ABSTRACT
Reactive uptake by ammonium (NH4+) salts is one of the major pathways for the gas-to-particle partitioning of alkyl amines. Recent studies using particles of individual ammonium salts and mixtures with hydrophilic organics have revealed that the degree of amine uptake depends on the phase state of ammonium salts, the particulate water contents and particle viscosity. The role of hydrophobic organic compounds, another important category of particulate organics commonly detected in the ambient atmosphere, in amine uptake remains unknown. Here we report the uptake of dimethylamine (DMA) by ammonium sulfate (AS) particles coated with fresh or ozone-aged bulk oleic acid (OA) at 60%, 30%, and <5% relative humidities (RHs) using an electrodynamic balance coupled with Raman spectroscopy. OA and DMA were selected to represent hydrophobic organics and alkyl amines, respectively. Over 74% of the original NH4+ ions were displaced due to DMA uptake, except those conditioned at <5% RH. On the other hand, the fresh or aged bulk OA coating retarded DMA uptake by preventing the particle surface from effectively accommodating gaseous DMA molecules. Judging from the estimated DMA uptake coefficients, the retardation gradually intensified as the weight percentage of coating increased before leveling off, likely when the particle surface was completely covered by fresh or aged bulk OA. We propose that the accommodation of DMA on the particle coating is the rate-limiting step of DMA uptake. Intensive aging of the OA coating had little effect on the equilibrium particle-phase compositions but retarded DMA uptake.
© 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Reactive uptake by ammonium (NH4+) salts is one of the major pathways for the gas-to-particle partitioning of short-chain alkyl amines, which are important alkaline gases in addition to ammonia (NH3) in the atmosphere. The reaction mechanism of NH4+ displacement by alkyl aminium has been confirmed using a wide range of gas-phase amine concentrations (Lloyd et al. Citation2009; Bzdek et al. Citation2010; Chan and Chan Citation2012; Sauerwein and Chan Citation2016). The phase state of individual ammonium salts can influence the extent of amine uptake: ammonium salts in aqueous or amorphous solid form lead to higher amine uptake than those in crystalline solid form (Chan and Chan Citation2013). Dawson et al. (Citation2014) observed a similar dependency of particle phase on the amine uptake by aminium methanesulfonate particles. Note that crystalline ammonium salts can still irreversibly absorb alkyl amines due to the presence of surface adsorbed water or surface defects, albeit at lower rates (Qiu et al. Citation2011; Chu and Chan Citation2017).
Ambient aerosol particles are composed of both organic and inorganic compounds (Murphy et al. Citation2006). Organics can alter the phase state of inorganic salts (Marcolli and Krieger Citation2006), hygroscopic growth (Drozd et al. Citation2014) and heterogeneous chemistry between reactive gases and inorganic particles (Abbatt et al. Citation2012). We have recently examined the role of particle-phase sucrose, a surrogate for hydrophilic viscous organics, in dimethylamine (DMA) uptake by ammonium sulfate (AS) – sucrose mixed particles. We found that the uptake of DMA also relied heavily on the particulate water content and particle viscosity (Chu and Chan Citation2017).
Extending the above findings, we investigate the effects of particle-phase hydrophobic organics on the reactive uptake of DMA by AS particles. Among the hydrophobic organics, fatty acids exist in abundance in the atmosphere and are emitted from natural (Mochida et al. Citation2002; Tervahattu et al. Citation2005) and anthropogenic (Rogge et al. Citation1991) sources. Unlike the hydrophilic secondary organic materials formed via the oxidation of volatile organic compounds (Renbaum-Wolff et al. Citation2013), fatty acids are highly immiscible with the aqueous phase and tend to form a coating over the particle core (Mochida et al. Citation2002).
We select oleic acid (OA) as the surrogate for fatty acids because it is widely detected in the ambient atmosphere (Feng et al. Citation2006; Dreyfus and Johnston Citation2008). The carbon-carbon double bond in the OA molecular structure allows us to investigate the influence of the ozone oxidative aging of OA, which is one of the model systems for studying the aging of organic molecules (Zahardis and Petrucci Citation2007; Yatavelli and Thornton Citation2010), on DMA uptake. OA is also considered as a marker compound for cooking aerosols (Robinson et al. Citation2006). DMA is chosen to represent alkyl amines due to its abundance in the ambient atmosphere (Müller et al. Citation2009; Yao et al. Citation2016). In the following, we present the mass change of OA-coated AS particles during DMA uptake and the kinetics of DMA uptake at 60%, 30%, and <5% relative humidities (RHs). In parallel experiments, after coating, OA is aged via ozonolysis before the introduction of DMA vapor. Different OA loadings and ozone exposure levels are applied. Experiments at different RHs are conducted in order to study the role of fresh or aged OA with AS in aqueous or crystalline phase rather than the role of RH. The physical properties of the coating are not expected to change appreciably under the above RH settings. Lastly, we summarize the roles of surrogates for particulate hydrophilic and hydrophobic organics in DMA uptake by AS particles.
2. Experimental method
All chemicals were used as received without further purification. AS (≥99%, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in Milli-Q water (18.2 MΩ cm) to prepare an AS bulk solution at ∼30 wt%. A single AS droplet was generated from the AS bulk solution and stably levitated in the electrodynamic balance (EDB) by passing it through a copper-made induction plate and setting the AC and DC electrical fields appropriately. A dry nitrogen stream (∼180 cm3 min−1) was introduced into the EDB to trigger the crystallization of the AS particle. Then, OA (≥99%, Sigma-Aldrich) was introduced to the EDB by passing the nitrogen stream over a heated OA reservoir so that it condensed onto the crystalline AS particle surface. During the coating procedure, light scattering was observed along the laser path, similar to what has been reported by Chan et al. (Citation2006), indicating that small OA particles were produced upon heating and collided with the AS particle to form a coating.
For experiments where the OA coating was aged via ozonolysis prior to DMA uptake, ozone was generated by passing zero air from a zero air generator (Teledyne Advanced Pollution Instrumentation [TAPI, San Diego, CA, USA], Model 701H) through a dynamic dilution calibrator (TAPI, Model T700), which controlled the ozone concentration of the airstream. The ozone-laden airstream was split into two branches. One branch (∼180 cm3 min−1) was directed into the EDB for OA aging and the rest went to the exhaust. To investigate the effects of the degree of OA aging on DMA uptake, we set two different levels of ozone exposure. For the mild level, the ozone concentration was ∼1 ppm and the processing of OA coating lasted 1 h. For the intensive level, the ozone concentration was ∼2 ppm and the processing lasted 12 h. Although the ozone concentrations in these two scenarios were comparable, we believe the difference in the duration of ozone processing led to very different degrees of OA aging. High ozone concentrations at ppm levels were applied with the aim of reducing the timescale of our experiments. Lee and Chan (Citation2007) did not observe any appreciable changes in the Raman spectra of oxidized OA under high (>10 ppm) and low (∼260 ppb) ozone concentrations, suggesting that the reaction pathways and products were unlikely affected by the high ozone concentrations used.
Upon the completion of OA coating (or aging, if necessary), the particle was dried with nitrogen in order to remove volatile compounds formed via ozonolysis (Zahardis and Petrucci Citation2007) until the particle mass became constant. The particle masses measured before and after coating (or aging, if necessary) were used to calculate the weight percentage of organic coating (wtorg) on the particle. We use the subscript “org” to represent both fresh and aged OA, since the chemical composition of the coating changed after aging.
The procedures for controlling the RH, generating the DMA vapor, measuring particle mass with the EDB and characterizing the particle Raman spectra were identical to those described in Chu and Chan (Citation2017). Note that OA was always coated and aged under dry conditions where AS was crystalline. For DMA uptake experiments at 30% and 60% RH, the particles were first conditioned at an RH well above the AS deliquescent point to ensure complete deliquescence of the particle core. Once the particle mass had become constant (indicating that the gas-particle water equilibrium had been reached), DMA vapor was introduced to the EDB. We followed De Gelder (Citation2007), Lee and Chan (Citation2007), and Chu et al. (Citation2015) to assign the Raman peaks.
3. Results and discussion
3.1. Reactive DMA uptake by AS particles coated with fresh or aged OA
The experimental conditions (wtorg, RH, ozone concentration, duration of ozone processing and DMA uptake) and the percentages of NH4+ displacement at the end of experiments are shown in . The displacement percentage of NH4+ was calculated based on particle mass measurements at <5% RH before and after DMA uptake and the fact that both the fresh OA and aged OA are inert to DMA vapor (see section S1 and Figure S1 in the online supplemental information [SI]).
Table 1. Summary of experimental conditions and NH4+ displacement percentages.
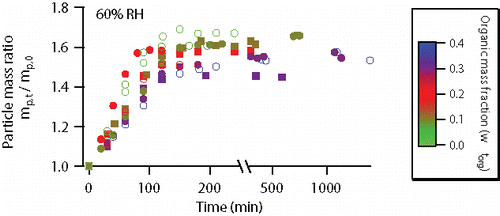
shows the mass changes of AS particles coated with fresh or aged OA during DMA uptake at 60% RH. AS was in aqueous form and particle mass increased effectively during the first 100–150 min due to DMA uptake. After that, the particle mass became constant within experimental uncertainty. Note that particle size could alter the slope of mass increase. Section 3.2 provides a detailed analysis on reaction kinetics. In addition, the higher particle mass ratios measured at equilibrium (the plateau in ) were generally consistent with lower wtorg. This is as expected because neither fresh nor aged OA reacted with DMA (see section S1 and Figure S1 in the SI). One exception was that the equilibrium mass ratios of the particle with wtorg = 0.31 (experiment #10) and 0.37 (experiment #4) were 1.46 and 1.53, respectively. In experiment #10, the >91% NH4+ displacement percentage (considering experimental uncertainty) was slightly lower than that in experiment #4 (>99%), and resulted in a lower particle mass ratio at DMA uptake equilibrium. Incomplete displacement of NH4+ was observed in experiments #2, 3 and 6 (), likely because the time allowed for DMA uptake in these experiments (∼3 h) was shorter than that in other experiments performed at 60% RH.
Figure 1. Particle mass changes of AS particles coated with fresh or aged OA at 60% RH. The color scale indicates the mass fraction (on a dry basis) of organic coating. Online color: Open circles—particles coated with fresh OA: light green—experiment #1 (in , same as below); dark green—experiment #2; red—experiment #3; blue—experiment #4. Solid circles—particles coated with mildly aged (1 ppm ozone, 1 h of processing) OA prior to DMA uptake: dark green – experiment #5; red – experiment #6; purple - experiment #7. Solid squares—particles coated with intensively aged (2 ppm ozone, 12 h of processing) OA prior to DMA uptake: dark green—experiment #8; red—experiment #9; purple—experiment #10. Experimental uncertainties are not shown because they are masked by the symbols.
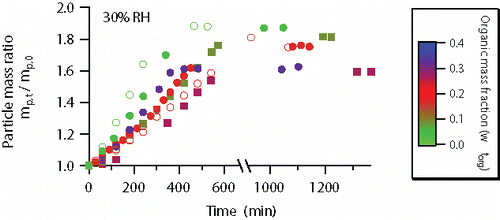
The particle mass changes during DMA uptake at 30% RH are shown in . AS crystallized prior to DMA uptake (, experiments #11–18) but it did not prevent the particles from absorbing DMA. The water adsorbed on the surface of the crystalline AS particles had triggered the initial DMA uptake and the formation of dimethylaminium sulfate (DMAS, more hygroscopic than AS) promoted further DMA uptake until NH4+ ions were depleted (Chu and Chan Citation2017). Before DMA uptake, we conditioned the particle at 30% RH for ∼30 min, much longer than the estimated characteristic time for water molecules to diffuse through the coating, which is up to 7.5 s (see section S2 in the SI). Therefore, the coating cannot rule out the presence of adsorbed water on crystalline AS at water equilibrium. The NH4+ ions originally in the particles were completely displaced under an exposure to 1 ppm DMA vapor for at least 16 h. Correspondingly, the particle core gradually transformed from crystalline AS to aqueous DMAS.
Figure 2. Particle mass changes of AS particles coated with fresh or aged OA at 30% RH. The color scale indicates the mass fraction (on a dry basis) of organic coating. Online color: Open circles—particles coated with fresh OA: light green—experiment #11 (in , same as below); dark green—experiment #12; red—experiment #13. Solid circles—particles coated with mildly aged (1 ppm ozone, 1 h of processing) OA prior to DMA uptake: light green—experiment #14; red—experiment #15; blue—experiment #16. Solid squares—particles coated with intensively aged (2 ppm ozone, 12 h of processing) OA prior to DMA uptake: dark green—experiment #17; purple—experiment #18. Experimental uncertainties are not shown because they are masked by the symbols.
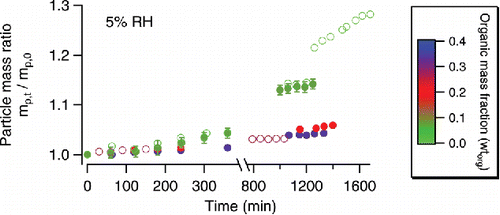
At <5% RH, the increase in particle mass during the course of DMA uptake was much smaller () than the increases at 60% and 30% RH. Incomplete displacement of NH4+ was observed for most particles (, experiments #19–24), except the uncoated AS particle (experiment #19). A higher wtorg generally resulted in less NH4+ displacement, within a comparable duration (∼20 h) of DMA uptake. The full displacement in experiment #19 did not occur until the AS particle had been exposed to 1 ppm DMA vapor for 28 hours. In our earlier paper, we observed a particle mass increase of only ∼3% (corresponding to an NH4+ displacement percentage of 8–12%) under similar conditions (1 ppm DMA, RH <5%) after ∼8 h (Chu and Chan Citation2017). The larger (∼28%) particle mass increase (due to full NH4+ displacement) observed here could be due to the longer period of DMA uptake.
Figure 3. Particle mass changes of AS particles coated with fresh or aged OA at <5% RH. The color scale indicates the mass fraction (on a dry basis) of organic coating. Online color: Open circles—particles coated with fresh OA: light green—experiment #19 (in , same as below); dark green—experiment #20; purple—experiment #21. Solid circles—particles coated with mildly aged (1 ppm ozone, 1 h of processing) OA prior to DMA uptake: dark green—experiment #22; red—experiment #23; blue—experiment #24. Experimental uncertainties are shown in error bars unless they are covered by the symbols.
OA aging did not affect the particle-phase equilibrium compositions at the end of the experiments by much. As long as the particle was levitated for long enough at 60% and 30% RH, all of the original NH4+ ions were displaced by DMA, regardless of the degree of OA aging. At the lowest RH (<5%), the displacement percentages were comparable with similar wtorg values, also regardless of the degree of OA aging. The effects of OA coating and the aging of OA coating on DMA uptake kinetics will be discussed in Section 3.2.
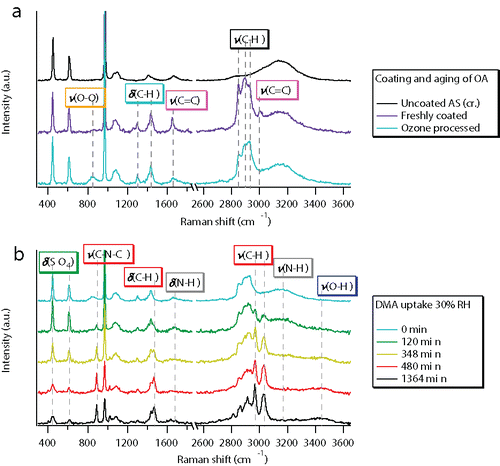
Temporal changes in particle-phase chemical compositions were reflected in the Raman spectra. We present the typical Raman spectral changes during OA coating and aging and DMA uptake for experiment #18. As shown in , C=C stretching peaks at 3005 and 1650 cm−1 and C–H bending peaks at 1460 and 1300 cm−1 appeared after bulk OA had condensed onto the crystalline AS particle. Intensive aging of OA by ozone significantly decreased the intensity of C=C stretching peaks and formed a broad O–O stretching band at 800–900 cm−1, indicating the formation of organic peroxides. These spectral variations were generally consistent with the Criegee mechanism (Zahardis and Petrucci Citation2007). Minor changes in the Raman intensities of the C–H stretching peaks at 2850–2950 cm−1 were also observed. In our experiments, the particle mass decreased by up to 4% as a result of aging, consistent with the findings by Lee and Chan (Citation2007). The intensity changes in C–H stretching peaks were attributed to the breaking of the OA C18 backbone, the formation of C9 compounds as well as the evaporation of 1-nonanal (Zahardis and Petrucci Citation2007). shows the spectral changes during DMA uptake at 30% RH. The decreased intensity of N–H peaks, increased intensity of C–H, C–N–C and O–H peaks and the broadening of the sulfate bending peak were in agreement with those in our earlier work (Chu and Chan Citation2017). As DMA uptake proceeded, particulate water accumulated. The decrease in Raman intensity of the O–O stretching peak at 800–900 cm−1 could be due to the hydration of hydrophilic functional groups.
3.2. DMA uptake kinetics
The estimation of DMA uptake coefficients (γ) has been described in detail in Chu and Chan (Citation2017). Minor changes were made in this study and are outlined below.
At 60% RH, the hygroscopic growth of OA has been reported to be negligible and the aging of OA via ozonolysis could enhance the particulate water contents by only 1–3% in mass (Lee and Chan Citation2007; Dennis-Smither et al. Citation2012). In fact, given the typical uncertainty of 1% in particle mass measurement using the EDB, a change of 3% in particle mass, especially for non-spherical particles, is considered comparable to the detection limit. Therefore, we treat both fresh OA and aged OA as non-hygroscopic constituents in this study. Additionally, the masses of fresh OA and aged OA were treated as constant during DMA uptake (see section S1 and Figure S1 in the SI).
Assuming that DMAS exists in the fully neutralized form during the course of DMA uptake, that the water equilibrium between particle and gas phases is achieved instantaneously and that the water uptake of AS and DMAS is independent, the amount of particle-phase water can be calculated using the Zdanovskii-Stokes-Robinson (ZSR) equation (Stokes and Robinson Citation1966):[1] where mw is the mass (kg) of particulate water in the multi-component solution with a water activity of aw; n(AS) and n(DMAS) are the moles of AS and DMAS, respectively; m0(AS) and m0(DMAS) are the molalities (mol kg−1) of AS and DMAS in their single-component solutions with the same aw as that in the multi-component solution, which are obtained from the Extended AIM Aerosol Thermodynamic Model (E–AIM, available at http://www.aim.env.uea.ac.uk/aim/aim.php) (Clegg et al. Citation1998) and Sauerwein et al. (Citation2015), respectively. Then, the particle mass (m, kg) is given by:
[2] where M(AS) and M(DMAS) are the molar masses of AS and DMAS, respectively; n(OA) and M(OA) are the moles and molar mass (kg mol−1) of OA, respectively. Note that we use the product of n(OA) and M(OA) as the upper limit of the mass of aged OA, as aging of OA can lead to a mass decrease of up to 5% mainly due to the formation of volatile compounds such as 1-nonanal (Zahardis and Petrucci Citation2007). Here, n(OA) and M(OA) should only be considered as the nominal moles and molar mass of aged OA, respectively. The initial particulate water contents (mw,0, kg) and total particle mass (m0, kg) are calculated using EquationEquations (3)
[3] and (Equation4
[4] ):
[3]
[4]
At <5% and 30% RH where AS crystallized prior to DMA uptake, the first term on the right-hand side of EquationEquation (1)[1] is removed and EquationEquation (3)
[3] becomes mw,0 = 0. The particle-phase composition was derived using particle mass ratios measured with the EDB, i.e., m/m0. Subsequent steps to estimate the pseudo-first-order reaction rate constant (k1) and γ are identical to those in Chu and Chan (Citation2017).
The γ at 60%, 30% and <5% RH at different wtorg are shown in . We focus on the trends of γ as wtorg increases instead of the absolute γ values. Sources of uncertainty include the derivation of particle-phase chemical composition using the ZSR equation, the determination of k1 using linear regression, and the measurements of particle mass with the EDB and particle diameter using an optical microscope. In this study, we measured the particle area (projected) on the 2D microscopic image with the ImageJ software and derived the area-equivalent particle diameter, regardless of the particle shape, in order to reduce the uncertainty in particle size measurements. Overall, typical γ uncertainty was 10–20% at 60% and 30% RH and could be up to 80% at <5% RH, because the uncertainty in particle size measurements influences the determination of k1 tremendously at <5% RH.
Figure 5. Measured DMA uptake coefficients at (a) 60% RH, (b) 30% RH, and (c) <5% RH against mass fractions of organic coating (on a dry basis). Open black circles—particles coated with fresh OA; solid black circles—particles coated with mildly aged OA with 1 ppm ozone processing for 1 h prior to DMA uptake; solid grey squares—particles coated with intensively aged OA with 2 ppm ozone processing for 12 h prior to DMA uptake.
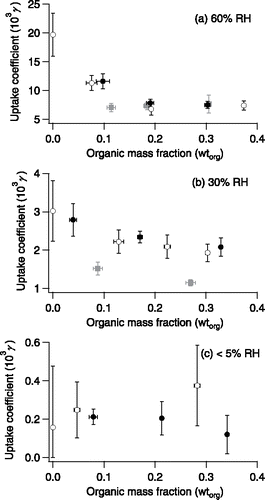
At 60% RH, the γ decreased by 60–70% as wtorg increased from 0 to 0.2 (), except the case where the OA coating was intensively aged. Afterwards, the γ leveled off and became independent of wtorg. This is likely because the fresh or aged bulk OA coating was less able to accommodate DMA than aqueous AS droplets and this had become the rate-determining step of the overall uptake. Based on the above discussions, we speculate that fresh or aged bulk OA completely covered the AS particle at wtorg = 0.2. Note that the amount of OA coated on the particle surface in our experiments greatly exceeded that equivalent to an OA monolayer. The detection limit of particle mass change with the EDB is 3 wt%. This corresponds to an OA molecule number density of 8.2 molecule Å−2 or that each OA molecule occupied an area of 0.123 Å2 on the particle surface assuming a uniform coating. The area covered by each OA molecule is significantly below the 48 Å2 molecular cross section required for an expanded liquid monolayer of OA (Langmuir Citation1917; McNeill et al. Citation2007; Stemmler et al. Citation2008). Hence, the coatings in our experiments consisted predominately of bulk OA.
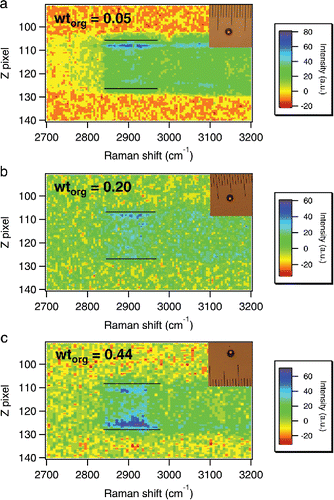
In order to verify the uniformity of organic distribution across the AS particle surface at different wtorg (before DMA uptake), we analyzed the Raman spectral intensities along the vertical axis of the particle using a charge-coupled device (CCD) detector coupled with a monochromator (Choi et al. Citation2004). Particles of different wtorg were equilibrated at 60% RH. shows the Raman intensities along the vertical direction. OA-related Raman signals are located between 2850 and 2950 cm−1 () and the Z pixel indicates the relative vertical position within the particle. The top and bottom of the particle showed stronger absolute intensities due to laser irradiation (Choi et al. Citation2004), so we focus on the changes in distribution and intensity (relative to N–H stretching band) of OA-related peaks instead. For a thin coating (e.g., wtorg = 0.05; ), organics clearly aggregated only at the top but did not appear at the bottom, implying an insufficient coverage of bulk OA. Note that an OA monolayer is too thin to be detected by our Raman system. As wtorg increased to 0.20 (), the distribution of organics became more uniform as OA-related Raman peaks were scattered along the vertical axis. These observations are consistent with our earlier assertion that the particle surface was fully covered by bulk OA at wtorg = 0.2. At wtorg = 0.44 (), OA-related Raman peaks intensified compared to the N–H stretching band at both poles of the particle, with higher intensities observed at ∼125 Z pixel and lower intensities at ∼110 Z pixel. This may suggest a thick coating at the bottom, since gravity can drive OA to the bottom of the particle.
Figure 6. Raman spectral intensities of AS particles coated with fresh or aged OA along the vertical direction. The Raman spectra were taken at 60% RH. The wtorg values (on a dry basis) are shown in the figure as annotations. The Z pixel represents the relative vertical position within the particle. The black bars indicate the top and bottom boundaries of the particle. The microscopic images of the particles are shown in the top right corner. One unit of the scale corresponds to a length of 20 μm.
Similar to our findings on reaction kinetics, Rouvière and Ammann (Citation2010) reported a 50% decrease in the ozone uptake coefficient by aqueous potassium iodide droplets as wtorg of nonanoic or lauric acid coating increased to 0.7. The retardation was more likely due to the bulk than the monolayer of nonanoic or lauric acid, because the amount of acid coated there greatly exceeded the equivalent amount for a monolayer. Furthermore, monolayers of nonanoic acid and lauric acid are in the expanded liquid state with high permeability and thus do not retard gas uptake (Stemmler et al. Citation2008; Rouvière and Ammann Citation2010).
Once a DMA molecule enters the particle, the diffusion through the fresh or mildly aged bulk OA coating does not impose a significant kinetic barrier due to the low viscosity (below 0.1 Pa s) (Valeri and Meirelles Citation1997). However, intensive aging of the OA coating increases its viscosity (from <0.1 Pa s up to 1.5 Pa s) (Hosny et al. Citation2016) and could considerably reduce the γ at wtorg = 0.11 (see the grey square in ). Here, we focused on the physical properties of bulk coating instead of a monolayer.
At 30% RH, the γ generally decreased as wtorg increased from 0 to 0.2, except the case where the OA coating was intensively aged (). As wtorg further increased, the γ became constant. The γ at 30% RH were 14–33% of those at 60% RH, because the major barrier was the crystalline AS. Although the effects of surface coverage of hydrophobic organics and interfacial accommodation of DMA molecules may still exist, their relative contribution to the retardation of DMA uptake became less important. This can partly explain why the dependence of γ on wtorg at 30% RH was not as clear as that at 60% RH. In addition, the intensively aged OA coating also reduced the γ at 30% RH (see grey squares in ) to values below even that corresponding to a full surface coverage by fresh or mildly aged bulk OA, indicating that the increased viscosity or weakened DMA accommodation became important. Furthermore, we believe that DMA uptake at 30% RH was limited by the dissolution of NH4+ from crystalline AS into the surface adsorbed water initially and into the bulk liquid water layer at later stages, as there was a gradual transition from crystalline AS to aqueous DMAS. However, the time-dependent uptake kinetics was beyond the scope of this article.
At <5% RH, the γ were on the order of 10−4 (). However, no clear trend could be seen, given the large γ uncertainties.
4. Conclusions and atmospheric implications
In this study, we used an EDB–Raman coupled system to study the reactive uptake of DMA by AS particles coated with fresh or aged bulk OA at 60%, 30%, and <5% RH. OA was aged via ozonolysis. Experiments performed at different RHs were aimed at investigating the effect of such a hydrophobic coating with AS in aqueous and crystalline forms rather than the effect of RH. Under these RH conditions, the physical properties and therefore the roles of fresh or aged bulk OA coating were not expected to change considerably (Lee and Chan Citation2007; Dennis-Smither et al. Citation2012). Since a continuous flow of 1 ppm DMA was applied in our experiments on a timescale of 1000 min, the measurements were not limited by the availability of DMA in the gas phase.
Our results showed that the coating of fresh or aged bulk OA did not change the equilibrium particle-phase compositions after DMA uptake. Although inert to DMA vapor, the coating did not stop NH4+ from being displaced at all of the RHs studied. This is likely because the loosely packed molecules within the coating (Stemmler et al. Citation2008) could not effectively inhibit the interaction between gaseous DMA and the AS particle core. For the coated particles at 60% and 30% RH, over 74% of the original NH4+ ions were displaced after 3 h of DMA uptake. At <5% RH, significantly less DMA was absorbed by the coated particles due to water scarcity.
On the other hand, the fresh or aged bulk OA coating reduced the γ due to inefficient surface accommodation of gaseous DMA molecules. This retarding effect gradually intensified as wtorg increased from 0 to 0.2 and then leveled off. Hence, a complete surface coverage of bulk OA was likely reached at wtorg = 0.2. In addition, the surface accommodation of DMA molecules was likely the rate-determining step for the whole uptake process. Mild aging of OA prior to DMA uptake did not change the γ, but an intensively aged OA coating reduced the γ at 60% and 30% RH, where the elevated viscosity or the even less efficient accommodation of DMA on the intensively aged OA coating may have provided some resistance to DMA uptake. Considering the large γ uncertainty at <5% RH, experiments with intensive aging of OA coating were not performed at <5% RH. Instruments that are able to determine the gas-phase DMA concentrations would help reduce experimental uncertainties (Davidovits et al., Citation2006). In this study, ozone concentrations at ppm levels were applied to reduce the timescale of our experiments. Lee and Chan (Citation2007) did not observe any appreciable changes in the Raman spectra of oxidized OA under high (>10 ppm) and low (∼260 ppb) ozone concentrations, suggesting that the reaction pathways and products were unlikely affected by the high ozone concentrations used. However, it is worth mentioning that the role of aging of organic coating in DMA uptake could depend on the nature of the organics.
We have previously reported that sucrose, a surrogate for hydrophilic viscous organics in mixtures with AS in the particle phase, can either accelerate DMA uptake by absorbing water or retard DMA uptake by increasing particle viscosity. Under the extreme condition where a glassy sucrose coating was formed at ≤10% RH, sucrose could inhibit the displacement of particulate NH4+ (Chu and Chan Citation2017). By contrast, the fresh or aged bulk OA coating consistently retarded but did not completely inhibit DMA uptake by AS, regardless of the AS phase state. The viscosity of OA after aging via ozonolysis can be up to 1.5 Pa s (Hosny et al. Citation2016), which may still be too low compared to that of sucrose (Power et al. Citation2013) to become a rate-determining factor in DMA uptake.
UAST_1323072_Supplemental_file.zip
Download Zip (113 KB)Funding
This work was supported by the Research Grants Council (RGC) of the Hong Kong Special Administrative Region (HKSAR), China (GRF 16300214). A grant from the Hong Kong RGC PhD Fellowship Scheme (HKPFS) is also gratefully acknowledged.
References
- Abbatt, J. P. D., Lee, A. K. Y., and Thornton, J. A. (2012). Quantifying Trace Gas Uptake to Tropospheric Aerosol: Recent Advances and Remaining Challenges. Chem. Soc. Rev., 41(19):6555–6581.
- Bzdek, B. R., Ridge, D. P., and Johnston, M. V. (2010). Amine Exchange Into Ammonium Bisulfate and Ammonium Nitrate Nuclei. Atmos. Chem. Phys., 10(8):3495–3503.
- Chan, L. P., and Chan, C. K. (2012). Displacement of Ammonium from Aerosol Particles by Uptake of Triethylamine. Aerosol Sci. Technol., 46(2):236–247.
- Chan, L. P., and Chan, C. K. (2013). Role of the Aerosol Phase State in Ammonia/Amines Exchange Reactions. Environ. Sci. Technol., 47(11):5755–5762.
- Chan, M. N., Lee, A. K. Y., and Chan, C. K. (2006). Responses of Ammonium Sulfate Particles Coated with Glutaric Acid to Cyclic Changes in Relative Humidity: Hygroscopicity and Raman Characterization. Environ. Sci. Technol., 40(22):6983–6989.
- Choi, M. Y., Chan, C. K., and Zhang, Y. H. (2004). Application of Fluorescence Spectroscopy to Study the State of Water in Aerosols. J. Phys. Chem. A, 108(7):1133–1138.
- Chu, Y., and Chan, C. K. (2017). Reactive Uptake of Dimethylamine by Ammonium Sulfate and Ammonium Sulfate–Sucrose Mixed Particles. J. Phys. Chem. A, 121(1):206–215.
- Chu, Y., Sauerwein, M., and Chan, C. K. (2015). Hygroscopic and Phase Transition Properties of Alkyl Aminium Sulfates at Low Relative Humidities. Phys. Chem. Chem. Phys., 17(30):19789–19796.
- Clegg, S. L., Brimblecombe, P., and Wexler, A. S. (1998). Thermodynamic Model of the System H+−NH4+−SO42-−NO3−−H2O at Tropospheric Temperatures. J. Phys. Chem. A, 102(12):2137–2154.
- Davidovits, P., Kolb, C. E., Williams, L. R., Jayne, J. T., and Worsnop, D. R. (2006). Mass Accommodation and Chemical Reactions at Gas-Liquid Interfaces. Chem. Rev., 106(4):1323–1354.
- Dawson, M. L., Varner, M. E., Perraud, V., Ezell, M. J., Wilson, J., Zelenyuk, A., Gerber, R. B., and Finlayson-Pitts, B. J. (2014). Amine-Amine Exchange in Aminium-Methanesulfonate Aerosols. J. Phys. Chem. C, 118(50):29431–29440.
- De Gelder, J., De Gussem, K., Vandenabeele, P., and Moens, L. (2007). Reference Database of Raman Spectra of Biological Molecules. J. Raman Spectrosc., 38(9):1133–1147.
- Dennis-Smither, B., Hanford, K. L., Kwamena, N. A., Miles, R. E. H., and Reid, J. P. (2012). Phase, Morphology, and Hygroscopicity of Mixed Oleic Acid/Sodium Chloride/Water Aerosol Particles before and after Ozonolysis. J. Phys. Chem. A, 116(24):6159–6168.
- Dreyfus, M. A., and Johnston, M. V. (2008). Rapid Sampling of Individual Organic Aerosol Species in Ambient Air with the Photoionization Aerosol Mass Spectrometer. Aerosol Sci. Technol., 42(1):18–27.
- Drozd, G., Woo, J., Häkkinen, S. A. K., Nenes, A., and McNeill, V. F. (2014). Inorganic Salts Interact with Oxalic Acid in Submicron Particles to form Material with Low Hygroscopicity and Volatility. Atmos. Chem. Phys., 14(10):5205–5215.
- Feng, J., Hu, M., Chan, C. K., Lau, P. S., Fang, M., He, L., and Tang, X. (2006). A Comparative Study of the Organic Matter in PM2.5 from Three Chinese Megacities in three Different Climatic Zones. Atmos. Environ., 40(21):3983–3994.
- Hosny, N. A., Fitzgerald, C., Vysniauskas, A., Athanasiadis, A., Berkemeier, T., Uygur, N., Poschl, U., Shiraiwa, M., Kalberer, M., Pope, F. D., and Kuimova, M. K. (2016). Direct Imaging of Changes in Aerosol Particle Viscosity Upon Hydration and Chemical Aging. Chem. Sci., 7(2):1357–1367.
- Langmuir, I. (1917). The Shapes of Group Molecules Forming the Surfaces of Liquids. Proc. Natl. Acad. Sci. U.S.A., 3(4):251–257.
- Lee, A. K. Y., and Chan, C. K. (2007). Single Particle Raman Spectroscopy for Investigating Atmospheric Heterogeneous Reactions of Organic Aerosols. Atmos. Environ., 41(22):4611–4621.
- Lloyd, J. A., Heaton, K. J., and Johnston, M. V. (2009). Reactive Uptake of Trimethylamine into Ammonium Nitrate Particles. J. Phys. Chem. A, 113(17):4840–4843.
- Marcolli, C., and Krieger, U. K. (2006). Phase Changes During Hygroscopic Cycles of Mixed Organic/Inorganic Model Systems of Tropospheric Aerosols. J. Phys. Chem. A, 110(5):1881–1893.
- McNeill, V. F., Wolfe, G. M., and Thornton, J. A. (2007). The Oxidation of Oleate in Submicron Aqueous Salt Aerosols: Evidence of a Surface Process. J. Phys. Chem. A, 111(6):1073–1083.
- Mochida, M., Kitamori, Y., Kawamura, K., Nojiri, Y., and Suzuki, K. (2002). Fatty Acids in the Marine Atmosphere: Factors Governing Their Concentrations and Evaluation of Organic Films on Sea‐Salt Particles. J. Geophys. Res.: Atmos., 107(D17):10.
- Müller, C., Iinuma, Y., Karstensen, J., van Pinxteren, D., Lehmann, S., Gnauk, T., and Herrmann, H. (2009). Seasonal Variation of Aliphatic Amines in Marine Sub-Micrometer Particles at the Cape Verde islands. Atmos. Chem. Phys., 9(24):9587–9597.
- Murphy, D. M., Cziczo, D. J., Froyd, K. D., Hudson, P. K., Matthew, B. M., Middlebrook, A. M., Peltier, R. E., Sullivan, A., Thomson, D. S., and Weber, R. J. (2006). Single-Particle Mass Spectrometry of Tropospheric Aerosol Particles. J. Geophys. Res.: Atmos., 111(D23):D23S32.
- Power, R. M., Simpson, S. H., Reid, J. P., and Hudson, A. J. (2013). The Transition from Liquid to Solid-Like Behaviour in Ultrahigh Viscosity Aerosol Particles. Chem. Sci., 4(6):2597–2604.
- Qiu, C., Wang, L., Lal, V., Khalizov, A. F., and Zhang, R. (2011). Heterogeneous Reactions of Alkylamines with Ammonium Sulfate and Ammonium Bisulfate. Environ. Sci. Technol., 45(11):4748–4755.
- Renbaum-Wolff, L., Grayson, J. W., Bateman, A. P., Kuwata, M., Sellier, M., Murray, B. J., Shilling, J. E., Martin, S. T., and Bertram, A. K. (2013). Viscosity of Alpha-Pinene Secondary Organic Material and Implications for Particle Growth and Reactivity. Proc. Natl. Acad. Sci. U.S.A., 110(20):8014–8019.
- Robinson, A. L., Subramanian, R., Donahue, N. M., Bernardo-Bricker, A., and Rogge, W. F. (2006). Source Apportionment of Molecular Markers and Organic Aerosol. 3. Food Cooking Emissions. Environ. Sci. Technol., 40(24):7820–7827.
- Rogge, W. F., Hildemann, L. M., Mazurek, M. A., Cass, G. R., and Simoneit, B. R. T. (1991). Sources of Fine Organic Aerosol. 1. Charbroilers and Meat Cooking Operations. Environ. Sci. Technol., 25(6):1112–1125.
- Rouvière, A., and Ammann, M. (2010). The Effect of Fatty Acid Surfactants on the Uptake of Ozone to Aqueous Halogenide Particles. Atmos. Chem. Phys., 10(23):11489–11500.
- Sauerwein, M., and Chan, C. K. (2016). Heterogeneous Uptake of Ammonia and Dimethylamine Into Sulfuric and Oxalic Acid Particles. Atmos. Chem. Phys. Discuss., 2016:1–28.
- Sauerwein, M., Clegg, S. L., and Chan, C. K. (2015). Water Activities and Osmotic Coefficients of Aqueous Solutions of Five Alkylaminium Sulfates and Their Mixtures with H2SO4 at 25°C. Aerosol Sci. Technol., 49(8):566–579.
- Stemmler, K., Vlasenko, A., Guimbaud, C., and Ammann, M. (2008). The Effect of Fatty Acid Surfactants on the Uptake of Nitric Acid to Deliquesced NaCl Aerosol. Atmos. Chem. Phys., 8(17):5127–5141.
- Stokes, R. H., and Robinson, R. A. (1966). Interactions in Aqueous Nonelectrolyte Solutions. I. Solute-Solvent Equilibria. J. Phys. Chem., 70(7):2126–2131.
- Tervahattu, H., Juhanoja, J., Vaida, V., Tuck, A. F., Niemi, J. V., Kupiainen, K., Kulmala, M., and Vehkamäki, H. (2005). Fatty acids on continental sulfate aerosol particles. J. Geophys. Res.: Atmos., 110:D06207.
- Valeri, D., and Meirelles, A. J. A. (1997). Viscosities of Fatty Acids, Triglycerides, and Their Binary Mixtures. J. Am. Oil Chem. Soc., 74(10):1221–1226.
- Yao, L., Wang, M., Wang, X., Liu, Y., Chen, H., Zheng, J., Nie, W., Ding, A., Geng, F., Wang, D., Chen, J., Worsnop, D. R., and Wang, L. (2016). Detection of Atmospheric Gaseous Amines and Amides by a High-Resolution Time-of-Flight Chemical Ionization Mass Spectrometer with Protonated Ethanol Reagent Ions. Atmos. Chem. Phys., 16(22):14527–14543.
- Yatavelli, R. L. N., and Thornton, J. A. (2010). Particulate Organic Matter Detection Using a Micro-Orifice Volatilization Impactor Coupled to a Chemical Ionization Mass Spectrometer (MOVI-CIMS). Aerosol Sci. Technol., 44(1):61–74.
- Zahardis, J., and Petrucci, G. A. (2007). The Oleic Acid-Ozone Heterogeneous Reaction System: Products, Kinetics, Secondary Chemistry, and Atmospheric Implications of a Model System – A Review. Atmos. Chem. Phys., 7(5):1237–1274.