ABSTRACT
A laser-induced breakdown spectrometer (LIBS) was developed for determining the elemental composition of individual airborne particles. The system employs two lasers focused on a narrow beam of particles. A continuous wave laser placed upstream scatters light from particles, while a pulse laser downstream ablates the particles. The scattered light from the upstream laser is used to trigger the downstream pulse laser, resulting in more accurate hitting of the particles than a free-firing laser system without the triggering signal (i.e., constant pulse laser firing). Various laboratory-generated aerosols (NaCl, MgCl2, KCl, and CaCl2) were used to evaluate the newly developed LIBS system. Particles were tightly focused into a center line with a sheath air focusing system using an optimum aerosol-to-sheath air velocity ratio. The locations of both the scattering laser and pulse laser beams were precisely controlled by a motorized X-Y stage controller. Data showed that for the LIBS with the triggering system, the hitting efficiency (%) of particles (200–600 nm) significantly increased (e.g., 350 nm particles had more than 26 times higher hitting efficiency at 1,000 particles/cm3), and much lower limits of particle size (∼200 nm) and number concentration (<100 particles/cm3) were achieved compared to the free-firing laser condition. Additionally, the hitting rate (hits/min) significantly increased with the triggering system. Our results suggest that the LIBS with the triggering system can be useful for real-time detection of elements of particles existing at low number concentrations (e.g., atmospheric particles) and for the determination of the variation of elemental composition among particles.
© 2017 American Association for Aerosol Research
EDITOR:
Introduction
Fine particulate matter (PM) in the ambient atmosphere are of current interest because they affect climate change (earth's radiation balance and cloud formation) (IPCC Citation2007), visibility impairment (Sloane and Richards Citation1991), and human health (Dockery and Pope Citation1994). It is essential to determine the chemical components of the ambient atmospheric aerosols to find their source and formation mechanisms and to better understand their effects on climate change and human health. To determine the elemental composition of the ambient particles, the filter-based technique has been widely used (Orsini et al. Citation2003). In the filter-based technique, the ambient particles passing through PM inlets were collected onto filters for 12–24 h and then the collected PM samples were pretreated for subsequent analysis by inductively coupled plasma-mass spectrometry (ICP-MS) or atomic absorption spectroscopy (AAS) (Tursic et al. Citation2008; Okuda et al. Citation2013). The long sampling time for the filter-based technique makes it difficult to determine the temporal variation of aerosol characteristics, which suffer from sampling artifacts due to evaporation of semi-volatile particles (negative artifacts) and condensation/adsorption of vapors (positive artifacts) (Chow et al. Citation2008). Additionally, particle-particle variation cannot be obtained due to the bulk analysis of collected particles. Atmospheric particles are often externally mixed, with each particle having different chemical composition. Particle-particle variation and mixing state can be determined by single particle elemental analysis.
A laser-induced breakdown spectrometer (LIBS) is a useful tool for the rapid detection of the elemental composition of particles (Madhavi et al. Citation1999; Cheng Citation2000; Hahn and Lunden Citation2000; Fisher et al. Citation2001; Mukherjee et al. Citation2006; Hettinger et al. Citation2006; Park et al. Citation2009; Gallou et al. Citation2011; Diwakar et al. Citation2011; Kwak et al. Citation2012; Kim et al. Citation2012, Citation2013). In the LIBS, a high power pulse laser hits a single particle or collected particles to generate microplasma (excited state).The excited atoms and ions emit light with specific wavelengths as they cool down to the ground state, where the light can be used to determine elements in a single particle or an ensemble of particles in real-time (Park et al. Citation2009). The broad range of emission lines (e.g., 200–900 nm) can be used for simultaneous identification of multiple elements after the single laser shot. No sample pretreatment is required for the LIBS technique, and the LIBS measurement can be performed nearly at atmospheric pressure (i.e., an expensive high vacuum system is not required unlike the mass spectrometry technique that uses laser ionization or other ionization methods) (Suess and Prather Citation1999). In our previous research (Park et al. Citation2009 2012), LIBS with a particle collection substrate was developed to determine hourly concentrations of elements (e.g., Al, Ca, Fe, Cr, Pb, and Zn) in the ambient atmospheric particles. However, this type of LIBS technique is not able to provide elemental composition of a single particle to determine particle-particle variation.
For a single particle detection, one particle must exist in the laser plasma. A low concentration of aerosols and narrow laser and particle beams are necessary for a single particle to be present in the laser plasma. By precisely controlling the diameters of the laser and particle beams, a single particle detection is possible under constant laser firing at 1–10 Hz (i.e., free-firing laser condition) (Park et al. Citation2009). However, at low concentration, which is typical for the ambient atmospheric aerosols, the hitting efficiency ( =the number of particle hits by pulse laser/the number of pulse laser shots) and the hitting rate ( =the number of particle hits by pulse laser/time) was significantly low under the free-firing laser condition (i.e., a number of particles were not hit by laser) (Park et al. Citation2009). Thus, a LIBS system that is designed to trigger the pulse laser at pre-determined delay time intervals based on particle velocity through a focusing nozzle can improve the hitting efficiency and the hitting rate of particles when air borne concentrations are low. The LIBS with a triggering system was used for single particle detection of bioaerosols (Tjärnhage et al. Citation2013; Larsson et al. Citation2016). They showed an improved hitting rate for micrometer particles with the triggering system. However, single particle detection of submicrometer was not conducted with a triggering LIBS system, to our best knowledge.
In this study, LIBS with a triggering system was developed for the detection of elements in a single particle in real-time. This system employs an additional continuous wave (CW) laser that is focused on the particle beam upstream from the pulse laser. When particles cross the scattering laser, the scattering light pulse is generated to trigger the downstream pulse laser to hit airborne particles at the pre-determined delay time. This triggering method can be useful for the detection of particles existing at low number concentrations, leading to the improvement of particle hitting efficiency and hitting rate as well as the determination of the elemental composition variation among single particles. Evaluation of the LIBS with the triggering system was conducted by using laboratory-generated particles (NaCl, MgCl2, KCl, and CaCl2) with varying number concentrations (100–100,000 particles/cm3) and sizes (200–600 nm). Additionally, a comparison of particle hitting efficiency and hitting rate between the triggering and free-firing conditions was conducted.
Experimental method
A schematic of the experimental setup is shown in . The LIBS system mainly consists of an aerosol chamber, a scattering laser, a pulse laser, and a spectrometer. Theaerosol chamber employs a sheath air focusing nozzle system to produce a narrow beam of particles (i.e., particle beam). The ratio of aerosol air to sheath air velocity was optimized to focus aerosols narrowly into the center of the chamber. This was evaluated by measuring the particle hitting efficiency with the varying air velocity ratio. During the triggering condition, particles were first detected by a continuous wave (CW) laser (642 nm, 35 mW, Excelsior Laser, Spectra Physics Inc., USA). The scattering light from the particles was measured with a photomultiplier tube (PMT) (H10722–20, Hamamatsu Inc., Iwata, Shizuoka, Japan). The scattered optical signal was used as a trigger source to fire the pulsed laser (1064 nm, 200 mJ/pulse, CFR 200, Quantel Inc., USA) (1064 nm, 650 mJ/pulse, Surelite II-10, Continuum Inc., USA) by synchronizing the arrival time of the particle at the pulsed laser plasma. To maximize the hitting efficiency, the flash lamp delay time was controlled while Q-switch delay time and spectrometer gate delay time were fixed. An oscilloscope (WaveRunner 104MXi, Lecroy Inc., USA) was used to send a pulse signal to the laser with a controlled time interval. The particle beam size is ∼1 mm. The focal volume of the scattering and pulse lasers are estimated to be 5 mm3 and 1 × 10−3 mm3, respectively. The time interval was adjusted to obtain the maximum hitting efficiency for 350 nm particles. The number of particle hits was determined by identifying emission peak in the spectrum. Values for the Q-switch delay time and the spectrometer gate delay time (=delay time to obtain emission spectrum after plasma generation) are summarized in .
Figure 1. A schematic of experiment setup including a top view of an aerosol chamber and a trigger/pulse timing diagram.
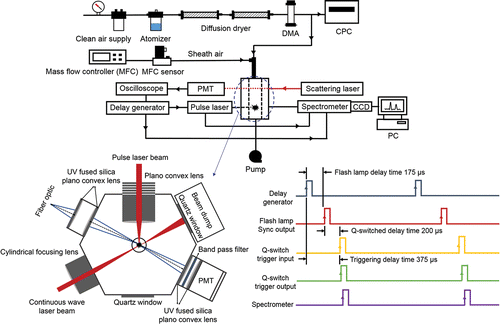
Table 1. A summary of parameters used for the triggering LIBS system.
The distance between the tip of the nozzle and the scattering laser beam is 2 mm, and the distance between the scattering laser beam and the pulse laser beam is 3 mm. The laser beam was fired, focusing narrowly into the center of the chamber through a plano-convex lens (focal length = 25 mm). After the laser shot, the emitted light was detected by collecting lens and was transmitted to the spectrometer by fiber optic cables. The broadband spectrometer (Aurora, Applied Spectra Inc., USA) with a charge-coupled device (CCD) detector with a spectral resolution of <0.1 nm for UV to VIS and <0.12 nm for VIS to NIR was used to measure the emitted light at wavelengths of 200–980 nm, simultaneously. A delay generator (BNC565, Berkeley Inc., USA) was used to provide a specific time delay before the spectrometer started to collect the emitted light from the microplasma. This minimizes the continuum emission lines which are typically produced at the initial stage after the microplasma and enhances the atomic emission lines of the LIBS spectra (Pasquini et al. Citation2007; Kwak et al. Citation2009). The LIBS spectra were analyzed by Aurora software (Applied Spectra Inc.). Under the free-firing condition, the scattering laser was off and the pulse laser fired under a constant frequency (1–10 Hz).
For the evaluation of the LIBS system, various laboratory-generated aerosols were used. A constant output atomizer (DS-A103, Dongsung Industry Inc., Korea) was used to produce particles from NaCl (Junsei Chemical, Japan), MgCl2 (Sigma-Aldrich, USA), KCl (Junsei Chemical), and CaCl2 (Sigma-Aldrich) in deionized (DI) water solutions. The aerosolized particles were dried out by a series of diffusion dryer before entering the LIBS chamber. To generate the monodisperse particles, a differential mobility analyzer (DMA) (Model 3081, TSI Inc., Shorview, MN, USA) was used to select particles of a certain size before they were introduced into the chamber. To measure the number concentration of particles, a condensation particle counter (CPC) (Model 3022, TSI Inc.) was used.
Results and discussion
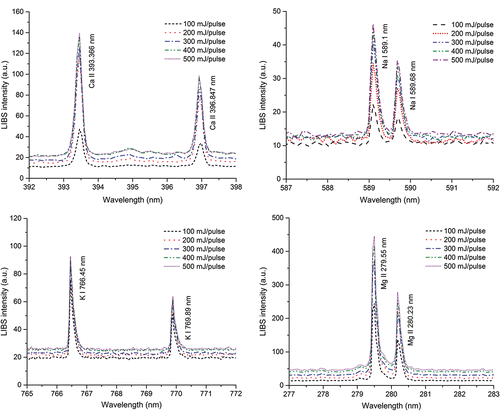
Examples of the LIBS spectra for particles (NaCl, MgCl2, KCl, and CaCl2) under the free-firing laser condition are shown in . Major elements in the particles (Mg, Na, Ca, and K) were successfully detected by the LIBS. No such elements were detected for clean air without particles (i.e., blank spectrum). The figure shows that the peak intensity in the LIBS spectra increased with increasing laser energy and the signal-to-noise ratio also increased. It was observed that the laser energy of 200 mJ/pulse provided the highest signal-to-noise ratio for most of the elements tested in this study as shown in the online supplementary information (SI, Figure S1). Hereafter, the laser pulse energy of 200 mJ/pulse was used for further experiments.
Figure 2. LIBS spectra for particles (NaCl, MgCl2, KCl, and CaCl2) with different values of the pulse laser energy under the free-firing laser condition (1 Hz).
Precise alignments of particle trajectory (i.e., particle beam), scattering laser beam, and pulse laser beam were essential for successful detection of a single particle by the LIBS under both the free-firing and triggering laser conditions. Particles were focused into the center line with a sheath air focusing system by controlling the aerosol-to-sheath air velocity ratio. Both locations of the scattering laser and pulse laser beams were controlled by a motorized X-Y stage controller with a 10 μm resolution. The best locations for both laser beams were determined by maximizing the hitting efficiency of the particles.
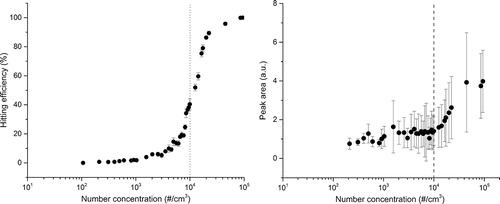
The hitting efficiency (%) of CaCl2 particles under the free-firing laser condition are shown in . As the particle number concentration increased, the hitting efficiency increased gradually over 1000∼10,000 #/cm3. In this range, the high concentration led to higher hitting efficiency (i.e., the probability that the pulse laser hit the particles increased). The hitting rate also increased with increasing concentration. At 1,000 #/cm3, the hitting efficiency was approximately 2%, and almost no particles were detected below 400 #/cm3. The hitting rate was 1 hits/min at 1,000 #/cm3. Above 10,000 #/cm3, the hitting efficiency increased at a much steeper rate. The peak area (Ca peak) in the LIBS spectra also increased significantly above 10,000 #/cm3 and below 10,000 #/cm3, the peak area was fairly constant, as shown in . Data suggest that no single particle existed in the pulse laser hitting area above 10,000 #/cm3. In other words, there were multiple particles in the pulse laser focal point, leading to the higher ablated mass and the higher peak area in the LIBS spectra. Thus, further experiments were performed at much lower concentrations than 10,000 #/cm3 to avoid the presence of multiple particles in the pulse laser hitting area.
Figure 3. (a) Hitting efficiency of particles (350 nm CaCl2 particles), and (b) peak area of Ca in LIBS spectra with varying number concentrations under the free-firing laser condition (1 Hz).
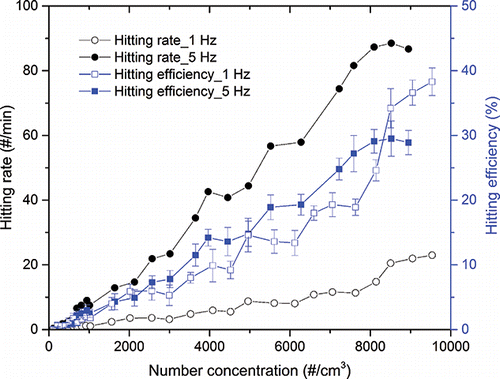
Hitting efficiencies (%) and hitting rate (hits/min) of CaCl2 particles under free-firing laser conditions with different laser frequencies (1 Hz versus 5 Hz) are shown in . The figure shows that the higher laser frequency did not significantly lead to higher hitting efficiency at low concentration. However, an improved hitting rate was observed for the higher laser frequency (8 hits/min and 1 hits/min for 5 Hz and 1 Hz at 1,000 particles/cm3, respectively). The hitting efficiencies at both frequencies were as low as 2–3% at a concentration of 1,000 particles/cm3, and almost no particles were detected at concentrations less than 400 particles/cm3. Our data suggest that the increase of the laser frequency under the free-firing laser condition should not have a significant effect on the enhancement of the particle hitting efficiency at low concentrations.
Figure 4. Comparison of hitting efficiency (%) and hitting rate of CaCl2 particles under free firing laser conditions (1 Hz versus 5 Hz) with varying number concentrations.
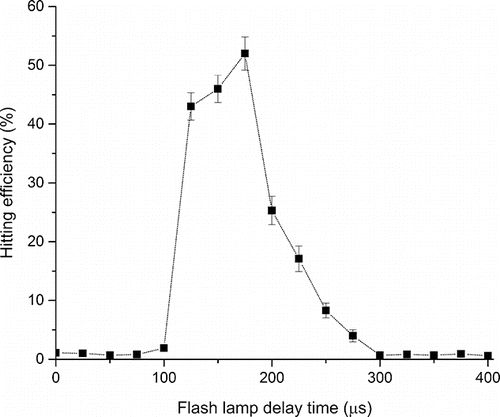
To increase the hitting efficiency of particles particularly at low concentrations (<1,000 particles/cm3), the triggering laser system as explained in the experimental section was employed. Under the triggering laser condition, the scattering laser was on and the scattering signal from particles was obtained. The amplitude of the scattering signal increased as particle size increased from 200 nm to 600 nm as shown in the SI (Figure S2a). The scattering light intensity can be used to infer the optical size which was not further explored in this study. In addition, the scattering rate ( = the number of particle hits by scattering laser/time) increased with the particle number concentration as shown in the SI (Figure S2b). Upon receiving the scattering signal, the flash lamp delay time was adjusted to hit a particle with a pulse laser at specific times. As shown in , the optimum flash lamp delay time for 350 nm CaCl2 particles was observed to be 175 μs. This time is close to the value (182 μs) calculated by assuming that the flow velocity through the nozzle is the same as the particle velocity. Since the distance from the scattering laser beam and the pulse laser beam was very short (3 mm), the velocity of the particles after the nozzle should be not significantly different for particles of other sizes. Thus, the delay time of 175 μs was used for further testing. The flash lamp delay time can be controlled or scanned to obtain the best hitting efficiency for particles with a wider size range than those reported here.
Figure 5. Hitting efficiency of 350 nm CaCl2 particles with different flash lamp delay times under the triggering laser condition.
compares the hitting efficiency of 350 nm particles between the triggering laser and free-firing laser condition at 1 Hz. As shown, the hitting efficiency significantly increased in the triggering laser condition. At 1,000 #/cm3, the hitting efficiency with the triggering laser condition is ∼26 times higher than that of the free-firing laser condition (52% versus 2%). In addition, the hitting rate increased to 15 hits/min compared to 1 hits/min with the free-firing laser condition. At particle concentrations less than 400 #/cm3 where almost no particles were detected with the free firing laser, the LIBS system with the triggering laser system successfully detected particles with hitting efficiencies of 8–25%. Theoretically, the hitting efficiency should be flat regardless of concentration with the triggering laser condition The long waiting time for pulse laser (i.e., time between pulse laser shots) was required to detect at least 1,000 particles at low concentration, probably causing instability of the pulse laser. Also, low counting accuracy at low concentrations may cause statistical uncertainty. However, it is not clear that they caused the steep decrease of the hitting efficiency at concentrations less than 1,000 particles/cm3.
Figure 6. Comparison of (a) hitting efficiency and (b) hitting rate of 350 nm CaCl2 particles between the trigger laser and free-firing laser conditions with varying number concentrations.
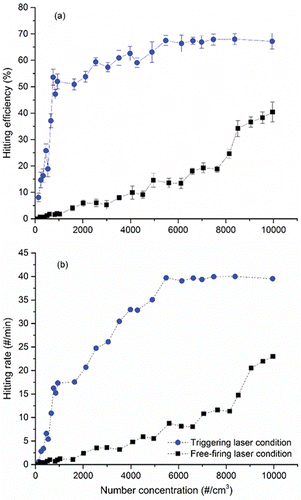
A similar hitting efficiency was obtained for particles of other sizes (250 nm and 300 nm). However, for 200 nm particles, the hitting efficiency at 1,000 #/cm3 decreased to 10% compared to 50% for 300 nm particles. Furthermore, the hitting rate decreased to 0.1 hits/min. This occurred because the scattering signal by the scattering laser for the 200 nm particles was too weak to send the triggering signal to the pulse laser. This limitation can be improved by reconstructing the optical system in the scattering regime or by installing a particle growth system using water or other condensing vapors before being detected by the scattering laser (Chai et al. Citation2009). Under the free-firing laser condition, particles as small as 200 nm were occasionally detected only when their concentration was considerably high. However, this would not be a single particle detection (i.e., multiple particles are present in the pulse laser hitting area). We have demonstrated that the LIBS with the triggering system was significantly useful for elemental detection of a single particle larger than 200 nm.
Conclusions
The LIBS technique with a triggering system was developed to determine the elemental composition of individual particles. A scattering laser and a pulse laser in series were employed to use a scattering signal produced by a single particle to fire the pulse laser at pre-determined time intervals, enabling higher accuracy when hitting airborne particles than for the constant laser firing condition. In addition, particles were focused into the center line with a sheath air focusing system and precise alignments of scattering laser and pulse laser beams were conducted. To evaluate the developed LIBS, various aerosols (NaCl, MgCl2, KCl, and CaCl2) with different sizes and concentrations were used. It was found that compared with free-firing LIBS, particle hitting efficiency and concentration limit were significantly improved with the triggering laser system. In particular, the LIBS with the triggering system showed more than 26 times higher hitting efficiency at 1,000 #/cm3 than in the free firing laser condition. It was concluded that the LIBS system with a triggering system is useful to determine the elemental composition of singe particles that exist at low number concentrations.
Supplementary_Material.docx
Download MS Word (307 KB)Funding
This work was supported by the National Leading Research Laboratory program (NRF- 2016R1A2A1A05005532) funded by the Ministry of Science, ICT, and Future Planning (MSIP), the National Research Foundation (NRF) of Korea and the Korea Meteorological Administration Research and Development Program under Grant KMIPA2014-21130. This study was also partially supported by Samsung electronics.
References
- Chai, K. B., Seon, C. R., and Choe, W. (2009). Dust Particle Growth in rf Silane Plasmas using Two-Dimensional Multi-Pass Laser Light Scattering. N. J. Phys., 11:103006–103014.
- Cheng, M. D. (2000). Real-Time Measurement of Trace Metals on Fine Particles by Laser-Induced Plasma Techniques. Fuel Process. Tehnol., 65:219–229.
- Chow, J. C., Watson, J. G., Lowenthal, D. H., Park, K., Doraiswamy, P., Bowers, K., and Bode, R. (2008). Continuous and Filter-Based Measurements of PM 2.5 Nitrate and Sulfate at the Fresno Supersite. Environ. Monitor. Assess., 144:179–189.
- Diwakar, P., Kulkarni, P., and Birch, M. E. (2012). New Approach for Near-Real-Time Measurement of Elemental Composition of Aerosol Using Laser-Induced Breakdown Spectroscopy. Aerosol Sci. Technol., 46:316–332.
- Dockery, D. W., and Pope, C. A. (1994). Mass Acute Respiratory Effects of Particulate Air Pollution. Ann. Rev. Publ. Health, 15:107–132.
- Fisher, B. T., Johnsen, H. A., Buckley, S. G., and Hahn, D. W. (2001). Temporal Gating for the Optimization of Laser-Induced Breakdown Spectroscopy Detection and Analysis of Toxic Metals. Appl. Spectrosc., 55:1312–1319.
- Gallou, G., Sirven, J. B., Dutouquet, C., Bihan, O. L., and Frejafon, E. (2011). Aerosols Analysis by LIBS for Monitoring of Air Pollution by Industrial Sources. Aerosol Sci. Technol., 45(8):918–926.
- Hahn, D. W., and Lunden, M. M. (2000). Detection and Analysis of Aerosol Particles by Laser-Induced Breakdown Spectroscopy. Aerosol Sci. Technol., 33:30–48.
- Hettinger, B., Hohreiter, V., Swingle, M., and Hahn, D. W. (2006). Laser-Induced Breakdown Spectroscopy for Ambient Air Particulate Monitoring: Correlation of Total and Speciated Aerosol Particle Counts. Appl. Spectrosc., 60:237–245.
- Intergovernmental Panel on Climate. (2007). Climate Change 2007. Cambridge Univ. Press, New York.
- Kim, G., Kwak, J., Choi, J., and Park, K. (2012). Detection of Nutrient Elements and Contamination by Pesticides in Spinach and Rice Samples Using Laser-Induced Breakdown Spectroscopy (LIBS). J. Agric. Food Chem., 60(3):718–724.
- Kim, G., Kwak, J., Kim, K. R., Lee, H., Kim, K. W., Yang, H., and Park, K. (2013). Rapid Detection of Soils Contaminated with Heavy Metals and Oils by Laser Induced Breakdown Spectroscopy (LIBS). J. Hazard. Mater., 263:754–760.
- Kwak, J. H., Kim, G., Kim, Y. J., and Park, K. (2012). Determination of Heavy Metal Distribution in PM10 During Asian Dust and Local Pollution Events Using Laser Induced Breakdown Spectroscopy (LIBS). Aerosol Sci. Technol., 46:1079–1089.
- Kwak, J. H., Kim, K. W., Park, M., Kim, J., and Park, K. (2012). Determination of Lead in Soil at a Historical Mining and Smelting Site using Laser-Induced Breakdown Spectroscopy. Environ. Technol., 33:2177–2184.
- Kwak, J. H., Lenth, C., Salb, C., Ko, E. J., Kim, K. W., and Park, K. (2009). Quantitative Analysis of Arsenic in Mine Tailing Soils using Double Pulse-Laser Induced Breakdown Spectroscopy. Spectrochimica Acta Part B, 64:1105–1110.
- Larsson, A., Karlsson, A., Gradmark, P. Å., and Landström, L. (2016). Bioaerosol Detection Using Single Particle Triggered LIBS, Proceedings of SPIE 9824, Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XVII, 9824:98240S–98248.
- Madhavi, Z. M., Cheng, M. D., and Martin, R. C. (1999). Aerosol Measurement by Laser-Induced Plasma Technique: A Review. Aerosol Sci. Technol., 31:409–421.
- Mukherjee, D., Rai, A., and Zachariah, M. R. (2006). Quantitative Laser-Induced Breakdown Spectroscopy for Aerosols via Internal Calibration: Application to the Oxidative Coating of Aluminum Nanoparticles. J. Aerosol Sci., 37:677–695.
- Okuda, T., Fujimori, E., Takada, H., Kumata, H., Nakajima, F., Hatakeyama, S., Uchida, M., Tanaka, S., He, H., Ma, Y., and Haraguchi, H. (2013). Rapid and Simple Determination of Multi-Elements in Aerosol Samples Collected on Quartz Fiber Filters by Using EDXRF Coupled with Fundamental Parameter Quantification Technique. Aerosol Air Qual. Res., 13(6):1864–1876.
- Orsini, D. A., Ma, Y., Sullivan, A., Sierau, B., Baumann, K., and Weber, R. J. (2003). Refinements to the Particle-Into-Liquid Sampler (PILS) for Ground and Airborne Measurements of Water Soluble Aerosol Composition. Atmos. Environ., 37:1243–1259.
- Park, K., Cho, G., and Kwak, J. H. (2009). Development of an Aerosol Focusing-Laser Induced Breakdown Spectroscopy (Aerosol Focusing-LIBS) for Determination of Fine and Ultrafine Metal Aerosols. Aerosol Sci. Technol., 43:375–386.
- Pasquini, C., Cortez, J., Silva, L. M. C., and Gonzaga, F. B. (2007). Laser Induced Breakdown Spectroscopy. J. Braz. Chem. Soc., 18:463–512.
- Sloane, C. S., and Richards, L. W. (1991). Size-Segregated Fine Particle Measurements by Chemical Species and Their Impact on Visibility Impairment in Denver. Atmos. Environ., 25:1013–1024.
- Suess, D. T., and Prather, K. A. (1999). Mass Spectrometry of Aerosols. Chem. Rev., 99:3007–3035.
- Tjärnhage, T., Gradmark, P. Å., Larsson, A., Mohammed, A., Landström, L., Sagerfors, E., Jonsson, P., Kullander, F., and Andersson, M. (2013). Development of a Laser-Induced Breakdown Spectroscopy Instrument for Detection and Classification of Single-Particle Aerosols in Real-Time. Optic. Commun., 296:106–108.
- Tursic, J., Radic, H., Kovacevic, M., and Verber, M. (2008). Determination of Selected Trace Elements in Airborne Aerosol Particles using Different Sample Preparation. Arch. Ind. Hyg. Toxicol., 59:111–116.
