ABSTRACT
This article introduces an aerosol-based technique to make aqueous suspension of hydrophobic nanomaterial without adding dispersant. The method is intended for making a test-sample for evaluating the toxicities of nanomaterial by intra-tracheal administration. The method can wet the surface of hydrophobic nanomaterial within a few seconds. After the wetting process five to ten minutes of sonication assisted with manual stirring can fully disperse the hydrophobic nanomaterials in water. Two types of TiO2 nanomaterial were used in this study; Tayca JMT-150IB whose surfaces are coated with negatively charged hydrophobic functional group, and P25 whose surfaces are naturally hydrophilic. Nanomaterials are aerosolized by a dry-method and become micrometer-sized agglomerates. Then supersaturated water vapor is condensed onto these airborne agglomerates by using a growth tube collector. The collected suspension (CS) of hydrophobic nanomaterial (JMT-150IB) is prepared in two steps; airborne agglomerates are collected onto a flat surface then transferred to liquid-water and subsequently sonicated for complete dispersion. This method works equally well for making the CS of hydrophilic nanomaterial. Size distribution measurements of the CS show that airborne agglomerates of TiO2 dissociate into smaller units of agglomerates once they are captured into water, and the sizes of the agglomerates are in the nanometer to sub-micrometer range. Light scattering technique is used to show that a short sonication process can reproduce the particle number concentration of the CS after long storage.
Copyright © 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Toxicological effects and environmental impacts of direct and indirect exposure to nanomaterials have been a great concern (Colvin Citation2003; Maynard et al. Citation2006). Since one important pathway of direct exposure to the nanomaterial is inhalation, inhalation exposure tests have been conducted to assess their toxicities including carbon nanotubes (Nakanishi et al. Citation2015; Oberdörster et al. Citation2015), metals (Yu et al. Citation2007; Sung et al. Citation2008), and metal oxides (Vicki et al. Citation2007; Hamilton et al. Citation2009). Intra-tracheal administration (ITA) tests have been investigated as a cost-effective strategy for toxicity-screening. ITA has been implemented to assess the toxicities of TiO2 (Sager et al. Citation2008; Kobayashi et al. Citation2009; Yoshiura et al. Citation2015), SiO2 (Kajiwara et al. Citation2007), CeO2 (He et al. Citation2010), and carbon nanotubes (Lam et al. Citation2004; Park et al. Citation2011; Teeguarden et al. Citation2011).
In order to perform ITA tests a nanomaterials powder needs to be suspended in water, and dispersants are generally added to slow the rate of agglomeration. Disodium phosphate was used to prepare aqueous suspension of TiO2 during ITA tests (Gamo Citation2011). Sodium pyrophosphate (Na4P2O7) was used to prepare aqueous suspension of P25 TiO2. Na4P2O7 dissociates into multiple charged ions in water and effectively suppresses the rate of agglomeration (Jiang et al. Citation2009).
Degree of dispersion in water varies among different nanomaterials. Repulsive forces among the agglomerates TiO2 and cupper (Cu) based nanomaterials are relatively weak while repulsion among the agglomerates of SiO2, Al, Al2O3, Ag, and hydrocarbon or polysaccharide-coated Ag are stronger (Li et al. Citation2007; Murdock et al. Citation2008). It appears that the surface of oxidized inorganic nanomaterial such as SiO2, CeO2, Al2O3, and NiO are relatively hydrophilic, and these nanomaterials form stable agglomerates or remain suspended as primary particles (Kajiwara et al. Citation2007; Li et al. Citation2007; Murdock et al. Citation2008; Morimoto et al. Citation2010). It is especially difficult to suspend carbon based nanoparticles in water; therefore, dispersants are almost always added (Vaisman et al. Citation2006; Buford et al. Citation2007). Non-ionic surfactants have been used more frequently than ionic surfactants for evaluating the toxicity of CNTs since they are less cytotoxic or irritating to living cells (Lifeng et al. Citation2008). Synthetic surfactants such as Triton X-100, Tween 80, and Pluronic® have been used to disperse CNTs in water due to their low cytotoxicity (Monteiro-Riviere et al. Citation2005; Vaisman et al. Citation2006; Nakanishi Citation2011). Natural lung surfactant Survanta® is used to disperse single-wall CNT in water and reported, and it does not affect the results of in-vitro studies (Wang et al. Citation2010).
While dispersants are routinely added to prepare aqueous suspension of nanomaterials it is desirable not to add them because dispersants penetrate into the biological system and can produce confounding results when the toxicity of nanomaterials are being assessed in vitro (Jones and Grainger Citation2009). It is still unknown whether enhanced dispersion of nanomaterial is truly helpful for evaluating its cytotoxicity. Experimental studies show that superior degree of bioavailability by addition of Pluronic F127 surfactant, which is non-toxic, does not necessarily enhance the cytotoxicity of CNT (Monteiro-Riviere et al. Citation2005).
There were several methods proposed in the past which did not add dispersants as a step of making aqueous suspension of fullerenes which is hydrophobic. These methods are extended mixing, mechanical grinding, and solvent exchange. Extended mixing adds fullerenes to deionized water, and the mixture is stirred continuously over several weeks (Cheng et al. Citation2004; Ma and Bouchard Citation2009). Although the time required to prepare suspension is rather long the method has been applied for environmental/toxicological studies on fullerenes (Andrievsky et al. Citation2005; Brant et al. Citation2005; Lyon et al. Citation2006; Isaacson et al. Citation2009). After mechanical grinding some fraction of fullerenes disperses well in water (Deguchi et al. Citation2006). However, most toxicological studies minimize applying strong mechanical forces (e.g., ball-milling, compacting) to nanomaterial powder since such treatments potentially change the particle morphology, effective density, surface area (Kim et al. Citation2002; Tao et al. Citation2004; Xing et al. Citation2013), and crystal structure of the original material (Li et al. Citation2011). Another method is solvent exchange, which first dissolves fullerene to aromatic solvents such as toluene. A stable aqueous suspension is obtained after intermediate steps of exchanging solvents by evaporation and addition of water (Scrivens et al. Citation1994; Andrievsky et al. Citation2005). A simplified process is to just add and evaporate tetrahydrofuran from water (Deguchi et al. Citation2001). However, it has been suggested that significant amounts of solvent (e.g., tetrahydrofuran) remain bound over the surface of fullerene, and the solvent-fullerene system has a stronger toxicological effect than fullerene itself (Andrievsky et al. Citation2005; Brant et al. Citation2005; Deguchi et al. Citation2006; Lyon et al. Citation2006).
This study introduces an aerosol based method for making aqueous suspension of strongly hydrophobic nanomaterial without adding dispersants. The method was primarily developed to prepare nanomaterial suspension for ITA tests. First, a nanomaterial powder is aerosolized by dry-dispersion. Dry-dispersion generates agglomerates. After removing larger agglomerates above a certain aerodynamic size, water vapor is condensed onto the surface of agglomerated nanomaterial. Then nanomaterial agglomerates, which are fully or partially covered with liquid-water, are aerodynamically collected onto a flat substrate. The collected nanomaterial is dispersed in water by stirring and sonicating for five to ten minutes. There are some precautions. This method does not guarantee long-term stability of the prepared aqueous suspension. This method does not intend to simulate the degree of aggregation found in a dry-dispersed nanomaterial, and the agglomerate sizes in a prepared aqueous suspension may not be the smallest possible size. The degree of stability and agglomeration of the suspension depends on the physical-chemical properties of the nanomaterial used.
We demonstrate this method by using two types of TiO2 nanomaterial powder; Tayca JMT-150IB and Aerosil® P25. In this study, Tayca JMT-150IB and P25 represent hydrophobic and hydrophilic nanomaterial, respectively. shows the original powder of JMT-150IB floating on de-ionized water since it repels liquid water. Neither sonication nor vigorous stirring enhances dispersion unless dispersant (e.g., tween 80) is added to ∼100 ppm. shows an aqueous suspension of JMT-150IB made by the aerosol-to-liquid collection method presented in this article. JMT-150IB are well dispersed in de-ionized water, and the suspension is made reproducibly without adding any dispersant or intermediary solvent. This new method has two major advantages. First, the time to wet the surface of hydrophobic nanomaterial is an order of seconds. Second, the subsequent procedure to fully disperse hydrophobic nanomaterials into water is simple, it is just five to ten minutes of sonication assisted with manual stirring.
2. Experiments
2.1. Nanomaterials used
summarizes relevant properties of Aerosil® P25 and Tayca JMT-150IB. The surface of JMT-150IB is coated with isobutyl functional group and negatively charged. The surface is hydrophobic. The surface of Aerosil® P25 is hydrophilic, and P25 is used primarily to compare hydrophobic and hydrophilic material with respect to the efficiency of condensation onto agglomerates and the stability of particle number concentration in the liquid suspension.
Table 1. Properties of Aerosil® P25 and Tayca JMT-150IB.
2.2. Aerosol generation and measurements of aerosolized nanomaterials
2.2.1. Dry dispersion system
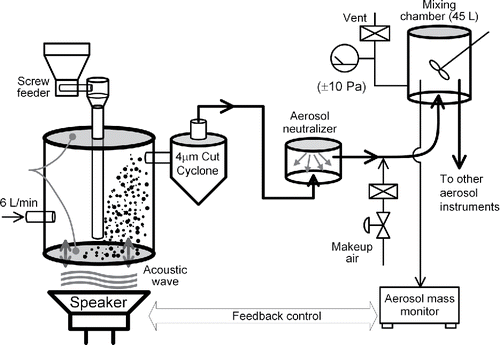
shows the schematic diagram of the dry-dispersion system in this study.
The acoustical aerosol generator (McKinney et al. Citation2009) is used to dry-disperse nanomaterial into carrier gas whose flowrate is set at 6 L/min. In this study, the device is called a vibrating-membrane powder disperser (VMPD). VMPD cannot aerosolize both types of TiO2 at sufficiently high concentrations; therefore, a powder feeder with a screw-feeding system (Nisshin Engineering, FC-uM-030F) is installed to intermittently drop small amounts of nanomaterial powder onto the bottom membrane.Footnote1
The aerosol exiting the VMPD is transported into a homemade cyclone. It collects particles whose aerodynamic diameter are greater than about 4 μm (Kenny and Gussman Citation1997). Subsequently, aerosol particles are passed through a homemade aerosol neutralizer to reduce the number of electrical charges on the aerosol particles. The neutralizer uses americium 241 nuclides as its bipolar ion source (3 MBq, Japan Radioisotope Association, AM162CE).
The electrically neutralized aerosol particles are mixed once in a stainless-steel container whose internal volume is 45 L. The aerosolized nanomaterials are sampled from the underside of the container. Two units of DC fans constantly mix the aerosol inside the container. Aerosol in the upper half region inside the container is sampled vertically downward by aerosol instruments.
2.2.2. Mass concentration
The mass concentration of aerosol particles in the mixing container is measured by an aerosol mass monitor (Thermo Scientific™ DataRAM4) and a filter-collection device. The measured values are fed back to the VMPD control program, so that the program adjusts the amplitude of the vibrating membrane to keep the concentration to a target value.
The aerosol mass monitor is a nephelometer and requires a calibration factor which depends on the optical properties and size distributions of the sampled aerosol. The calibration factor was set to unity in this study. True mass concentration in the mixing chamber is measured using the filter-collection method. Zeflour™ PTFE membrane filter (pore size 2 μm, Pall Life Sciences) was used in this study. The flowrate through the filter is set to equalize the inlet velocity of the filter-assembly and the inlet velocity of the growth tube collector.Footnote2
A mass flow controller (MFC; Lintec, Ōtsu, Shiga, Japan, Model MC-3102F-NC) regulates the flowrate through the filter, and the MFC is installed downstream of the filter. The aerosol mass concentration, , in the mixing chamber is calculated by
[1] where
is the particle mass collected on the filter during the collection time
, and
is the flowrate set to the MFC. A microbalance (Mettler Toledo, UMX2) was used to measure the mass of the filter. By weighing the filter before and after sampling, the value of
is calculated.
2.2.3. Particle size distributions
The particle size distributions of aerosolized nanomaterials are measured using a scanning mobility particle sizer (SMPS; Wang and Flagan Citation1990), an airborne optical particle counter (hereafter airborne OPC), and an aerodynamic particle sizer spectrometer (APS). The SMPS was used to measure the size distributions ranging from 14 nm to 670 nm. The SMPS classifies aerosol particles according to their mobility. The SMPS consists of an aerosol neutralizer (SOKKEN 0412M4-101) whose ionization source is 241Am, a long differential mobility analyzer (hereafter DMA, Model 3080, TSI Inc., Shoreview, MN, USA), a condensation particle counter (hereafter CPC, Model 3022A, TSI), and a platform for regulating the flowrate and controlling the voltages applied to the DMA under a scanning mode. An airborne OPC (Model KC-01E, RION) measured the distributions ranging from 0.3 μm to 10 μm. The diameter measured by airborne OPC is optically equivalent to the diameter of polystyrene latex (PSL) spheres. An APS (Model 3321, TSI) measured the size distributions ranging from 0.55 μm to 20 μm. The diameter measured by APS is aerodynamically equivalent to the diameter of PSL spheres.
2.3. Liquid-phase collection and particle measurements in liquid phase
2.3.1. Growth tube collector (GTC)
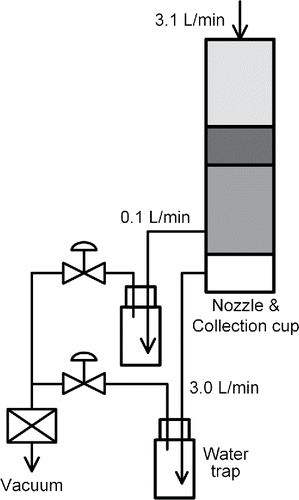
The aerosol particles of the dry dispersed nanomaterials are collected into liquid phase by using a growth tube collector (hereafter GTC, Aerosol Dynamics). A paper written by its manufacturer describes its operating principle in detail (Hering et al. Citation2014). shows the flow diagram of the GTC. The inlet flowrate was set at 3.1 L/min, and the flow is equally distributed among three growth tubes (GT) by inducing pressure drop through an acceleration nozzle at exit of each GT. The inner diameter of the nozzle is 1.3 mm. The liquid condensed on the GT-walls is extracted at the bottom of the third stage. The flowrate for extracting the condensed water into a trap was set to 0.1 L/min.
In this study, the GTC was used differently to prepare water-based suspension of hydrophobic and hydrophilic nanomaterials, the online supplementary information (SI) explains in detail. Aerosol particles of hydrophilic nanomaterial, such as P25, can be collected onto a liquid water surface by inertial-impaction of droplets after condensation. Simultaneously, the collection liquid is constantly mixed using a magnetic stirring bar to fully disperse the collected hydrophilic nanomaterial. Hydrophobic nanomaterial, such as JMT-150IB, requires a higher degree of supersaturation to activate condensational growth. Collected JMT-150IB accumulate over the surface of liquid water, and simple magnetic stirring cannot disperse them in water; therefore, JMT-150IB are first collected onto a substrate. All of the collected material is washed off from the substrate using de-ionized water and transferred to a glass beaker. After sonication and additional manual stirring in the beaker JMT-150IB becomes fully suspended. We found that the two-step procedure used for JMT-150IB was more robust and easier to reproduce than the one-step procedure used for P25. Accordingly, the two-step procedure is also recommended for collecting hydrophilic nanomaterial in future studies.
2.3.2. Evaluation of particle collection efficiencies of the GTC
The mass-based particle collection efficiency of the GTC, , was evaluated for JMT-150IB and P25. The value of
is defined as the ratio of the aerosol mass concentration based on the particle mass collected by the GTC,
, to the actual aerosol mass concentration given by Equation (Equation1
[1] ).
[2a] where the value of
is defined by
[2b] where
is the flowrate at the inlet of GTC. The mass of the powder collected and suspended in the liquid phase,
, is evaluated by filtering the collected suspension (CS). The CS was filtered through a hydrophilic membrane filter (Whatman 50). The filter was attached to the Buchner funnel with a flask to collect the suspended particles in the CS. After completing the filtration, the filter was dried for 3 to 8 h in an oven whose temperature was set to 80°C. The filter was left to cool naturally, and the mass of filter was measured using a microbalance (Mettler Toledo, UMX2). By weighing the filter before and after sampling, the value of
is calculated.
2.3.3. Particle size distribution in the collected suspension
Measured size distribution of the CS is based on particle number. A liquid-borne optical particle counter (hereafter liquid-borne OPC) was used to measure size distribution ranging from 0.25 μm to 10 μm, while the aerosol measurement technique was applied to measure size distribution ranging from 0.015 to 0.28 μm. The particles in the CS are re-dispersed into the air, and these aerosolized-particles are sampled by a SMPS.
2.3.3.1. Measurements using a liquid-borne OPC
To measure the particle number concentration accurately using a liquid-borne OPC, the suspension needs to be diluted down to a relatively low particle number concentration of approximately 1,000 particles/mL or less. The original CS, whose volume is typically about 40 mL, is diluted by a factor of 104 to 107 using ultrapure-water. Larger dilution factors are generally needed to measure smaller particle size range, and the dilution factor is defined as the sample mass before dilution to the sample mass after dilution, and the sample-mass was measured using a microbalance (Model XPE-504DR, Mettler Toledo). The liquid-borne OPC consists of three components; a particle sensor (Model KS-28B,Footnote3 RION), a syringe pump (Model KZ-30W1, RION), and a control box with readouts (Model KL-11A, RION). The readout has six size channels, and each channel displays cumulative counts. Manufacturer of the liquid OPC calibrates the threshold size of each size channel using PSL spheres. The size of each particle measured by the liquid OPC is PSL equivalent optical diameter; therefore, the measured PSD does not accurately represent the PSD of TIO2 agglomerates. However, it is more important to accurately measure the number concentration of micrometer-sized agglomerates in the CS, to study whether the micrometer-sized agglomerates found in the dry-dispersed powder are still present in the CS or not. Single particle counting instrument such as OPC is an appropriate choice for this purpose. The sampling flowrate of the syringe pump is 10 mL/min, and the syringe pump is located downstream of the sensor, and it pulls a liquid sample through the sensor.
2.3.3.2. Measurements using aerosol instrumentations
It has been proven that SMPS can accurately measure the size distribution of agglomerates in liquid suspension when the agglomerates are made of granular primary particles. A study proves that the size distributions of NiO agglomerates measured by microscopic method using SEM and the present approach using SMPS generally are in good agreement (Shimada et al. Citation2009). On the other hand, several studies showed that the agreement between the above two methods are poor for fibrous particles (Kubo et al. Citation2014; Ku and Kulkarni Citation2015). P25 and JMT-150IB are both granular; therefore, the present approach using SMPS is expected to yield accurate particle size distribution.
shows the experimental setup for measuring number based size-distributions of the particles, , in the CS within 0.015 μm to 0.28 μm particle diameter range. A constant output atomizer (TSI Model 3076) was used to aerosolize the particles in the CS. A MFC (Lintec, Model MC-3102F-NC) regulates the flowrate into the atomizer at 2.5 L/min. Atomized droplets are passed through a heated tube whose length and wall temperature are 73 cm and 120°C, respectively, to remove water content in the droplets. The aerosol is subsequently passed through a diffusion dryer (SOKKEN, Japan) to reduce water vapor content. At the inlet of SMPS the relative humidity ranges from 6 to 10%, and the temperature ranges from 21 to 25°C.
Figure 4. (a) Experimental setup for measuring the number based size-distributions, , in a CS. Measured particle diameter range is from 0.015 μm to 0.28 μm. (b) Experimental setup for collecting DMA-classified particles on a TEM grid.
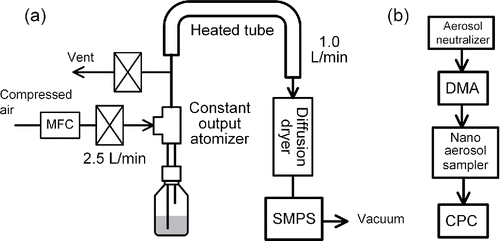
The volume of the CS is increased to 250 mL by adding ultrapure-water since the atomizer requires at least that volume. After first measurement set is completed the suspension is diluted further by a factor of four to five, and another measurement is carried out to check that the modal size of the particle size distributions does not change significantly due to the change in the probability of multiple particles being present in the atomized droplets under different dilutions. The measured values of are multiplied by a conversion factor which converts the particle number concentration of the aerosol at the inlet of SMPS to the particle number concentration of the aqueous suspension in the nebulizer bottle. The value of the conversion factor is 1.44 × 105 (particles mL−1)/(particles cm−3), and the SI explains the procedure to obtain the conversion factor.
Atomized droplets which do not contain nanomaterial become residue particles whose mobility diameters are below 20–30 nm. The size distributions of residue particles and nanomaterial always overlap in the size range; therefore, two functions are utilized to separate the size distribution of these two species.Footnote4
Particles near the peak region of the measured PSDs are collected onto a TEM grid by using a nanometer aerosol sampler (NAS, TSI-3089, Dixkens and Fissan 1999, Li et al. Citation2011). shows the experimental setup. The NAS is placed between DMA and CPC of the SMPS system, and classified mobility diameter is held constant during the collection. The images of the collected particles were taken by using a transmission electron microscope (TEM, TOPCON EM-002B).
2.3.4. Zeta potential of the collected suspension
Zeta potential of a suspension is an important parameter for qualitatively evaluating the electrical repulsive forces among particles in the liquid suspension. Zeta potential of the CS were measured using Model ZPW388 (Particles Sizing Instruments) or Malvern Zetasizer-Nano, respectively.Footnote5
2.3.5. Reproducing the particle number concentration after storage
The particles in the CS drift toward the lower half of a 100-mL bottle, and a concentration gradient is seen when the bottle is left for more than a few weeks. However, the concentration gradient readily disappears after the CS-bottles are sonicated for a few minutes. Light scattering technique was used to qualitatively evaluate whether the original state in terms of particle number concentration reproduces after a mild sonication. Zetasizer-Nano (Malvern) gives the light scattering intensity as one of the output data. It measures backward scattering (173°) from a crowd of particles in a liquid suspension to minimize the scattered light from large agglomerates in the suspension. The wavelength and power of the incident light are 632.8 nm and 4 mW, respectively. illustrates the procedure for this evaluation. The CS in the original bottle is subdivided into five different bottles on the day after a collection experiment. Then the concentration in each CS is measured at different times; 4 days, 7 days, 11 days, and 14 days later. Each CS is sonicated in an ultrasonic bath (70 W 42 kHz) for five minutes before measurement.
Figure 5. Illustration of the procedure for evaluating the stability of a CS by measuring the light scattering signals from the CS.
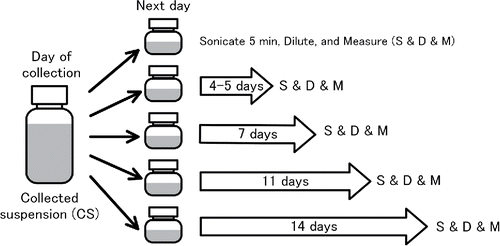
The intensity of the light scattering signals from a CS, , are not calibrated as a function of known particle number concentration; therefore, absolute value cannot be measured. Instead the concentrations of a CS at different times,
, are evaluated with respect to its initial concentration,
.
[3] where
is normalized particle number concentration at time
. The parameter
is the sensitivity coefficient between
and
, and the value was empirically evaluated. The values of
for P25 and JMT-150IB are 1.31 ± 0.14 and 1.013 ± 0.0078, respectively. It was also found that CS needs to be diluted before measurement of
to avoid the effect of multiple light scattering, and the dilution factor is set to 64. The procedure for determining the factor
is given in the SI.
3. Results and discussion
3.1. Mass concentration measurement of aerosolized nanomaterials
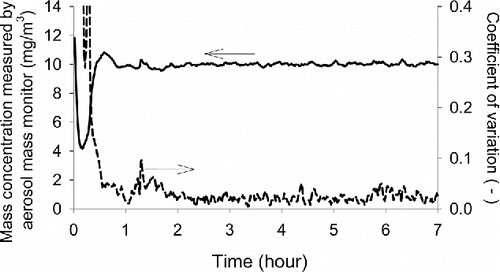
shows the time profile of the aerosol mass concentration of dry-dispersed P25 powders as measured by the aerosol mass monitor. Mass concentration is regulated at 10 mg/m3. The results for JMT-150IB are very similar to P25 and therefore are not shown here. The average value over different days and the coefficient of variation among these days are presented. It takes approximately 30 min from the start of aerosol generation until the concentration stabilizes. Once the concentration stabilizes, the VMPD with a feeding system can sustain the concentration at a target value for many hours. True mass concentration measured by a filter assembly is compared to the target value as set on the aerosol mass monitor. Then the ratio of the measured value to the true value is defined as the calibration factor in this study. For a given particle material the calibration factor changed significantly before and after cleaning the optics of DataRAM4, and the value changed from 1.27 to 0.64. On the other hand, as long as no maintenance is performed to the optics the calibration factor for P25 and JMT-150IB agreed within a few percent.
Figure 6. Aerosol mass concentration of dry-dispersed P25 powders as a function of time. An aerosol mass monitor is used to measure the concentration. The average value over the reproduced experiments and the coefficient of variation among these experiments are presented. The concentration is regulated at 10 mg/m3. The results for JMT-150IB are similar to P25 therefore not shown here.
3.2. Particle size distributions of aerosolized powders
3.2.1. Particle number concentration
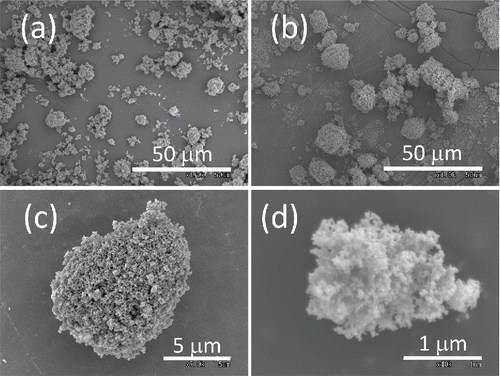
shows the particle size distribution of dry-dispersed P25. The size distributions of JMT-150IB are similar to those of P25 and therefore are not shown here. The error bars are the standard deviations among different days of experiments. The size distributions of dry-dispersed P25 or JMT-150IB are reproducible over different days. shows the scanning electron microscope (SEM) images of P25. and show the leftover on the bottom membrane of VMPD. Long hours of operation increase the size of agglomerates on the membrane. These “mature” agglomerates on the membrane look more densely packed than those of raw powder, suggesting that the number density of primary particles of an agglomerate increases after long hours of operation. and show an example of aerosolized agglomerates of P25 collected on a cascade impactor. The overall shape of airborne agglomerates is rather spherical than randomly shaped due to the packing effect of the vibrating membrane.
Figure 7. Particle number based size distribution of dry-dispersed P25 measured by SMPS, Airborne OPC, and APS. The error bars are the standard deviations among the reproduced experiments. Inset figure is the size distribution of JMT-150IB when the upper end of the measurement size range of SMPS is extended to 1 μm.
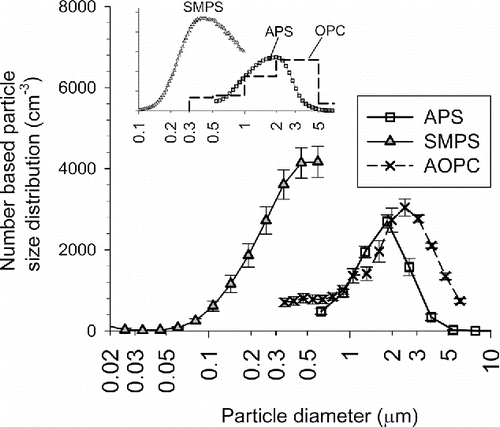
Figure 8. Scanning electron microscope (SEM) images of P25 TiO2: (a) the original powder, (b) the leftover on vibrating membrane after dry-dispersed for 15 h, (c, d) the typical agglomerates of dry-dispersed aerosol particles.
The size distributions of TiO2 agglomerates measured by SMPS, OPC, and APS do not agree well. The size distributions measured by OPC and APS indicate that the optical diameter of TiO2 agglomerates are larger than their aerodynamic diameter. Particle number concentration of TiO2 agglomerates measured by the SMPS are significantly higher than the OPC within the overlapping size range. Inset figure shows the size distribution of JMT-150IB when the upper end of the measurement size range of SMPS was set to 1 μm.Footnote6 The modal sizes are significantly different between SMPS and OPC (or SMPS and APS). The fraction of particles which have multiple electrical charges is significant in sub-micrometer range and above. Accordingly, the number concentrations measured by SMPS tend to be higher than the value measured by OPC in the overlapping size range; however, the presence of particles having multiple electrical charges cannot entirely explain the reason for the difference. The main reason for the large discrepancies among the three instruments is not investigated further in this study.
3.3. Liquid-phase collection
3.3.1. Collection efficiencies of the GTC for dry-dispersed powders
summarizes the particle collection efficiencies of the GTC for dry-dispersed P25 TiO2 and JMT-150IB TiO2. The value of evaluated for P25 TiO2 is 82% on average. These values are sufficiently high for practical use and highly reproducible with a coefficient of variation around 10%. Meanwhile, the value of
decreases to 30% when the dry-dispersed JMT-150IB TiO2 is sampled into the GTC. As shown in the aerodynamic particle size distributions of P25 and JMT-150IB are similar indicating that aerodynamic properties of these two materials are similar. The observed difference in the values of
between these two materials is most likely caused by the presence of hydrophobic coatings on JMT-150IB.
Table 2. Summary of collection efficiencies and corresponding experimental conditionsFootnote*.
also gives the particle mass concentration of CS assuming the final volume of the CS is 40 mL. The concentration ranges from 0.16 to 0.30 mg/mL. The table also shows the collection speed of the GTC used in this study; the value is expressed as the rate of particle mass collected divided by the airborne particle mass concentration. The values are around 0.15 mg/hr/(mg/m3) for P25 TiO2, and the value decreases to 0.065 mg/hr/(mg/m3) for JMT-150IB.
3.3.2. Size distribution of particles in the collected suspension
and show the size distribution of P25 and JMT-150IB particles in the CS, respectively. The number concentration is calculated assuming the final volume of the CS is 40 mL, and the collection time is 6 hours. The error bar includes the standard deviation among different collection experiments and the standard uncertainty of the conversion factor for obtaining the number concentration in liquid phase. The results show that the particle number concentrations measured by aerosol technique and liquid-borne OPC agree within their uncertainties. gives the total particle number concentration, modal size, and geometric standard deviations of the CS. Particle number concentrations are on the order of 1011 particles/mL. Particles in sub-micrometer range dominate in terms of particle number.
Figure 9. Particle size distribution (PSD) of agglomerates in a CS: (a) P25 and (b) JMT-150IB. SEMS and Liquid-borne OPC are used to measure 0.015–0.28 μm and 0.25–10 μm diameter range, respectively. The particles in the CS is aerosolized, and its PSD is measured using the SEMS.
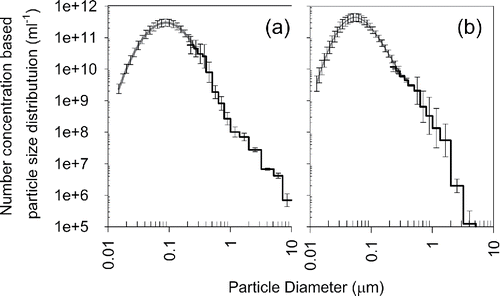
Table 3. Particle size distribution parameters, zeta potential, and pH of the collected suspension.
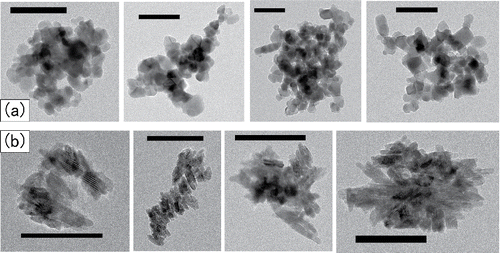
and show TEM images of P25 and JMT-150IB in the CS whose mobility diameters are near the peak of the measured PSDs. These results indicate that the micrometer-sized agglomerates in aerosol phase dissociate into smaller units of agglomerates once these agglomerates are captured into liquid. Images show that overall shape of these agglomerates is more spherical than fractal-like. Primary particles of JMT-150IB are aligned side-by-side in these agglomerates. It has been previously observed that fractal-like soot agglomerates restructure into spherical shape when water droplets containing these soot agglomerates evaporate (Ma et al. Citation2013). It is possible that agglomerates of P25 and JMT-150IB in CS have restructured when the aerosolized droplets of these CS evaporated in the diffusion dryer.
Figure 10. TEM images of the agglomerates in a CS: (a) P25 and (b) JMT-150IB. The black ribbons indicate 100 nm in length.
also shows zeta-potential and pH of the CS. The zeta potentials of P25 and JMT-150IB are significantly away from zero indicating that the surface of the agglomerate surface is ionized. Zeta potentials show that agglomerates of P25 and JMT-150IB are positively and negatively charged, respectively. The polarity of JMT-150IB indicates that negatively charged functional group are still intact on the surface of collected particles. Previous study shows that pH and zeta potential of the liquid suspension of original P25 powder. Isoelectric point of the suspension is around 6.3, and the zeta potential of the suspension is about 33 mV when its pH is around 5 (Jiang et al. Citation2009). The pH of the P25 liquid suspension made by the aerosol-to-liquid collection method ( = 4.7) is away from the reported isoelectric point indicating that the particles are not expected to rapidly agglomerate. Zeta potential of the P25 liquid suspension made by the present method ( = 25 mV) is appreciably lower than the reported value; however, it has the same polarity implying that the present method does not significantly affect the polarity and amount of surface charge.
3.3.3. Reproducing the particle number concentration after storage
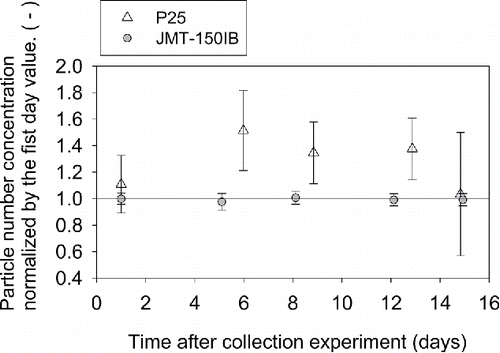
shows the particle number concentration of the CS (of P25 and JMT-150IB) as a function time which is the number of days after the collection experiment. It is noted that measured light scattering signals are normalized by the first day value, and raised to which is the sensitivity coefficient between the concentration and light scattering intensity on a logarithmic scale. The error bars represent the expanded uncertainty (k = 2) which includes the uncertainty of measured light scattering intensities and the sensitively coefficient. The concentration of CS containing JMT-150IB ranges from × 0.97 to × 1.01 of initial value, and the uncertainties are less than 5%. Concentration can be easily reproduced after sonicating the CS since the agglomerates of JMT-150IB are electrostatically repelling each other. The concentration of CS containing P25 ranges from × 1.03 to × 1.5 of the initial value, and the uncertainties ranged from 21% to 46%. It is possible that agglomerates of P25 may dissociate or agglomerate further when the CS is stored over days. This result suggests that the aerosol-to-liquid collection method proposed in this article is particularly useful for making aqueous suspension of nanomaterial whose surface is hydrophobic and electrically charged toward one polarity.
3.4. Potential mechanism of condensation
Dry-dispersed TiO2 nanomaterial consists of a large number of primary particles, and each agglomerate is expected to have large number sites where the width of the gap between adjacent primary particles ranges from zero to the size of primary particles. These sites can induce capillary condensation of water vapor (Dobbs and Yeomans Citation1992; Butt and Kappl Citation2009). Supersaturated water-vapor heterogeneously nucleate on about 30% of JMT-150IB agglomerates within the residence time (<0.5 s) in GTC implying that supersaturated water vapor diffuses into the sites which can induce capillary condensation. Then supersaturated water vapor preferentially condenses onto the sites where capillary condensation holds liquid water. Consequently, the surface of hydrophobic agglomerates holds sufficient amount of water to disperse the collected JMT-150IB in liquid water. A similar mechanism has been suggested by recent studies on the restructuring of soot aggregate exposed to super-saturated vapor (Ma et al. Citation2013). Previous experimental studies on capillary condensation set up an environment where the saturation ratio of condensing vapor ranges from below saturation to slightly below unity (Kohonen et al. Citation1999; Kohonen and Christenson Citation2000; Kim and Ehrman Citation2007; Morishige et al. Citation2014), and heterogeneous nucleation of supersaturated vapor usually assumes a single spherical particle (Fletcher Citation1958; Kulmala et al. Citation2007). More studies are needed to understand the kinetics of heterogeneous nucleation of supersaturated vapor onto the agglomerates of nanomaterials.
Conclusion
We have developed an aerosol-based method for making water-based suspension of hydrophobic nanomaterial without adding dispersants. This new method has two major advantages. First, the time to wet the surface of hydrophobic nanomaterial is an order of seconds. Second, the subsequent procedure to fully disperse hydrophobic nanomaterials into water is simple, it is just five to ten minutes of sonication assisted with manual stirring. The method is applied to make a test-sample for evaluating the toxicities of nanomaterials by intra-tracheal administration (ITA). ITA requires water-based nanomaterial suspension, and it is always better that the suspension contains minimum amounts of additives to avoid unpredictable interferences caused by the dispersant.
As an example of hydrophobic nanomaterial, JMT-150IB TiO2 whose surfaces are coated with negatively charged hydrophobic functional group is used. As an example of hydrophilic nanomaterial, P25 TiO2 whose surfaces are naturally hydrophilic is used. The method first aerosolizes nanomaterial powder by dry-dispersion. The aerosol particles of these nanomaterials are agglomerates of primary particles with the most frequent size range from 1.0 μm to 3.0 μm. Then supersaturated water vapor is condensed onto these aerosol particles to wet the particle surfaces with water. Water-condensation also increases the overall particle sizes since the aerosol particles are fully or at least partially covered with condensed water. Aerosol particles of hydrophilic nanomaterial (e.g., P25) can be collected and dispersed in water simultaneously. However, it is recommended that nanomaterials are collected in two steps regardless of its affinity to water; aerosol particles are first collected onto a flat substrate then transferred to liquid-water, and the collected particles dispersed in water separately after the collection experiment.
The most frequent sizes of particles in the collected suspension (CS) ranged from 54–80 nm showing that once agglomerates of these TiO2 nanomaterials are collected into water they dissociate into smaller units of agglomerates. The particle number concentration in the CS reproduces after being stored up to two weeks and re-dispersed just before use. Particle number concentration in the CS of JMT-150IB varies only within a few percent indicating that surface coating of JMT-150IB is preventing further agglomeration and dissociation. The number concentration in the CS of P25 varied by up to a factor of 1.5 after storage and re-dispersion indicating that further agglomeration or dissociation might be occurring in the CS of P25.
UAST_1337868_Supplementary_File.zip
Download Zip (2.8 MB)Acknowledgments
The first author would like to express his special thanks to Dr. Kazumasa Honda for giving him the opportunity to participate in this wonderful project.
Funding
This work is part of the research program “Development of innovative methodology for safety assessment of industrial nanomaterials” supported by the Ministry of Economy, Trade and Industry (METI) of Japan.
Notes
1 TiO2 powder is dropped for 2 seconds in every 30 or 60 s through a stainless-steel tube whose inner and outer diameters are 21.2 mm and 25.4 mm, respectively. The feeding rate was evaluated using a microbalance (A&D, 4212B-101), and the rate varied between 55 to 110 mg/s.
2 The flowrate through the filter was set to 3.1 L/min and 1.4 L/min when the outer diameter of the sampling tube of the filter assembly was 9.53 mm and 6.35 mm, respectively.
3 Two KS-28B sensors with different dynamic range are used to cover the size range from 0.2 μm to 10 μm: one has a range from 0.25 μm to 2.0 μm, and the other has a range from 0.5 μm to 20 μm.
4 Either an exponentially decaying function or a lognormal distribution is used for residue particles, and a lognormal distribution is used for nanomaterials. Extracted PSD of nanomaterial below 20–30 nm are merged to the measured PSD above the size range.
5 Unfortunately, the same instrument was not available to analyze the CS of P25 and JMT-150IB during the course of this study. However, at one point, zeta potentials measured by two instruments were compared by analyzing the CS of JMT-150IB. The measured values agreed within 30%.
6 The sheath flow of the differential mobility analyzer (DMA) inside the SMPS was set to 1.5 L/min while keeping the aerosol flowrate at 0.3 L/min.
References
- Andrievsky, G., Klochkov, V., and Derevyanchenko, L. (2005). Is the C60 Fullerene Molecule Toxic?! Fullerenes, Nanotubes Carbon Nanostructures, 13(4):363–376.
- Brant, J., Lecoanet, H., Hotze, M., and Wiesner, M. (2005). Comparison of Electrokinetic Properties of Colloidal Fullerenes (n-C60) Formed Using Two Procedures. Environ. Sci. Technol., 39(17):6343–6351.
- Buford, M. C., Hamilton Jr, R. F., and Holian, A. (2007). A Comparison of Dispersing Media for Various Engineered Carbon Nanoparticles. Part. Fibre Toxicol., 4(1):1–9.
- Butt, H.-J., and Kappl, M. (2009). Normal Capillary Forces. Adv. Colloid Interface Sci., 146(1):48–60.
- Cheng, X., Kan, A. T., and Tomson, M. B. (2004). Naphthalene Adsorption and Desorption from Aqueous C60 Fullerene. J. Chem. Eng. Data, 49(3):675–683.
- Colvin, V. L. (2003). The potential environmental impact of engineered nanomaterials. Nat. Biotech., 21(10):1166–1170.
- Deguchi, S., Alargova, R. G., and Tsujii, K. (2001). Stable Dispersions of Fullerenes, C60 and C70, in Water. Preparation and Characterization. Langmuir, 17(19):6013–6017.
- Deguchi, S., Mukai, S., Tsudome, M., and Horikoshi, K. (2006). Facile Generation of Fullerene Nanoparticles by Hand-Grinding. Adv. Mater., 18(6):729–732.
- Dobbs, H. T., and Yeomans, J. M. (1992). Capillary Condensation and Prewetting Between Spheres. J. Phys.: Condens. Matter, 4(50):10133.
- Fletcher, N. H. (1958). Size Effect in Heterogeneous Nucleation. J. Chem. Phys., 29(3):572–576.
- Gamo, M. (2011). Risk Assessment of Manufactured Nanomaterials: Titanium dioxide (TiO2). NEDO project (P06041) “Research and Development of Nanoparticle Characterization Methods.” 2017, Available from https://en.aist-riss.jp/assessment/2721/
- Hamilton, R. F., Wu, N., Porter, D., Buford, M., Wolfarth, M., and Holian, A. (2009). Particle Length-Dependent Titanium Dioxide Nanomaterials Toxicity and Bioactivity. Part Fibre Toxicol., 6.
- He, X., Zhang, H., Ma, Y., Bai, W., Zhang, Z., Lu, K., Ding, Y., Zhao, Y., and Chai, Z. (2010). Lung Deposition and Extrapulmonary Translocation of Nano-Ceria After Intratracheal Instillation. Nanotechnology, 21(28):285103.
- Hering, S. V., Spielman, S. R., and Lewis, G. S. (2014). Moderated, Water-Based, Condensational Particle Growth in a Laminar Flow. Aerosol Sci. Technol., 48(4):401–408.
- Isaacson, C. W., Kleber, M., and Field, J. A. (2009). Quantitative Analysis of Fullerene Nanomaterials in Environmental Systems: A Critical Review. Environ. Sci. Technol., 43(17):6463–6474.
- Jiang, J., Oberdörster, G., and Biswas, P. (2009). Characterization of Size, Surface Charge, and Agglomeration State of Nanoparticle Dispersions for Toxicological Studies. J. Nanopart. Res., 11(1):77–89.
- Jones, C. F., and Grainger, D. W. (2009). In Vitro Assessments of Nanomaterial Toxicity. Adv. Drug Deliv. Rev., 61(6):438–456.
- Kajiwara, T., Ogami, A., Yamato, H., Oyabu, T., Morimoto, Y., and Tanaka, I. (2007). Effect of Particle Size of Intratracheally Instilled Crystalline Silica on Pulmonary Inflammation. J. Occup. Health, 49(2):88–94.
- Kajiwara, T., Ogami, A., Yamato, H., Oyabu, T., Morimoto, Y., and Tanaka, I. (2007). “Effect of Particle Size of Intratracheally Instilled Crystalline Silica on Pulmonary Inflammation. J. Occup. Health, 49(2):88–94.
- Kenny, L. C., and Gussman, R. A. (1997). Characterization and Modelling of a Family of Cyclone Aerosol Preseparators. J. Aerosol Sci., 28(4):677–688.
- Kim, S., and Ehrman, S. H. (2007). Capillary Condensation onto Titania (TiO2) Nanoparticle Agglomerates. Langmuir, 23(5):2497–2504.
- Kim, Y. A., Hayashi, T., Fukai, Y., Endo, M., Yanagisawa, T., and Dresselhaus, M. S. (2002). Effect of Ball Milling on Morphology of Cup-Stacked Carbon Nanotubes. Chem. Phys. Lett., 355(3,4):279–284.
- Kobayashi, N., Naya, M., Endoh, S., Maru, J., Yamamoto, K., and Nakanishi, J. (2009). Comparative Pulmonary Toxicity Study of Nano-TiO2 Particles of Different Sizes and Agglomerations in Rats: Different Short- and Long-Term Post-Instillation Results. Toxicology, 264(1,2):110–118.
- Kohonen, M. M., and Christenson, H. K. (2000). Capillary Condensation of Water between Rinsed Mica Surfaces. Langmuir, 16(18):7285–7288.
- Kohonen, M. M., Maeda, N., and Christenson, H. K. (1999). Kinetics of Capillary Condensation in a Nanoscale Pore. Phys. Rev. Lett., 82(23):4667–4670.
- Ku, B. K., and Kulkarni, P. (2015). Measurement of Transport Properties of Aerosolized Nanomaterials. J. Aerosol Sci., 90:169–181.
- Kubo, M., Nakaoka, A., Morimoto, K., Shimada, M., Horie, M., Morimoto, Y., and Sasaki, T. (2014). Aerosol Generation by a Spray-Drying Technique Under Coulomb Explosion and Rapid Evaporation for the Preparation of Aerosol Particles for Inhalation Tests. Aerosol Sci. Technol., 48(7):698–705.
- Kulmala, M., Mordas, G., Petäjä, T., Grönholm, T., Aalto, P. P., Vehkamäki, H., Hienola, A. I., Herrmann, E., Sipilä, M., Riipinen, I., Manninen, H. E., Hämeri, K., Stratmann, F., Bilder, M., Winkler, P. M., Birmili, W., and Wagner, P. E. (2007). The Condensation Particle Counter Battery (CPCB): A New Tool to Investigate the Activation Properties of Nanoparticles. J. Aerosol Sci., 38:289–304.
- Lam, C.-W., James, J. T., McCluskey, R., and Hunter, R. L. (2004). Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days After Intratracheal Instillation. Toxicol. Sci., 77(1):126–134.
- Li, L. H., Chen, Y., Behan, G., Zhang, H., Petravic, M., and Glushenkov, A. M. (2011). Large-Scale Mechanical Peeling of Boron Nitride Nanosheets by Low-Energy Ball Milling. J. Mater. Chem., 21(32):11862–11866.
- Li, X., Zhu, D., and Wang, X. (2007). Evaluation on Dispersion Behavior of the Aqueous Copper Nano-Suspensions. J. Colloid Interface Sci., 310(2):456–463.
- Lifeng, D., Katherine, L. J., Colette, M. W., and Michael, M. C. (2008). Cytotoxicity of Single-Walled Carbon Nanotubes Suspended in Various Surfactants. Nanotechnology, 19(25):255702.
- Lyon, D. Y., Adams, L. K., Falkner, J. C., and Alvarez, P. J. J. (2006). Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size. Environ. Sci. Technol., 40(14):4360–4366.
- Ma, X., and Bouchard, D. (2009). Formation of Aqueous Suspensions of Fullerenes. Environ. Sci. Technol., 43(2):330–336.
- Ma, X., Zangmeister, C. D., Gigault, J., Mulholland, G. W., and Zachariah, M. R. (2013). Soot Aggregate Restructuring During Water Processing. J. Aerosol Sci., 66:209–219.
- Maynard, A. D., Aitken, R. J., Butz, T., Colvin, V., Donaldson, K., Oberdörster, G., Philbert, M. A., Ryan, J., Seaton, A., Stone, V., Tinkle, S. S., Tran, L., Walker, N. J., and Warheit, D. B. (2006). Safe Handling of Nanotechnology. Nature, 444(7117):267–269.
- McKinney, W., Chen, B., and Frazer, D. (2009). Computer Controlled Multi-Walled Carbon Nanotube Inhalation Exposure System. Inhal. Toxicol., 21(12):1053–1061.
- Monteiro-Riviere, N. A., Inman, A. O., Wang, Y. Y., and Nemanich, R. J. (2005). Surfactant Effects on Carbon Nanotube Interactions with Human Keratinocytes. Nanomedicine: Nanotechnology, Biology Med., 1(4):293–299.
- Morimoto, Y., Ogami, A., Todoroki, M., Yamamoto, M., Murakami, M., Hirohashi, M., Oyabu, T., Myojo, T., Nishi, K., Kadoya, C., Yamasaki, S., Nagatomo, H., Fujita, K., Endoh, S., Uchida, K., Yamamoto, K., Kobayashi, N., Nakanishi, J. and Tanaka, I. (2010). Expression of Inflammation-Related Cytokines Following Intratracheal Instillation of Nickel Oxide Nanoparticles. Nanotoxicology, 4(2):161–176.
- Morishige, K., Kawai, T., and Kittaka, S. (2014). Capillary Condensation of Water in Mesoporous Carbon. J. Phys. Chem. C, 118(9):4664–4669.
- Murdock, R. C., Braydich-Stolle, L., Schrand, A. M., Schlager, J. J., and Hussain, S. M. (2008). Characterization of Nanomaterial Dispersion in Solution Prior to in Vitro Exposure Using Dynamic Light Scattering Technique. Toxicol. Sci., 101(2):239–253.
- Nakanishi, J. (2011). Risk Assesment of Manufactured Nanomaterials: Carbon Nanotubes (CNT). NEDO project (P06041) “Research and Development of Nanoparticle Characterization Methods.” 2017, Available from https://en.aist-riss.jp/assessment/2721/
- Nakanishi, J., Morimoto, Y., Ogura, I., Kobayashi, N., Naya, M., Ema, M., Endoh, S., Shimada, M., Ogami, A., Myojyo, T., Oyabu, T., Gamo, M., Kishimoto, A., Igarashi, T., and Hanai, S. (2015). Risk Assessment of the Carbon Nanotube Group. Risk Anal., 35(10):1940–1956.
- Oberdörster, G., Castranova, V., Asgharian, B., and Sayre, P. (2015). Inhalation Exposure to Carbon Nanotubes (CNT) and Carbon Nanofibers (CNF): Methodology and Dosimetry. J. Toxicol. Environ. Health, Part B, 18(3,4):121–212.
- Park, E. J., Roh, J., Kim, S. N., Kang, M. S., Han, Y. A., Kim, Y., Hong, J. T., and Choi, K. (2011). A Single Intratracheal Instillation of Single-Walled Carbon Nanotubes Induced Early Lung Fibrosis and Subchronic Tissue Damage in Mice. Arch Toxicol., 85:1121–1131.
- Sager, T. M., Kommineni, C., and Castranova, V. (2008). “Pulmonary Response to Intratracheal Instillation of Ultrafine Versus Fine Titanium Dioxide: Role of Particle Surface Area. Part Fibre Toxicol., 5(17):19046442.
- Scrivens, W. A., Tour, J. M., Creek, K. E., and Pirisi, L. (1994). Synthesis of 14C-Labeled C60, Its Suspension in Water, and Its Uptake by Human Keratinocytes. J. Am. Chem. Soc., 116(10):4517–4518.
- Shimada, M., Wang, W.-N., Okuyama, K., Myojo, T., Oyabu, T., Morimoto, Y., Tanaka, I., Endoh, S., Uchida, K., and Ehara, K. (2009). Development and Evaluation of an Aerosol Generation and Supplying System for Inhalation Experiments of Manufactured Nanoparticles. Environ. Sci. Technol., 43(14):5529–5534.
- Sung, J. H., Ji, J. H., Park, J. D., Yun, J. U., Kim, D. S., Jeon, K. S., Song, M. Y., Jeong, J., Han, B. S., and Han, J. H. (2008). Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci., 109(2):452–461.
- Tao, Z., Geng, H., Yu, K., Yang, Z., and Wang, Y. (2004). Effects of High-Energy Ball Milling on the Morphology and the Field Emission Property of Multi-Walled Carbon Nanotubes. Mater. Lett., 58(27,28):3410–3413.
- Teeguarden, J. G., Webb-Robertson, B. J., Waters, K. M., Murray, A. R., Kisin, E. R., Varnum, S. M., Jacobs, J. M., Pounds, J. G., Zanger, R. C., and Shvedova, A. A. (2011). Comparative Proteomics and Pulmonary Toxicity of Instilled Single-Walled Carbon Nanotubes, Crocidolite Asbestos, and Ultrafine Carbon Black in Mice. Toxicol. Sci., 120(1):123–135.
- Vaisman, L., Wagner, H. D., and Marom, G. (2006). The Role of Surfactants in Dispersion of Carbon Nanotubes. Adv. Colloid Interface Sci., 128–130:37–46.
- Vicki, H. G., Patrick, T. O. S., Andrea, A.-D., John, M. P., and Thorne, P. S. (2007). Inhalation Exposure Study of Titanium Dioxide Nanoparticles with a Primary Particle Size of 2 to 5 nm. Environ. Health Perspect., 115(3):397–402.
- Wang, L., Castranova, V., Mishra, A., Chen, B., Mercer, R. R., Schwegler-Berry, D., and Rojanasakul, Y. (2010). Dispersion of Single-Walled Carbon Nanotubes by a Natural Lung Surfactant for Pulmonary in Vitro and in Vivo Toxicity Studies. Part Fibre Toxicol., 7:31–41.
- Wang, S. C., and Flagan, R. C. (1990). Scanning Electrical Mobility Spectrometer. Aerosol Sci. Technol., 13(2):230–240.
- Xing, T., Sunarso, J., Yang, W., Yin, Y., Glushenkov, A. M., Li, L. H., Howlett, P. C., and Chen, Y. (2013). Ball Milling: A Green Mechanochemical Approach for Synthesis of Nitrogen Doped Carbon Nanoparticles. Nanoscale, 5(17):7970–7976.
- Yoshiura, Y., Izumi, H., Oyabu, T., Hashiba, M., Kambara, T., Mizuguchi, Y., Lee, B. W., Okada, T., Tomonaga, T., and Myojo, T. (2015). Pulmonary Toxicity of Well-Dispersed Titanium Dioxide Nanoparticles Following Intratracheal Instillation. J. Nanopart. Res., 17(6):1–11.
- Yu, L. E., Lanry Yung, L.-Y., Ong, C.-N., Tan, Y.-L., Suresh Balasubramaniam, K., Hartono, D., Shui, G., Wenk, M. R., and Ong, W.-Y. (2007). Translocation and Effects of Gold Nanoparticles After Inhalation Exposure in Rats. Nanotoxicology, 1(3):235–242.