ABSTRACT
Our objective was to evaluate the suitability of using a capillary aerosol generator (CAG) instead of using e-cigarette devices in 90-day or longer inhalation studies. Aerosol characteristics for both the CAG (which uses heat to produce a condensation aerosol) and e-cigarette generators have been previously reported, but a side-by-side comparison with the identical formulation has not been reported. Aerosols from both devices were analyzed immediately after generation for chemicals in the formulation (propylene glycol [PG], glycerin, water, and nicotine), selected carbonyls (acetaldehyde, acrolein, and formaldehyde) by ultra-performance liquid chromatography with ultraviolet detection (UPLC-UV), and a chemical fingerprint analysis using gas chromatography-mass spectroscopy (GC-MS). Aerosol capture methods for chemical analysis included Cambridge filter pads or two impingers in series each containing solution to trap and stabilize selected carbonyl compounds. Particle size distribution (cascade impactor) and exposure port uniformity (gravimetric) was measured in four rodent inhalation exposure chambers under inhalation study conditions. The aerosol of both generators contained the same known and unknown chemicals. Similar levels of compounds in the formula except for PG were detected in the aerosol of both generators. CAG produced more consistent particulate aerosol than e-cigarette generator and had lower levels of carbonyls primarily due to lower levels of formaldehyde. Exposure port concentrations were consistent and closer to target values with the CAG compared to the e-cigarette aerosol generator. CAG was easier to operate on a daily basis although more difficult to maintain because it required daily cleaning compared to single-use e-cigarettes. CAG was determined to be suitable for use in 90-day or longer inhalation studies.
Copyright © 2016 American Association for Aerosol Research
EDITOR:
1. Introduction
Electronic cigarettes (e-cigarettes) were first introduced into the global marketplace in 2004 and have been steadily gaining popularity among adult tobacco consumers. E-cigarettes are battery operated devices designed to deliver nicotine and flavors in a fine particulate aerosol via inhalation. Regulatory requirements are still evolving for e-cigarettes with the European Tobacco Product Directive (European Commission Citation2014) and UK standard for e-cigarettes as a medical product (MHRA Citation2015) being recent examples. In the realm of e-cigarettes, safety testing must involve evaluation of e-cigarette aerosols using in vitro and in vivo models designed to assess potential aerosol toxicity. Experience has shown over many years that inhalation exposure testing is perhaps the shortest, most-direct, and straight-forward of possible evaluation approaches for inhaled products. This view is echoed by NIOSH in their recent commentary to FDA related to e-cigarette evaluations (CDC NIOSH Citation2015). A well-designed inhalation exposure study can be used to assess the potential toxicity at elevated aerosol exposure concentrations, repeated exposures, and elevated doses over a significant proportion of the animals' lifetime and provide a thorough in-life and post-mortem evaluation of the impact upon all the exposed tissues and organs.
The preparation for laboratory inhalation tests is extensive, involving the selection and evaluation of appropriate aerosol generators that will simulate the aerosol exposures that humans might experience. For e-cigarettes, previous work (Werley et al. Citation2015) demonstrated that approximately 27,000 e-cigarettes were required for a 90-day rodent inhalation study. For e-cigarettes that generate a condensation aerosol, the most common approach uses a fluid-filled cartomizer containing a formulation, a battery to heat a coil to produce a vapor that quickly cools prior to the mouthpiece to yield a condensed particulate aerosol (also called vapor; Marple and Rubow Citation1980). The aerosol characteristics are related to many factors including the composition of the formulation, battery current and heat of the coil, device materials, airflow, vent-hole placement, configuration of the outlet such as diameter, length, composition, and shape, all of which contribute to cooling and condensation of the resulting aerosol (Brown and Cheng Citation2014; Herrington and Myers Citation2015; Flora et al. Citation2016). In some cases, design features of these devices may contribute to unwanted chemical exposures such as elevated levels of formaldehyde and other carbonyls from overheated coils, low airflow from blocked vent holes, and “dry puffing” nearly empty cartomizers (Ohta et al. Citation2011; Uchiyama et al. Citation2013; Kosmider et al. Citation2014; Jensen et al. Citation2015; Nitzkin et al. Citation2015; Flora et al. Citation2016). The simplicity and reported lack of a quality control (Cheng Citation2014; Williams et al. Citation2015) aspect for individual e-cigarette devices may also contribute to variability in the aerosol output, as subtle differences across devices have been shown to contribute to changes in composition, aerosol output, formation of contaminants, and even duration of use for filled cartomizers (Williams and Talbot Citation2011; Bekki et al. Citation2014).
In order to assess e-cigarette formulations separate from their respective devices and simplify the logistics of potential future 90-day or longer inhalation studies, other aerosol generators must be considered. Previously, we and others had reported on the characteristics and use of a laboratory bench-top generator called the capillary aerosol generator (CAG) (Gupta et al. Citation2003; Werley et al. Citation2011). This device produces a large volume of condensation aerosol, which is quite similar to e-cigarette aerosol but with very different setup and handling requirements. As previously described (Gupta et al. Citation2003; Hindle Citation2003; Werley et al. Citation2011), the CAG uses a heated tube to create a condensation aerosol. Werley et al. Citation(2015) describes that e-cigarette aerosol generation required a long lead time, a large number of prefilled cartomizers, and a purpose-built smoking machine for study conduct, whereas the CAG is a stand-alone device connected only to a test-material reservoir and temperature controller with minimal daily setup requirements except for daily cleaning.
The objective of the experiments reported here was to evaluate the suitability of using a CAG condensation aerosol generator instead of using e-cigarette devices in 90-day or longer inhalation studies. This comparative study involved chemical analysis of e-cigarette type formulations, chemical byproducts of the heated formulation, particle size measurements, and port-to-port variability in nose-only exposure equipment.
2. Materials and methods
A series of tests was performed to evaluate the CAG aerosol generator and compare it with the MarkTen® prototype e-cigarette (prototype e-cigarette; CVR 1.3 & CVR 1.4; 3.9 W; not user adjustable). The difference in prototype e-cigarettes CVR 1.3 and CVR 1.4 was a change in three adhesive labels and the connector between the cartomizer and battery, none of which affected aerosol generation or delivery.
This series of tests included quantitative chemical evaluation of an aerosolized e-cigarette type formulation for the principal ingredients (propylene glycol [PG], glycerin, water and nicotine) using gas chromatography-mass spectroscopy (GC-MS), gas chromatography-thermal conductivity detector (GC-TCD), and gas chromatography with flame ionization detector (GC-FID); a fingerprint analysis performed with GC-MS; measurement of selected carbonyls (formaldehyde, acrolein, and acetaldehyde) using ultra-performance liquid chromatography with ultraviolet detection (UPLC-UV); and measurement of the mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), and port-to-port variability in rodent nose-only exposure apparatus.
2.1. Chemical evaluation
A standardized e-cigarette type formulation was created that consisted of 40% PG and 60% glycerin to which 15% water, 2% nicotine, and 8.6% nicotine bitartrate by weight were added. The resulting formulation consisted of 29.8% PG, 44.7% glycerin, 15% water, 5% nicotine, and 5.5% tartaric acid. All chemicals were US Pharmacopeia grade except the nicotine bitartrate, which was analytical grade, and the water, which was potable. This formulation was loaded in the prototype e-cigarettes that were puffed (55 mL puff volume, 5-s puff duration, and 5-s interpuff interval) using a 20-port linear smoking machine (Cerulean, Milton Keynes, UK). A total of six puffs from each of nine prototype e-cigarettes were used to generate aerosol, which was collected from the smoking machine using a 44 mm Cambridge filter pad. The CAG was set to a temperature of 275°C, which matched the coil temperature measured during puffing of the prototype e-cigarette, a fluid flow rate of 1 mL/min, and an airflow rate of 5 L/min. The CAG-generated aerosol was sampled from an enclosure using a Cambridge filter pad at 1.35 standard liters per minute (SLM) for 43 s to match the aerosol mass of the prototype e-cigarettes. The Cambridge filter pads were extracted with n-propanol and analyzed using GC-MS in scan mode. The GC-MS scan for the aerosol collected from each device provided the quantitative comparison of principal components (PG and glycerin) and chemical aerosol fingerprint analysis. Nicotine was analyzed using GC-FID and water was analyzed using GC-TCD.
Carbonyl analysis (formaldehyde, acetaldehyde, and acrolein) was performed by collecting aerosol using two impingers in series. Each impinger contained 30 mL solution containing 2,4-dinitrophenylhydrazine to trap and stabilize the carbonyls. Five replicates were performed, with each replicate consisting of six puffs from each of three prototype e-cigarettes for a total of 18 puffs/replicate. Prototype e-cigarettes were puffed (55 mL puff volume, 4-s puff duration, and 5-s interpuff interval) using a 5-port linear SC5 smoking machine (KC Automation, Richmond, VA, USA). The CAG-generated aerosol was sampled from an enclosure using two impingers in series at 1.35 SLM for 138 s to ensure an adequate sample. The sampling time was three times longer than required to match the aerosol mass of the prototype e-cigarettes so results were adjusted accordingly. An aliquot from the 60 mL mixture of both impingers was analyzed by UPLC-UV.
All aerosol generation, sampling, and chemical analysis were performed in a standardized laboratory environment (temperature 22°C ± 2°C and relative humidity [RH] 60% ± 5%). A paired t-test was used to compare results (CAG to prototype e-cigarette) that were above the limit of quantification for statistical significance (p ≤ 0.05) with the realization that any detected statistically significant differences would likely be diminished if measured at the exposure port of the nose-only exposure systems.
2.2. Particle size distribution measurements
A seven-stage stainless-steel cascade impactor (Model 01-130, IN-TOX Products, Moriarty, NM, USA) was used to determine the MMAD and GSD of the aerosol in each of four separate nose-only exposure towers (48-port, directed-flow, nose-only exposure systems, CH Technologies, Westwood, NJ, USA). Aerosol was sampled at 1 L/min for 3 min from two nose-only exposure ports. Mass from each stage was determined gravimetrically using pre-weighed, 22 mm stainless-steel collection substrates. A pre-weighed, 25 mm glass fiber filter was used as the final collection substrate. Each nose-only exposure tower was supplied by a dedicated modified smoking machine (Werley et al. Citation2015) when measuring prototype e-cigarette-generated aerosol or by one CAG generator. The generated aerosol was diluted with 38–42 L/min of HEPA-filtered air to provide a target exposure concentration of 1 mg/L. Consistent with Good Laboratory Practices (GLP) for animal studies, the chamber humidity was maintained at 40–60% RH, 24–28°C chamber temperature with 0.5 L/min/animal chamber airflow.
2.3. Exposure port homogeneity
For each of the four nose-only exposure towers, homogeneity between exposure ports was measured gravimetrically using pre-weighed 44 mm glass-fiber filters held in an in-line filter holder. Following sample collection, the filter was weighed again, and the concentration was calculated as the filter weight difference divided by the sample volume. Three replicate samples were taken with a target exposure concentration of 1 mg/L. A reference port near the top of the chamber aerosol inlet (reference port) was compared with the exposure concentration at various other ports on different tiers, and port positions in the exposure chambers. As noted above, each nose-only exposure tower was supplied by a dedicated modified smoking machine (Werley et al. Citation2015) when measuring prototype e-cigarette generated aerosol or one CAG generator. Consistent with GLP for animal studies, the chamber humidity was maintained at 40–60% RH, 24–28°C chamber temperature with 0.5 L/min/animal chamber airflow.
3. Results
3.1. Chemistry
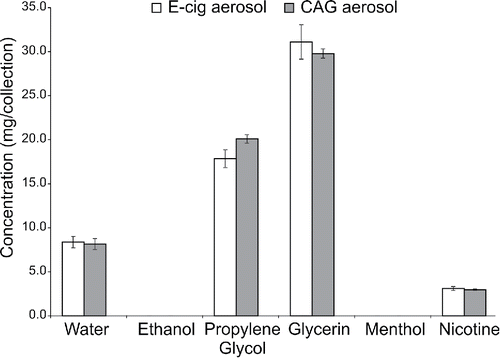
Quantitative evaluations of the aerosol for the major components of the formulation show relatively close agreement for both aerosol generators (). The only statistically significant difference was in the amount of PG detected in the aerosol.
Figure 1. Major component chemical analyte comparisons (mean ± 1SD; N = 3) using prototype e-cigarette and CAG aerosol generators. A statistically significant difference in the concentration of PG (p < 0.05) was measured.
The chemical fingerprint comparison of chemical analytes of the prototype e-cigarette and CAG aerosols are comparable (). The appearances of the major peaks (retention time) and their identification for both generators are also similar (); however, the different peak area counts suggest that the aerosol sampling was not perfectly matched.
Figure 2. Chemical “fingerprint” for an identical formulation using (a) prototype e-cigarette and (b) CAG aerosol generators.
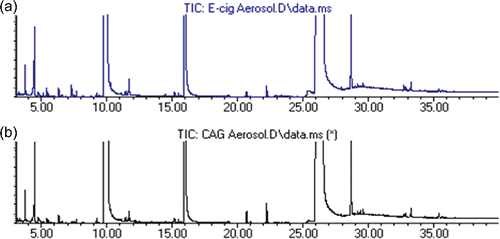
Table 1. Chemical identification, retention times, and peak area count comparisons for prototype e-cigarette and CAG aerosol generation.
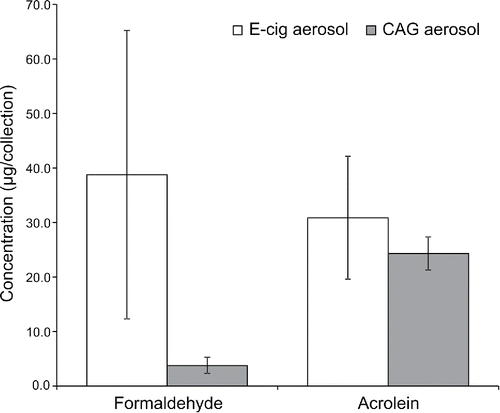
For carbonyls, all values for acetaldehyde in aerosols generated by both the prototype e-cigarette and CAG were below the limit of quantification (137 µg/collection). A statistically significant difference (p < 0.05) in the level of formaldehyde was observed between the prototype e-cigarette and CAG aerosol generators (). The variability in the measurements for formaldehyde was large for the prototype e-cigarette and relatively small for the CAG. Overall, the level of total carbonyls was approximately double for the prototype e-cigarette compared with the CAG aerosol generator, with the most significant difference due to the formaldehyde levels. Acrolein levels were similar for both the prototype e-cigarette and CAG aerosol generators ().
Figure 3. Quantitative comparison of carbonyl level (mean ± 1-SD; N = 3) using UPLC-UV for prototype e-cigarette and CAG aerosol generators. All values for acetaldehyde were below the limit of quantification of 137 µg/collection. A statistically significant difference in the concentration of formaldehyde (p < 0.05) was measured.
3.2. Particle size distribution measurements
Using test conditions that simulated GLP study conditions, the MMAD for prototype e-cigarette generated aerosols ranged from 0.8 to 1.3 µm, with the GSD ranging from 1.4 to 1.63 (). The MMAD of the CAG-generated aerosol was slightly more consistent, ranging from 1.0 to 1.3 µm and the GSD ranging from 1.47 to 1.72 (). The MMAD and GSD from the third impactor run for two of the nose-only exposure towers using the CAG were not provided due to technical difficulties.
Table 2. Particle size distribution data for prototype e-cigarette aerosol sampled from two nose-only exposure ports of each exposure chamber.
Table 3. Particle size distribution data for CAG aerosol sampled from two nose-only exposure ports of each exposure chamber.
3.3. Exposure port homogeneity
For the four nose-only exposure towers port-to-port evaluations were performed to assure a minimal difference in aerosol exposure from each device. For the prototype e-cigarette test atmospheres, the percent differences for each “test” port compared with the reference port were generally less than 3% and ranged from −5.7% to +6.9% of the target values () with most values much lower than these. In the CAG exposure systems, the test atmosphere percent differences for each “test” port compared with the reference port were generally around 1% and ranged from −10.5% to +4.1% of the targeted aerosol value (). Port-to-port exposure concentrations were usually more consistent with the CAG, were closer to the target values, and generally showed less variability than comparable data for the prototype e-cigarette exposures.
Table 4. Exposure port homogeneity for prototype e-cigarette-generated aerosol.
Table 5. Exposure port homogeneity for CAG generated aerosol.
4. Discussion
Data reported here were collected during qualification studies of a prototype e-cigarette and the CAG aerosol generator in order to qualify the CAG for use in definitive 90-day rodent inhalation studies. Ideally, both devices or aerosol generators should produce very similar aerosols under the same study conditions in order to be able to compare biological results across studies. The CAG was qualified as a surrogate device to generate and test e-liquid formulations in 90-day rodent inhalation studies. To this end, we used analytical “fingerprint” chemical analysis to identify differences in chemical composition of the aerosols, and targeted analytical methods to compare differences in vehicle generation, water, nicotine concentration, and carbonyls in the test atmospheres under temperature and humidity conditions and exposure concentrations to which animals would be exposed.
The prototype e-cigarette and the CAG use heat to generate an aerosol. A heated coil is used for the prototype e-cigarette and a heated metal block in the CAG creates a superheated vapor that quickly condenses outside the device to form a condensation aerosol. Given similarities in heating temperatures, the number of known and unknown compounds determined using GC-MS were nearly identical (; ). The chemical identity of the unknowns however was not completely determined. Comparison of the aerosol levels of formulation constituents from the respective prototype e-cigarette and CAG generators () shows good correlation with only a statistically significant difference found in levels of PG. Given the sample collection time (prototype e-cigarette = 60 s and CAG = 43 s) and the amount of dilution air (38–42 L/min of HEPA-filtered air) required for nose-only inhalation studies, this difference in levels of PG will not be seen at the nose-only exposure ports in the exposure towers. The chromatogram of chemical analytes from the prototype e-cigarette and CAG aerosols is also comparable ().
There was an observed difference in levels of carbonyls. In general, the prototype e-cigarette produced about double the concentration of carbonyls (mainly formaldehyde) compared with the CAG under similar exposure conditions. This difference was attributable to differences in the level of formaldehyde measured for each aerosol generator. While the level of acetaldehyde from both aerosol generators was below the limit of quantification and the level of acrolein was not statistically significantly different between the two aerosol generators, the level of formaldehyde was about eight times higher for the prototype e-cigarette compared with the CAG. From a post-hoc perspective, additional puffs/e-cigarette or e-cigarettes/device could have been used to increase the number of puffs collected per replicate so that the measured level of acetaldehyde for both aerosol generators exceed the LOQ. It is unclear why the difference in the level of formaldehyde exists between the two aerosol generators.
When tested under animal-exposure conditions at 40–60% humidity, the particle size generated by both devices was approximately the same with a similar GSD, but increased from laboratory benchtop conditions (data not shown). This change is likely due to a combination of factors that include coagulation and hygroscopic growth of the aerosol particles in a more humid environment. The similarity in MMAD and GSD of aerosols from each generator indicated that both devices were appropriate for the generation of test aerosols for exposure since both yielded particles of sizes inhalable by the rat (Raabe et al. Citation1988; Menache et al. Citation1995).
The exposure port homogeneity for aerosols generated from prototype e-cigarettes and the CAG over the four separate exposure systems were generally not significantly different and under ±10% except for one port with the CAG (10.5%). Reviews of exposure chambers (Tillery et al. Citation1976; MacFarland Citation1983; Cheng and Moss Citation1995; Phalen Citation1997, Citation2009) indicate whole body chamber uniformity ranging from ±2.1 to ±12.8% and varied depending on the tracer gas used (MacFarland Citation1983) and, as would be expected, vary as function of particle size (Yeh et al. Citation1986). Oldham et al. Citation(2009) reported nose-only exposure port uniformity of ±10% for a single system that compares well with the exposure port uniformity results for prototype e-cigarette or CAG generated aerosols in the four exposure systems.
The only significant difference noted in our evaluation were the levels of carbonyls generated by the prototype e-cigarette compared with the levels produced by the CAG (approximately 65% less). The majority of the difference in levels appeared to be related to an approximate two- to ten-fold difference in the level of formaldehyde generated depending upon the prototype e-cigarette device being evaluated. Apparently prototype e-cigarettes are inherently more variable than other standardized inhalation laboratory methods, and the aerosols they produce reflect that device characteristic.
In general, interpretation of inhalation study results is only meaningful with some certainty that animals were exposed to a consistent, stable aerosol. Therefore, laboratories expend considerable effort to develop analytical methods and monitor chemical analytes to be able to show that the test atmospheres are stable and can be repeatedly generated. Thus, these evaluations demonstrated to us that an alternative aerosol generation method was not only useful, but also that it was essential to be able to compare formulation differences and potential biological effects across studies and over time. The CAG satisfied all of our qualification criteria with the only exception that carbonyls needed to be closely monitored in any future inhalation evaluations so as not to underestimate the potential biological responses that might be due to lower levels of formaldehyde, specifically, generated by the CAG. Our further expectation at the time was that eventually most, if not all, of the early e-cigarette prototypes would develop and evolve to the point that excess generation of aerosolized carbonyls was no longer an issue, and such comparison with CAG might become more straightforward. Furthermore, we considered that while formaldehyde is believed to be an acute toxicant among others in the class of carbonyls, acrolein is reported to be more important toxicologically (Haussmann Citation2012), with respect to respiratory tract irritation and pathological findings in longer-term studies. The prototype e-cigarette and CAG were closely matched with respect to the generation of acrolein in the test atmospheres evaluated in these qualification studies (). And while the e-cigarette prototype produced more, and with greater variability than the CAG, the differences were not even close to being statistically significant. Finally, we are aware that formaldehyde () is classified by the International Agency for Research on Cancer as a carcinogen (IARC Citation2015), and special attention must be paid with respect to inhalation exposure evaluations due to that fact. We considered the available data that showed that formaldehyde produced toxicological effects in nasal and olfactory epithelium in rats when dosed up to 20 ppm in a subchronic inhalation study (Woutersen et al. Citation1987). In a six-month study in rats, hamsters, and nonhuman primates exposed for 22 h/day, the only significant finding was squamous metaplasia in the nose of rats and nonhuman primates at about 3 ppm, while hamsters appeared to be less sensitive than the other species (Rusch et al. Citation1983). Appleman et al. Citation(1988) reported similar findings in the nose of rats treated with up to 10 ppm inhaled formaldehyde for 6 h/day, 5 days/week for one year, with more pronounced effects in rats with artificially damaged nasal mucosa. Nevertheless, the animal and human exposure data related to inhaled formaldehyde are voluminous, and it's clear that formaldehyde vapor is a biological toxicant particularly to the nose and lower respiratory tract. Such findings must obviously be considered in the evaluation of any inhalation study results, particularly those that may pose an unusually low formaldehyde exposure and risk such as may occur with the CAG.
The major limitation of this work is that the CAG was only compared to one specific e-cigarette device, the MarkTen® prototype e-cigarette. To mimic potential thermal degradation conditions, the CAG temperature was matched to the measured coil temperature of the MarkTen® prototype e-cigarette. Since the temperature of the CAG can be adjusted to match any measured coil temperature, it could mimic other e-cigarette devices with different coil temperatures, but additional work would be required to substantiate this hypothesis.
Based upon our pre-study inhalation characterization of both the prototype e-cigarette and CAG devices, we determined that aerosol output and performance of the two devices were similar enough to justify using both in 90-day subchronic rat inhalation studies to assess the relative safety of commercial e-cigarette formulations given the cautions with respect to the relatively small differences in the formaldehyde levels already described. As always, expert judgment by the inhalation toxicologist is required to identify and appropriately weigh the limitations of the test material generation and exposure methods when interpreting the results. In these studies, the differences were relatively small, and the further use of the CAG is well justified based upon improved logistics, consistency, and subchronic study throughput, allowing us to consider inhalation testing of future commercial e-cigarette formulations before commercial launch.
Acknowledgments
The authors would like to thank Dan J. Kirkpatrick and James Randazzo of WIL Research Laboratories for the exposure system-related data. The authors acknowledge the editorial assistance of Eileen Y. Ivasauskas of Accuwrit Inc.
References
- Appleman, J. R., Howell, E. E., Kraut, J., Kuhl, M., and Blakley, R. L. (1988). Role of Aspartate 27 in the Binding of Methotrexate to Dihydrofolate Reductase from Escherichia Coli. J. Biol. Chem., 263(19):9187–9198.
- Bekki, K., Uchiyama, S., Ohta, K., Inaba, Y., Nakagome, H., and Kunugita, N. (2014). Carbonyl Compounds Generated from Electronic Cigarettes. Int. J. Environ. Res. Public Health, 11(11):11192–11200. doi:10.3390/ijerph111111192
- Brown, C. J., and Cheng, J. M. (2014). Electronic Cigarettes: Product Characterization and Design Considerations. Tob. Control., 23:ii4–ii10.
- CDC NIOSH. (2015). Comments of the National Institute of Safety and Health to the Food and Drug Administration (FDA) in Response to Establishment of Public Docket; Electronic Cigarettes and the Public Health Workshop. Docket no. FDA-2014-N-1936, May 8. Available at http://www.regulations.gov/#!documentDetail;D=FDA-2014-N-1936-0431. Accessed 9 September 2015.
- Cheng, T. (2014). Chemical Evaluation of Electronic Cigarettes. Tob. Control., 23(Suppl 2):ii11–ii17. doi:10.1136/tobaccocontrol-2013-051482
- Cheng, Y. S., and Moss, O. R. (1995). Inhalation Exposure Systems, in Concepts in Inhalation Toxicology, R. O. McClellan and R. F. Henderson, eds, Taylor and Francis, Washington, DC, pp. 25–61.
- European Commission. (2014). Tobacco Products Directive. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014. Available at http://ec.europa.eu/health/tobacco/docs/dir_201440_en.pdf. Accessed 9 September 2015.
- Flora, J. W., Meruva, N., Huang, C. B., Wilkinson, C. T., Ballentine, R., Smith, D. C., Werley, M. S., and McKinney, W. J. (2016). Characterization of Potential Impurities and Degradation Products in Electronic Cigarette Formulations and Aerosols. Regulat. Toxicol. Pharmacol., 74:1–11.
- Gupta, R., Hindle, M., Byron, P. R., Cox, K. A., and McRae, D. D. (2003). Investigation of a Novel Aerosol Condensation Generator; Solute and Solvent Effects. Aerosol. Sci. Technol., 37:672–681. doi:10.1080/02786820300910
- Haussmann, H. J. (2012). Use of Hazard Indices for a Theoretical Evaluation of Cigarette Smoke Composition. Chem. Res. Toxicol., 25(4):794–810. doi:10.1021/tx200536w
- Herrington, J. S., and Myers, C. (2015). Electronic Cigarette Solutions and Resultant Aerosol Profiles. J. Chromatograph., 1418:192–199.
- Hindle, M. (2003). Aerosolization of Human Insulin Using the Capillary Aerosol Generator (CAG). AAPS Pharm. Sci., 5: Abstract T3234.
- IARC. (2015). Agents Classified by the IARC Monographs. Volumes 1–112. Available at http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf. Accessed 9 September 2015.
- Jensen, R. P., Luo, W., Pankow, J. F., Strongin, R. M., and Peyton, D. H. (2015). Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med., 372(4):392–394. doi:10.1056/NEJMc1413069
- Kosmider, L., Sobczak, A., Fik, M., Knysak, J., Zaciera, M., Kurek, J., and Goniewicz, M. L. (2014). Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage. Nicotine Tob. Res., 16(10):1319–1326. doi:10.1093/ntr/ntu078
- MacFarland, H. N. (1983). Designs and Operational Characteristics of Inhalation Exposure Equipment–A Review. Fundam. Appl. Toxicol., 3(6):603–613. doi:10.1093/toxsci/3.6.603
- Marple, V. A., and Rubow, K. L. (1980). Aerosol Generation Concepts and Parameters, in Generation of Aerosols and Facilities for Exposure Experiments, K. Willeke, ed, Ann Arbor Science Publishers, Ann Arbor, MI, p. 22.
- Menache, M. G., Miller, F. J., and Raabe, O. G. (1995). Particle Inhalability Curves for Humans and Small Laboratory Animals. Ann. Occup. Hyg., 39(3):317–328. doi:10.1016/0003-4878(95)00002-v
- MHRA. (2015). New Rules about Tobacco, E-cigarettes and Smoking: 1 October 2015, Department of Health, Medicines and Healthcare Products Regulatory Agency, 9 July 2015. Available at https://www.gov.uk/government/publications/new-rules-about-tobacco-e-cigarettes-and-smoking-1-october-2015/new-rules-about-tobacco-e-cigarettes-and-smoking-1-october-2015. Accessed 9 September 2015.
- Nitzkin, J. L., Farsalinos, K., and Siegel, M. (2015). More on Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med., 372(16):1575. doi:10.1056/NEJMc1502242#SA1
- Ohta, K., Uchiyama, S., Inaba, Y., Nakagome, H., and Kunugita, N. (2011). Determination of Carbonyl Compounds Generated from the Electronic Cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine. Bunseki Kagaku, 60:791–797.
- Oldham, M. J., Phalen, R. F., and Budimen, T. (2009). Comparison of Predicted and Experimentally Measured Aerosol Deposition Efficiency in Balb/c Mice in a New Nose-Only Exposure System. Aerosol Sci. Technol., 43:970–977. doi:10.1080/02786820903082029
- Phalen, R. F. (1997). Inhalation Exposure Methods, in Methods in Inhalation Toxicology, R. F. Phalen, ed, CRC Press, Boca Raton, FL, pp. 137–138.
- Phalen, R. F. (2009). Methods for Exposing Subjects, in Inhalation Studies: Foundations and Techniques. Informa Healthcare, New York, pp. 109–134.
- Raabe, O. G., Al-Bagati, M. A., Teague, S. V., and Rasolt, A. (1988). Regional Deposition of Inhaled Monodisperse Coarse and Fine Aerosol Particles in Small Laboratory Animals. Ann. Occup. Hyg., 32(Suppl 1):53–63. doi:10.1093/annhyg/32.inhaled_particles_VI.53
- Rusch, G. M., Clary, J. J., Rinehart, W. E., and Bolte, H. F. (1983). A 26-Week Inhalation Toxicity Study with Formaldehyde in the Monkey, Rat, and Hamster. Toxicol. Appl. Pharmacol., 68(3):329–343. doi:10.1016/0041-008X(83)90276-4
- Tillery, M. I., Wood, G. O., and Ettinger, H. J. (1976). Generation and Characterization of Aerosols and Vapors for Inhalation Experiments. Environ. Health Perspect., 16:25–40. doi:10.1289/ehp.761625
- Uchiyama, S., Ohta, K., Inaba, Y., and Kunugita, N. (2013). Determination of Carbonyl Compounds Generated from the E-Cigarette Using Coupled Silica Cartridges Impregnated with Hydroquinone and 2,4-Dinitrophenylhydrazine, Followed by High-Performance Liquid Chromatography. Anal. Sci., 29(12):1219–1222. doi:10.2116/analsci.29.1219
- Werley, M. S., Kirkpatrick, D. J., Oldham, M. J., Jerome, A. M., Langston, T. B., Lilly, P. D., Smith, D. C., and Mckinney Jr., W. J. (2015). Toxicological Assessment of a Prototype E-Cigarette Device and Three Flavor Formulations: A 90-Day Inhalation Study. Inhal. Toxicol., 28(1):22–38. doi: 10.3109/08958378.2015.1130758
- Werley, M. S., McDonald, P., Lilly, P., Kirkpatrick, D., Wallery, J., Byron, P., and Venitz, J. (2011). Non-Clinical Safety and Pharmacokinetic Evaluations of Propylene Glycol Aerosol in Sprague-Dawley Rats and Beagle Dogs. Toxicology, 287(1–3):76–90. doi:10.1016/j.tox.2011.05.015
- Williams, M., Ghai, S., and Talbot, P. (2015). Disposable Electronic Cigarettes and Electronic Hookahs: Evaluation of Performance. Nicotine Tob. Res., 17(2):201–208. doi:10.1093/ntr/ntu118
- Williams, M., and Talbot, P. (2011). Variability among Electronic Cigarettes in the Pressure Drop, Airflow Rate, and Aerosol Production. Nicotine Tob. Res., 13(12):1276–1283. doi:10.1093/ntr/ntr164
- Woutersen, R. A., Appelman, L. M., Wilmer, J. W., Falke, H. E., and Feron, V. J. (1987). Subchronic (13-Week) Inhalation Toxicity Study of Formaldehyde in Rats. J. Appl. Toxicol., 7(1):43–49. doi:10.1002/jat.2550070108
- Yeh, H. C., Newton, G. J., Barr, E. B., Carpenter, R. L., and Hobbs, C. H. (1986). Studies of the Temporal and Spatial Distribution of Aerosols in Multi-Tiered Inhalation Exposure Chambers. Am. Ind. Hyg. Assoc. J., 47(9):540–545. doi:10.1080/15298668691390197
