ABSTRACT
Nanosized silica size standards produced with a sol–gel synthesis process were evaluated for particle size, effective density, and refractive index in this study. Particle size and effective density measurements were conducted following protocol from the National Institute of Advanced Industrial Science and Technology (AIST) in Japan. Particle sizes were measured via electrical mobility analysis using a differential mobility analyzer (DMA) at sheath flow rates (Qsh) of 3.0 and 6.0 L/min and a constant aerosol flow rate (Qa) of 0.3 L/min. The measured mean and mode diameters agreed well with the labeled sizes in the size range 40–200 nm, with differences ranging from 0.03% to 0.8%, well within the labeled expanded uncertainties (95% confidence intervals) of 1.8%–2.2%. The coefficient of variation (CV) of the size distribution was 0.012–0.027 for 40–200 nm. Particle sizes measured for 20 nm and 30 nm standards showed size differences with respect to the certified sizes of 1.7% and 8.3% at Qsh = 6.0 L/min, but the size distributions were narrow, with CV = 0.047–0.064. The average effective density for the range 40–200 nm measured with an aerosol particle mass analyzer (APM) was 1.9 g/cm3. The real component of the refractive index measured with an optical particle counter (OPC) was 1.41 at a wavelength of 633 nm. All properties (size, effective density, and refractive index) were stable and could be measured with good repeatability. From these evaluations, it was found that the nanosized silica size standards have good characteristics for use as size standards and constitute a feasible alternative to PSL particles.
© 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Polystyrene latex (PSL) spheres are well accepted as particle size standards for instrument calibration (John Citation1993) because they have known composition (and hence, known optical properties), ideal shape, and are generally uniform in size. In a typical aerosol instrument calibration application, PSL spheres are dispersed into gas by atomization (nebulization), and after appropriate aerosol conditioning, are measured by the instrument to determine its response to particles of the stated mean or modal size.
In the past several years, silica (SiO2) particles have found increasing use as size standards for calibration of scanning surface inspection system (SSIS) tools used in the semiconductor manufacturing industry via deposition on silicon wafers (Syedain et al. Citation2013). SiO2 nanoparticles have also been developed for a wide range of applications in the life sciences (Barahona et al. Citation2015). Commercial silica particles are often polydisperse, with wider size distributions than those of PSL spheres with comparable mean size (Yook et al. Citation2008; Wang et al. Citation2008; Rudzevich et al. Citation2014). To obtain size distributions that are sufficiently narrow for SSIS calibration, the polydispersions can be classified by a differential mobility analyzer (DMA; Liu and Pui Citation1974; Knutson and Whitby Citation1975) that is calibrated with NIST traceability (Mulholland et al. Citation1996; Yook et al. Citation2008; Wang et al. Citation2008). Electrical mobility analysis using a DMA is also a very useful technique for measuring size distributions of size standards. An accurate wafer calibration standard can be produced for SSIS calibration when the modal diameter of each deposited size standard is precisely known.
Unfortunately, confusion can arise within an industry when size standards are not accurately characterized. For example, Mulholland et al. (Citation1999) reported that PSL particles with a stated diameter of 100 nm produced by JSR Corporation were about 10% larger than the NIST standard reference material (SRM) 1963, PSL spheres with a certified mean diameter of 100.7 nm. The size difference was determined to be 11.2% by electrical mobility analysis and 9.6% by TEM measurement. JSR Corporation found that the PSL size standards with mean diameter smaller than 150 nm that were characterized by TEM had measurable errors when reevaluated using a DMA. From these studies, it was determined that electrical mobility analysis is the most certain method for evaluating particle standards of smaller size. Since then, PSL size standards offered by JSR Corp. have been released with the stated mean/modal diameter value measured by electrical mobility analysis (Nanahara Citation2003).
If silica particles have characteristics similar to PSL (narrow size distribution, uniform particle shape, etc.), they may have potential for use as particle size standards in semiconductor manufacturing and other industries, and in government and university research laboratories. However, basic properties of silica particles (including density and refractive index) need to be adequately defined for the particles to be accepted and used as size standards.
In this work, properties of novel monodisperse silica particles were evaluated including particle size, effective density, and refractive index. These are the first measurements of effective density and refractive index of these nanosized silica particles, which were recently introduced for commercial sale. The manufacturer characterized the size distribution of each silica particle suspension using electrical mobility analysis, determining the modal diameter with NIST traceability and approximating the size distribution width. To verify the original size measurements, particle size was evaluated in this study by another method prescribed by the National Metrology Institute of Japan (NMIJ), a division of the National Institute of Advanced Industrial Science and Technology (AIST). Their size measurement method (Takahata Citation2011) was used for evaluation of silica particles with on-line measurements in an inter-laboratory study (Motzkus et al. Citation2013). NMIJ also developed a protocol (Sakurai Citation2011) for characterizing particle mass using an aerosol particle mass analyzer (APM). In this work, we measured size (mean and modal diameters) and effective density of the new silica size standards using a DMA and an APM calibrated and characterized with PSL particles. We were interested in mean and modal diameters because agreement between them is an indicator of the degree of symmetry in the number size distribution. Refractive index was characterized using an optical particle counter (OPC). We use the terms “effective density” and “effective refractive index” because these quantities were not directly measured; they were inferred from measurements of mean particle mass and light-scattering intensity, respectively, for particles classified on the basis of electrical mobility.
2. Nanosized silica size standards
MSP Corporation recently developed a series of highly monodisperse silica nanoparticle size standards (Syedain et al. Citation2013) using a sol–gel synthesis process. These size standards are currently available in 16 standard sizes ranging from 15 to 200 nm, with size distribution relative full-width at half-maximum (RFWHM, based on modal diameter) ranging from 3% for the largest sizes to 12% for the smallest sizes. Eight sizes ranging from 20 to 200 nm were used in this study (Table S1, see the online supplemental information [SI]). Assuming normal distributions of particle size, the size distribution width expressed as the standard deviation is σ = RFWHM/2.355, ranging from 0.013 to 0.051 (Table S2a).
Each size standard is packaged in a low density polyethylene (LDPE) bottle with 5 mL of concentrated suspension. The number concentration is on the order of 1013 particles/mL for the largest sizes to 1015 particles/mL for the smallest sizes. For typical aerosol generation applications, a few drops (∼0.04 mL volume per drop) of the concentrated suspension may be mixed in 100 mL of ultra-pure water (UPW) to obtain suitable number concentrations of airborne silica particles. The synthesized silica particles are quasi-spherical in shape with moderate surface roughness, as shown in Figure S1 in the SI.
Each batch of silica suspension (typically 15 bottles) was certified by measuring the modal diameter of a small sample diluted with UPW. The modal diameter measurements were performed with a particle deposition system (MSP Model 2300NPT), which includes aerosol generation and size classification subsystems. The diluted suspension was dispersed into air using a Collison-type atomizer for nominal particle sizes of 80 nm and greater or a proprietary nanoparticle atomizer designed for generating particles smaller than 80 nm. The silica particles were dried, charge-neutralized with bipolar ions generated by an AC corona, and drawn into one of two DMAs for electrical mobility analysis of the size distribution.
The DMAs were short and long variations of the design developed at the University of Minnesota (Liu and Pui Citation1974; Knutson and Whitby Citation1975), which were calibrated with NIST SRMs prior to characterization of the silica size standards used in the current work. Details of the methodology for calibrating these DMAs and their use for modal diameter determination are provided in the SI.
Analysis of the expanded uncertainty (95% confidence interval) for the modal diameter of each size standard was adapted from the work of Mulholland and coworkers (Mulholland et al. Citation1999, Citation2006). The expanded uncertainty (coverage factor k = 2) ranged from 1.8% to 2.5% of the measured modal diameter for the size standards evaluated in this study (Table S1).
3. Calibration/measurement methods
3.1. Particle size
Particle size measurements were conducted following the protocol established by the National Institute of Advanced Industrial Science and Technology (AIST) in Japan (Takahata Citation2011). Polystyrene latex (PSL) spheres (STADEX SC-010-S, JSR Corporation, Tokyo, Japan; “SC-010-S” in this article), with a mean diameter of 100.8 ± 0.66 nm (expanded uncertainty; k = 2), defined as the number-mean diameter as measured by the electro-gravitational aerosol balance method, were used as a calibration size standard for calibrating a DMA (Ehara et al. Citation2006b). The measurement apparatus is shown schematically in Figure S2 in the SI. In the protocol, it is critical that the sheath flow rate and the voltage source (DC power supply) are calibrated before the DMA column itself is calibrated. It is also important that the CPC used for measuring concentration downstream of the DMA has relatively constant counting efficiency over the entire range of measured particle size. Both of these requirements were met.
Test silica particles with labeled modal diameters ranging from 20 nm to 201 nm (Tables S1 and S2a) and SC-010-S PSL spheres (100.8 nm) were generated with an electrospray aerosol generator (Model 3480; TSI Inc., Shoreview, MN, USA). For comparison, PSL spheres from Microgenics Corporation (Thermo Scientific, Fremont, CA, USA) with labeled mean diameters ranging from 22 nm to 203 nm (Table S2b) were also generated by electrospray. A suspension of each size standard was made by mixing 10 μL of the original concentrated suspension with 990 μL of 2 mM ammonium acetate solution.
The inner diameter of the electrospray capillary tube was approximately 100 μm (TSP100375; Polymicro Technologies, Molex, LLC), which is larger than the ID of the original tube and thereby helps prevent clogging. The optimum air flow, pressure, and applied voltage were set to 2.0 L/min, 0.6 psi, and 2.0–3.0 kV, respectively. When the desired cone-jet mode was not achieved with these settings, pressure and applied voltage were changed to 0.2–1.0 psi and 1.3–1.9 kV, respectively, and a new buffer was made, consisting of 20 mM of ammonium acetate in pure water (990 μL) and ethanol (5–500 μL).
Particles were sampled by the DMA-CPC combination (Figure S2) after passing through a neutralizer with a Po-210 ionization source. For DMA operation, rates of sheath flow (Qsh) and aerosol flow (Qa) were set to 3.0 L/min and 0.3 L/min, respectively, for some measurements, and 6.0 L/min and 0.3 L/min for the remaining measurements. To measure the apparent electrical mobility of PSL and silica particles, size distributions of singly charged particles were scanned with the DMA using the stepping mode described by Takahata (Citation2011) and Motzkus et al. (Citation2013). The DMA was set to 12 different voltages, alternating between lower and higher values of voltage with respect to the expected peak voltage (based on the expected modal diameter). For each step, particle concentration was measured 20 s after setting the voltage and averaged over a time period of 10 s. After the voltage stepping, the voltage was set to the first voltage level, and the respective concentrations were compared to check the stability of aerosol generation (Motzkus et al. Citation2013). The DMA (TSI Model 3081) was calibrated with SC-010-S using a DMA-CPC in this stepping mode. TSI Models 3022A and 3025A condensation particle counters were used for measuring particle number concentrations.
The number-mean diameters (Dpmean) of SC-010-S (reference particles), test silica, and PSL (Thermo Scientific) were obtained from least-square best fitting of the DMA-CPC scan data to asymmetric Gaussian curves and subsequent analysis using a moment method (Ehara et al. Citation2000), a summary of which is provided in the SI (Section S3). Modal diameter (Dpmode) was determined for each DMA-CPC scan by using DMA theory to calculate the diameter associated with the peak voltage (electrical mobility) obtained from the asymmetric Gaussian curve.
Calibrated particle sizes were obtained by application of Equations (Equation1[1] ) and (Equation2
[2] ). The slip correction factor by Kim et al. (Citation2005) and Wiedensohler's approximation to the equilibrium charge distribution (Wiedensohler Citation1988) were used in the calculations:
[1]
[2] where Ztest denotes the true electrical mobility of the test particles [m2/V/s],
denotes the apparent electrical mobility of test particles [m2/V/s], Zreference denotes the true electrical mobility of reference particles [m2/V/s],
denotes the apparent electrical mobility of reference particles [m2/V/s], n denotes the number of charges [-], e denotes the elementary charge [ = 1.602 × 10−19 C], Cc denotes the Cunningham slip correction factor [-], η denotes the viscosity of the gas [Pa·s], and Dp denotes the particle mobility diameter [m].
3.2. Effective density
Effective density measurements were carried out based on the protocol by AIST (Sakurai Citation2011). Effective density is defined as the density determined from the DMA-APM (aerosol particle mass analyzer; Ehara et al. Citation1996) method, in which the particle mass distribution is measured by an APM for particles classified with known mean electrical mobility diameter by a DMA (McMurry et al. Citation2002; Tajima et al. Citation2011, Citation2013). Experiments were conducted using the experimental apparatus shown in Figure S2. PSL and silica particles were generated by electrospray and classified with a calibrated DMA. Classified particles were counted by a CPC to determine the classification voltage at the peak size (i.e., peak voltage) following the procedure described in Section 3.1. After size measurement with the DMA, particles were introduced to the APM-CPC instrumentation (Figure S2) to measure effective density. The first-generation APM (KANOMAX JAPAN, Inc., Suita City, Osaka, Japan) was utilized for this study. The governing equation can be expressed in the form of Equation (Equation3[3] ) when the centrifugal and the electrostatic forces are balanced in the center of electrode gap:
[3] where M denotes the particle mass [g], ω denotes the rotation speed [s−1], rc denotes the center of electrode gap [m], E denotes the electric field strength [V/m], V denotes the applied voltage [V], and r1 and r2 denote the radii of the inner and outer electrodes, respectively. The variables n and e are the same as described in Section 3.1.
To obtain the mass distribution, the APM and CPC were operated like a differential mobility particle sizing system (DMA and CPC) by changing the voltage applied to the APM and measuring the particle concentration for each applied voltage with the CPC. The number-average mass was obtained by analyzing the classification voltage and particle concentration data assuming they fit an asymmetric Gaussian curve. Finally, the effective density was determined based on the number-mass distribution according to the following equation:[4] where the mobility-equivalent volume is calculated as πDp3/6.
3.3. Refractive index
A schematic diagram of the apparatus used for measurement of particle refractive index is shown in Figure S3. Reference (PSL) and test (silica) particles were generated by electrospray and classified with a calibrated DMA. A suspension of each size was made by mixing 10 μL of original suspension with 990 μL of 2 mM ammonium acetate solution. Classified particles were sampled by an optical particle counter (OPC; PMS LASAIR 1002; Particle Measuring Systems, Boulder, CO, USA) after dilution to maintain the concentration below the maximum level of 1.77 particles/cm3 (50,000 particles/ft3). Light-scattering intensities were obtained from pulse height distributions of the OPC operated at high gain (100–203 nm at 0.19–10 V). A multi-channel analyzer (MCA; ORTEC EASY-MCA-2K; ORTEC, Oak Ridge, TN, USA) and associated software (ORTEC, MAESTRO ver.7.1) were used to measure the distribution of pulse height from the OPC. The OPC was first calibrated with PSL and then the test particles were measured. Refractive index was determined by inverting the response of the OPC with light-scattering theory assuming known values of particle size (as determined from DMA measurements).
In this study, only the real component n of the complex refractive index (m = n ± i·k) was evaluated. The imaginary component k was assumed to be zero for PSL and silica because these materials are transparent for visible wavelengths. Real refractive index was evaluated using Lorenz-Mie light scattering theory (Bohren and Huffman Citation1983). Theoretical OPC response can be calculated with Equation (Equation5[5] ) assuming all of the scattered light for given values of the scattering angle θ and the azimuth angle ϕ enters the detector:
[5]
The laser used for particle illumination in the LASAIR 1002 is coherent and unpolarized. Therefore, Equation (Equation5[5] ) can be expressed as (Wang Citation2002)
[6]
[7]
[8] where IA and IB denote the scattered irradiances for vertically and horizontally polarized incident light, respectively. I0 denotes the intensity of the incident light, θ and ϕ the scattering and azimuthal angles [°], r the particle radius [m], λ the wavelength of incident light (633 nm), β the collection semi-angle (18°–53°), and η the angle between axes of incident light and the collection aperture (90°). S1 and S2 are the Mie scattering amplitudes (infinite series that relate the scattered and incident electric fields), which were calculated using BHMIE code from Bohren and Huffman (Citation1983).
4. Measurement results and discussions
4.1. Particle size
The average measured mean diameter (Dpmean) and the associated standard deviation (k = 1) of the repeated measurements are reported in Table S1 for each silica size standard evaluated in this study. These values were derived from measurements made with the two DMA flow combinations, i.e., Qsh = 3.0 L/min and Qa = 0.3 L/min, and Qsh = 6.0 L/min and Qa = 0.3 L/min. The standard deviation of Dpmean ranged from 0.05 to 0.70 nm. The measured modal diameters (Dpmode) of silica particles at the two conditions are also reported in Table S1. Dpmean and Dpmode agreed within 1% (0.07%–0.95%) of each other for all nominal sizes for the condition Qsh = 3.0 L/min. Dpmean and Dpmode for the condition Qsh = 6.0 L/min had differences of 0.03%–0.85% down to 30 nm in nominal size and 2.1% for 20 nm.
Measured sizes (mean diameter) are compared to labeled sizes (modal diameter) for the silica size standards in . The measured sizes (Dpmean) agreed well with the labeled sizes. Although the differences between the measured mean and modal diameters are minor, indicating a high degree of symmetry in the silica size distributions, the measured mean diameters were in slightly better overall agreement with the labeled modal sizes than the measured modal diameters. Values of the coefficient of determination R2 between labeled size and measured size at Qsh = 3.0 L/min and Qsh = 6.0 L/min were 0.99998 and 0.99990, respectively. Sometimes, particle suspensions labeled as size standards show significant differences between labeled and measured sizes and have wider size distributions (Rudzevich et al. Citation2014), in contrast to the results from this study. shows relative size differences (measured mean size/labeled size) as a function of labeled size. Measured mean diameters agreed with labeled sizes down to 40 nm nominal size (NS-0040A; 39.1 nm labeled modal diameter) with relative differences of 0.04%–0.91%. For particle size smaller than 40 nm, the ratios of measured size to labeled size increased with decreasing size and with increasing ratio of sheath flow rate to aerosol flow rate. For 30 nm nominal (NS-0030A), measured mean and modal diameters differed from labeled sizes by 0.04%–2.0% for both flow conditions. For NS-0020A, the difference between measured mean diameter and the labeled modal diameter for the condition Qsh = 6.0 L/min was 8.4%.
Figure 1. Comparison between labeled and measured sizes of monodisperse silica particles from 20 to 201 nm for DMA Qsh = 3.0 L/min and Qsh = 6.0 L/min: 1:1 comparison (a), and relative difference (b).
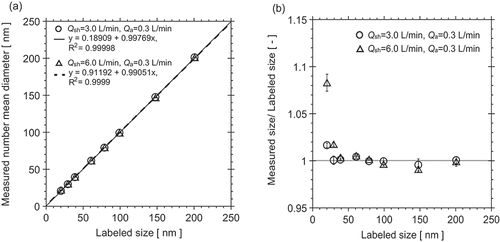
A possible reason for the larger differences between measured and labeled diameters for the smallest sizes may be a limitation of the design of the DMA that was used in this study. The DMA (TSI Model 3081) is based on the original University of Minnesota design (Liu and Pui Citation1974; Knutson and Whitby Citation1975) with a length of 44.4 cm and radii of inner and outer electrodes of 0.94 cm and 1.96 cm. From Chen et al. (Citation1999), reducing the aerosol flow rate while keeping the sheath flow rate constant enlarges the recirculation zone in the aerosol entrance section of the DMA, which moves the aerosol/sheath flow-matching position toward the upper portion of the wide entrance slit, effectively increasing the classification length of the DMA, and thereby requiring a higher voltage to classify a given particle size. Inverting the higher voltage level results in an artificially greater particle size. The size differences for 20 nm and 30 nm nominal silica sizes may stem from this problem, which may be exacerbated by diffusional broadening of the DMA transfer function at these smaller sizes.
MSP characterized silica size standards smaller than 80 nm using their 2300 Short DMA, which included the inlet modification proposed by Chen et al. (Citation1999). Measurements with this DMA were therefore not affected by the ratio of aerosol flow rate to sheath flow rate. The shorter classification length (11.9 cm) of this DMA also reduced any errors incurred from diffusional broadening and reduced the relative uncertainty in the applied voltages, which were higher than those applied by the University of Minnesota to their DMA. For 20 nm with Qsh = 3.0 L/min, the peak voltage applied to the University of Minnesota DMA was about 25 V, whereas the voltage applied to the MSP Short DMA (Qsh = 8.0 L/min) was about 250 V. Another possible contribution to the differences observed for 20 nm and 30 nm sizes may be differences in particle concentration generated at MSP and at the University of Minnesota. Much higher concentrations of 20 nm and 30 nm particles were generated for Qsh = 6.0 L/min at the University of Minnesota.
compares size distributions of silica size standards for small (20, 30 nm) and large sizes (99–201 nm) with those of comparable PSL size standards for the Qsh = 6.0 L/min DMA condition. For small sizes, silica size standards are observed to have substantially narrower size distributions than PSL size standards. Larger PSL and SiO2 size standards were found to have similar distribution widths. The data points plotted in do not represent actual size distributions obtained from deconvolution of the DMA-CPC scan data; they were generated by simply converting the applied voltage to particle size via electrical mobility. Note that the 20 nm silica particle size distribution is not shown above 24 nm, because a secondary peak was observed in the DMA scans, presumably associated with dimers. The effect of the omission of these data points on the mean diameter has not been quantified.
Figure 2. Typical size distributions measured for small and large sizes of PSL and silica for DMA Qsh = 6.0 L/min and Qa = 0.3 L/min. Plotted particle diameters were calculated from simple conversion of the applied voltages, i.e., these are not deconvoluted size distributions.
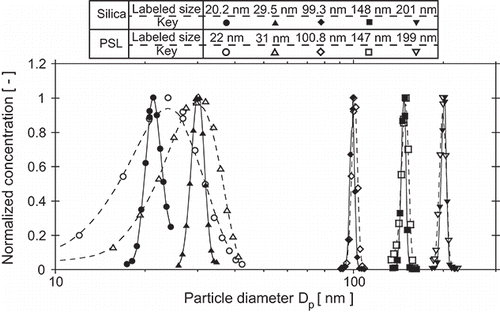
The raw data exemplified in were deconvoluted for all silica and PSL measurements for quantitative information on properties reported in Table S2, including size distribution width. Naturally, deconvoluted size distributions are narrower than those shown in . Details of the deconvolution methods and results are provided in the SI.
Focusing on 100 nm nominal size, the mean particle size and uncertainty (standard deviation; k = 1) for SC-010-S and NS-0100A were 100.8 ± 0.44 nm and 99.2 ± 0.37 nm, respectively, for Qsh = 3.0 L/min, and 100.5 ± 0.50 nm and 98.9 ± 0.20 nm, respectively, for Qsh = 6.0 L/min. These results are compared to historic measurements of 100 nm NIST Standard Reference Materials (SRM 1963 and SRM 1963a) in Table S3.
In this work, we have presumed that measured mobility diameters can be equated with actual geometric diameters. However, in careful measurements with monomobile ions in the 1 nm range, Fernandez de la Mora and coworkers (Ku and Fernandez de la Mora Citation2009; Larriba et al. Citation2011) found that geometric sizes are 0.3 nm smaller than mobility diameters (at room temperature) calculated with Equation (Equation2[2] ) of this article. Applying a 0.3 nm offset to our mobility diameters is not a valid approach, though, because the DMAs used for measurement were calibrated with standard reference particles whose certified sizes are traceable back to standard reference particles measured by other methods. For example, the 2300 Short DMA used by MSP Corporation was calibrated with SRM 1964 (60.4 nm) and SRM 1963a (101.8 nm), which were characterized by Mulholland et al. (Citation2006) using a DMA calibrated with SRM 1963 (100.7 nm). SRM 1963 was characterized by Mulholland et al. (Citation1999) using a DMA calibrated with SRM 1690, the mean diameter of which (895 nm) was measured by laser light scattering (Mulholland et al. Citation1985). Characterizations of these SRMs by Mulholland and coworkers were made before the discovery by Fernandez de la Mora and coworkers, hence the 0.3 nm difference between mobility diameter and geometric diameter was not considered in the SRM data analyses. Considering that a 0.3 nm difference is only 0.03% of 895 nm and less than 4% of the expanded uncertainty of ±8 nm, the impact of neglecting this difference when using the measured mobility of the 895 nm standard to calibrate the DMA (via sheath flow correction) for measurement of the SRM 1963 mean diameter is negligible; one can argue that the measured mean diameter of 100.7 nm ± 1 nm (expanded uncertainty) is equivalent to the geometric diameter. The same argument, in turn, can be applied to measurement of SRM 1963a and SRM 1964 modal diameters, and subsequently to measurements of the silica size standards made with the 2300 Short DMA, which was calibrated with these SRMs. However, there may be second-order nonlinear errors from the use of two SRMs for DMA calibration, which has not been considered explicitly.
Barahona et al. (Citation2015) characterized the hydrodynamic size of six silica size standards manufactured by MSP Corporation (nominal sizes of 20, 40, 60, 80, 100, 150 nm) using TEM, DLS, centrifugal liquid sedimentation (CLS), and asymmetric flow field-flow fractionation (AF4) coupled with inductively coupled plasma mass spectrometry (ICP-MS). All of the standards with the exception of the 60 nm standard were from the same batches from which suspensions were prepared for the current work. Z-average diameters measured with DLS were just 1%–4% larger than labeled modal diameters larger than 20 nm. Sizes measured by CLS were all within ±3% of the labeled sizes. They concluded that the silica nanoparticle materials “are highly monodispersed” and that appropriate dilutions remain stable for at least 8 weeks.
4.2. Effective density
Effective density was measured with the APM, with the dimensionless constant λ ranging from 0.28 to 1.17, depending on particle size. The dimensionless parameter λ depends on the mass-to-charge ratio via the relaxation time and can be interpreted as the ratio of the axial and radial traversal times. To characterize the classification performance of the APM, λ is an important parameter (Ehara et al. Citation1996), as it affects the resolution of and the particle penetration within the APM (Tajima et al. Citation2011).
Measurements of mean mass were obtained by scanning the particle mass distribution manually, because response of the APM with Qa = 0.3 L/min was too slow when the applied voltage was changed automatically. Samples of 10–300 μL of SiO2 and PSL were drawn from the original suspension bottles and diluted with appropriate volumes of ammonium acetate solution for electrospray aerosol generation to obtain sufficient particle concentrations at the outlet of the APM. Silica particles (NS-060A, NS-0100A, NS-0150A, and NS-0200A) and PSL spheres from JSR (SC-010-S) and Thermo Scientific (3100A, 3200A, 3300A) were generated and characterized in terms of size before the density measurements were performed. It was difficult to measure mass distributions of particles smaller than 60 nm due to particle losses in the APM. The effective density was determined from analysis of experimental conditions and acquired data (APM dimensions, temperature, pressure, particle counts vs. applied voltage, particle size, APM flow rate, and rotation speed) using the program “FIT_APM” provided by AIST. This program provides the number-averaged mass and the modal mass (Tajima et al. Citation2011).
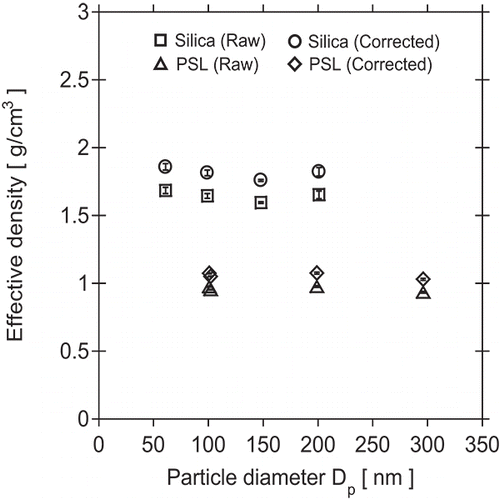
Particle density results obtained for silica and PSL, corrected for instrumentation errors, are shown in . Effective densities obtained for PSL standards were relatively constant with respect to particle size. However, PSL densities were 15.8% lower, on average, than the value of 1.05 g/cm3 specified by the manufacturer. A possible reason may be error in control of the APM rotation speed, which was not monitored. The actual rotation speed may have been higher than the set speed. Therefore, a correction factor of 1.158 was used for calculating effective densities of SiO2, resulting in values ranging from 1.84 to 1.97 g/cm3. The effective densities of the various SiO2 sizes were consistent, though the value for NS-0060A was 3.4% higher than the average value for the other sizes. In previous studies using the APM by Kanomax-Japan (Tajima et al. Citation2011, Citation2013), it was found that effective densities determined for particles smaller than around 100 nm decreased with decreasing particle size. Density measurements for particle sizes larger than around 100 nm were stable. Considering this phenomenon, the effective density of SiO2 was determined from results of NS-0100A, NS-0150A, and NS-0200A. It was found that the effective density of SiO2 created by the sol–gel synthesis process is 1.87 g/cm3. This effective density is somewhat smaller than the true material density because the volumes calculated from mobility diameters for nonspherical particles are greater than the true volume-equivalent diameters for perfect spheres.
4.3. Refractive index
PSL size standards, including JSR Catalog No. SC-010-S (100.8 nm certified size) and Thermo Scientific Catalog Nos. 3125A (125 nm), 3150A (147 nm), and 3200A (203 nm), and SiO2 size standards, including MSP Catalog Nos. NS-0150A (148 nm) and NS-0200A (201 nm), were generated by electrospray for evaluation of refractive index. Thermo Scientific 3100A and MSP NS-0100A were not evaluated because they did not provide adequate signal-to-noise ratios for accurate results. SC-010-S was generated to calibrate the DMA for classification of the other size standards. A DMA was operated at Qsh = 6.0 L/min and Qa = 0.3 L/min. PSL and SiO2 aerosols were classified at their respective peak voltages.
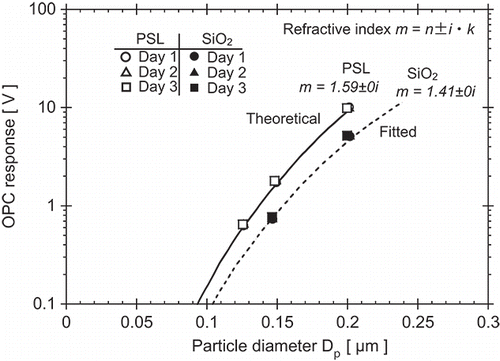
PSL and SiO2 particles were measured with a PMS Model LASAIR 1002 optical (laser) particle counter (OPC) for determination of the real component of the refractive index n. Analog signals from the OPC were inverted from negative amplitude to positive amplitude and the amplitudes (pulse heights) were measured using a multi-channel analyzer (MCA). The real part of the refractive index of SiO2 was determined from best fit of theoretical response of the OPC for variant n to the average pulse heights measured for the two SiO2 sizes. Measurements were performed on three different days. OPC response was calibrated using measurements of PSL and assuming m = 1.59 ± 0i at a wavelength of 633 nm to model the theoretical light-scattering response.
OPC response and refractive index measurement results are presented in . For SiO2, the OPC response for NS-0150A and NS-0200A was measured as 0.75 ± 0.019 V and 5.12 ± 0.070 V, respectively. From these results, the complex refractive index was determined from fitting to be m = 1.41 ± 0i. Silica is known to be transparent in the visible, so the imaginary component k was assumed to be zero. Amorphous silica (glass) and quartz have real components of 1.46 and 1.55, respectively in this wavelength range (Skinner and Appleman Citation1963). Hence, the refractive index of these SiO2 particles created by sol–gel synthesis is lower than natural forms of SiO2. The repeatability of the refractive index measurements was good for both SiO2 and PSL.
Figure 4. Experimental results of silica particle refractive index measurements using a PMS Model LASAIR 1002 calibrated with PSL.
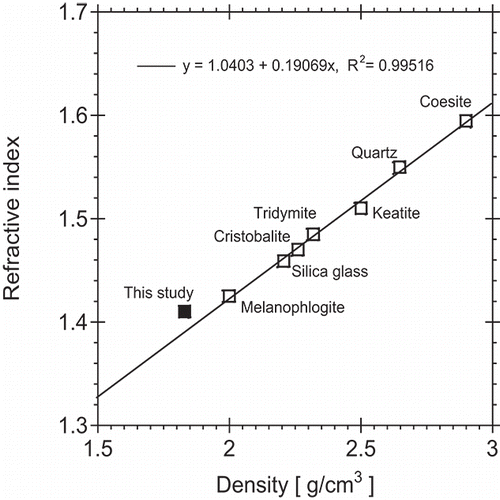
Coincidentally, the average effective density of 1.87 g/cm3 (Section 4.2) for three SiO2 size standards (nominal diameters of 100, 150, and 200 nm) is also substantially lower than density values for natural forms of SiO2, typically ranging from about 2.2 to 2.7 g/cm3 (Skinner and Appleman Citation1963). As a result, the relationship between the measured effective density and effective refractive index of the amorphous silica particles evaluated in this study agrees well with the trend for natural silica forms shown in .
Figure 5. Relationship between density and refractive index measured for amorphous silica particles in this study and comparison with values from other forms of silica from Skinner and Appleman (Citation1963).
5. Conclusions
Nanosized silica size standards made from a sol–gel synthesis process were characterized in terms of mean and modal particle size, size distribution width, effective density, and effective refractive index in this study. For particle size and effective density measurements, evaluation was conducted following protocol specified by the National Institute of Advanced Industrial Science and Technology (AIST) in Japan. Particle sizes were evaluated by electrical mobility analysis using a differential mobility analyzer operating at sheath flow rates of 3.0 and 6.0 L/min and a constant aerosol flow rate of 0.3 L/min. Measured mean and modal diameters agreed very well with the labeled modal diameters in the nominal size range 40–200 nm and size distributions were determined to be narrower than those of comparable PSL size standards for all of the investigated sizes. The relationship between the average effective density of the silica particles, measured as 1.87 g/cm3 by an aerosol particle mass analyzer, and the real component of the refractive index, determined to be 1.41 from optical particle counter measurements, was found to be consistent with the relationship between density and refractive index observed for natural forms of silica. All properties (size, effective density, and refractive index) were stable; the associated measurements were repeatable. From these evaluations, it was found that the nanosized silica size standards have good performance as size standards and are suitable alternatives to PSL spheres.
UAST_1335388_Supplementary_File.zip
Download Zip (1.8 MB)Acknowledgments
The authors thank Prof. Xiaoliang Wang (Desert Research Institute) and Drs. Kensei Ehara, Hiromu Sakurai, and Keiji Takahata (National Institute of Advanced Industrial Science and Technology) for their help on refractive index measurement, electrical mobility analysis, and the effective density measurement/calibration technique. The authors would also like to acknowledge the expert TEM sample preparation and imaging work performed by Prof. Wei Zhang at the Characterization Facility, University of Minnesota.
Funding
The authors thank the support of members of the Center for Filtration Research (CFR) at the University of Minnesota: 3M Corporation, BASF Corporation, Boeing Commercial Airplanes, Cummins Filtration Inc., Donaldson Company, Inc., Entegris, Inc., Ford Motor Co., H.B. Fuller Company, W.L. Gore & Associates, Inc., Mann + Hummel GmbH, MSP Corporation, Samsung Electronics Co., Ltd, Shigematsu Works Co., Ltd, and TSI Inc.
References
- Barahona, F., Geiss, O., Urbán, P., Ojea-Jimenez, I., Gilliland, D., and Barrero-Moreno, J. (2015). Simultaneous Determination of Size and Quantification of Silica Nanoparticles by Asymmetric Flow Field-Flow Fractionation Coupled to ICPMS Using Silica Nanoparticles Standards. Anal. Chem., 87:3039–3047.
- Bohren, C. F., and Huffman, D. R. (1983). Absorption and Scattering of Light by Small Particles. John Wiley & Sons, New York.
- Chen, D.-R., Pui, D. Y. H., Mulholland, G. W., and Fernandez, M. (1999). Design and Testing of an Aerosol/Sheath Inlet for High Resolution Measurements with a DMA. J. Aerosol Sci., 30:983–999.
- Ehara, K., Hagwood, C., and Coakley, K. J. (1996). Novel Method to Classify Aerosol Particles According to Their Mass-to-Charge Ratio—Aerosol Particle Mass Analyzer. J. Aerosol Sci., 27:217–234.
- Ehara, K., Mulholland, G. W., and Hagwood, R. C. (2000). Determination of Arbitrary Moments of Aerosol Size Distributions from Measurements with a Differential Mobility Analyzer. Aerosol Sci. Technol., 32:434–452.
- Ehara, K., Takahata, K., and Koike, M. (2006a). Absolute Mass and Size Measurement of Monodisperse Particles Using a Modified Millikan's Method: Part I—Theoretical Framework of the Electro-Gravitational Aerosol Balance. Aerosol Sci. Technol., 40:514–520.
- Ehara, K., Takahata, K., and Koike, M. (2006b). Absolute Mass and Size Measurement of Monodisperse Particles Using a Modified Millikan's Method: Part II—Application of Electro-Gravitational Aerosol Balance to Polystyrene Latex Particles of 100 nm to 1 μm in Average Diameter. Aerosol Sci. Technol., 40:521–535.
- Ehara, K., Takahata, K., Mulholland, G. W., and Hagwood, R. C. (2002). A Method to Determine Size Distribution Moments of Nano-particles from Electrical Mobility Analysis, in Proc. 6th Int. Aerosol Conf., Taipei, Taiwan, p. 1011.
- John, W. (1993). The Characteristics of Environmental and Laboratory-Generated Aerosols, in Aerosol Measurement: Principles, Techniques, and Applications, K. Willeke, and P. A. Baron, eds., Van Nostrand Reinhold, New York, p. 63.
- Kim, J. H., Mulholland, G. W., Kukuck, S. R., and Pui, D. Y. H. (2005). Slip Correction Measurements of Certified PSL Nanoparticles Using a Nanometer Differential Mobility Analyzer (Nano-DMA) for Knudsen Number from 0.5 to 83. J. Res. Natl. Inst. Stand. Technol., 110:31–54.
- Knutson, E. O., and Whitby, K. T. (1975). Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications. J. Aerosol Sci., 6:443–451.
- Ku, B. K., and Fernandez de la Mora, J. (2009). Relation between Electrical Mobility, Mass, and Size for Nanodrops 1–6.5 nm in Diameter in Air. Aerosol Sci. Technol., 43(3):241–249.
- Larriba, C., Hogan, C. J., Attoui, M., Borrajo, R., Fernandez Garcia, J. F., and Fernandez de la Mora, J. (2011). The Mobility-Volume Relationship Below 3 nm Examined by Tandem Mobility-Mass Measurement. Aerosol Sci. Technol., 45:453–467.
- Liu, B. Y. H., and Pui, D. Y. H. (1974). A Submicron Aerosol Standard and the Primary, Absolute Calibration of the Condensation Nuclei Counter. J. Colloid Interf. Sci., 47(1):155–171.
- McMurry, P. H., Wang, X., Park, K., and Ehara, K. (2002). The Relationship Between Mass and Mobility for Atmospheric Particles: A New Technique for Measuring Particle Density. Aerosol Sci. Technol., 36:227–238.
- Motzkus, C., Macé, T., Gaie-Levrel, F., Ducourtieux, S., Delvallee, A., Dirscherl, K., Hodoroaba, V.-D., Popov, I., Popov, O., Kuselman, I., Takahata, K., Ehara, K., Ausset, P., Maillé, M., Michielsen, N., Bondiguel, S., Gensdarmes, F., Morawska, L., Johnson, G. R., Faghihi, E. M., Kim, C. S., Kim, Y. H., Chu, M. C., Guardado, J. A., Salas, A., Capannelli, G., Costa, C., Bostrom, T., Jämting, Å. K., Lawn, M. A., Adlem, L., and Vaslin-Reimann, S. (2013). Size Characterization of Airborne SiO2 Nanoparticles with On-Line and Off-Line Measurement Techniques: An Interlaboratory Comparison Study. J. Nanopart. Res., 15:1919–1954.
- Mulholland, G. W., Bryner, N., Liggett, W., Scheer, B. W., and Goodall, R. K. (1996). Selection of Calibration Particles for Scanning Surface Inspection Systems, in Proc. SPIE., Flatness, Roughness, and Discrete Defect Characterization for Computer Disks, Wafers, and Flat Panel Displays, Vol. 2862, pp. 104–118.
- Mulholland, G. W., Bryner, N. P., and Croarkin, C. (1999). Measurement of the 100 nm NIST SRM 1963 by Differential Mobility Analysis. Aerosol Sci. Technol., 31:39–55.
- Mulholland, G. W., Donnelly, M. K., Hagwood, C. R., Kukuck, S. R., and Hackley, V. A. (2006). Measurement of 100 nm and 60 nm Particle Standards by Differential Mobility Analysis. J. Res. Natl. Inst. Stand. Technol., 111:257–312.
- Mulholland, G. W., Hartmann, A. W., Hembree, G. G., Marx, E., and Lettieri, T. R. (1985). Development of a One-Micrometer-Diameter Particle Size Standard Reference Material. J. Res. Natl. Bureau Standards, 90(1):3–26.
- Nanahara, S. (2003). Peripheral Technologies Supporting the Next Generation Semiconductor Factory. Latest Airborne Particle Measuring Instrument. Electron. Mater. Parts., 42:36–40 (in Japanese).
- Rudzevich, Y., Lin, Y., Wearne, A., Ordonez, A., Lupan, O., and Chow, L. (2014). Characterization of Liposomes and Silica Nanoparticles Using Resistivepulse Method. Colloids Surfaces A: Physicochem. Eng. Aspects., 448:9–15.
- Sakurai, H. (2011). Testing and Calibration protocol of Aerosol Particle Mass and an Effective Density Measurement Instrument. Available at: https://www.aist-riss.jp/projects/nedo-nanorisk/nano_rad2/docs/preparation/nano_airborne-prep_4.pdf (in Japanese).
- Skinner, B. J., and Appleman, D. E. (1963). Melanophlogite, A Cubic Polymorph of Silica. Am. Mineral., 48:854–867.
- Syedain, Z., Hunt, B., Ho, I. C., Beckwitt, K., and Dick, W. D. (2013). DMA Characterization of Sub-50 nm Silica Nanoparticle Size Standards and Comparison with PSL Size Standards, in AAAR 32nd Annual Conference, Portland, Oregon, 8NM.5.
- Tajima, N., Fukushima, N., Ehara, K., and Sakurai, H. (2011). Mass Range and Optimized Operation of the Aerosol Particle Mass Analyzer. Aerosol Sci. Technol., 45:196–214.
- Tajima, N., Sakurai, H., Fukushima, N., and Ehara, K. (2013). Design Considerations and Performance Evaluation of a Compact Aerosol Particle Mass Analyzer. Aerosol Sci. Technol., 47:1152–1162.
- Takahata, K. (2011). Relative Measurement Protocol of Particle Size by Electrical Mobility Analysis. Available at: https://www.aist-riss.jp/projects/nedo-nanorisk/nano_rad2/docs/preparation/nano_airborne-prep_1.pdf (in Japanese).
- Wang, J., Pui, D. Y. H., Qi, C., Yook, S. J., Fissan, H., Ultanir, E., and Liang, T. (2008). Controlled Deposition of NIST-Traceable Nanoparticles as Additional Size Standards for Photomask Applications. Proc. SPIE., 6922:69220G.
- Wang, X. (2002). Optical Particle Counter (OPC) Measurements and Pulse Height Analysis (PHA) Data Inversion, M.S. Thesis, University of Minnesota.
- Wiedensohler, A. (1988). An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range. J. Aerosol Sci. 19:387–389.
- Yook, S.-J., Fissan, H., Engelke, T., Asback, C., Zwaag, T., van der Kim, J. H., Wang, J., and Pui, D. Y. H. (2008). Classification of Highly Monodisperse Nanoparticles of NIST-Traceable Sizes by TDMA and Control of Deposition Spot Size on a Surface by Electrophoresis. J. Aerosol Sci., 39:537–548.