ABSTRACT
It is now recognized that some organic components of ambient aerosols absorb light with a spectrum distinct from that of other absorbers such as black carbon and mineral components. The most common method for isolating this light-absorbing organic fraction, or “brown carbon,” is to collect particulate matter on filters and extract in a solvent, usually water or methanol. Here, we compare the absorption spectra of water-soluble (WS) and methanol-soluble (MS) extracts from ambient samples collected in Athens, Georgia. We find that despite syringe filtering the MS extracts, extinction by suspended particles is evident in the spectra leading to an overestimation of absorption by a factor of two on average. No such particle extinction is evident in the WS extracts. We demonstrate that it is possible to subtract the extinction contribution in the MS extracts by fitting the spectrum to the sum of two power-law functions, one describing the absorption spectrum and the other describing the extinction spectrum. With extinction thus removed, we find that integrated absorption (300–800 nm) by the MS brown carbon extract is highly correlated with the WS extract and is on average 1.55× larger. The wavelength dependence of the WS and MS spectra are also correlated and very similar with average absorption Ångström exponents of 6.1 (±0.7) and 6.7 (±1.1), respectively. This study demonstrates that for the samples collected: (1) brown carbon absorption can be overestimated if scattering in MS spectra is not accounted for, (2) there is no spectral evidence that the WS and MS chromophores are different, and (3) it may be possible to use WS spectra to represent total brown carbon absorption using a simple scaling factor. These findings may differ for other types of aerosol samples and analytical methods.
© 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Aerosols in the atmosphere affect the climate both directly by interacting with sunlight (i.e., absorption and scattering) and indirectly by altering cloud properties. The magnitude and even the sign of the impact of particle scattering and absorption, in particular, is not very well constrained, in part because they depend on many factors including particle size, shape, composition, and mixing state (Cazorla et al. Citation2013; Laskin et al. Citation2015; Fierce et al. Citation2016; Saleh et al. Citation2016). It is known that black carbon (BC) particles, for example, efficiently absorb solar radiation throughout the UV-visible region of the spectrum with an estimated anthropogenic contribution to positive radiative forcing second only to carbon dioxide (Bond et al. Citation2013). However, internal mixing of organic constituents can alter this effect (Lack and Cappa Citation2010), and some organic species also absorb light further complicating estimates of radiative transfer in the atmosphere.
This absorbing organic fraction, often termed “brown carbon” (BrC) because of its more pronounced wavelength dependence compared to BC, can contribute significantly to aerosol absorption in the UV and near-UV region of the spectrum. Measurements of both the magnitude and shape of the BrC spectrum have varied substantially making it difficult to correlate with specific types of sources and processes in the atmosphere as well as quantitatively incorporate into radiative transfer modules of climate models. This BrC spectrum is usually derived either by: (Equation1[1] ) measuring the absorption spectrum of the entire aerosol (including BC and BrC) and subtracting the BC contribution (Lack et al. Citation2012; Guo et al. Citation2014; Saleh et al. Citation2014; Wiegand et al. Citation2014; Zhang et al. Citation2016), or (Equation2
[2] ) extracting collected particulate matter samples in a solvent thereby separating the solvent-extractable BrC fraction from the insoluble BC (Kirchstetter et al. Citation2004; Chen and Bond Citation2010; Hecobian et al. Citation2010; Liu et al. Citation2013). The latter approach has the advantage that the particle mixing state need not be known to measure the BrC absorption spectrum. Additionally, collection of particulate matter over an extended period of time makes it possible to study aerosols even when loadings are low and makes a variety of offline analyses possible.
Most commonly, water is used as the extraction solvent, but it does not extract all BrC. Organic solvents, and methanol in particular, have been found to extract even more absorption than water does, and it is believed that the water-soluble (WS) fraction of BrC is a subset of the methanol-soluble (MS) fraction (Chen and Bond Citation2010; Liu et al. Citation2013). Some studies have suggested that differences in the wavelength dependence of the two extracts indicate different classes of chromophores present (Chen and Bond Citation2010; Zhang et al. Citation2013). However, relatively few studies have extracted in both solvents much less compared them systematically. Here, we analyze a collection of ambient particulate matter samples using a split-filter approach in which we extract half of each filter in water and the other half in methanol. Through such direct comparison we find that the WS and MS BrC spectra display many similarities but also with some important differences, including extinction by suspended insoluble particles in MS extracts, which has not previously been reported.
2. Materials and methods
2.1. Particulate matter sampling and extraction
Ambient aerosols were collected out of a window in the Chemistry Building at the University of Georgia (20 m above ground level) in Athens, Georgia (33.9488° N, 83.3747° W) for a total of 22 days during the months of July–October 2015 and May–June 2016. The samples were collected on 47 mm diameter polytetrafluoroethylene (PTFE, Teflon) filters (0.2 μm pore size, Sterlitech, Kent, WA, USA) at 16.7 L/min for separate 24 h periods. A Very Sharp Cut Cyclone inlet (BGI, Inc., Butler, NJ, USA) was used to size select for particles with diameters less than 2.5 μm. The filters were cut in half for extraction in either water or methanol, thereby allowing a direct comparison of the water-soluble (WS) and methanol-soluble (MS) absorption spectra for the same sample. shows an image of a typical split filter after extraction in water (top half) and methanol (bottom half).
Figure 1. Picture of split filter after extraction in water (top) and methanol (bottom). Greater absorption visible on the water half is believed to be associated with insoluble particles remaining on the filter. Note the absence of such absorption on the methanol half. The radial pattern is imposed by the substrate supporting the filter.

Solvent-extractable species were extracted, similarly to Hecobian et al. (Citation2010), by sonication of the filter for 20 min in 10 mL of either Milli-Q water (<18.2 MΩ·cm) or methanol (Sigma Aldrich, St. Louis, MO, USA; purity). Unless otherwise noted, extracted solutions were filtered using a 13 mm, 0.45 μm PTFE disposable syringe filter (28145-493, VWR, Radnor, PA, USA). Some samples were filtered using a 13 mm, 0.22 μm PTFE disposable syringe filter (SFTF13RB, Omicron Scientific, Alpharetta, GA, USA). The syringe filters were pre-wetted with methanol and in the case of the WS extractions were subsequently rinsed with water several times prior to filtering. The pH of the water and methanol extracts was adjusted to 7 using sodium hydroxide or sodium methoxide, respectively, before spectra were measured. This pH adjustment had no discernable impact on the extinction by insoluble particles.
2.2. UV-visible absorption spectra measurements and fitting
UV-visible absorption spectra (200–800 nm) of the extracts were measured in a 1 cm cuvette on a Cary 60 UV−vis spectrophotometer (Agilent). Spectra were blanked with cuvettes containing the appropriate solvent (water or methanol) and were fit using either a single power-law function[1] or a dual power-law function:
[2] where a and b are scaling coefficients, AAE is the absorption Ångström exponent, and EAE is the extinction Ångström exponent.
3. Results and discussion
3.1. Methanol-soluble spectra contain extinction by suspended particles
3.1.1. Extinction by particles can be accounted for with a power-law function
Particulate matter extracted in methanol consistently demonstrated larger absorbance at all wavelengths compared to the corresponding water extracts, similar to observations from other studies (Chen and Bond Citation2010; Liu et al. Citation2013; Kim et al. Citation2016; Kirillova et al. Citation2016). This behavior can be seen in in which the average absorbance spectra of all 22 filters analyzed are shown for both solvents. To account for day-to-day variations in absorbance intensity, each day's spectrum has been normalized to the methanol extract value at 300 nm. The long-wavelength () “tail” evident in the methanol extract is particularly interesting, as BrC is typically not believed to absorb significantly at these wavelengths (Kirchstetter et al. Citation2004; Lack et al. Citation2012; Saleh et al. Citation2013). We considered the possibility that this tail is the result of baseline drift, however it is not clear why the MS spectra would suffer from baseline drift while the WS spectra clearly do not. Furthermore, baseline drift would not be expected to result in systematically positive offsets in the spectra for nearly every filter sample.
Figure 2. Average absorption spectra for methanol-soluble (black line), water-soluble (dark gray [blue] line), and methanol-soluble BrC with particle extinction removed (light gray [red] line). The gray area represents extinction by particles in the methanol extract, while the orange area represents the contribution by BrC absorption.
![Figure 2. Average absorption spectra for methanol-soluble (black line), water-soluble (dark gray [blue] line), and methanol-soluble BrC with particle extinction removed (light gray [red] line). The gray area represents extinction by particles in the methanol extract, while the orange area represents the contribution by BrC absorption.](/cms/asset/65faa0b0-1b66-46ce-ae9f-c4776ba94344/uast_a_1334109_f0002_oc.gif)
Alternatively, the long-wavelength tail could result from scattering (and possibly absorption) by suspended particles in the extract. Not all of the particulate matter collected on the filter is soluble in water or methanol, so it stands to reason that some particles may remain in the extracts. In fact, the extract is passed through the 0.45 μm syringe filter to remove these particles. Yet, the apparent absorption at long wavelengths suggests that this removal is incomplete. To account for this particle extinction, we performed a nonlinear fit of the apparent MS absorption spectrum to a dual power-law function (Equation (Equation2[2] )) in which one power-law function describes the soluble extract absorption and the other describes the insoluble particle extinction. Such a fit is similar to fits used by others to separate contributions from BC and BrC (Wiegand et al. Citation2014; Liu et al. Citation2015; Massabò et al. Citation2015; Olson et al. Citation2015).
This dual power-law function (green dashed line in ) fits the MS spectrum well with a coefficient of determination, R2, of 1.00 and values of AAE = 6.43 and EAE = 1.07. The residual spectrum, which remains after the extinction component (gray area) has been subtracted from the MS spectrum, represents just the MS BrC absorption spectrum (light gray shaded [orange] area), which is also plotted as the red curve. We point out that we use the term “MS BrC” to distinguish the soluble absorbing component of the entire MS extract, which includes extinction by insoluble particles as well.
The spectra are also plotted in a log-transformed plot in in which a single power-law function is represented by a straight line. Single power-law functions fit the WS (dark gray [blue] line, AAE = 6.07, R2 = 1.00) and MS BrC (mid-gray [red] line, AAE = 6.49, R2 = 1.00) curves well over the entire range 300–700 nm. A single power-law does not fit the MS spectrum (black line) as well yielding an AAE = 3.48 (R2 = 0.96), which clearly reflects the influence of the particle extinction at longer wavelengths.
Figure 3. Log-transformed plot of absorption spectra for WS and MS extracts. Insoluble particle extinction contributes significantly to apparent MS absorption (black line), but the dual power-law fit (dashed [green] line) accounts for it and the BrC absorption.
![Figure 3. Log-transformed plot of absorption spectra for WS and MS extracts. Insoluble particle extinction contributes significantly to apparent MS absorption (black line), but the dual power-law fit (dashed [green] line) accounts for it and the BrC absorption.](/cms/asset/c33f2739-d5d8-4daa-be20-1728793713ad/uast_a_1334109_f0003_oc.gif)
Also shown in is the MS spectrum that would be obtained by subtracting the value at 700 nm to account for a presumed baseline offset (lightest gray [blue] line); this fit yields an AAE = 5.43 and appears to fit well (R2 = 1.00), though it systematically underestimates absorption at >450 nm. And, compared to the MS BrC spectrum it overestimates integrated absorption (300–800 nm) by 34%. In essence, the baseline subtraction has served to remove some of the influence of the particle extinction but in doing so assumes that contribution is wavelength independent (i.e., with an equivalent EAE = 0 in the dual power-law formalism). Not surprisingly, this helps but does so incompletely.
3.1.2. MS BrC absorption spectra are similar with different degrees of filtration
To further confirm that extinction by suspended particles is present in the spectra, we applied the dual power-law approach to an ambient sample that had been filtered to various degrees. Specifically, we measured the apparent absorption spectra of a single methanol extract that was either unfiltered, filtered using a 0.45 μm pore size syringe filter, or filtered using a 0.22 μm pore size syringe filter (). There should be a consistent contribution from the MS BrC absorption in each of these samples despite different particle concentrations and size distributions, thereby providing a good test of the dual power-law approach.
Figure 4. Comparison of filtration of a sample collected on 8 June 2016 in Athens, GA. (a) Apparent MS absorption spectra either not filtered (black lines), filtered with a 0.45 μm syringe filter (dark gray [blue] lines), or filtered with a 0.22 μm syringe filter (light gray [red] lines). Dashed [green] lines are dual power-law fits and gray/black areas represent suspended particle extinction. (b) Residual MS BrC spectra are similar regardless of extent of filtering. Green dashed lines are single power-law fits. (c) WS absorption spectra with different extents of filtering, as in (a), showing no evidence of particle extinction.
![Figure 4. Comparison of filtration of a sample collected on 8 June 2016 in Athens, GA. (a) Apparent MS absorption spectra either not filtered (black lines), filtered with a 0.45 μm syringe filter (dark gray [blue] lines), or filtered with a 0.22 μm syringe filter (light gray [red] lines). Dashed [green] lines are dual power-law fits and gray/black areas represent suspended particle extinction. (b) Residual MS BrC spectra are similar regardless of extent of filtering. Green dashed lines are single power-law fits. (c) WS absorption spectra with different extents of filtering, as in (a), showing no evidence of particle extinction.](/cms/asset/c29e6e6d-01fd-4931-94c1-e93339476dc2/uast_a_1334109_f0004_oc.gif)
Each of the spectra is fit well by the dual power-law function (dashed [green] lines), and the fraction of apparent absorption attributable to particle extinction (gray/black shaded areas) decreases exactly as would be expected from 91% for the unfiltered to 71% and 38% for the 0.45 μm and 0.22 μm syringe filters, respectively (). What is more, the MS BrC absorption spectra in each case appear very similar () with the AAE values obtained nearly indistinguishable () despite the varying contributions from particle extinction. Importantly, even when the smallest pore size syringe filter (0.22 μm) was used, extinction by suspended particles was evident indicating that fitting to the dual power-law function is likely always needed, at least for the types of particles we sampled and the sampling and extraction methods that we employed. We note that such particle extinction may not be present to the same degree with the use of a narrow-bore liquid waveguide capillary cell (LWCC) as others have used (Hecobian et al. Citation2010; Cheng et al. Citation2011; Liu et al. Citation2013; Zhang et al. Citation2013; Liu et al. Citation2015; Cheng et al. Citation2016; Kirillova et al. Citation2016).
Table 1. Comparison of spectral fits for different extents of particle filtration.
The finding that particle extinction remains despite syringe filtering is not surprising if we assume that filters with a 0.22 μm pore size permit particles 0.22 μm in diameter (and smaller to pass through); for example, the 550 nm extinction cross-section for a 0.22 μm black carbon particle (m = 1.95 + 0.79i) is about 20% of that of a 0.45 μm particle, as calculated using Mie theory. Since the ability to obtain the MS BrC spectrum relies on an accurate parameterization of the particle extinction spectrum, we recommend that the 0.45 μm syringe filter offers the best compromise between reducing the magnitude of the particle extinction and retaining enough particle extinction to fit well.
By comparison, the spectra from the same sample extracted in water, instead, showed no discernible differences regardless of which syringe filter was used or even if one was used (). Clearly, then, the use of the syringe filters is not affecting the soluble BrC spectra. The AAE values from these WS spectra are similar to the values obtained from the MS BrC spectra () indicating very similar spectral shapes despite the different magnitudes of absorption and presence of particle extinction in the MS extracts. Furthermore, the fact that particle extinction is present only in the MS spectra suggests that the insoluble particles are only dislodged from the sample filters (not the syringe filters) by methanol and not by water.
This distinction is corroborated by the observation of a visible difference in the appearance of the filter after extraction in the two solvents (); the filter half extracted in methanol appears white indicating a substantial removal of particles while the half extracted in water clearly shows visible absorption remaining. It is not clear why the insoluble particles would be removed from the filter in methanol but not in water; perhaps hydrophobic compounds, such as polyaromatic hydrocarbons, present on the particles inhibit their dissolution in water.
3.2. MS BrC is more absorbing than WS BrC but correlated with it
As the average spectra in show, MS BrC absorption is greater than that of WS BrC at all wavelengths. Here, we explore the correlation between MS and WS BrC absorption by comparing integrated absorption (300–800 nm) for pairs of filter halves each taken from the same original filter. In this way, we can directly compare the effects of the extraction solvents on the spectra from the same sample.
shows the observed relationship between the integrated spectral areas for the two solvents. If particle extinction in the MS extract is not accounted for (open squares), there is a lot of scatter and correlation with WS absorption is weak with an adjusted R2 of 0.75 and a slope of 4.21 (black dashed line). Despite having used the 0.45 μm syringe filter to remove particles, enough extinction remains that it appears as if the methanol extracts significantly more absorption than does water. Even subtracting the absorption at 700 nm (open triangles), ostensibly accounting for possible baseline drift, does not change the relationship much; the slope is 3.83 with an adjusted R2 = 0.76 (dashed light gray [green] line) and the data are still scattered. Once the particle extinction is removed using the dual power-law approach (dark gray [red] circles), however, the MS absorption is reduced substantially and the correlation improves, with the adjusted R2 = 0.90 and a slope of 1.81 (dashed dark gray [red] line). Removing the apparent outlier (open dark gray [red] circle) improves the correlation substantially with an adjusted R2 = 0.98 and a slope of 1.55 (solid dark gray [red] line).
Figure 5. Correlation between integrated absorption (300–800 nm) for WS and MS BrC extracts (dark gray [red] circles). Insoluble particle extinction has been subtracted using a dual power-law function. Linear fits are shown excluding the outlier point (dark gray [red] solid line, point indicated by open dark gray [red] circle) or not (dashed dark gray [red] line). Data without particle extinction subtracted (open squares) and with baseline subtraction (open triangles) and respective fits (black and light gray [green] dashed lines) are also shown for comparison.
![Figure 5. Correlation between integrated absorption (300–800 nm) for WS and MS BrC extracts (dark gray [red] circles). Insoluble particle extinction has been subtracted using a dual power-law function. Linear fits are shown excluding the outlier point (dark gray [red] solid line, point indicated by open dark gray [red] circle) or not (dashed dark gray [red] line). Data without particle extinction subtracted (open squares) and with baseline subtraction (open triangles) and respective fits (black and light gray [green] dashed lines) are also shown for comparison.](/cms/asset/153cfcf7-284c-46de-bb07-e28a91c6178b/uast_a_1334109_f0005_oc.gif)
The systematically larger absorption MS absorption compared to the WS absorption could result from the extraction of more chromophores or more highly absorbing chromophores. Alternatively, it is also possible that the nature of the solvents, themselves, could change the magnitude and shape of the absorption spectrum. To investigate this possibility, we extracted a filter sample in water and then diluted it in a 10:90 ratio in either water or in methanol. Comparison of these two spectra (Figure S1 in the online supplemental information [SI]) shows very little difference with the 90% methanol spectrum 5% larger than the water spectrum at all wavelengths, thus confirming no significant solvent effect on the chromophores. We conclude, then that the differences in the WS and MS BrC spectra originate from differences in extraction.
The large number of split-filter samples (22) represented in and summarized in Table S1 (in the SI) allows several important conclusions to be drawn. First, most MS spectra contain particle extinction as evidenced by the differences in the integrated areas with (dark gray [red] circles) and without (open squares) the use of the dual power-law approach. In fact, of the 22 samples only two contain no particle extinction, i.e., are fit better by a single power-law function. Of the other 20 samples, particle extinction accounted for an average of 56% () of integrated apparent absorption (300–800 nm) ranging from 13% to 86%. Even though the samples were filtered with the syringe filter, extinction by suspended particles is not insignificant and is present in nearly every sample.
Second, the degree of scatter in the data would suggest that there is not much correlation between MS BrC and WS absorption despite the fact that the initial split filter samples were nearly identical. Such a conclusion would be difficult to rationalize, as it is commonly believed that the WS BrC fraction is a subset of the MS BrC fraction. Indeed, the correlation observed after removing particle extinction (dark gray [red] circles) suggests that the WS BrC fraction may, indeed, be a subset of the MS BrC fraction.
Third, the high degree of correlation observed in the data corrected for particle extinction (dark gray [red] circles) is highly suggestive that the dual power-law approach is effective for single filter analysis (i.e., not just for spectra averaged from all filters). The correlation was observed for filter samples taken throughout the year in Athens, Georgia, indicating that the observed relationship may be independent of season, at least for the background aerosols sampled at a site such as this one. Furthermore, it may be possible to estimate the magnitude of MS BrC absorption, believed to be more representative of total BrC, from WS absorption (or vice versa).
3.3. MS BrC and WS BrC spectral shapes are similar
We can also take advantage of the split-filter dataset to explore the degree to which the MS and WS BrC spectra are similar. In , we compare the absorption Ångström exponents (AAE's) derived from power-law fits to the MS and WS spectra. We fit the WS spectra to a single power-law function (300–500 nm), while we fit the MS spectra (300–800 nm) using both a single power-law function (black squares) and the dual power-law function (gray [red] circles), for which the AAE represents the absorbing component after particle extinction is removed. For comparison, we also include AAE values reported in the limited number of studies that have measured the absorption spectra of both WS and MS ambient particulate matter (Liu et al. Citation2013; Zhang et al. Citation2013; Liu et al. Citation2015; Cheng et al. Citation2016; Kim et al. Citation2016; Kirillova et al. Citation2016) (see open symbols in ).
Figure 6. Correlation between WS and MS BrC absorption Ångström exponents (AAE's) when insoluble particle extinction is subtracted using a dual power-law function (gray [red] points). Values fall close to the 1:1 line indicating correlation between MS and WS AAE values. AAE values for MS spectra fit to a single power-law function (black squares) show more scatter and less correlation with WS AAE values. Measurements from other studies in which AAE's from both WS and MS are shown for comparison (open symbols).
![Figure 6. Correlation between WS and MS BrC absorption Ångström exponents (AAE's) when insoluble particle extinction is subtracted using a dual power-law function (gray [red] points). Values fall close to the 1:1 line indicating correlation between MS and WS AAE values. AAE values for MS spectra fit to a single power-law function (black squares) show more scatter and less correlation with WS AAE values. Measurements from other studies in which AAE's from both WS and MS are shown for comparison (open symbols).](/cms/asset/97daa415-09b3-49b4-8012-ea9014c31f2d/uast_a_1334109_f0006_oc.gif)
We find that the WS AAE values range from 4.96 to 7.54 with an average of 6.13 (). This value is typical of WS AAE's reported by many others for particulate matter sampled from a variety of locations, with many falling in the range of 6–8 (Hoffer et al. Citation2006; Hecobian et al. Citation2010; Cheng et al. Citation2011; Liu et al. Citation2013; Bosch et al. Citation2014; Du et al. Citation2014; Kirillova et al. Citation2014; Liu et al. Citation2015; Chen et al. Citation2016; Cheng et al. Citation2016; Fan et al. Citation2016; Kim et al. Citation2016; Kirillova et al. Citation2016). The quality of the single power-law fits to the WS spectra is good, with the majority of the R2 values at least 0.999. We tried to fit the WS spectra to a dual power-law function, but none of the 22 samples was fit better than with a single power-law function. This finding is highly suggestive that insoluble particles are not liberated from the filters when extracted in water, or if they are they do not scatter and absorb light efficiently enough to be detected.
Fitting the MS spectra to a single power-law function (i.e., including particle extinction) is not nearly as good, however, with AAE values ranging from 2.04 to 6.80 (black squares) with an average of 4.00 () and most R2 values well below 0.99. Considerable scatter is evident with little correlation between the MS and WS AAE's. Conversely, the MS BrC AAE values (i.e., with particle extinction subtracted) demonstrate less scatter. These values span a range of 5.20–9.09 with an average of 6.68 (
). The average EAE of the particle extinction from these dual power-law fits is 1.05 (
), which is in the range of values found for extinction by ambient particles suspended in air (0–2) (Cappa et al. Citation2016; Hamill et al. Citation2016; Liu et al. Citation2016).
Clearly, fitting the MS spectra to a single power-law function inadequately describes the wavelength dependence of the BrC absorption even though it may appear to capture the shape of the spectrum (see , for example). The consequence of such a fitting is to overestimate absorption at the longer wavelengths thereby erroneously lowering the value of AAE. What is more, this error obscures the correlation between the MS BrC and WS BrC spectral shapes because it originates from particle extinction, which is only present in the MS extracts. Upon subtraction of this extinction, there appears to be little evidence for much spectral difference with average WS and MS BrC AAE's of 6.13 and 6.68, respectively. This small difference, however, appears to reflect real differences in the absorption of the extracted chromophores as opposed to changes induced by the different solvents. Comparison of a WS extract diluted 10:90 either in water or in methanol (Figure S1 in the SI) shows no discernable difference in the spectral shape with AAE values of 7.13 and 7.15, respectively.
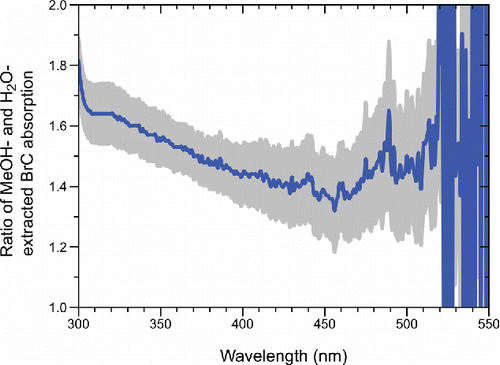
The small difference in the WS and MS BrC spectral shapes is easily seen in the plot of the average ratio of the MS BrC and WS absorption as a function of wavelength from the 22 individual filters (). While the MS BrC absorption is greater than the WS absorption at all wavelengths, this enhancement is seen to decrease monotonically with increasing wavelength. This trend illustrates the fact that the MS BrC spectra systematically decrease with wavelength more rapidly (i.e., have a larger AAE) than do WS spectra even though they are more highly absorbing. Such spectral differences could be indicative of chromophores that are more soluble in methanol than in water and which tend to absorb in the UV and near-UV of the spectrum, such as aromatic species. Recognition of this difference, though slight, could be useful for understanding the chemical sources and evolution of BrC and may have implications for how it is implemented in radiative transfer calculations in climate models. This ability, however, requires the ability to resolve the difference in spectral shape, which is only possible in the present work because the insoluble particle extinction is removed using the dual power-law fit.
4. Conclusions
We have compared split filters of ambient particulate matter extracted in water and methanol and found that most (19 out of 22) MS spectra are significantly impacted by extinction of suspended insoluble particles liberated from the filter even after filtering with a syringe filter. It appears as if particles are not liberated from the filter by water, and thus the WS spectra do not include particle extinction. A dual power-law fit to the MS spectra is able to separate the MS BrC absorption from the particle extinction adequately. Comparing the WS and MS BrC spectra we find that:
| • | WS and MS BrC integrated absorption (300–800 nm) is highly correlated; | ||||
| • | MS BrC integrated absorption (300–800 nm) is a factor of 1.55× larger than WS integrated absorption; | ||||
| • | The spectral shapes of WS and MS BrC absorption are similar, with average AAE's of 6.13 and 6.68, respectively; | ||||
| • | MS BrC spectra display a slight absorption enhancement at UV and near-UV wavelengths. | ||||
Based on these findings, we recommend that a dual power-law function (Equation (Equation2[2] )) be used in analyzing methanol-soluble spectra obtained by extraction from filters if: (Equation1
[1] ) extinction is evident at wavelengths longer than 600 nm and/or (Equation2
[2] ) a single power-law function does not fit the spectrum adequately.
UAST_1334109_Supplemental_File.zip
Download Zip (83.5 KB)Funding
Funding was provided by the National Science Foundation through the Atmospheric and Geospace Sciences Division of the Geosciences Directorate (AGS-1241621 and AGS-1638307).
References
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., Ghan, S., Kaercher, B., Koch, D., Kinne, S., Kondo, Y., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S. (2013). Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res.: Atmos., 118(11):5380–5552. Available at: https://doi.org/10.1002/jgrd.50171
- Bosch, C., Andersson, A., Kirillova, E. N., Budhavant, K., Tiwari, S., Praveen, P. S., Russell, L. M., Beres, N. D., Ramanathan, V., and Gustafsson, Ö. (2014). Source-Diagnostic Dual-Isotope Composition and Optical Properties of Water-Soluble Organic Carbon and Elemental Carbon in the South Asian Outflow Intercepted Over the Indian Ocean. J. Geophys. Res.: Atmos., 119(20):11,743–11,759. Available at: https://doi.org/10.1002/2014JD022127
- Cappa, C. D., Kolesar, K. R., Zhang, X., Atkinson, D. B., Pekour, M. S., Zaveri, R. A., Zelenyuk, A., and Zhang, Q. (2016). Understanding the Optical Properties of Ambient Sub- and Supermicron Particulate Matter: Results from the CARES 2010 Field Study in Northern California. Atmos. Chem. Phys., 16(10):6511–6535. Available at: https://doi.org/10.5194/acp-16-6511-2016
- Cazorla, A., Bahadur, R., Suski, K. J., Cahill, J. F., Chand, D., Schmid, B., Ramanathan, V., and Prather, K. A. (2013). Relating Aerosol Absorption Due to Soot, Organic Carbon, and Dust to Emission Sources Determined from in-situ Chemical Measurements. Atmos. Chem. Phys., 13(18):9337–9350. Available at: https://doi.org/10.5194/acp-13-9337-2013
- Chen, Q., Ikemori, F., and Mochida, M. (2016). Light Absorption and Excitation-Emission Fluorescence of Urban Organic Aerosol Components and Their Relationship to Chemical Structure. Environ. Sci. Technol., 50(20):10859–10868. Available at: https://doi.org/10.1021/acs.est.6b02541
- Chen, Y., and Bond, T. C. (2010). Light Absorption by Organic Carbon from Wood Combustion. Atmos. Chem. Phys., 10(4):1773–1787.
- Cheng, Y., He, K.-B., Du, Z.-Y., Engling, G., Liu, J.-M., Ma, Y.-L., Zheng, M., and Weber, R. (2016). The Characteristics of Brown Carbon Aerosol During Winter in Beijing. Atmos. Environ., 127:355–364.
- Cheng, Y., He, K. B., Zheng, M., Duan, F. K., Du, Z. Y., Ma, Y. L., Tan, J. H., Yang, F. M., Liu, J. M., Zhang, X. L., Weber, R., Bergin, M. H., and Russell, A. G. (2011). Mass Absorption Efficiency of Elemental Carbon and Water-Soluble Organic Carbon in Beijing, China. Atmos. Chem. Phys., 11(22):11497–11510. Available at: https://doi.org/10.5194/acp-11-11497-2011
- Du, Z., He, K., Cheng, Y., Duan, F., Ma, Y., Liu, J., Zhang, X., Zheng, M., and Weber, R. (2014). A Yearlong Study of Water-Soluble Organic Carbon in Beijing II: Light Absorption Properties. Atmos. Environ., 89:235–241.
- Fan, X., Wei, S., Zhu, M., Song, J., and Peng, P. (2016). Comprehensive Characterization of Humic-Like Substances in Smoke PM2.5 Emitted from the Combustion of Biomass Materials and Fossil Fuels. Atmos. Chem. Phys., 16(20):13321–13340. Available at: https://doi.org/10.5194/acp-16-13321-2016
- Fierce, L., Riemer, N., and Bond, T. C. (2016). Toward Reduced Representation of Mixing State for Simulating Aerosol Effects on Climate. Bull. Amer. Meteor. Soc., 98:971–980. Available at: https://doi.org/10.1175/BAMS-D-16-0028.1
- Guo, X., Nakayama, T., Yamada, H., Inomata, S., Tonokura, K., and Matsumi, Y. (2014). Measurement of the Light Absorbing Properties of Diesel Exhaust Particles using a Three-Wavelength Photoacoustic Spectrometer. Atmos. Environ., 94:428–437. Available at: https://doi.org/10.1016/j.atmosenv.2014.05.042
- Hamill, P., Giordano, M., Ward, C., Giles, D., and Holben, B. (2016). An AERONET-Based Aerosol Classification Using the Mahalanobis Distance. Atmos. Environ., 140:213–233. Available at: https://doi.org/10.1016/j.atmosenv.2016.06.002
- Hecobian, A., Zhang, X., Zheng, M., Frank, N., Edgerton, E. S., and Weber, R. (2010). Water-Soluble Organic Aerosol Material and the Light-Absorption Characteristics of Aqueous Extracts Measured Over the Southeastern United States. Atmos. Chem. Phys., 10(13):5965–5977. Available at: https://doi.org/10.5194/acp-10-5965-2010
- Hoffer, A., Gelencser, A., Guyon, P., and Kiss, G. (2006). Optical Properties of Humic-Like Substances (HULIS) in Biomass-Burning Aerosols. Atmos. Chem. Phys., 6(11):3563–3570. Available at: https://doi.org/10.5194/acp-6-3563-2006
- Kim, H., Kim, J. Y., Jin, H. C., Lee, J. Y., and Lee, S. P. (2016). Seasonal Variations in the Light-Absorbing Properties of Water-Soluble and Insoluble Organic Aerosols in Seoul, Korea. Atmos. Environ., 129:234–242. Available at: https://doi.org/10.1016/j.atmosenv.2016.01.042
- Kirchstetter, T. W., Novakov, T., and Hobbs, P. (2004). Evidence That the Spectral Dependence of Light Absorption by Aerosols is Affected by Organic Carbon. J. Geophys. Res., 109(D21):D21208. Available at: https://doi.org/10.1029/2004JD004999
- Kirillova, E. N., Andersson, A., and Han, J. (2014). Sources and Light Absorption of Water-Soluble Organic Carbon Aerosols in the Outflow from Northern China. Atmos. Chem. Phys., 14:1413–1422.
- Kirillova, E. N., Marinoni, A., and Bonasoni, P. (2016). Light Absorption Properties of Brown Carbon in the High Himalayas. J. Geophys. Res.: Atmos., 121:1–19. Available at: https://doi.org/10.1002/2016JD025030
- Lack, D. A., and Cappa, C. D. (2010). Impact of Brown and Clear Carbon on Light Absorption Enhancement, Single Scatter Albedo and Absorption Wavelength Dependence of Black Carbon. Atmos. Chem. Phys., 10(9):4207–4220. Available at: https://doi.org/10.5194/acp-10-4207-2010
- Lack, D. A., Langridge, J. M., Bahreini, R., Cappa, C. D., Middlebrook, A. M., and Schwarz, J. P. (2012). Brown Carbon and Internal Mixing in Biomass Burning Particles. Proc. Natl. Acad. Sci. USA, 109(37):14802–14807. Available at: https://doi.org/10.1073/pnas.1206575109
- Laskin, A., Laskin, J., and Nizkorodov, S. A. (2015). Chemistry of Atmospheric Brown Carbon. Chem. Rev., 115(10):4335–4382. Available at: https://doi.org/10.1021/cr5006167
- Liu, C., Chung, C. E., Zhang, F., and Yin, Y. (2016). The Colors of Biomass Burning Aerosols in the Atmosphere. Sci. Rep., 6(1):28267. Available at: https://doi.org/10.1038/srep28267
- Liu, J., Bergin, M. H., Guo, H., King, L., Kotra, N., Edgerton, E. S., and Weber, R. (2013). Size-Resolved Measurements of Brown Carbon in Water and Methanol Extracts and Estimates of Their Contribution to Ambient Fine-Particle Light Absorption. Atmos. Chem. Phys., 13:12389–12404.
- Liu, J., Scheuer, E., Dibb, J., Diskin, G. S., Ziemba, L. D., Thornhill, K. L., Anderson, B. E., Wisthaler, A., Mikoviny, T., Devi, J. J., Bergin, M. H., Perring, A. E., Markovic, M. Z., Schwarz, J. P., Campuzano-Jost, P., Day, D. A., Jimenez, J. L., and Weber, R. (2015). Brown Carbon Aerosol in the North American Continental Troposphere: Sources, Abundance, and Radiative Forcing. Atmos. Chem. Phys., 15(14):7841–7858. Available at: https://doi.org/10.5194/acp-15-7841-2015
- Massabò, D., Caponi, L., Bernardoni, V., Bove, M. C., Brotto, P., Calzolai, G., Cassola, F., Chiari, M., Fedi, M. E., Fermo, P., Giannoni, M., Lucarelli, F., Nava, S., Piazzalunga, A., Valli, G., Vecchi, R., and Prati, P. (2015). Multi-Wavelength Optical Determination of Black and Brown Carbon in Atmospheric Aerosols. Atmos. Environ., 108:1–12. Available at: https://doi.org/10.1016/j.atmosenv.2015.02.058
- Olson, M. R., Victoria Garcia, M., Robinson, M. A., Van Rooy, P., Dietenberger, M. A., Bergin, M., and Schauer, J. J. (2015). Investigation of Black and Brown Carbon Multiple‐Wavelength‐Dependent Light Absorption from Biomass and Fossil Fuel Combustion Source Emissions. J. Geophys. Res.: Atmos., 120(13):6682–6697. Available at: https://doi.org/10.1002/2014JD022970
- Saleh, R., Adams, P. J., Donahue, N. M., and Robinson, A. L. (2016). The Interplay Between Assumed Morphology and the Direct Radiative Effect of Light‐Absorbing Organic Aerosol. Geophys. Res. Lett., 43(16):8735–8743. Available at: https://doi.org/10.1002/2016GL069786
- Saleh, R., Hennigan, C. J., McMeeking, G. R., Chuang, W. K., Robinson, E. S., Coe, H., Donahue, N. M., and Robinson, A. L. (2013). Absorptivity of Brown Carbon in Fresh and Photo-Chemically Aged Biomass-Burning Emissions. Atmos. Chem. Phys., 13(15):7683–7693. Available at: https://doi.org/10.5194/acp-13-7683-2013
- Saleh, R., Robinson, E. S., Tkacik, D. S., Ahern, A. T., Liu, S., Aiken, A. C., Sullivan, R. C., Presto, A. A., Dubey, M. K., Yokelson, R. J., Donahue, N. M., and Robinson, A. L. (2014). Brownness of Organics in Aerosols from Biomass Burning Linked to Their Black Carbon Content. Nature Geosci., 7(9):647–650. Available at: https://doi.org/10.1038/ngeo2220
- Wiegand, J. R., Wiegand, J. R., Mathews, L. D., Mathews, L. D., and Smith, G. D. (2014). A UV-Vis Photoacoustic Spectrophotometer. Anal. Chem., 86(12):6049–6056. Available at: https://doi.org/10.1021/ac501196u
- Zhang, X., Kim, H., Parworth, C. L., Young, D. E., Zhang, Q., Metcalf, A. R., and Cappa, C. D. (2016). Optical Properties of Wintertime Aerosols from Residential Wood Burning in Fresno, CA: Results from DISCOVER-AQ 2013. Environ. Sci. Technol., 50(4):1681–1690. Available at: https://doi.org/10.1021/acs.est.5b04134
- Zhang, X., Lin, Y.-H., Surratt, J. D., and Weber, R. (2013). Sources, Composition and Absorption Ångström Exponent of Light-Absorbing Organic Components in Aerosol Extracts from the Los Angeles Basin. Environ. Sci. Technol., 47(8):3685–3693. Available at: https://doi.org/10.1021/es305047b