ABSTRACT
The recent development of an Aerosol Extinction Differential Optical Absorption Spectrometer (AE-DOAS) has allowed for the retrieval of wavelength dependent complex refractive indices for polystyrene latex spheres (PSL). The AE-DOAS is a white-type multi-pass gas cell coupled to a UV-Vis spectrometer. Refractive index values are retrieved for wavelengths between 220 and 420 nm by minimizing the χ2 goodness-of-fit between measured extinction for five diameters of PSL and model Mie Theory predictions. Comparison to literature shows agreement at wavelengths >360 nm demonstrating the validity of this new instrumental approach while expanding the known refractive index range for PSLs further into the UV where it is previously unreported. In the studied wavelength range, coefficients for the general Cauchy dispersion relationship (A = 1.538(11); B = 0.0043(16) μm2; C = 0.00094(5) μm4) for PSLs were determined using the retrieved real portion of the refractive index and the wavelength in microns. In addition, this work indicates that the precision of retrieved values is impacted by the particle diameters chosen for the experiment where retrievals for shorter wavelengths of light benefit from the study of smaller sized particles.
Copyright © 2017 American Association for Aerosol Research
EDITOR:
1. Introduction
Aerosol interact with solar radiation directly through absorption and scattering as well as indirectly by acting as cloud condensation or ice nuclei (Charlson et al. Citation1992; IPCC Citation2014). Currently, the uncertainty associated with direct interactions limits the ability of models to accurately predict radiative forcing thus constraining improvements in our understanding of climate change (IPCC Citation2014). Particle-light interactions depend on the wavelength of light, the size and shape of the particle, and the complex refractive index (CRI, m = n ± ik) (Bohren and Huffman Citation1983). The CRI is a wavelength (λ) dependent physical property of each chemical species. The real portion of the refractive index (nD) for some chemicals are reported in the CRC Handbook of Chemistry and Physics at λ = 589 nm, which is the sodium D-line denoted as the subscript (Haynes Citation2016). Other general references exist for nD values, such as Lange's Handbook of Chemistry (Speight Citation2005), but complete CRI are usually reported in the literature only as a result of specific experiments and even then are limited. Despite being more common, nD values are still not available within these reference books for some common chemicals present in ambient aerosols such as malonic acid, adipic acid, and pyrene (Rogge et al. Citation1993). In some cases the range of CRI for a chemical has been expanded by applying the Cauchy dispersion equation. This equation relates the wavelength of light to the real portion of the CRI through a series of constants which are characteristic of the chemical. While the theoretical reasoning of the equation no longer holds true, calculated indices in regions of normal dispersion are still satisfactory (Jenkins and White Citation2001).
Knowledge of the CRI, along with the size distribution and shape, of a type of aerosol are used to predict the influence on Earth's energy balance by calculating the single scattering albedo (SSA). Particularly the CRI provides information on the scattering and absorption ability of a type of aerosol. SSA is a ratio of the scattered light to the total attenuance where the dividing line between cooling and warming aerosol is 0.85 (Hansen et al. Citation1980; Ackerman and Toon Citation1981). Pure scatterers like particles composed of ammonium sulfate have a value of k = 1 × 10−7 in the visible region and represent aerosol that would have an SSA >0.85 and a net cooling effect on the atmosphere (Toon et al. Citation1976; Charlson et al. Citation1991). Particles composed of black carbon have a higher value of k, reported in the literature as k = 0.63 – 0.79 at λ = 550 nm, resulting in the absorption of solar radiation, an SSA <0.85, and ultimately a net warming effect (Bond and Bergstrom Citation2006). Often the CRI for specific chemicals, or types of aerosol, is only reported at a single wavelength, is only reported for nD, or may even be unknown and thus climate impact is uncertain.
Measurement, or retrieval, of CRIs or portions of the CRI has been done in several ways. For example, ellipsometry can be used to directly determine the CRI by collecting an aerosol sample to be prepared into a thin film where a change in polarization of light reflecting off the surface of the film is measured (Humlicek Citation2005; Liu et al. Citation2013). Another method for determining the CRI is through retrievals from other optical properties. In specific, Lack et al. (Citation2006) describes an experiment where: (Equation1[1] ) the aerosol extinction was measured with cavity ring-down spectroscopy (CRDS) and absorption was measured with photoacoustic spectroscopy (PAS) for absorbing nigrosin particles at a series of sizes, (Equation2
[2] ) the CRI was retrieved exclusively from the extinction efficiency results and then (Equation3
[3] ) a Mie theory curve was generated from the retrieved CRI to compare with the measured absorption cross section. The agreement is very good between the Mie Theory based on extinction retrieved CRI and the PAS measured absorption data (Lack et al. Citation2006). Others have followed a similar procedure for retrieving CRI for absorbing and non-absorbing aerosol which when possible compare favorably with other methods and literature values (Abo Riziq et al. Citation2007; Spindler et al. Citation2007; Dinar et al. Citation2008; Freedman et al. Citation2009; Lang-Yona et al. Citation2009; Kim et al. Citation2010; Mack et al. Citation2010; Miles et al. Citation2010; Erlick et al. Citation2011; Bluvshtein et al. Citation2012; Washenfelder et al. Citation2013; Flores et al. Citation2014). For individual refractive index components, the Abbe refractometer has been used to determine the real portion, n, of the refractive index for chemical samples by measuring diffraction of light traveling through two prisms sandwiching the sample. The imaginary part of the refractive index, k, describes the light attenuation by the bulk and is related to the absorption coefficient (Horvath Citation1993; Sun et al. Citation2007; Moosmuller et al. Citation2009). The absorption coefficient has been measured in many ways and these techniques have been reviewed recently and include the following instruments: aethalometer, particle soot absorption photometer (PSAP), micro soot sensor, multi-angle absorption photometer (MAAP), multi-filter rotating shadow band radiometer, PAS, single particle soot photometer (SP2), integrating sphere, plate or sandwich, and the subtraction method via extinction and scattering measurements (Horvath Citation1993; Moosmuller et al. Citation2009). A number of these instruments and techniques have potential filter interference requiring corrections, complicated sample preparation requirements and/or operate at a single or limited number of wavelengths.
The recent development of a broadband aerosol extinction differential optical absorption spectrometer (AE-DOAS) has allowed for the measurement of light extinction by aerosol over the spectral range of 220–1050 nm, with optimal results from 235–700 nm (Chartier and Greenslade Citation2012). The AE-DOAS can be used for the retrieval of CRI over nearly the entire solar spectrum when coupled with Mie Theory and an iterative minimization routine. To validate this application, measurements of extinction were made for polystyrene latex spheres (PSL), a common standard used to calibrate aerosol research instruments. PSLs are widely used since they are manufactured to be spherical and of a known diameter allowing Mie Theory to accurately predict extinction cross sections. The AE-DOAS measures extinction, so it is important to measure multiple diameters in order to minimize the uncertainty on the retrieved value, as scattering and absorption can respond differently to changes in particle size (Zarzana et al. Citation2014). The measurement of extinction alone represents a limitation to instruments like the AE-DOAS or CRDS in retrieving the CRI. To the best of our knowledge only French et al. have previously measured the CRI of thin film polystyrene below 360 nm (French et al. Citation2007). Washenfelder et al. retrieved CRI for polystyrene spheres between 360–420 nm based on similar optical measurements (Washenfelder et al. Citation2013) and Ma et al. measured suspensions of polystyrene spheres in water from 370–1610 nm (Ma et al. Citation2003). Two other recent studies retrieved CRI outside of the wavelength range presented in this work, specifically in the visible and IR regions with references to additional studies contained within (Miles et al. Citation2010; Zhao et al. Citation2014).
In this article, we present retrieved CRI values for PSLs in the wavelength range from 220–420 nm, where we expect larger uncertainties where there is less lamp intensity below 235 nm. Further, we investigate the relationship between particle diameter and wavelength of light with respect to the retrievals, where the differences between Rayleigh and Mie scattering become important at small particle diameters.
2. Experimental
2.1. AE-DOAS instrument description
The AE-DOAS manufactured by Cerex Monitoring Solutions (CMS UV-5000, Cerex Monitoring Solutions, Atlanta, GA, USA) consists of a xenon lamp and a detector as part of a standard UV-Vis spectrometer, plus a white-type multi-pass gas cell (Chartier and Greenslade Citation2012). The Xe lamp produces photons spanning the range from the ultraviolet to near infrared (∼220–1050 nm). The emission intensity of this lamp has a large broad peak, with substantial intensity from 275–800 nm. Lower energy wavelengths in the IR are characterized by sharp and intense emission peaks making this wavelength range challenging for measurement. Lamp variability can have a substantial effect on the measured extinction, but this variability is more significant from day to day as opposed to over the time of an experiment (Jordan et al. Citation2015). In this work, we focus on the 220–420 nm range and we minimize deviations in lamp intensity by using background measurements made before sampling. In a recent redesign of the AE-DOAS, which has also been referred to as Spectral Aerosol Extinction (SpEx), higher flow rates are used to exchange the volume within the gas cell in as little as 40 s. In addition, backgrounds are taken both before and after sampling to better understand any lamp drift, where these modifications could be used in future AE-DOAS experiments (Jordan et al. Citation2015).
In more detail, the gas cell contains one fixed (primary) and two movable (secondary) concave mirrors of equal curvature. Slight adjustments, of the secondary mirrors, allow for the path length to range in 2.44 m increments from 2.44 to 19.51 m by changing the number of internal reflections within the cell. These mirrors are not protected by any purge flow avoiding dilution corrections but degradation of mirror reflectivity could introduce uncertainty to measurements, yet this is minimized with the timing of the background measurements. The cell is made from stainless steel with dimensions of 73.0 cm × 19.2 cm × 7.5 cm creating an internal volume of around 5.8 L; it was kept grounded during experiments. Finally, the spectrometer detector consists of a grating and a 3078 linear diode array. Fiber optic cables (Ocean Optics QP600–2-SR-BX, Ocean Optics, Dunedin, FL, USA) connect the gas cell to the lamp and spectrometer. For a more detailed explanation of instrumental design and validation, see previous work by Chartier and Greenslade (Citation2012).
2.2. Sample generation and extinction measurement
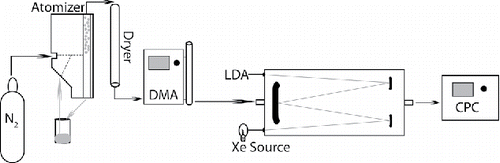
Samples were prepared from 10% (w/w) polystyrene latex sphere suspensions (Thermo Scientific, formerly Duke Scientific, Thermo Scientific, Waltham, MA, USA) by further diluting 15 drops with 20 mL of deionized water (J. T. Baker, HPLC water, J. T. Baker a brand name of Avantor Performance Materials, Center Valley, PA, USA). PSLs of five different sizes were used for the experiment including diameters (d) of 150 (3150A), 220 (HF22), 300 (HF30, 5030A), 430 (5043A), and 600 (5060A) nm. No attempts were made to remove the proprietary surfactant included during manufacturing. The sample is aerosolized in a Collison-type atomizer using nitrogen (N2) gas (May Citation1973; Liu and Lee Citation1975). The aerosol generation and further experimental design are depicted in . This aerosol entrained in nitrogen is dried to a relative humidity of <3% by passing over molecular sieves housed in a diffusion drier before entering into a differential mobility analyzer (DMA, TSI model 3080L, TSI Inc., Shoreview, MN, USA). Within the DMA, a minimally polydisperse aerosol sample is refined by first establishing a charge on the particles with a krypton source and then separating by electrical mobility within a concentric electric field between an inner rod and outer cylinder. Corrections for multiply charged particles exiting the DMA typically need to be applied to the particle number concentrations and the extinction results; however in this case, due to the nature of the sample, such a correction is not applied. Further information on multiply charged PSL particle and DMA size selection is included in the results section. This now monodisperse aerosol sample enters into the AE-DOAS where extinction is continuously measured. For these experiments, the AE-DOAS was operated at a path length between 17.00 and 19.51 m. The flow of aerosol then exits the cell and terminates at a condensation particle counter (CPC, TSI model 3775) where concentration is measured. The CPC has an uncertainty of ± 10% and this dominates the uncertainty of the extinction cross section measurements. The aerosol flow within the DMA, AE-DOAS, and CPC is kept at 0.3 L/min to promote laminar flow within the setup. Importantly, a time offset between the AE-DOAS and the CPC is determined. This is done by correlating the highest concentration measured with the CPC to the highest extinction measured with the AE-DOAS, and applying this time offset to all other measurements; the observed offset agrees with times estimated from the flow and instrument dimensions between the two locations. Typically, an experiment takes about an hour from measuring the blank before sample to purging the cell of aerosol following measurement.
Figure 1. The complete experimental design used for broadband extinction measurements. The path through the system starts on the left with aerosol generation from a sample solution using nitrogen in an atomizer block. The aerosol is then dried before size selection with a DMA. Optical extinction is measured next with the AE-DOAS and finally number concentration is measured with the CPC. The abbreviations used are: DMA: differential mobility analyzer; AE-DOAS: aerosol extinction differential optical absorption spectrometer; LDA: linear diode array; Xe source: xenon lamp; CPC: condensation particle counter.
3. Results
Collected data from individual experiments includes light intensity measurements of dry, filtered, particle free nitrogen bath gas from within the gas cell for background (I0) and of the aerosol sample (I), as well as aerosol number concentration (N). Aerosol size is controlled by manufacturing and DMA selection. This data allows for the determination of experimental extinction cross sections, Cext (cm2/particle), which can be compared to Mie Theory based calculations. First experimental extinction, σext (cm−1), is calculated using Equation (Equation1[1] ) below where l is the path length.
[1]
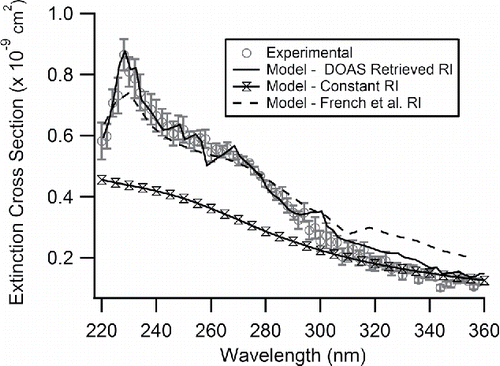
Extinction cross sections (Cext) are then calculated using the number concentration, N (particles/cm3), from the CPC using Equation (Equation2[2] ). Number concentrations are found by averaging 2.5 min of data from the CPC which corresponds to the time for the AE-DOAS to collect the light intensity data. Since there is an offset between intensity and concentration measurements from the travel time of the aerosol, a time offset described above is used. An experimentally obtained spectrum of the measured extinction cross section for PSLs with a diameter of 150 nm is represented by the open grey circles in .
[2]
Figure 2. The measured extinction cross section for 150 nm PSLs using the AE-DOAS shown as open grey circles. The error bars on the measured values represent 1 σ of the mean. Shown for comparison are Mie Theory calculations based on various CRI. The solid black line represents the CRI retrieved in this manuscript, the dashed black line represents the CRI for polystyrene-568 measured by French et al. (Citation2007), and the open hour glasses represent a constant RI supplied by the manufacturer at the wavelength of 589 nm. Points are connected by lines to guide the eye where the French et al. (Citation2007) data is reported at larger intervals than the AE-DOAS data.
In addition, the dimensionless extinction efficiency, Qext, is calculated for use in the CRI retrievals. A graph of this quantity versus the dimensionless size parameter (χ) produces a uniquely shaped curve that is distinctive for different CRI. The size parameter, defined as πd/λ, is useful when comparing the extinction of two spheres with the same CRI. Even if the spheres have different diameters as long as χ is the same, the extinction efficiency will be equal. Qext is calculated using Equation (Equation3[3] ) below where r (cm) is the radius of the particle.
[3]
Final manipulation of the data includes averaging Qext for replicate trials as well as calculating the standard deviation on Qext for each diameter. The number of trials and the experimental path lengths for each sized PSL are shown in .
Table 1. Path length and number of trials averaged to calculate Qext for the various sized PSLs. The uncertainty on the diameter of the PSL in solution ranges from ≤3% to ≤5%. Fewer trials of 600 nm spheres were completed as it was more challenging to obtain the necessary particle number concentration.
The experimental Qext average and standard deviation along with the sphere diameter and size uncertainty from the manufacturer are used for the retrieval algorithm (Washenfelder et al. Citation2013). This algorithm compares the measured extinction efficiency calculated with Equations Equation(1)–(3) to those calculated with Mie Theory for a particular CRI (Washenfelder et al. Citation2013). Both the real and imaginary portions of the CRI are varied within the retrieval to minimize the χ2 in Equation (Equation4[4] ), where NDp is an index for a particular PSL diameter, Qext is the experimental extinction efficiency, and QMie is the calculated Mie Theory extinction efficiency for some CRI:
[4]
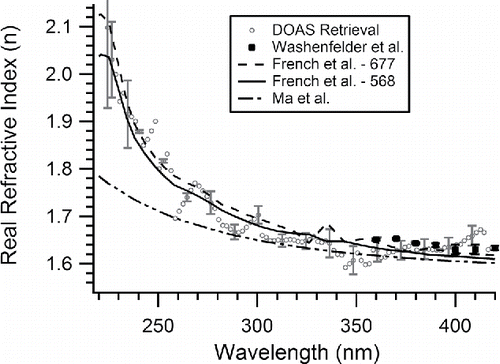
The uncertainty in the retrieved real and imaginary portions of the CRI is obtained empirically by considering the range of Qext values bounded by the uncertainty, which is on average ± 11% of Qext; the method used here is based on that described by (Washenfelder et al. Citation2013). This retrieval is repeated for the wavelength range 220–420 nm in 2 nm steps. The retrieved CRI values and uncertainties are given in Table S1 and are displayed graphically in (for the real portion (n)) and 4 (for the imaginary portion (k)). When using a DMA to size select particles for optical measurements, it is generally necessary to correct for multiply charged particles where larger sizes would have a similar mobility ratio so they could pass through the DMA and contribute to the observed number concentration and optical extinction (Hoppel Citation1978). We have considered several literature reports of the necessity of such corrections for PSL in analyzing our results. A two DMA method has been used to show polydisperse samples like ammonium sulfate can have measured extinction cross sections 17–47% greater than those predicted with Mie Theory whereas PSLs are typically only 2–12% greater (Khalizov et al. Citation2009). This small difference for PSLs was hypothesized to result from surfactant residue which prevents agglomeration and minimizes doublets but may yield small changes in diameter (Khalizov et al. Citation2009). Other researchers have noted that PSL conglomerates were observed in experiments, but when simplified and advanced correction approaches were compared, no systematic differences were observed (Petzold et al. Citation2013). Further, high linear correlation between Mie Theory including number concentration data and experimentally measured extinction values were observed yielding slopes between the two correction methods which were not statistically different, thus it was concluded no corrections were needed (Petzold et al. Citation2013). Another report noted that the DMA in use for the experiment removed aggregate and surfactant particles from the optically interrogated flow (Miles et al. Citation2010). Ultimately, PSLs are nearly monodisperse with a size distribution smaller than the DMA transfer function, they contain surfactant to prevent aggregation, and other researchers have noted low impact to their data from multiply charged particles, so we have therefore not applied corrections for multiply charged particles in this work (Khalizov et al. Citation2009; Miles et al. Citation2010; Petzold et al. Citation2013).
Figure 3. The real portion (n) of the CRI versus wavelength retrieved from this work with literature values. The open grey circles represent the retrieved values from the AE-DOAS with error bars representing the retrieval uncertainty, the solid black squares represent the data from Washenfelder et al. (Citation2013), the dashed line represents polystyrene-677 from French et al. (Citation2007), the solid black line represents polystyrene-568 from French et al. (Citation2007), and the dashed-dotted line represents the values generated from the Cauchy coefficients extrapolated below 370 nm from Ma et al. (Citation2003). Lines are used for the French et al. (Citation2007) and Ma et al. (Citation2003) data to guide the eye where data is available at larger intervals than the AE-DOAS data. Only ∼20% of the data is shown with error bars for clarity. Numerical values and uncertainties for the retrieved refractive index are presented in Table S1 in the online supplementary information.
Coefficients (A, B, and C) for the Cauchy dispersion equation were found using Equation (Equation5[5] ) where λ is the wavelength in microns and n(λ) is the real refractive index at that λ:
[5]
The retrieved PSL real refractive index data from Table S1 in the online supplementary information is fit to Equation (Equation5[5] ) using an iterative χ2 minimization in Igor Pro. The resulting Cauchy coefficients are given in with values from the literature for comparison. Others have published results for similar relationships such as the Cauchy relationship based on a few wavelengths, a modified Cauchy relationship with additional coefficients, or Sellmeier's formula. A recent and comprehensive review of the literature can be found in Miles et al. (Citation2010).
Table 2. Cauchy dispersion equation coefficients determined for polystyrene latex spheres based on fitting Equation (Equation5[5] ). This work focuses on utilizing the retrieved real refractive index at UV-Vis wavelengths from 220–420 nm. Whereas, the Ma et al. (Citation2003) values are based on the 390–1310 nm wavelength range and the Matheson and Saunderson (Citation1952) values are based on measurements from 436–767 nm.
4. Discussion
The retrieved values of the real and imaginary CRI as a function of wavelength for PSL are shown in and , respectively, and Table S1. In , the real portion (n) shows normal dispersion by an increase in the index with increasing energy (Bohren and Huffman Citation1983). Agreement with previous literature results is also shown for most regions of the spectrum. This is especially true for the Washenfelder et al. (Citation2013) data which uses a similar technique to that presented here. Briefly, Washenfelder et al. (Citation2013) aerosolized PSLs and measured extinction with either a custom broadband cavity enhanced spectrometer (BBCES) or a CRDS both of which are similar to our optical interrogation of suspended PSL but differ in the wavelengths available for measurement. The French et al. (Citation2007) results also show general agreement for the real portion over the spectrum. This study employed spectroscopic ellipsometry to interrogate thin films of two different formulations of polystyrene. These two formulations of the polymer return CRI values with some differences suggesting that if the PSLs used in this work, or by Washenfelder et al. (Citation2013), are a different formulation, then differences would be observed (Liu et al. Citation2013). Further, the manufacturer of the PSLs uses a proprietary surfactant which may stay on the surface of the aerosols after generation and this may contribute to the CRI retrieved in this work (Khalizov et al. Citation2009). The Ma et al. (Citation2003) data shows more deviation compared with all three studies, particularly below λ = 300 nm. It is important to note that the data from Ma et al. (Citation2003) has been extrapolated beyond the intended region which ends at λ = 390 nm using the reported Cauchy coefficients and the agreement with other data sets is good above 390 nm. To improve the accuracy of the Cauchy model for PSLs based on the newly retrieved real refractive index at UV wavelengths, new coefficients have been calculated and are shown in . The values compare well with the literature. The differences in coefficients may result from the significant increase in n(λ) into the UV observed in and the different wavelength ranges considered. Further, the Ma et al. (Citation2003) results may also differ because solution phase measurements of suspended PSLs were the basis of the coefficient determination and solvent effects may be possible. Whereas, the Matheson and Saunderson (Citation1952) work was completed by measuring the diffraction of light through polystyrene prisms and are bulk measurements.
Figure 4. (a) The imaginary portion (k) of the CRI versus wavelength retrieved from this work with literature values. The open grey circles represent the retrieved values from the AE-DOAS with error bars representing the retrieval uncertainty, the solid black squares represent the data from Washenfelder et al. (Citation2013), the dashed line represents polystyrene-677 from French et al. (Citation2007), and the solid black line represents polystyrene-568 from French et al. (Citation2007). Lines are used for the French et al. (Citation2007) data to guide the eye. (b) An expanded view of panel (a) in the wavelength range from 240–420 nm to show detail. Only ∼20% of the data is shown with error bars for clarity. Numerical values and uncertainties for the retrieved refractive index are presented in Table S1 in the online supplementary information.
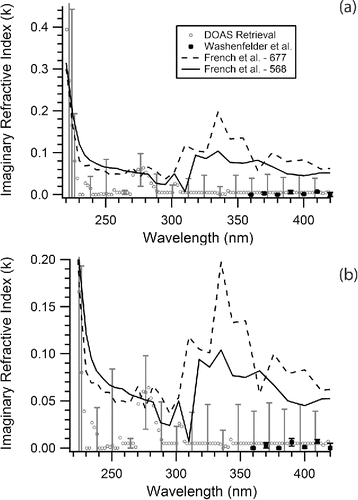
In , the retrieved imaginary portion of the CRI shows excellent agreement with the similar work of Washenfelder et al. (Citation2013). The uncertainty on a number of these retrieved values is larger than the values suggesting that the k+Δk represents an upper limit for k. Future refinements to k values for PSL in this wavelength region may benefit from a supplementary absorption measurement. PSLs show two absorption bands, one below λ = 240 nm and one centered at λ = 280 nm. While these compare well with the imaginary portion measured by French et al. (Citation2007) the band centered at 280 nm may also be a result of additional gas phase absorption, mainly acetone, which was found to occasionally persist in the lab during measurement. There are noticeable differences however between the polymers of French et al. (Citation2007) and this work. The disagreement is increased at wavelengths greater than 300 nm and is most likely caused by differences in the techniques used or the polymer formulation, as discussed above. With regard to the technique, French et al. (Citation2007) note that based on spectral differences for a single polystyrene film measured with two different spectroscopic techniques used in the study, the uncertainty of the imaginary portion is quite large at the absorption maximum and could be at other wavelengths as well. The authors also demonstrate that absorption spectra have differences between bulk and surface sampling. The films used by French et al. (Citation2007) may not replicate the same surface to bulk ratio of the PSLs causing the measured values to disagree. A final consideration is that birefringence, a measure of how the CRI varies with the direction of light propagation, has been shown to increase for polystyrene in the visible region with decreasing wavelength (Inoue et al. Citation1998). The birefringence is induced by strain from preparing the material and may not be the same between the films used by French et al. (Citation2007) and the PSLs used here.
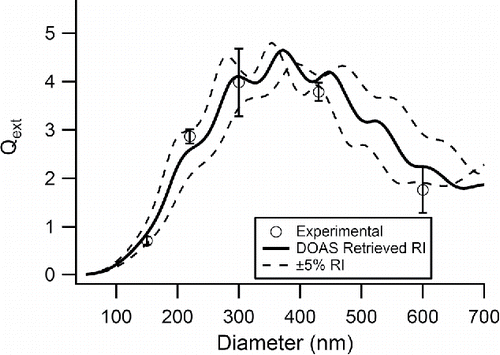
The diameter of the PSLs is an important variable depending on the wavelength of interest for a CRI retrieval. The uncertainty of the retrieved values generally increases as the wavelength of light decreases particularly into the UV region as seen in and . This increase in the uncertainty magnitude can be explained using a different representation of the experimental data. Here, and show a comparison of the measured extinction efficiency (Qext) for PSLs and calculated Mie Theory extinction efficiency (QMie) for the retrieved CRI values versus particle diameter at two different wavelengths, 228 and 351 nm, respectively. In both and , the measured Qext values are represented by open circles and the calculated Mie Theory model using the retrieved CRI is shown with a solid black line. Also included are two other Mie Theory models, shown as dashed lines calculated using ±5% of both portions of the retrieved CRI. While Qext agrees with the model at λ = 228 nm better than at λ = 351 nm, the resulting uncertainty of the retrieved real portion of the CRI for 228 nm is 0.04 which is greater than the uncertainty for 351 nm, 0.03. This is due to the fact that for each λ, the Qext becomes more sensitive to small changes of the CRI as the particle size decreases reflected by the decreasing area between the dashed lines towards the smallest diameters in and . In order to improve the precision of the retrieved CRI, it is crucial to select particles with diameters in the steep beginning portion of the curve where the variability in CRI causes only small differences in the calculated Qext. For example, at λ = 228 nm, the PSL diameters chosen for this experiment do not fall in the steep portion of the curve before d = 110 nm whereas at λ = 351 nm three of the particle diameters measured fall in this region. In this work, the extinction measurements were always completed with the same particle diameters no matter the wavelength of interest. By failing to choose sizes in the steep region, the retrieved CRI will have more uncertainty thus our results yield increases in both portions of the CRI uncertainty with decreasing wavelength. Previous literature has shown that careful selection of particle diameters allows for increased precision in retrieval of CRI with as few as two diameters (Bluvshtein et al. Citation2012).
Figure 5. Extinction efficiency versus diameter for PSLs measured at λ = 228 nm with the AE-DOAS. The open circles represent the measured values with errors bars representing 1 σ of the mean, the solid black line is the Mie Theory extinction calculated using the retrieved CRI from this work, and the dashed line represents Mie Theory extinction calculated for ±5% of both portions of the retrieved CRI.
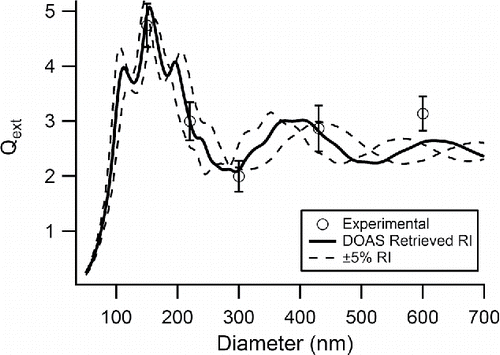
Figure 6. Extinction efficiency versus diameter for PSLs measured at λ = 351 nm with the AE-DOAS. The open circles represent the measured values with errors bars representing 1 σ of the mean, the solid black line is the Mie Theory extinction calculated using the retrieved CRI from this work, and the dashed line represents Mie Theory extinction calculated for ±5% of both portions of the retrieved CRI.
This research on PSLs demonstrates how much the CRI can vary over a range of wavelengths. The CRI variation with λ will have a large impact on light extinction as shown in . Specifically, when using the nD = 1.59 as supplied by the manufacturer at other wavelengths there is quite poor agreement between model and experiment. The model agreement looks good at wavelengths longer than 300 nm, but then begins deviating at shorter wavelengths and clearly fails to reproduce the peak in the experimental data around 220 nm. This demonstrates a need to measure the CRI for atmospherically relevant aerosol chemicals particularly in the UV region. In the visible region, it is generally the case that the variation in CRI is much less. However, at λ = 420 nm near the division between visible and UV regions, while the retrieved CRI from this work, n = 1.64, is only different from the manufacturer constant nD by 3%, the Mie Theory modeled extinction cross section varies by 5–20% depending on the particle diameter. Since the solar spectrum peaks within the visible region and still has significant intensity at wavelengths around 420 nm, this is further evidence that limited knowledge of the CRI can contribute added uncertainty in model predictions of radiative forcing.
The AE-DOAS expands the wavelength range accessible for aerosol extinction measurements into the UV to 220 nm. For the wavelength range from 235–700 nm, the AE-DOAS average detection limit based on 3σ baseline noise is 32.5 Mm−1 for a 3 min average with an order of magnitude better detection limit at specific wavelengths. A few of the other instruments which have different advantages but have been designed and used for aerosol extinction measurements are discussed here for comparison. CRDS instruments can use a variety of laser sources to measure aerosol extinction including but not limited to an Nd:YAG at λ = 355, 532, and/or 1064 nm (Sappey et al. Citation1998, Smith and Atkinson Citation2001). CRDS is incredibly sensitive and time responsive with detection limits around 0.17 Mm−1 for 1 s averaging owing to the long path lengths and fast lasers (Pettersson et al. Citation2004). Washenfelder et al. (Citation2013) developed a broadband cavity enhanced instrument, the BBCES, which measures aerosol extinction in the wavelength ranges from 360–390 nm and 385–425 nm with a precision of ∼0.19 Mm−1 for 1 min averages at the central wavelengths of 365 and 405 nm, respectively. The CAPS PMex instrument is based on the cavity attenuated phase shift technique and operates at 630 nm (and other wavelengths; see Massoli et al. Citation2010) with a detection limit of less than 0.3 Mm−1 for 60 s averaging (Kebabian and Freedman Citation2007; Kebabian et al. Citation2007). BBCES, CAPS PMex and CRDS all have versions or were specifically designed for field measurements (Baynard et al. Citation2007; Petzold et al. Citation2013; Washenfelder et al. Citation2013). Still other instruments exist for laboratory measurements of aerosol extinction and we discussed others in a previous article (Chartier and Greenslade Citation2012). The AE-DOAS is unique because it expands extinction data collection and the potential for subsequent CRI retrieval further into the UV than any of these other instruments and provides closer to continuous spectral results.
In conclusion, we have shown the ability of the AE-DOAS to retrieve CRI values at wavelengths from the visible into the UV for PSLs with good agreement with similar measurements by other researchers. We have importantly extended the wavelength range of known PSLs CRI values further into the UV. With regards to PSL characterization, these new results should allow for more accurate calibrations when used as a standard for a variety of optical measurements. We note that some differences in CRI may arise due to differences in material and our work is specifically concerned with commercially available polystyrene spheres.
In the future, this instrument can be used to optically characterize an expanded range of atmospherically relevant aerosol especially into the UV where work by others indicates light absorption by carbonaceous and secondary organic species (Hecobian et al. Citation2010; Flores et al. Citation2014). The ability of the AE-DOAS to retrieve CRI allows the balance between scattering and absorption to be quantified and the warming or cooling of a particular aerosol species to be assessed.
UAST_1339014_Supplementary_File.zip
Download Zip (126.8 KB)Acknowledgments
The authors would like to thank J. Michel Flores for supplying the refractive index retrieval algorithm. We also wish to thank Roger H. French for sharing the numerical values for the refractive indices retrieved in his 2007 publication.
Funding
This work was funded by the University of New Hampshire, College of Engineering and Physical Sciences.
References
- Abo Riziq, A., Erlick, C., Dinar, E., and Rudich, Y. (2007). Optical Properties of Absorbing and Non-Absorbing Aerosols Retrieved by Cavity Ring Down (CRD) Spectroscopy. Atmos. Chem. Phys., 7:1523–1536.
- Ackerman, T. P., and Toon, O. B. (1981). Absorption of Visible Radiation in Atmosphere Containing Mixtures of Absorbing and Nonabsorbing Particles. Appl. Optics., 20:3661–3668.
- Baynard, T., Lovejoy, E. R., Pettersson, A., Brown, S. S., Lack, D., Osthoff, H., Massoli, P., Ciciora, S., Dube, W. P., and Ravishankara, A. R. (2007). Design and Application of a Pulsed Cavity Ring-Down Aerosol Extinction Spectrometer for Field Measurements. Aerosol Sci. Tech., 41:447–462.
- Bluvshtein, N., Flores, J. M., Abo Riziq, A., and Rudich, Y. (2012). An Approach for Faster Retrieval of Aerosols' Complex Refractive Index Using Cavity Ring-Down Spectroscopy. Aerosol Sci. Tech., 46:1140–1150.
- Bohren, C., and Huffman, D. (1983). Absorption and Scattering of Light by Small Particles. John Wiley & Sons Inc, Weinheim, Germany, p. 232.
- Bond, T. C., and Bergstrom, R. W. (2006). Light Absorption by Carbonaceous Particles: An Investigative Review. Aerosol Sci. Tech., 40:27–67.
- Charlson, R. J., Langner, J., Rodhe, H., Leovy, C. B., and Warren, S. G. (1991). Perturbation of the Northern Hemisphere Radiative Balance by Backscattering from Anthropogenic Sulfate Aerosols. Tellus A, 43:152–163.
- Charlson, R. J., Schwartz, S. E., Hales, J. M., Cess, R. D., Coakley, J. A., Hansen, J. E., and Hofmann, D. J. (1992). Climate Forcing by Anthropogenic Aerosols. Science, 255:423–430.
- Chartier, R. T., and Greenslade, M. E. (2012). Initial Investigation of the Wavelength Dependence of Optical Properties Measured with a New Multi-Pass Aerosol Extinction Differential Optical Absorption Spectrometer (AE-DOAS). Atmos. Meas. Tech., 5:709–721.
- Dinar, E., Abo Riziq, A., Spindler, C., Erlick, C., Kiss, G., and Rudich, Y. (2008). The Complex Refractive Index of Atmospheric and Model Humic-Like Substances (HULIS) Retrieved by a Cavity Ring Down Aerosol Spectrometer (CRD-AS). Faraday Discuss, 137:279–295.
- Erlick, C., Haspel, M., and Rudich, Y. (2011). Simultaneous Retrieval of the Complex Refractive Indices of the Core and Shell of Coated Aerosol Particles from Extinction Measurements Using Simulated Annealing. Appl. Optics, 50:4393–4402.
- Flores, J. M., Washenfelder, R. A., Adler, G., Lee, H. J., Segev, L., Laskin, J., Laskin, A., Nizkorodov, S. A., Brown, S. S., and Rudich, Y. (2014). Complex Refractive Indices in the Near-Ultraviolet Spectral Region of Biogenic Secondary Organic Aerosol Aged with Ammonia. Phys. Chem. Chem. Phys., 16:10629–10642.
- Freedman, M. A., Hasenkopf, C. A., Beaver, M. R., and Tolbert, M. A. (2009). Optical Properties of Internally Mixed Aerosol Particles Composed of Dicarboxylic Acids and Ammonium Sulfate. J. Phys. Chem. A, 113:13584–13592.
- French, R. H., Winey, K. I., Yang, M. K., and Qiu, W. M. (2007). Optical Properties and Van Der Waals-London Dispersion Interactions of Polystyrene Determined by Vacuum Ultraviolet Spectroscopy and Spectroscopic Ellipsometry. Aust. J. Chem., 60:251–263.
- Hansen, J. E., Lacis, A. A., Lee, P., and Wang, W. C. (1980). Climatic Effect of Atmospheric Aerosols. Ann. NY Acad. Sci., 338:575–587.
- Haynes, W. M. (2016). Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data. CRC Press, Boca Raton, FL.
- Hecobian, A., Zhang, X., Zheng, M., Frank, N., Edgerton, E. S., and Weber, R. J. (2010). Water-Soluble Organic Aerosol material and the light-absorption characteristics of aqueous extracts measured over the Southeastern United States. Atmos. Chem. Phys., 10:5965–5977.
- Hoppel, W. A. (1978). Determination of the Aerosol Size Distribution from the Mobility Distribution of the Charged Fraction of Aerosols. J. Aerosol Sci., 9:41–54.
- Horvath, H. (1993). Atmospheric Light Absorption - A Review. Atmos. Environ. A-Gen., 27:293–317.
- Humlicek, J. (2005). Handbook of Ellipsometry. William Andrew Inc, Norwich, New York. pp. 3–91.
- Inoue, T., Kuwada, S., Ryu, D. S., and Osaki, K. (1998). Effects of Wavelength on Strain-Induced Birefringence of Polymers. Polym. J., 30:929–934.
- IPCC. (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Core Writting Team, R. K. Pachauri, and L. A. Meyer, eds., IPCC, Geneva, Switzerland, p. 151.
- Jenkins, F. A., and White, H. E. (2001). Fundamentals of Optics. McGraw-Hill Companies, Inc, New York, New York. p. 479.
- Jordan, C. E., Anderson, B. E., Beyersdorf, A. J., Corr, C. A., Dibb, J. E., Greenslade, M. E., Martin, R. F., Moore, R. H., Scheuer, E., Shook, M. A., Thornhill, K. L., Troop, D., Winstead, E. L., and Ziemba, L. D. (2015). Spectral Aerosol Extinction (SpEx): A New Instrument for in Situ Ambient Aerosol Extinction Measurements Across the UV/Visible Wavelength Range. Atmos. Meas. Tech., 8:4755–4771.
- Kebabian, P. L., and Freedman, A. (2007). System and Method for Trace Species Detection Using Cavity Attenuated Phase Shift Spectroscopy with an Incoherent Light Source. US Patent No. 7301639 (issued 27 November 2007).
- Kebabian, P. L., Robinson, W. A., and Freedman, A. (2007). Optical Extinction Monitor Using cw Cavity Enhanced Detection. Rev. Sci. Instrum., 78(6):063102.
- Khalizov, A. F., Xue, H. X., Wang, L., Zheng, J., and Zhang, R. Y. (2009). Enhanced Light Absorption and Scattering by Carbon Soot Aerosol Internally Mixed with Sulfuric Acid. J. Phys. Chem. A, 113:1066–1074.
- Kim, H., Barkey, B., and Paulson, S. E. (2010). Real Refractive Indices of α- and β-Pinene and Toluene Secondary Organic Aerosols Generated from Ozonolysis and Photo-Oxidation. J. Geophys. Res.-Atmos., 115(D24):D24212.
- Lack, D. A., Lovejoy, E. R., Baynard, T., Pettersson, A., and Ravishankara, A. R. (2006). Aerosol Absorption Measurement Using Photoacoustic Spectroscopy: Sensitivity, Calibration, and Uncertainty Developments. Aerosol Sci. Tech., 40:697–708.
- Lang-Yona, M., Rudich, Y., Segre, E., Dinar, E., and Abo-Riziq, A. (2009). Complex Refractive Indices of Aerosols Retrieved by Continuous Wave-Cavity Ring Down Aerosol Spectrometer. Anal. Chem., 81:1762–1769.
- Liu, B. Y. H., and Lee, K. W. (1975). Aerosol Generator of High Stability. Am. Ind. Hyg. Assoc. J., 36:861–865.
- Liu, P. F., Zhang, Y., and Martin, S. T. (2013). Complex Refractive Indices of Thin Films of Secondary Organic Materials by Spectroscopic Ellipsometry from 220 to 1200 nm. Environ. Sci. Technol., 47:13594–13601.
- Ma, X. Y., Lu, J. Q., Brock, R. S., Jacobs, K. M., Yang, P., and Hu, X. H. (2003). Determination of complex refractive index of polystyrene microspheres from 370 to 1610 nm. Phys. Med. Biol., 48:4165–4172.
- Mack, L. A., Levin, E. J. T., Kreidenweis, S. M., Obrist, D., Moosmuller, H., Lewis, K. A., Arnott, W. P., McMeeking, G. R., Sullivan, A. P., Wold, C. E., Hao, W. M., Collett, J. L., and Malm, W. C. (2010). Optical Closure Experiments for Biomass Smoke Aerosols. Atmos. Chem. Phys., 10:9017–9026.
- Massoli, P., Kebabian, P. L., Onasch, T. B., Hills, F. B., and Freedman, A. (2010). Aerosol Light Extinction Measurements by Cavity Attenuated Phase Shift (CAPS) Spectroscopy: Laboratory Validation and Field Deployment of a Compact Aerosol Particle Extinction Monitor. Aerosol Sci. Tech., 44:428–435.
- Matheson, L. A., and Saunderson, J. L. (1952). Optical and Electrical Properties of Polystyrene. in Styrene: Its Polymers, Copolymers, and Derivatives, R. H. Boundy, and R. F. Boyer, Reinhold Publishing Corp, New York, p. 517.
- May, K. R. (1973). The Collison Nebulizer: Description, Performance and Application. J. Aerosol Sci., 4:235–243.
- Miles, R. E. H., Rudic, S., Orr-Ewing, A. J., and Reid, J. P. (2010). Influence of Uncertainties in the Diameter and Refractive Index of Calibration Polystyrene Beads on the Retrieval of Aerosol Optical Properties Using Cavity Ring Down Spectroscopy. J. Phys. Chem. A, 114:7077–7084.
- Miles, R. E. H., Rudic, S., Orr-Ewing, A. J., and Reid, J. P. (2010). Measurements of the Wavelength Dependent Extinction of Aerosols by Cavity Ring Down Spectroscopy. Phys. Chem. Chem. Phys., 12:3914–3920.
- Moosmuller, H., Chakrabarty, R. K., and Arnott, W. P. (2009). Aerosol Light Absorption and its Measurement: A Review. J. Quant. Spectrosc. Ra., 110:844–878.
- Pettersson, A., Lovejoy, E. R., Brock, C. A., Brown, S. S., and Ravishankara, A. R. (2004). Measurement of Aerosol Optical Extinction at 532 nm with Pulsed Cavity Ring Down Spectroscopy. J. Aerosol Sci., 35:995–1011.
- Petzold, A., Onasch, T., Kebabian, P., and Freedman, A. (2013). Intercomparison of a Cavity Attenuated Phase Shift-Based Extinction Monitor (CAPS PMex) with an Integrating Nephelometer and a Filter-Based Absorption Monitor. Atmos. Meas. Tech., 6:1141–1151.
- Rogge, W. F., Mazurek, M. A., Hildemann, L. M., Cass, G. R., and Simoneit, B. R. T. (1993). Quantification of Urban Organic Aerosols at a Molecular Level: Identification, Abundance and Seasonal Variation. Atmos. Environ. A-Gen., 27:1309–1330.
- Sappey, A. D., Hill, E. S., Settersten, T., and Linne, M. A. (1998). Fixed-Frequency Cavity Ringdown Diagnostic for Atmospheric Particulate Matter. Opt. Lett., 23:954–956.
- Smith, J. D., and Atkinson, D. B. (2001). A Portable Pulsed Cavity Ring-Down Transmissometer for Measurement of the Optical Extinction of the Atmospheric Aerosol. Analyst, 126:1216–1220.
- Speight, J. G. (2005). Lange's Handbook of Chemistry. McGraw-Hill Education, New York, New York.
- Spindler, C., Abo Riziq, A., and Rudich, Y. (2007). Retrieval of Aerosol Complex Refractive Index by Combining Cavity Ring Down Aerosol Spectrometer Measurements with Full Size Distribution Information. Aerosol Sci. Tech., 41:1011–1017.
- Sun, H. L., Biedermann, L., and Bond, T. C. (2007). Color of Brown Carbon: A Model for Ultraviolet and Visible Light Absorption by Organic Carbon Aerosol. Geophys. Res. Lett., 34:L17813.
- Toon, O. B., Pollack, J. B., and Khare, B. N. (1976). The Optical Constants of Several Atmospheric Aerosol Species: Ammonium Sulfate, Aluminum Oxide, and Sodium Chloride. J. Geophys. Res., 81:5733–5748.
- Washenfelder, R. A., Flores, J. M., Brock, C. A., Brown, S. S., and Rudich, Y. (2013). Broadband Measurements of Aerosol Extinction in the Ultraviolet Spectral Region. Atmos. Meas. Tech., 6:861–877.
- Zarzana, K. J., Cappa, C. D., and Tolbert, M. A. (2014). Sensitivity of Aerosol Refractive Index Retrievals Using Optical Spectroscopy. Aerosol Sci. Tech., 48:1133–1144.
- Zhao, W., Xu, X., Dong, M., Chen, W., Gu, X., Hu, C., Huang, Y., Gao, X., Huang, W., and Zhang, W. (2014). Development of a Cavity-Enhanced Aerosol Albedometer. Atmos. Meas. Tech., 7:2551–2566.
