured by the CPC was low enough (<0.01 particles/cm3) that counting statistics were poor, producing a noisy concentration signal. (This can be improved by using a longer SMPS scan time). This is magnified upon weighting by mass. For the high OC setting, the SMPS scan was unable to capture the upper tail of the distribution. The high OC soot has a very significant amount of surface-condensed volatile material (22%
) to complement its non-volatile component, along with some purely volatile particles (around 5%
, increasing at lower sizes).
It may be noted that the mass distribution for high EC shows a greater proportion of purely volatile particles compared to the distribution for high OC, even though they have very similar volatile particle fractions above 100 nm. This is due to the fact that the density of the purely volatile material is assumed to be constant at 1000 kg/m3, while the density of the non-volatile material is decreasing with size. Thus, the particles from high OC are around 750 kg/m3 near the mode of its distribution, and the particles from high EC are only around 450 kg/m3 near the mode of its distribution. The purely volatile particles therefore form a greater proportion of the mass relative to their number fraction at the high EC condition.
also gives the average particle diversity , bulk population diversity
, and mixing state parameter
calculated according to Equations (Equation12)–(14) for each condition.
is higher in the high OC condition because the total proportion of volatile material is higher, that is, the total mass of the population is more evenly divided between the two species.
is higher in the high OC case, and closer to the value of
, because the mass of the average particle is more evenly divided between the two species. The values of
for the two cases show that the high EC condition can be thought of as almost entirely “externally mixed,” while the high OC condition can be thought of as mostly “internally mixed.” While these measures capture and summarize some of the information in the mass distributions of , the interpretation of the mass distributions is more direct and immediately meaningful.
4. Conclusion
A methodology to quantify the mixing state of an aerosol with volatile material has been described, and demonstrated using miniCAST-generated soot at high-EC and high-OC settings. The methodology calculates the proportions of aerosol mass at each size, which are in each component of the aerosol: non-volatile particles, volatile material condensed onto non-volatile material, and purely volatile particles. In this study, material is considered to be volatile if it is removed by a certain catalytic denuder heated to 350°C; different values for the temperature and residence time of the denuder could produce different results, and this is important to note when comparing these results to others. The mass distribution is found by measuring the volatile mass fractions and volatile number fractions of the aerosol at different sizes, using a DMA to classify by size and a CPMA to classify by mass, and comparing measurements of the sample with and without removing volatile material in a denuder.
The experimental demonstration of this methodology shows that it successfully quantifies the differences between an aerosol with a low amount of independent volatile material, and next to no surface-condensed volatile material (the high-EC setting), and an aerosol with a significant amount of volatile material, much of which is condensed onto non-volatile material (the high-OC setting). The method also detects another difference in the morphology of these aerosols, namely, that the effective density of the high-OC soot is higher. The method is limited by several factors. To simplify calculations, the method assumes a unique relationship between mass and size, which is valid for particles with a uniform morphology like spheres, but only an approximation for soot aggregates. Furthermore, the distribution of masses together with the low number of independent volatile particles in this experiment meant that the density of the volatile material could not be measured, and so a value had to be assumed. In applications with a significant amount of volatile material, the mass of the volatile particles could be directly measured, but in cases with very high proportions of volatile material, the opposite problem could be encountered where it then becomes difficult to measure the mass of the non-volatile particles.
The aerosol used to demonstrate the methodology had multiple-species particles where the volatile material is thought to coat or envelop the non-volatile material, but it is also appropriate for other kinds of mixing that may not have a coated structure (e.g., a salt-water aerosol where the non-volatile material dissolves in the volatile material). Furthermore, while it is not observed in the presented data, the method also has the potential to distinguish between different states of non-volatile material when it is present in both independent (pure) particles and multiple-species particles. Future work on this methodology could explore these possibilities, and experiment with measuring the mixing states of different kinds of aerosols.
Acknowledgments
We wish to acknowledge Catalytic Instruments for providing the catalytic denuders for these experiments.
Funding
Funding for this research was provided by Transport Canada, National Sciences and Engineering Research Council, and Alberta Innovates Technology Futures.
Notes
1 Refractory black carbon is used to refer to measurements derived from incandescence methods (Petzold et al. Citation2013).
2 Graves Citation(2015) shows the soot from several diesel, gasoline direct injection, gasoline port-injection, and natural gas direct injection compression-ignition engines follows a mass mobility relationship of
where dm is the mobility equivalent diameter of the particle.
3 A different miniCAST burner was used for these measurements as the one used in the main part of the experiment was unavailable.
4 Because the measured mass distributions are relatively narrow and symmetrical, the median, mean, and mode are expected to be fairly close together; the median was selected for its resistance to outliers.
5 More generally, they range from 1 to the number of different species present.
6 This is why there is no direct contribution from the diversity of the purely volatile particles in the expression for ; they would contribute factors to the product of the form
, which do not change the result.
References
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., Ghan, S., Karcher, B., Koch, D., Kinne, S., Kondo, Y., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S. (2013). Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atm., 118(11):5380–5552. http://doi.org/10.1002/jgrd.50171
- Burnett, R. T., Pope, C., Arden, I., Ezzati, M., Olives, C., Lim, S. S., Mehta, S., Shin, H. H., Singh, G., Hubbell, B., Brauer, M., Anderson, H. R., Smith, K. R., Balmes, J. R., Bruce, N. G., Kan, H., Laden, F., Prüss-Ustün, A., Turner, M. C., Gapstur, S. M., Diver, W. R., and Cohen, A. (2014). An Integrated Risk Function for Estimating the Global Burden of Disease Attributable to Ambient Fine Particulate Matter Exposure. Environ. Health Persp., 122(4):397–403.
- Cappa, C. D., Onasch, T. B., Massoli, P., Worsnop, D. R., Bates, T. S., Cross, E. S., Davidovits, P., Hakala, J., Hayden, K. L., Jobson, B. T., Kolesar, K. R., Lack, D. A., Lerner, B. M., Li, S.–M., Mellon, D., Nuaaman, I., Olfert, J. S., Petaja, T., Quinn, P. K., Song, C., Subramanian, R., Williams, E. J., and Zaveri, R. A. (2012). Radiative Absorption Enhancements Due to the Mixing State of Atmospheric Black Carbon. Sci.(New York, N.Y.), 337(6098):1078–1081. http://doi.org/10.1126/science.1223447
- Chan, T. W., Brook, J. R., Smallwood, G. J., and Lu, G. (2011). Time-Resolved Measurements of Black Carbon Light Absorption Enhancement in Urban and Near-Urban Locations of Southern Ontario, Canada. Atmos. Chem. Phys., 11(20):10407–10432. http://doi.org/10.5194/acp-11-10407-2011
- China, S., Mazzoleni, C., Gorkowski, K., Aiken, A. C., and Dubey, M. K. (2013). Morphology and mixing state of individual freshly emitted wildfire carbonaceous particles. Nat. Commun., 4:2122. http://doi.org/10.1038/ncomms3122
- China, S., Scarnato, B., Owen, R. C., Zhang, B., Ampadu, M. T., Kumar, S., Dzepina, K., Dziobak, J. P., Fialho, P., Perlinger, J. A., Hueber, J., Helmig, D., Mazzoleni, L. R., and Mazzoleni, C. (2015). Morphology and Mixing State of Aged Soot Particles at a Remote Marine Free Troposphere Site: Implications for Optical Properties. Geophys. Res. Lett., 42(4):1243–1250. http://doi.org/10.1002/2014GL062404
- Cross, E. S., Onasch, T. B., Ahern, A., Wrobel, W., Slowik, J. G., Olfert, J., Lack, D. A., Massoli, P., Cappa, C. D., Schwarz, J. P., Spackman, J. R., Fahey, D. W., Sedlacek, A., Trimborn, A., Jayne, J. T., Freedman, A., Williams, L. R., Ng, N. L., Mazzoleni, C., Dubey, M., Berm, B., Kok, G., Subramanian, R., Freitag, S., Clarke, A., Thornhill, D., Marr, L. C., Kolb, C. E., Worshop, D. R., and Davidovits, P. (2010). Soot Particle Studies—Instrument Inter-Comparison—Project Overview. Aerosol Sci. Technol., 44(8):592–611. http://dx.doi.org/10.1080/02786826.2010.482113
- Ehara, K., Hagwood, C., and Coakley, K. J. (1996). Novel Method to Classify Aerosol Particles According to Their Mass-to-Charge Ratio - Aerosol Particle Mass Analyser. J. Aerosol Sci., 27:217–234.
- Ghazi, R., and Olfert, J. S. (2013). Coating Mass Dependence of Soot Aggregate Restructuring due to Coatings of Oleic Acid and Dioctyl Sebacate. Aerosol Sci. Technol., 47(2):192–200. http://doi.org/10.1080/02786826.2012.741273
- Ghazi, R., Tjong, H., Soewono, A., Rogak, S. N., and Olfert, J. S. (2013). Mass, Mobility, Volatility, and Morphology of Soot Particles Generated by a Mckenna and Inverted Burner. Aerosol Sci. Technol., 47(4):395–405.
- Graves, B. (2015). Characterization of Particulate Matter Morphology and Volatility for Two Direct-Injection Engines. MSc. thesis, University of Alberta.
- Graves, B., Olfert, J. S., Patychuk, B., Dastanpour, R., and Rogak, S. (2015). Characterization of Particulate Matter Morphology and Volatility from a Compression-Ignition Natural-Gas Direct-Injection Engine. Aerosol Sci. Technol., 49(8):589–598. http://doi.org/10.1080/02786826.2015.1050482
- Healy, R. M., Riemer, N., Wenger, J. C., Murphy, M., West, M., Poulain, L., Wiedensohler, A., O'Connor, I. P., McGillicuddy, E., Sodeau, J. R., and Evans, G. J. (2014). Single Particle Diversity and Mixing State Measurements. Atmos. Chem. Phys., 14:6289–6299. doi:10.5194/acp-14-6289-2014.
- Jayne, J. T., Leard, D. C., Zhang, X., Davidovits, P., Smith, K. A., Kolb, C. E., and Worsnop, D. R. (2000). Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles. Aerosol Sci. Technol., 33(1–2):49–70. http://doi.org/10.1080/027868200410840
- Kinney, P. D., Pui, D. Y. H., Mulholland, G. W., and Bryner, N. P. (1991). Use of the Electrical Classification Method to Size 0.1 mm SRM Particles—A Feasibility Study. J. Res. Natl. Inst. Stan. Technol., 96:147–176.
- Kuwata, M., Kondo, Y., and Takegawa, N. (2009). Critical Condensed Mass for Activation of Black Carbon as Cloud Condensation Nuclei in Tokyo. J. Geophys. Res., 114(D20):D20202. http://doi.org/10.1029/2009JD012086
- Lee, A. K. Y., Willis, M. D., Healy, R. M., Onasch, T. B., and Abbatt, J. P. D. (2015). Mixing State of Carbonaceous Aerosol in an Urban Environment: Single Particle Characterization Using the Soot Particle Aerosol Mass Spectrometer (SP-AMS). Atmos. Chem. Phys., 15(4):1823–1841. http://doi.org/10.5194/acp-15-1823-2015
- Liu, D., Allan, J., Whitehead, J., Young, D., Flynn, M., Coe, H., McFiggans, G., Flemming, Z. L., and Bandy, B. (2013). Ambient Black Carbon Particle Hygroscopic Properties Controlled by Mixing State and Composition. Atmos. Chem. Phys., 13(4):2015–2029. http://doi.org/10.5194/acp-13-2015-2013
- Mamakos, A., Khalek, I., Giannelli, R., and Spears, M. (2013). Characterization of Combustion Aerosol Produced by a Mini-CAST and Treated in a Catalytic Stripper. Aerosol Sci. Technol., 47(8):927–936. doi: 10.1080/02786826.2013.802762
- McFiggans, G., Artaxo, P., Baltensperger, U., Coe, H., Facchini, M. C., Feingold, G., Fuzzi, S., Gysel, M., Laaksonen, A., Lohmann, U., Mentel, T. F., Murphy, D. M., O'Dowd, C. D., Snider, J. R., and Weingartner, E. (2006). The Effect of Physical and Chemical Aerosol Properties on Warm Cloud Droplet Activation. Atmos. Chem. Phys., 6(9):2593–2649. http://doi.org/10.5194/acp-6-2593-2006
- McMeeking, G. R., Morgan, W. T., Flynn, M., Highwood, E. J., Turnbull, K., Haywood, J., and Coe, H. (2011). Black Carbon Aerosol Mixing State, Organic Aerosols and Aerosol Optical Properties Over the United Kingdom. Atmos. Chem. Physics, 11(17):9037–9052. http://doi.org/10.5194/acp-11-9037-2011
- Momenimovahed, A., and Olfert, J. S. (2015). Effective Density and Volatility of Particles Emitted from Gasoline Direct Injection Vehicles and Implications for Particle Mass Measurement. Aerosol Sci. Technol., 49(11):1051–1062. http://doi.org/10.1080/02786826.2015.1094181
- Moteki, N., and Kondo, Y. (2007). Effects of Mixing State on Black Carbon Measurements by Laser-Induced Incandescence. Aerosol Sci. Technol., 41(4):398–417. http://doi.org/10.1080/02786820701199728
- Myhre, G., Shindell, D., Breon, F.-M., Collins, W., Fuglestvedt, J., Huang, J., Koch, D., Lamarque, J.-F., Lee, D., Mendoza, B., Nakajima, T., Robock, A., Stephens, G., Takemura, T., and Zhang, H. (2013). Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, and P. M. Midgley, eds., Cambridge University Press, Cambridge, UK and New York, NY, USA. Retrieved from http://www.climatechange2013.org/images/report/WG1AR5_Chapter08_FINAL.pdf
- O'Brien, R. E., Wang, B., Laskin, A., Riemer, N., West, M., Zhang, Q., Sun, Y., Yu, X.-Y., Alpert, P., Knopf, D. A., et al. (2015). Chemical Imaging of Ambient Aerosol Particles: Observational Constraints on Mixing State Parameterization. J. Geophys. Res. Atmos., 120:9591–9605. doi:10.1002/2015JD023480.
- Olfert, J. S., and Collings, N. (2005). New Method for Particle Mass Classification—The Couette Centrifugal Particle Mass Analyzer. J. Aerosol Sci., 36(11):1338–1352. http://doi.org/10.1016/j.jaerosci.2005.03.006
- Onasch, T. B., Trimborn, A., Fortner, E. C., Jayne, J. T., Kok, G. L., Williams, L. R., Davidovits, P., and Worsnop, D. R. (2012). Soot Particle Aerosol Mass Spectrometer: Development, Validation, and Initial Application. Aerosol Sci. Technol., 46(7):804–817. http://doi.org/10.1080/02786826.2012.663948
- Pagels, J., Khalizov, A. F., McMurry, P. H., and Zhang, R. Y. (2009). Processing of Soot by Controlled Sulphuric Acid and Water Condensation—Mass and Mobility Relationship. Aerosol Sci. Technol., 43(7):629–640. http://doi.org/10.1080/02786820902810685
- Petzold, A., Fiebig, M., Fritzsche, L., Stein, C., Schumann, U., Wilson, C. W., Hurley, C. D., Arnold, F., Katragkou, E., Baltensperger, U., Gysel, M., Nyeki, S., Hitzenberger, R., Giebl, H., Hughes, K., and Technology, C. (2005). Particle Emissions from Aircraft Engines – A Survey of the European Project PartEmis. Meteorol. Z., 14(4):465–476. http://doi.org/10.1127/0941-2948/2005/0054
- Petzold, A., Ogren, J., Fiebig, M., Laj, P., Li, S.-M., Baltensperger, U., Holzer-Popp, T., Kinne, S., Pappalardo, G., Sugimoto, N., Wehrli, C., Wiedensohler, A., and Zhang, X.-Y. (2013). Recommendations for Reporting “Black Carbon” Measurements. Atmos. Chem. Phys., 13:8365–8379. doi:10.5194/acp-13-8365-2013
- Radney, J. G., and Zangmeister, C. D. (2016). Practical Limitations of Aerosol Separation by a Tandem Differential Mobility Analyzer-Aerosol Particle Mass Analyzer. Aerosol Sci. Technol., 50(2):160–172.
- Rawata, V. K., Buckleya, D. T., Kimotoa, S., Leeb M.-H., Fukushimac, N., and Hogan, C. J. (2016). Two Dimensional Size–Mass Distribution Function Inversion from Differential Mobility Analyzer–Aerosol Particle Mass Analyzer (DMA–APM) Measurements. J. Aerosol Sci., 92:70–82. doi:10.1016/j.jaerosci.2015.11.001
- Riemer, N., and West, M. (2013). Quantifying Aerosol Mixing State with Entropy and Diversity Measures. Atmos. Chem. Phys., 13:11423–11439. doi:10.5194/acp-13-11423-2013
- Rissler, J., Nordin, E., Eriksson, A., Nilsson, P., Frosch, M., Sporre, M., and Swietlicki, E. (2014). Effective Density and Mixing State of Aerosol Particles in a Near-Traffic Urban Environment. Environ. Sci. Technol., 48(11):6300–6308. doi: 10.1021/es5000353
- SAE. (2013). Aerospace Information Report (AIR) 6241, Procedure for the Continuous Sampling and Measurement of Non-Volatile Particle Emissions from Aircraft Turbine Engines. SAE International, Warrendale, PA
- Sakurai, H., Park, K., McMurry, P. H., Zarling, D. D., Kittleson, D. B., and Ziemann, P. J. (2003). Size-Dependent Mixing Characteristics of Volatile and Nonvolatile Components in Diesel Exhaust Aerosols. Environ. Sci. Technol., 37:5487–5495.
- Schnitzler, E., Dutt, A., Charbonneau, A., Olfert, J. S., and Jaeger, W. (2014). Soot Aggregate Restructuring due to Coatings of Secondary Organic Aerosols Derived from Aromatic Precursors. Environ. Sci. Technol., 48:14309–14316.
- Schwarz, J. P., Spackman, J. R., Gao, R. S., Perring, A. E., Cross, E., Onasch, T. B., Ahern, A., Wrobel, W., Davidovits, P., Olfert J., Dubey, M. K., Mazzoleni C., and Fahey D. W. (2010). The Detection Efficiency of the Single Particle Soot Photometer. Aerosol Sci. Technol., 44(8):612–628. http://doi.org/10.1080/02786826.2010.481298
- Sorensen, C. M. (2011). The Mobility of Fractal Aggregates: A Review. Aerosol Sci. Technol., 45(7):765–779. doi: 10.1080/02786826.2011.560909
- Stephens, M., Turner, N., and Sandberg, J. (2003). Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity. Appl. Optics, 42(19):3726–3736. http://doi.org/10.1364/AO.42.003726
- Stolzenburg, M. R., and McMurry, P. H. (2008). Equations Governing Single and Tandem DMA Configurations and a New Lognormal Approximation to the Transfer Function. Aerosol Sci. Technol., 42(6):421–432. http://doi.org/10.1080/02786820802157823
- Symonds, Jonathan P. R., Reavell, Kingsley St. J., and Olfert, Jason S. (2013). The CPMA-Electrometer System - A Suspended Particle Mass Concentration Standard. Aerosol Sci. Technol., 47(8):i–iv.
- UNECE Regulation No. 83 (Emissions of M1 and N1 categories of vehicles). Retrieved from http://www.unece.org/trans/main/wp29/wp29regs81-100.html
- Wang, Q., Huang, R.-J., Cao, J., Han, Y., Wang, G., Li, G., Wang, Y., Dai, W., Zhang, R., and Zhou, Y. (2014). Mixing State of Black Carbon Aerosol in a Heavily Polluted Urban Area of China: Implications for Light Absorption Enhancement. Aerosol Sci. Technol., 48(7):689–697. http://doi.org/10.1080/02786826.2014.917758
- Wang, S. C., and Flagan, R. C. (1990). Scanning Electrical Mobility Spectrometer. Aerosol Sci. Technol., 13(2):230–240. http://doi.org/10.1080/02786829008959441
- Zhang, R., Khalizov, A. F., Pagels, J., Zhang, D., Xue, H., and McMurry, P. H. (2008). Variability in Morphology, Hygroscopicity, and Optical Properties of Soot Aerosols during Atmospheric Processing. Proc. Natl. Acad. Sci. USA., 105(30):10291–10296. Retrieved from http://www.pnas.org/cgi/content/abstract/105/30/10291.