ABSTRACT
The aim of this study was to provide a description and characterize the performance of a volatility hygroscopicity tandem differential mobility analyzer (VH-TDMA) by calibration measurements. In our investigations, we used the two calibration standards most often used by the TDMA community: ammonium sulfate ((NH4)2SO4) and sodium chloride (NaCl) particles. The hygroscopic growth factors, volatility factors, and the hygroscopic growth factors of the nonvolatile particle core were measured in different relative humidity and thermal denuder temperature conditions. The measured hygroscopic growth and deliquescence relative humidities for ammonium sulfate and sodium chloride particles are in line with theory and results from previous measurements, as are the measurements for volatility and hygroscopicity of the particle core. We summarize the measurement campaigns, where the VH-TDMA has been deployed, and show the instrument is capable of measuring high quality data of atmospheric particle properties in various environments, in the laboratory and in the field.
Copyright © 2017 American Association for Aerosol Research
EDITOR:
Introduction
The size, shape, concentration, and chemical composition of the particles are the most important parameters while studying the effect of aerosols to health and climate. The size of the aerosol particles depend on the relative humidity (RH) of the surrounding air (Swietlicki et al. Citation2008; Birmili et al. Citation2009). This dependence is called the hygroscopicity of the particle, which is a function of the size and the chemical composition of the particle. For soluble particles, RH also dictates, whether the particles are in solid or liquid phase (Pajunoja et al. Citation2015). Some particles, such as black carbon and fresh organic aerosol particles are hydrophobic, and the RH below supersaturation has little or no effect on their size (Weingartner et al. Citation1997; Duplissy et al. Citation2011). Particles consisting of water soluble salts are highly hygroscopic and they grow rapidly when RH approaches supersaturated conditions (Tang and Munkelwitz Citation1994; Tang et al. Citation1997). To estimate the effect of aerosol population to health and climate, one has to know the behavior of the aerosol population in varying atmospheric RH conditions (Cappa et al. Citation2012).
In the atmosphere, volatile compounds mainly exist in the gas phase, while compounds with low volatility, which are important for new particle formation and growth of nanoparticles (Kulmala et al. Citation1998; Riipinen et al. Citation2011; Ehn et al. Citation2014), readily nucleate and condense into particle phase. Semi-volatile compounds are detected both in the gas and particle phases (Donahue et al. Citation2011). Recent studies show that a large fraction of organic compounds found in the aerosols are semi-volatile under atmospheric conditions (Robinson et al. Citation2007). Whether these compounds condense on or evaporate from the particle phase is determined by their volatility. Hence, volatility is an important parameter when it comes to gas-particle partitioning of semi-volatile and low volatile aerosol compounds. Therefore, it is necessary to examine the volatility properties of aerosols for the estimation of their atmospheric lifetimes (Huffman et al. Citation2009), and understand how these semi-volatile and low-volatile compounds affect the formation of new particles. Atmospheric aerosols consist of a large number of different organic and inorganic compounds with different volatilities (Philippin et al. Citation2003; Burtscher et al. Citation2001). By studying hygroscopic properties of aerosols after heating, or the non-volatile fraction of the aerosol at a given temperature, one can estimate aerosol composition and hygroscopic properties for both the evaporated and remaining fraction (Häkkinen et al. Citation2012; Johnson et al. Citation2005).
H-TDMA and V-TDMA (hygroscopicity tandem differential mobility analyzer and volatility tandem differential mobility analyzer, respectively) systems have been used widely, and here they are combined into one instrument. The VH-TDMA (volatility hygroscopicity tandem differential mobility analyzer) (Liu et al. Citation1978; McMurry and Stolzenburg Citation1989; Johnson et al. Citation2004; Villani et al. Citation2008) is an instrument for investigating the volatility and hygroscopicity of aerosol particles, and the hygroscopicity of the particle core, i.e., what is left of the particle after the volatilization. The VH-TDMA is capable of measuring volatility of particles down to 20 nm in diameter, and hygroscopicity of particles down to 10 nm. An instrument capable of measuring the particle volatility and separately quantify the hygroscopicity of volatile and nonvolatile material is a useful tool to estimate the particle chemical composition, especially below the aerosol mass spectrometer (AMS) measuring range (<40 nm). Also, whether or not the aerosol is externally mixed in terms of volatility and hygroscopicity can be detected using the VH-TDMA technique. Our instrument has a simplified, robust design, and improved time resolution compared to the previous designs by Johnson et al. (Citation2004) and Villani et al. (Citation2008). With a slight change in the configuration, it can also double as a SMPS to measure the ambient aerosol number size distribution. In this study we characterize the performance of a VH-TDMA instrument by calibration measurements and verify its usability for ambient measurements. We also summarize briefly the outcomes of the VHTDMA field studies.
Theory
In our instrument, particles are conditioned by vaporizing or humidifying them, or both. The hygroscopic growth of the particles is governed by saturation ratio S. An aqueous solution droplet is in equilibrium with the surrounding water vapor when[1] where aw is the water activity of the salt solution, σ is the surface tension of the droplet, vw is the partial molar volume of water in the solution, R is the gas constant, T is temperature and d is the diameter of the spherical droplet. The concentration dependent values for aw, σ and vw are calculated using the polynomial fits to the experimental data for (NH4)2SO4 and NaCl, and they are presented in Table S1. The growth factor for the particle is
[2] where Ddry and Dcond are the electrical mobility diameters of the dry and conditioned particles, respectively. Three types of growth factors with different conditionings are measured in this study. The conditioning for the hygroscopic growth factor (HGF) is humidifying, the conditioning for the volatility factor (VF) is volatilization, and for the hygroscopic growth factor of the particle core (HGFC), the conditioning is volatilization followed by humidifying. For soluble particles the hygroscopic GF can be expressed as
[3] where ρs is the density of the salt, ρsol is the density of the solution droplet, m is the molality of the solution and Ms is the molar weight of the salt (Swietlicki et al. Citation1999). For nonspherical particles it is reasonable to use volume equivalent diameter instead of the mobility diameter, especially when measuring the hygroscopicity of cubical NaCl particles (Krämer et al. Citation2000).
Instrument description
The main components of the VH-TDMA are the custom made Nafion dryer containing five 12 inch Permapure tubes, aerosol neutralizer, three Vienna-type differential mobility analyzers (DMAs; Winkmayr et al. Citation1991); a humidifier constructed of a short Gore-Tex tube in a heated water bath, a thermal denuder (TD), and two CPCs for particle counting. The first DMA is for selecting a monodisperse fraction of the sample aerosol and the following two DMAs operated in parallel are to measure the effect of conditioning on the aerosol.
The instrument was constructed keeping in mind the special requirements for measuring small particles (Hämeri et al. Citation2000). Diffusion transports small particles towards the walls by Brownian motion. The diffusion losses for particles below the diameter of 20 nm are especially substantial, so the distance the particles travel inside the instrument is minimized. Charged particles can also be lost by electrostatic forces. When deposited on the surface of non-conductive tubing, the charged particles form an electric field, which forces other charged particles towards the walls. To prevent this from happening, the aerosol sample line was made of conductive material, either stainless steel, copper or conductive silicone tubing. The Nafion dryer was specially designed for low pressure difference over the dryer and for minimal diffusion losses. Also the humidifier was constructed to minimize particle losses: the Gore-Tex tube through which the particles travel is only 2.5 cm long.
A detailed flow diagram is presented in . The sample is drawn into the instrument at a rate of 2.2 l min−1. It is dried below the RH of 20% with a Nafion dryer. A 0.2 l min−1 purge flow for the dryer is separated from the sample. The drying is driven by the pressure and RH differences between the inside and outside of the nafion tube. The sample is then neutralized (charged) with a radioactive 14C aerosol neutralizer. Before the size selection with DMA-1, the temperature and RH are measured with a temperature and humidity sensor (Rotronic HC2-CO4). After DMA-1, the sample either goes straight to the second set of DMAs, or passes through TD. The path of the aerosol is controlled by a 3-way solenoid valve. The sample flow is then separated in two equal flows, a and b. The size distribution of aerosol a is measured with DMA-2a and CPC (TSI 3772). The size distribution of aerosol b is measured with DMA-2b and CPC (TSI 3010) after conditioning it to a desired RH with the humidifier. The TSI 3772 has a water removal feature, so it is used for particle detection of the humidified aerosol. The humidifier can be bypassed with a 3-way valve for dry calibration. The temperature and the RH of the flows a and b are monitored with Rotronic humidity sensors before entering the second set of DMAs.
Figure 1. A detailed flow diagram of the VH-TDMA. Optional configuration for SMPS size distribution measurement marked in gray (red).
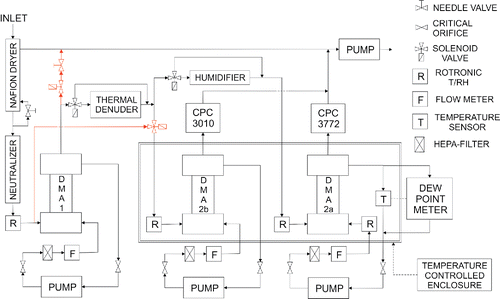
The configuration used in this study uses a short DMA-1 and medium DMAs-2a and b. All of the DMAs are operated with a closed loop sheath flow system. The sheath air flows for the DMAs are 20 l min−1 for DMA-1, 10 l min−1 for DMA-2a and 12 l min−1 for DMA-2b. Lower sheath air flow requires lower voltages for particle classification, and thus, the DMA-2a sheath air flow is slightly lower than the flow of the DMA-2b because of the possible arcing in the DMA at high voltage and high humidity. The flow rates of the sheath air flows are constantly monitored with a TSI 4000 Series mass flow meter for DMA-1 and a TSI 4100 Series mass flow meters for DMAs-2 a and b. The temperature, RH and the dew point of the sheath flow of DMA-2a are monitored with an Edge Tech Dew Master dew point meter and a Rotronic sensor for reference.
We also present an optional configuration marked in red in , which allows a SMPS size distribution measurement. A 3-way solenoid valve bypasses DMA-1, and DMA-2b is used to classify the particles from 15 nm to 500 nm. Another solenoid valve and a needle valve ensure a constant sample flow through DMA-1.
The hygroscopic growth factor (HGF, measured at RH of 90%, unless stated otherwise) calibration measurements were done by atomizing the solutions of ammonium sulfate ((NH4)2SO4, 99.999% purity) and sodium chloride (NaCl, 99.99% purity), both widely used standards within the H-TDMA community. Synthetic air 5.0 was used as carrier gas to minimize the possible contamination. The performance of a H-TDMA is usually verified with two characteristic values for the salt under the study: the growth factor at RH of 90% and a deliquescence RH (DRH), or the RH, in which the particle undergoes a phase transition from solid particle to liquid droplet. We measured the hygroscopic growth factors of 20 nm, 50 nm, 100 nm, and 145 nm (NH4)2SO4 and NaCl particles in several RHs. The results of our experiments compared to the theory are presented in and for (NH4)2SO4, and and for NaCl. The comparisons to the results by other groups are also presented in and . Both constant and size dependent shape correction factors were used with the NaCl HGF data (De Carlo et al. Citation2004; Biskos et al. Citation2006a). Shape correction for (NH4)2SO4 particles was not used, as the particles are considered almost spherical.
Figure 2. The Hygroscopic Growth Factors (HGF) of 20 nm, 50 nm, 100 nm, and 145 nm (NH4)2SO4 particles in different relative humidities (RH). The dots are experimental results and the continuous lines represent the theory. The sudden jump in the GFs near RH 80% denotes the Deliquescence RHs (DRH).
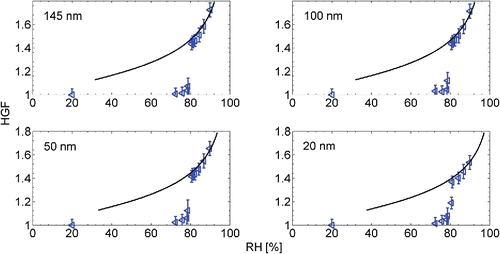
Table 1. The measured and theoretical growth factors, and measured deliquescence RH of ammonium sulfate particles with comparison to the results by other groups (1Gysel et al. Citation2002; 2Hämeri et al. Citation2000).
Figure 3. The Hygroscopic Growth Factors (HGF) of 20 nm, 50 nm, 100 nm, and 145 nm NaCl particles in different relative humidities (RH). The triangles (blue) and circles (red) are the experimental results with constant and size dependent shape factor corrections, respectively. The stars (green) take into account the correction for shape caused by volatile impurities and the continuous black lines represent the theory.
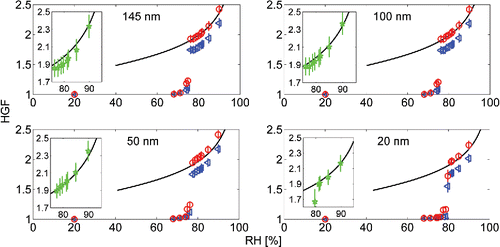
Table 2. The measured and theoretical growth factors, and measured deliquescence RH of sodium chloride particles with comparison to the results by other groups. (1Gysel et al. Citation2002; 2Hämeri et al. Citation2000; 3Biskos et al. Citation2006c).
Table 3. The growth factors for cubical thermally denuded NaCl particles with their size dependent shape correction factors and effective diameters and shape correction factors for particles without denuding.
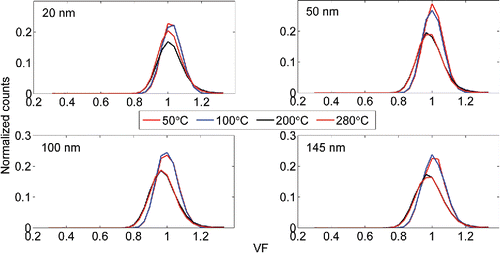
The volatility (or volatility factor, VF) of (NH4)2SO4 and NaCl particles were also examined with several TD temperatures ranging from 50°C to 280°C. represent the growth factor distributions for (NH4)2SO4 and NaCl particles gone through TD. As can be seen from , the ammonium sulfate particles are starting to volatilize when the TD temperature reaches 150°C. The smaller particles volatilize at lower temperatures. At the volatilization temperature range (150°C–180°C) the growth factor distributions are bimodal. The bimodality is most probably caused by radial temperature gradient in the TD. The temperature is highest on the surface of the heated tube, and the particle residence time in the TD is not long enough for the particles to volatilize evenly. At 180°C the growth factor distributions have evolved to unimodal, peaking at GF = 0.45. An interesting observation is that the mode near GF = 0.45 is larger in diameter and has more particles at 180°C than at 170°C. We suspect that this might be caused by nucleation or vapors re-condensing on residual particles. At the TD temperature of 200°C all the particles have been decomposed or dropped out of the range. The plateauing of the distribution for 20 nm particles below VF = 0.5 is caused by DMA2a's high voltage source reaching its lower limit. The NaCl particles are non-volatile at temperatures below 280°C. However, some shrinking due to evaporation of volatile impurities (e.g., water) can be seen in at temperatures higher than 200°C. If NaCl was volatile, the shrinking would be most prominent in 20 nm particles, which is not the case here. The slight decrease in particle count with higher temperatures is due to increasing diffusional losses.
Figure 4. The Volatility Factor (VF) distributions of (NH4)2SO4 particles with different thermal denuder temperatures.
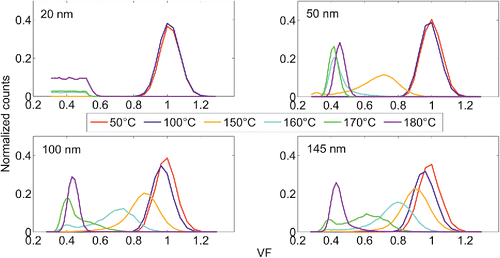
Figure 5. The Volatility Factor (VF) distributions of NaCl particles with different thermal denuder temperatures.
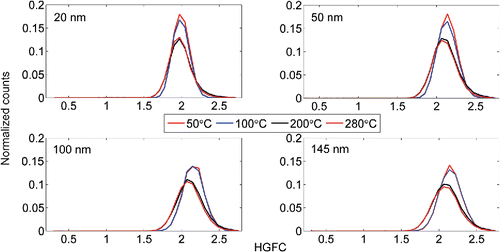
The hygroscopicity of the particle core (HGFC, measured at RH of 90%, unless stated otherwise), or what is left from the particle after passing through the thermal denuder, was also measured. The HGFC distributions for (NH4)2SO4 and NaCl are presented in and , respectively. The results duplicate the VF distributions shown in , if multiplied with the corresponding HGFs from . In can be seen, that some residual particles are left, even with high thermal denuder temperatures. These may be due to the impurities in the water supply and in the atomizer, even though special care had been taken to prepare as pure sample as possible.
Figure 6. The Hygroscopic Growth Factor (HGFC) distributions of the core with different thermal denuder temperatures for (NH4)2SO4 particles.
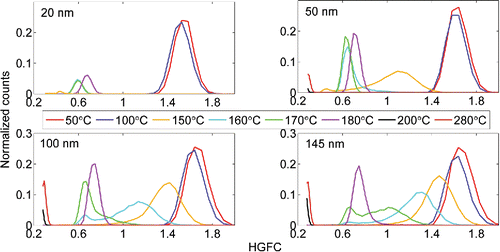
Figure 7. The Hygroscopic Growth Factor (HGFC) distributions of the core with different thermal denuder temperatures for NaCl particles.
The slight shrinking of the NaCl particles raises a question about the shape correction factor. As we can see in and , using a constant shape and size dependent shape correction factors give slightly lower and higher HGFs than the theory, respectively. We can calculate the volume equivalent diameter of the thermally denuded particle (Dve,V)s by multiplying Ddry with the corresponding average VF at 280°C, and applying size dependent correction of a cubical particle for the resulting diameter. If we multiply the corresponding HGFC by the ratio of denuded particle diameter and volume equivalent diameter (DV/Dve,V), we get a HGF (HGFV) very close to the theoretical value. This suggests that the particles, when sampled through the TD at 280°C, are pure cubical NaCl crystals. The effective particle diameter (Deff) can be calculated from the ratio of the original measured HGFM and HGFV: Deff = HGFM/HGFV*DDry. From Deff we calculate a new effective shape correction factor χeff. This shape correction factor, when applied to DDry, results in Deff, and it takes into account both the impurities and the shape deviant from cubical. It can be then used throughout the whole RH range above the deliquescence RH to correct HGFM for impurities and shape. Effective particle diameters, corresponding shape factors and corrected HGFs are presented in , and the shape correction is applied to the measured HGFs in .
The same reasoning was applied to (NH4)2SO4 particles. In we can see slight decrease in particle size at 100°C. We believe that this is also caused by volatile impurities evaporating from the crystal, which means that the shape of the particle is most likely more spherical than a pure (NH4)2SO4 particle. If we assume the particle to be pure (NH4)2SO4 at 100°C and calculate the HGF from corresponding VFs and HGFCs, and use the shape correction factor of 1.02 as suggested by Biskos et al. (Citation2006b), we get the same results as without using any shape correction at all.
To highlight the versatility of the instrument, we generated two component NaCl/(NH4)2SO4 particles. We atomized NaCl solution and size selected 100 nm NaCl particles with a DMA after drying them out with silica gel diffusion dryer. We used evaporation/condensation method to coat these particles with (NH4)2SO4 by running them through a tube furnace (T = 265°C) with a ceramic container filled with (NH4)2SO4 crystals. The VF and HGFC at RH of 90% of 130 nm particles were measured, and the results are presented in . As expected, the particle size decreased and the hygroscopicity of the remaining particle increased as the TD temperature increased, as the less hygroscopic (NH4)2SO4 gradually volatilized from the surface of the particles.
Figure 8. The Volatility Factors (VF) and the Hygroscopic Growth Factors of the particle core (HGFC) for a (NH4)2SO4-coated NaCl particles with a comparison of measured and calculated Hygroscopic Growth Factors (HGF = HGFC/VF) of the volatilized particles at different temperatures.
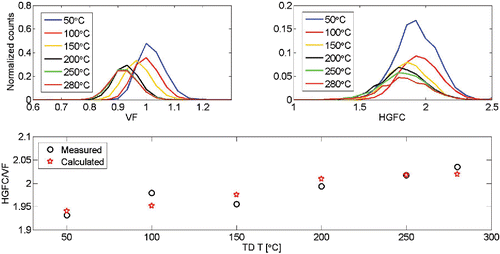
Furthermore, from the results we can quantify several qualities: the quantity of NaCl and (NH4)2SO4 in the particles and the HGFs for the volatilized and residual compounds. At TD temperature of 50°C we didn't observe any shrinking and we measured a HGF of 1.93. If we assume cubical NaCl particle with mobility diameter of 100 nm, and that the 130 nm NaCl/(NH4)2SO4 particle is spherical, we will result in a particle containing 40% NaCl and 60% (NH4)2SO4 by volume. The HGF of such particle using the measured HGFs from for (NH4)2SO4 and NaCl would be 1.94. Similarly, if we assume that the NaCl core will remain intact over the whole TD temperature range and the coating is (NH4)2SO4, we can calculate the particle composition and corresponding HGF at each TD temperature. The results are shown in and plotted in . Also listed in are the HGFs for the coating and for the volatilized material. The HGFs for the coating were calculated from the volume fractions of the NaCl core and the coating. The HGFs for the volatilized material was calculated from the volume fractions of the core, the coating and the evaporated volume.
Table 4. The volume fractions of NaCl and (NH4)2SO4, the measured and calculated HGFs for the residual particle, and calculated HGFs for the coating and volatilized material at different TD temperatures.
An interesting observation was that the (NH4)2SO4 coating did not volatilize completely even at TD temperature of 280°C. The explanation for this behavior may be that in between the NaCl core and (NH4)2SO4 coating there is a transition layer containing Na2SO4 and NH4Cl. The formation of these compounds is energetically more favorable (Clegg et al. Citation1998) and they are less volatile than (NH4)2SO4 with melting points above 280°C. The presence of these compounds does not make a difference in HGF compared to pure NaCl/(NH4)2SO4 mixture, since these salts are also water soluble and the ion balance remains the same in the formed droplet. The upper limit for the thickness of the Na2SO4/NH4Cl layer calculated from the difference between the diameter of the NaCl core and the diameter of the residual particle at TD temperature of 280°C was 11 nm. A considerable source of error in these calculations is that we cannot be certain about the particle shape.
Field deployments
The VH-TDMA has been deployed in several successful campaigns in different environments around the world, both in a laboratory and in the field (Massoli et al. Citation2010; Bates et al. Citation2012; Cappa et al. Citation2012; Hong et al. Citation2014; Pajunoja et al. Citation2015). As an example in we present the hygroscipicity and volatility properties of 145 nm ambient marine aerosol particles measured on board R/V Atlantis on the coast of California in 2010 during the CalNex campaign (Bates et al. Citation2012). The time series for HGF, VF and HGFC distributions show the probability density, which is a normalized spread of the GF distribution with no indication for the actual concentration of the particles. To calculate the probability density functions we used an inversion program by Gysel et al. (Citation2009). The HGF time series shows a narrow almost unimodal distribution throughout the data set with the mode around HFG = 1.5. The occasional multimodal distributions show a little bit of external mixing, with particles with HGFs higher and/or lower than 1.5. From the VF and HGF time series we can see that the vast majority of aerosol particles are volatile at TD temperatures above 200°C.
Figure 9. The time series of Hygroscopic Growth Factors (HGF), Volatility Factors (VF) and Hygroscopic Growth Factors of the particle Core (HGFC) for 145 nm ambient marine aerosol particles and normalized average VF and HGFC distributions at different temperature ranges.
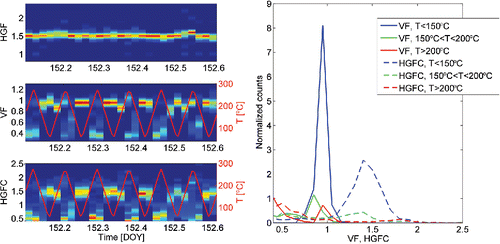
Also in are presented the normalized average GF distributions at different temperature ranges. The normalization was done by scaling the average probability density functions by NTD/N, where NTD is the average for total particles measured using TD at given temperature range, and N is the corresponding average of total particles measured without TD. This, normalization enables the comparison between the average distributions when the ambient particle size distribution in not constant over the measuring period.
From we can make following observations:
The particles are mostly internally mixed with respect to hygroscopic properties with an average HGF of 1.5.
The majority of particles are mostly non-volatile below TD temperatures of 150°C, and the residual particles are hygroscopic with an average HGF of 1.5.
The majority of particles are beginning to evaporate above 150°C at least some of the residual particles are hygroscopic with HGF close to 1.5.
Above 200°C most of the particles are evaporated, but some residual particles are still left, some with high hygroscopicity.
Now, if we compare these to the calibration measurements presented in , we can conclude that these observations are strikingly similar to the properties of ammonium sulfate. The lower hygroscopicity is most likely due to internally mixed organics, which are at least partly volatile at higher TD temperatures (Ehn et al. Citation2007). The nonvolatile mode in VF distribution at TD > 200°C close to 1 is probably made of two components: black carbon and sea salt. The presence of sea salt is furthermore backed up by the HGFC distribution, in which we can see residual particles with very high hygroscopicity. The small residual mode at 1 points towards black carbon, which is hydrophobic. Our interpretation for this data set is that the aerosol is mostly composed of ammonium sulfate internally mixed with a small fraction of organics. There is also a hint of small externally mixed black carbon and sea salt fractions. This interpretation is confirmed by the Aerosol Mass Spectrometer (AMS) data from the same aerosol: high sulfate loading with a small fraction of organics. Black carbon and sea salt cannot be properly detected by an AMS due to their non-volatile nature.
Maintaining stable temperature conditions is essential for keeping the VH-TDMA at constant RH. Sudden changes in the room temperature, an A/C cooling cycle for example, affect the RH significantly, which must be taken into account while deploying the VH-TDMA. The semi volatile organic compounds can contaminate the instrument and condense onto the particles, thus changing the hygroscopic and volatility properties. This was only observed after a high concentration of laboratory generated aerosol was measured, and it rarely lead into inaccuracy in the ambient measurements.
Discussion
Johnson et al. (Citation2004) and Villani et al. (Citation2008) have previously provided descriptions of VH-TDMAs similar to ours. Both use a separate humidification for the sheath flow of their humidified DMAs. We have a simplified approach, and only humidify the sample. The only use for a separate humidifier in the sheath air loop would be for reaching the desired RH faster. Our VH-TDMA is designed to be used at constant DMA-2a RH, which makes the sheath air humidifier unnecessary. It takes less than one hour to reach a stable RH of 90% from dry (RH < 20%) conditions. We have aimed for simplicity and robustness throughout the whole design, and combined the most practical solutions in our VH-TDMA.
Villani et al. uses only one SMPS system after initial size selection. The obvious improvement to their design is that we use two. For a complete measurement (unperturbed GF, HGF, VF, and HGFC distribution measurements) of one diameter it would take at least twice as long, i.e., our design improves the time resolution by a factor of two or more. Unperturbed GF measurements are important when measuring semi-volatile particles, because they may shrink after (and during) the initial size classification (Khlystov Citation2014). Unperturbed GF is also a good indicator for instrument contamination, as particles grow when contaminants condense on particles.
Johnson et al., like us, uses two SMPS systems after initial size classification, but their instrument lacks the possibility to bypass the thermal denuder. There are two reasons why the bypass feature is an improvement: (Equation1[1] ) Better time resolution for HGF and unperturbed GF measurements, as it will take time to cool off the TD to ambient temperature and heat it up to volatilization temperature again. (Equation2
[2] ) If the possible particle evaporation (or instrument contamination) is deemed to have only a minor effect, DMA-2b can be used in a SMPS mode to measure the ambient size distribution by bypassing DMA-1. This feature makes the VH-TDMA a rather versatile stand-alone unit. We present this optional configuration in , and demonstrate the feature with an example of simultaneous SMPS and HGF scans in Figure S1, and compare the measured size distribution with the one measured at the nearby SMEAR III station (Järvi et al. Citation2009).
Conclusions
The verification of the VH-TDMA performance was successful. The hygroscopic GFs of ammonium sulfate and sodium chloride agreed very well with theory and the results of other groups. The DRHs were close to previously measured values. The decomposing temperature for ammonium sulfate was as predicted, and the smaller particles decomposed more readily than the bigger ones, as expected. Sodium chloride proved to be non-volatile in the TD temperature region used in this study, but some shrinking due to evaporation of volatile impurities was observed with TD temperatures higher than 100°C. Comparing , one can see that the VF and the HGFC of NaCl particles shrink identically (i.e., HGFC/VF remains constant) as a function of temperature. This suggests that the volatile compound has a HGF of 1.0, which makes water the most probable candidate.
We conclude that the VH-TDMA can be confidently used to measure the hygroscopicity and volatility properties of particles from 20 nm to 145 nm with RH up to 90%. The instrument is easily converted for measuring even smaller particles by changing the DMAs 2a and 2b to the short DMA model. An optional configuration also allows for SMPS size distribution measurement simultaneously with the HGF scan. The sample humidifying coupled with closed loop DMA sheath flow arrangement proved to provide extremely stable RH conditions, given that temperature of the space housing the VH-TDMA was constant. With stable temperature conditions very accurate results can be obtained. In field conditions extra consideration is needed to provide stable enough temperature conditions to get the best results.
UAEM_1255712_Supplementary_File.zip
Download Zip (109.5 KB)References
- Bates, T. S., Quinn, P. K., Frossard, A. A., Russell, L. M., Hakala, J., Petäjä, T., Kulmala, M., Covert, D. S., Cappa, C. D., Li, S.-M., Hayden, K. L., Nuaaman, I., McLaren, R., Massoli, P., Canagaratna, M. R., Onasch, T. B., Sueper, D., Worsnop, D. R., and Keene, W. C. (2012). Measurements of Ocean Derived Aerosol off the Coast of California. J. Geophys. Res., 117: doi:10.1029/2012JD017588.
- Birmili, W., Schwirn, K., Nowak, A., Petäjä, T., Joutsensaari, J., Rose, D., Wiedensohler, A., Hämeri, K., Aalto, P., Kulmala, M., and Boy, M. (2009). Measurements of Humidified Particle Number Size Distributions in a Finnish Boreal Forest: Derivation of Hygroscopic Particle Growth Factors. Boreal Env. Res., 14:458–480.
- Biskos, G., Russell, L. M., Buseck, P. R., and Martin, S. T. (2006a). Nanosize Effect on the Hygroscopic Growth Factor of Aerosol Particles. Geophys. Res. Lett., 33:L07801. doi:10.1029/2005GL025199.
- Biskos, G., Paulsen, D., Russell, L. M., Buseck, P. R., and Martin, S. T. (2006b). Prompt Deliquescence and Efflorescence of Aerosol Nanoparticles. Atmos. Chem. Phys., 6:4633–4642.
- Biskos, G., Manlinowski, A., RussellL. M., Buseck, P. R., and Martin, S. T. (2006c). Nanosize Effect on the Deliquescence and the Efflorescence of Sodium Chloride Particles. Aerosol Sci. Technol., 40:97–106.
- Burtscher, H., Baltensperger, U., Bukowiecki, N., Cohn, P., Huglin, C., Mohr, M., Matter, U., Nyeki, S., Scmatloch, V., Streit, N., and Weingartner, E. (2001). Separation of Volatile and Non-Volatile Aerosol Fractions by Thermodesorption: Instrumental Development and Applications. J. Aerosol Sci., 32:427–442.
- Cappa, C. D., Onasch, T. B., Massoli, P., Worsnop, D. R., Bates, T. S., Cross, E. S., Davidovits, P., Hakala, J., Hayden, K. L., Jobson, B. T., Kolesar, K. R., Lack, D. A., Lerner, B. M., Li, S.-M., Mellon, D., Nuaamaan, I., Olfert, J. S., Petäjä, T., Quinn, P. K., Song, C., Subramanian, R., Williams, E. J., and Zaveri, R. A. (2012). Radiative Absorption Enhancement due to the Mixing State of Atmospheric Black Carbon. Science, 337:1078–1081.
- Clegg, S. L., Brimblecombe, P., and Wexler, A. S. (1998). Thermodynamic Model of the System H+−NH4+−Na+−SO42−−NO3−−Cl−−H2O at 298.15 K. J. Phys. Chem. A., 102:2155–2171.
- De Carlo, P. F., Slowik, J. E., Worsnop, D. R., Davidovits, P., and Jimenez, J. L. (2004). Particle Morphology and Density Characterization by Combined Mobility and Aerodynamic Diameter Measurements. Part 1: Theory. Aerosol Sci. Technol., 38:1185–1205.
- Donahue, N. M., Epstein, S. A., Pandis, S. N., and Robinson, A. L. (2011). A Two Dimensional Volatility Basis Set: 1. Organic Aerosol Mixing Thermodynamics. Atmos. Chem. Phys., 11:3303–3318.
- Duplissy, J., DeCarlo, P. F., Dommen, J., Alfarra, M. R., Metzger, A., Barmpadimos, I., Prevot, A. S. H., Weingartner, E., Tritscher, T., Gysel, M., Aiken, A. C., Jimenez, J. L., Canagaratna, M. R., Worsnop, D. R., Collins, D. R., Tomlinson, J., and Baltensperger, U. (2011). Relating Hygroscopicity and Composition of Organic Aerosol Particulate Matter. Atmos. Chem. Phys., 11:1155–1165.
- Ehn, M., Petäjä, T., Aufmhoff, H., Aalto, P., Hämeri, K., Arnold, F., Laaksonen, A., and Kulmala, M. (2007). Hygroscopic Properties of Ultrafine Aerosol Particles in the Boreal Forest: Diurnal Variability, Solubility and the Influence of Sulfuric Acid. Atmos. Chem. Phys., 7:211–222.
- Ehn, M., Thornton, J. A., Kleist, E., Sipilä, M., Junninen, H., Pullinen, I., Springer, M., Rubach, F., Tillmann, R., Lee, B. et al. (2014). A Large Source of Low-Volatility Secondary Organic Aerosol. Nature, 506:476–479.
- Gysel, M., McFiggans, G. B., and Coe, H. (2009). Inversion of Tandem Differential Mobility Analyser (TDMA) Measurements. J. Aerosol Sci., 40:134–151.
- Gysel, M., Weingartner, E., and Baltensberger, U. (2002). Hygroscopicity of Aerosol Particles at low Temperatures. 2. Theoretical and Experimental Hygroscopic Properties of Laboratory Generated Aerosols. Environ. Sci. Technol., 36:63–68.
- Hong, J., Häkkinen, S. A. K., Paramonov, M., Äijälä, M., Hakala, J., Nieminen, T., Mikkilä, J., Prisle, N., Kulmala, M., Riipinen, I., Bilde, M., Kerminen, V.-M., and Petäjä, T. (2014). Hygroscopicity, CCN and Volatility Properties of Submicron Atmospheric Aerosol in a Boreal Forest Environment During the Summer of 2010. Atmos. Chem. Phys., 14:4733–5748.
- Huffman, J. A., Docherty, K. S., Aiken, A. C., Cubison, M. J., Ulbrich, I. M., DeCarlo, P. F., Sueper, D., Jayne, J. T., Worsnop, D. R., Ziemann, P. J., and Jimenez, J. L. (2009). Chemically-Resolved Aerosol Volatility Measurements from Two Megacity Field Studies. Atmos. Chem. Phys., 9:7161–7182.
- Häkkinen, S. A. K., Äijälä, M., Lehtipalo, K., Junninen, H., Backman, J., Virkkula, A., Nieminen, T., Vestenius, M., Hakola, H., Ehn, M., Worsnop, D. R., Kulmala, M., Petäjä, T., and Riipinen, I. (2012). Long-Term Volatility Measurements of Submicron Atmospheric Aerosol in Hyytiälä, Finland. Atmos. Chem. Phys., 12:10771–10786.
- Hämeri, K., Väkevä, M., Hansson, H.-C., and Laaksonen, A. (2000). Hygroscopic Growth of Ultrafine Ammonium Sulphate Aerosol Measured with an Ultrafine Tandem Differential Mobility Analyser. J. Geophys. Res., 105:22231–22242.
- Hämeri, K., Väkevä, M., Aalto, P. P., Kulmala, M., Swietlicki, E., Zhou, J., Seidl, W., Becker, E., and O'Dowd, C. (2001). Hygroscopic and CCN Properties of Aerosol Particles in Boreal Forests. Tellus B, 53:359–379.
- Johnson, G. R., Ristovski, Z., and Morawska, L. (2004). Method for Measuring the Hygroscopic Behaviour of Lower Volatility Fractions in an Internally Mixed Aerosol. J. Aerosol. Sci., 35:443–445.
- Johnson, G. R., Ristovski, Z., D'Anna, B., and Morawska, L. (2005). Hygroscopic Behavior of Partially Volatilized Coastal Marine Aerosols Using the Volatilization and Humidification Tandem Differential Mobility Analyzer Technique. J. Geophys. Res., 110:D20203.
- Järvi, L., Hannuniemi, H., Hussein, T., Junninen, H., Aalto, P. P., Hillamo, R., Mäkelä, T., Keronen, P., Siivola, E., Vesala, T., and Kulmala, M. (2009). The Urban Measurement Station SMEAR III: Continuous Monitoring of Air Pollution and Surface-Atmosphere Interactions in Helsinki, Finland. Boreal Environ. Res., 14:86–109.
- Khlystov, A. (2014). Effect of Aerosol Volatility on the Sizing Accuracy of Differential Mobility Analyzers. Aerosol Sci. Technol., 48:604–619.
- Krämer, L., Pöschl, U., and Niessner, R. (2000). Microstructural Rearrangement of Sodium Chloride Condensation Aerosol Particles on Interaction with Water Vapor. J. Aerosol Sci., 31:8200–8205.
- Kulmala, M., Toivonen, A., Mäkelä, J. M., and Laaksonen, A. (1998). Analysis of the Growth of Nucleation Mode Particles Observed in Boreal Forest. Tellus B, 50:449–462.
- Liu, B. Y. H., Pui, D. Y. H., Whitby, K. T., Kittelson, D. B., Kousaka, Y., and McKenzie, R. L. (1978). The Aerosol Mobility Cromatograph: A New Detector for Sulfuric Acid Aerosols. Atmos. Environ., 12:99–104.
- Massoli, P., LambeA. T., Ahern, A. T., Williams, L. R., Ehn, M., Mikkilä, J., Canagaratna, M. R., Brune, W. H., Onasch, T. B., Jayne, J. T., Petäjä, T., Kulmala, M., Laaksonen, A., Kolb, C. E., Davidovits, P., and Worsnop, D. R. (2010). Relationship Between Aerosol Oxidation Level and Hygroscopic Properties of Laboratory Generated Secondary Organic Aerosol (SOA) particles. Geophys. Res. Lett., 37:L24801.
- McMurry, P. H. and Stolzenburg, M. R. (1989). On the Sensitivity of Particle Size to Relative Humidity for Los Angeles Aerosols. Atmos. Environ., 23:4979–507.
- Pajunoja, A., Lambe, A. T., Hakala, J., Rastak, N., Cummings, M. J., Brogan, J. F., Hao, L., Paramonov, M., Hong, J., Prisle, N. L., Malila, J., Romakkaniemi, S., Lehtinen, K. E. J., Laaksonen, A., Kulmala, M., Massoli, P., Onasch, T. B., Donahue, N. M., Riipinen, I., Davidovits, P., Worsnop, D. R., Petäjä, T., and Virtanen, A. (2015). Adsorptive Uptake of Water by Semisolid Secondary Organic Aerosols. Geophys. Res. Lett., 42:3063–3068.
- Philippin, S., Wiedensohler, A., and Stratmann, F. (2003). Measurements of Non-Volatile Fractions of Pollution Aerosols with an Eight-Tube Volatility Differential Mobility Analyzer (VTDMA-8). J. Aerosol Sci., 35:185–203.
- Riipinen, I., Pierce, J. R., Yli-Juuti, T., Nieminen, T., Häkkinen, S., Ehn, M., Junninen, H., Lehti- palo, K., Petäjä, T., Slowik, J., Chang, R., Shantz, N. C., Abbatt, J., Leaitch, W. R., Kerminen, V.-M., Worsnop, D. R., Pandis, S. N., Donahue, N. M. and Kulmala, M. (2011). Organic Condensation: A Vital Link Connecting Aerosol Formation to Cloud Condensation Nuclei (CCN) Concentrations. Atmos. Chem. Phys., 11:3865–3878.
- Robinson, A. L., Donahue, N. M., Shrivastava, M. K., Weitkamp, E. A., Sage, A. M., Grieshop, A. P., Lane, T. E., Pierce, J. R., and Pandis, S. N. (2007). Rethinking Organic Aerosols: Semivolatile Emissions and Photochemical Aging. Science, 315:1259–1262.
- Swietlicki, E., Hansson, H.-C., Hämeri, K., Svenningsson, B., Massling, A., McFiggans, G., McMurry, P. H., Petäjä, T., Tunved, P., Gysel, M., Topping, D., Weingartner, E., Baltensberger, U., Rissler, J., Wiedensohler, A. and Kulmala, M. (2008). Hygroscopic Properties of Submicrometer Particles Measured with H-TDMA Instruments in Various Environments—A Review. Tellus B, 60:432–469.
- Swietlicki, E., Zhou, J., Berg, O. H., Martinsson, B. G., Frank, G., Cederfelt, S. I., Dusek, U., Berner, A., Birmili, W., Wiedensohler, A., Yuskiewicz, B., and Bower, K. N. (1999). A Closure Study of Sub-Micrometer Aerosol Particle Hygroscopic Behaviour. Atmos. Res., 50:205–240.
- Tang, I. N. and Munkelwitz, H. R. (1994). Water Activities, Densities, and Refractive Indices of Aqueous Sulfates and Sodium Nitrate Droplets of Atmospheric Importance. J. Geophys. Res., 99:18801–18808.
- Tang, I. N., Tridico, A. C., and Fung, K. H. (1997). Thermodynamic and Optical Properties of Sea Salt Aerosols. J. Geophys. Res., 102:23–269.
- Villani, P., Picard, D., Michaud, V., Laj, P., and Wiedensohler, A. (2008). Design and Validation of a Volatility Hygroscopic Tandem Differential Mobility Analyzer (VH-TDMA) to Characterize the Relationships Between the Thermal and Hygroscopic Properties of Atmospheric Aerosol Particles. Aerosol Sci. Technol., 42:729–41.
- Weingartner, E., Burtscher, H., and Baltensperger, U. (1997). Hygroscopic Properties of Carbon and Diesel Soot Particles. Atmos. Environ., 31:2311–2327.
- Winkmayr, W., Reischl, G. P., Linder, A.O., and Berner, A. (1991). A New Electromobility Spectrometer for the Measurement of Aerosol Size Distributions in the Size Range from 1 to 1000 nm. J. Aerosol Sci., 22:289–296.
