ABSTRACT
The aerosol dynamics model ADiC was extended to include chemical reactions. It is used to computationally replicate denuder tube experiments where freshly generated cigarette smoke is drawn through a vertically arranged, acid covered tube to capture alkaline substances. The calculated deposition rates and total deposition are compared to experimental findings from several studies that investigated respective quantities for nicotine (and ammonia). Further, the form of deposition, vaporous and condensed phase, is considered. The model does not apply any parameters changing physico-chemical properties to fit simulation and experimental findings.
The only variable parameter used in all simulations is the choice of the amount of acid initially in the system to establish a certain pH value. An initial pH of 5.9 to 6.25 (i.e. the baseline scenarios) allows for replicating the nicotine deposition rate and total deposition in the lower tube sections. For the same simulation, ammonia deposition rate and total deposition are of the order of the experimental data. For the simulation featuring the initially lower pH value, the deposition of ammonia is lower than the experimental data – in the other case it is higher. Increasing the molality of alkaline substances initially in the system by roughly 20% drastically reduces the differences between simulated and measured nicotine deposition rate.
The present model describes some aspects of the dynamics of the complex cigarette smoke in a simplified manner; however, since it is independent of experiment specific parameters it may be applied to other environments such as deposition in the respiratory tract.
EDITOR:
Introduction
Tobacco smoke is a complex aerosol featuring thousands of chemical substances which are distributed between the particle and the vapor phase (Perfetti and Rodgman Citation2014). Those substances may undergo chemical reactions or quick phase transition spontaneously or when surrounding conditions change. Their volatilities range over many orders of magnitude. As a result, the partition between particle and vapor phase ranges from almost pure gas to nonvolatile particulate materials. Further, fresh and undiluted cigarette smoke shows considerable internal dynamics. Particle concentrations are of the order of 109 particles per cm3 which causes quick coagulation transforming the aerosol (Ingebrethsen et al. Citation2011). This makes an unequivocal description of the aerosol difficult to achieve. Thus many measurements regarding cigarette smoke are standardized to enable intercomparison of experimental results dealing with specific aspects of the aerosol. However, due to the high complexity, even those particular aspects are an issue of an ongoing discussion (Wright Citation2015).
A complete description of tobacco smoke is still not possible as current experimental methods often require some manipulation of the aerosol:
The determination of the smoke particle size distribution (which is of importance for the calculation of deposition e.g. in human lungs) requires immediate dilution to prevent coagulation. This causes an undefined loss of volatiles from the particle to the vapor phase (Alderman and Ingebrethsen Citation2011).
Total particulate mass is often measured by sampling particles on a filter pad (e.g. so-called Cambridge glass fiber filters; Roemer et al. Citation2012) for further analysis. The sampled aerosol is therefore already aged.
Various parameters of an aerosol are determined under different experimental conditions and for different lifetimes of a highly dynamic aerosol. Therefore, a full description (which would include a particle size dependent distribution of volatiles amongst the condensed and the vapor phase) at a given point in time is currently not possible. Even more difficult might be the description of the chemical states of substances which are relevant for the description of phase transition.
A possible solution to this problem is the use of computer simulation. Computer models could on the one hand help to describe the initial aerosol as well as its evolution based on carefully measured data. The present work aims to computationally replicate experiments from literature by applying the aerosol dynamics model ADiC (Aerosol Dynamics in Containments, Pichelstorfer and Hofmann Citation2015) which was further developed and now includes a chemistry sub-model as well as a new particle and vapor phase deposition sub-model. The goal is to provide a process based simulation without the usage of any parametrization of physical parameters to fit the simulation to measurement data.
Those experiments, often referred to as denuder tube studies, are probably the best defined ones dealing with cigarettes and capturing some of its dynamics. No uncontrolled manipulation (with respect to impact on the aerosol) is included. The experiments were first described by Lewis et al. (Citation1994) and later on repeated by others (Ingebrethsen et al. Citation2001; Lewis et al. Citation1995; Lipowicz and Piadé Citation2004). The principle is very simple: freshly generated cigarette smoke is drawn through a vertically arranged glass tube which inner surface is coated with some acidic material to capture nicotine. The tube is cut in pieces and the coating is washed out and analyzed for nicotine and, in the case of Ingebrethsen et al. (Citation2001), ammonia (NH3) deposition is also determined.
Earlier attempts to theoretically analyze the findings have often used exponential functions like the Ingham equation (Ingham Citation1975) which automatically produces a curve that is shaped similarly compared to the experimental findings. However, this function may only be applied where some concentration or temperature equilibrates with a boundary and no source or sink has to be considered. In the present experiment, nicotine and probably also ammonia are almost solely present in the particle phase when the smoke enters the tube (Lipowicz and Piadé Citation2004). Thus most of the substance deposited evaporates from the particle as deposition in condensed form can be neglected (< 1% according to Lewis (Citation1994) who measured the retention of solanesol, which is practically non-volatile, in the denuder tube).
The main objective of the present work is to provide a process based description of the experiment that does not apply any parametrizations (unless otherwise stated) in order to be able to apply the model to other situations (e.g. aerosol dynamics and deposition of smoke from combustion type cigarettes or e-cigarettes in the human lung (Pichelstorfer et al. Citation2016)).
In the following text, the method to solve the aerosol dynamics equations including coagulation, heat transport, vapor transport, particle transport, multi-component phase transition, particle phase chemistry and particle deposition will be described.
Material and methods
The aim of this work is to compare experimental findings from literature to simulation results. This is done by numerically solving a set of equations describing the transport of the aerosol and vapor molecules through the denuder tube, the changes within the aerosol (i.e. the aerosol dynamics) and interaction with the boundaries of the system (i.e. the tube walls). This is graphically depicted by .
Figure 1. Draft of the experimental setup. Particles, gaseous molecules (acids, bases, water and inert) travel along the parabolic flow profile (thin arrows stand for flow velocity and direction) in the vertically arranged denuder tube. Bases and particles are supposed to be captured when hitting the acid coated tube walls (i.e. we assume the wall to be a perfect sink for particles and bases).
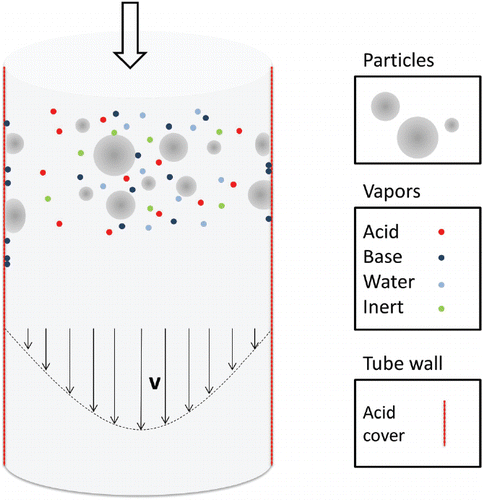
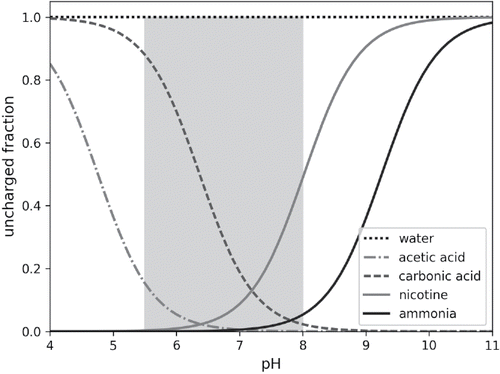
Figure 2. Uncharged fraction against solution pH for substances in considered thermodynamic equilibrium. The greyish area represents the experimentally determined range of cigarette smoke pH obtained from literature (Lauterbach et al., Citation2010; Pankow et al., Citation2003; Wayne et al., Citation2006).Footnote1
The denuder tube experiment is simulated by solving a discrete version of the general dynamics equation (Hidy Citation1984):[1] ni is the particle concentration contained in size bin i, t represents time, q is the convective related velocity, the binary diffusion coefficient of a particle of size bin I within the carrier gas is depicted by DIi and velocities related to external forces are denoted as qF. Thus the change of particle number concentration in size bin i with respect to time is represented by the left hand side of the equation. Dynamic processes altering the bin concentration are listed on the right hand side of the equation, namely: convective transport, diffusive particle transport, phase transition, coagulation, nucleation and transport due to external forces (e.g. gravitation). The solution of the equation using the aerosol dynamics model ADiC is described in detail elsewhere (Pichelstorfer and Hofmann Citation2015).
The ADiC model is a box model describing the evolution of an aerosol within a certain volume along a prescribed trajectory. Note that the aerosol is assumed to be homogeneously distributed within this volume and thus across the tube cross section. However, some mechanisms, such as diffusion, may assume a gradient of particles or gas molecules. There is only one degree of freedom, which is time that is integrated by means of the Eulerian forward method.
The aerosol dynamics model solves equations describing the individual processes in a sequential manner. To minimize resulting errors due to interaction of the processes, integration time steps are automatically adjusted to ensure that all changes are below a certain limiting maximum relative change. These limiting quantities are characteristic for each of the processes described by means of sub-models:
| • | Coagulation | ||||
| • | Phase transition | ||||
| • | Heat/ Vapor transport | ||||
| • | Deposition | ||||
| • | Particle phase chemistry | ||||
Coagulation
Coagulation is described by solving a discrete version of the so-called Smoluchowski equation (Smoluchowski Citation1917):[2] where t is the time, nk is the particle concentration within size bin k, βik is the coagulation coefficient of colliding particles i and k which varies its form according to the coagulation mechanism considered. A description of the solution of this equation can be found elsewhere (Pichelstorfer et al. Citation2013). In the present work only thermal coagulation of spherical particles carrying no charge is considered. Further, the probability of adherence after each collision, also known as accommodation coefficient, is considered to be one.
Phase transition
In the present work two different phase transition models are applied. The first is a transport based formulation which is applied for all species with considerable mass fractions in the particle phase. The second is an equilibrium phase transition model which is applied for traces (fraction in the particle phase < 10−8). The transport based phase transition model applied in this work is based on Mattila et al. (Citation1997) and Vesala et al. (Citation1997). It describes the coupled transport of heat and vapor along a fully established gradient towards and away from the particle surface. The transport is driven by differences between vapor pressure pi,d of species i at the particle surface and the surrounding which are calculated in two different ways:[3] where Ke represents the Kelvin term taking into account the curvature of the surface, nfrac is the fraction of the substance being in its neutral form (since we assume that only the uncharged fraction of a substance has a considerable vapor pressure). The upper option including the activity coefficient (act), the mole fraction (χi) and the pure substance vapor pressure represents the Raoult law (see chemistry textbooks, e.g.: Chang (Citation2010) representation of the vapor pressure which, together with the activity correction for non-idealities (regarding the chemical potential) is valid for all mole fraction values of a substance. For small mole fractions of the substance (i.e. close to 0) a vapor pressure calculated by means of Henry's law (see chemistry textbooks, e.g.: Chang (Citation2010) might be more realistic for non-ideal mixtures and in case an activity coefficient is not available. For ideal solutions, Raoult's law representation and Henry's law representation are identical. In the present work, non-idealities of the solution are considered by the activity coefficient (act) which allows for the application of Raoult's law for the whole range of mole fractions. Saturation vapor pressures and Henry's law coefficients used for the simulations are listed in the supplementary material. The description of mass and heat fluxes towards and away from the particles are described in (Pichelstorfer and Hofmann Citation2015).
The equilibrium phase transition model redistributes the total mass mi,tot of a substance i between the particle and the vapor phase using the following equations:[4]
[5] where pi,v is the vapor pressure of substance i in the vapor phase, mi,dis is the mass of substance i which is dissolved in water, Hi,j is Henry's law coefficient for dissolution of substance i in substance j, and Vj is the liquid volume of substance j; mi,tot and mi,v are the total mass of substance i and the vapor phase mass of substance i.
Note that phase transition of all substances except for NH3 (Henry's law) and carbon dioxide (CO2; equilibrium phase transition) apply the Raoult representation.
Heat/vapor transport
The present model considers convective heat/vapor transport in direction of the flow. Further, conductive heat transport and diffusive vapor transport, both perpendicular to the direction of gas flow in a laminar flow field, are described.
For conductive transport, transport velocity and mean residence time of a given gas volume within the tube was determined by considering the experimentally determined volume flow and the tube cross section (both obtained from literature).
The determination of conductive heat transport and diffusive vapor transport is more complex. The application of an analytical solution (Equation (Equation6[6] ) describing axial transport within a cylindrical tube that features a laminar flow field (Jacob Citation1950), which (or a similar equation) was used by previous authors addressing the denuder tube experiments, is not appropriate for the present situation. The reason is that the particle phase of the aerosol represents a source or sink for heat and vapor molecules. This, however, is not considered by the equation and thus may cause significant errors. The error gets more pronounced the higher the ratio of particle phase mass versus gas phase mass of a substance is.
[6] where the temperature (in the case of heat transfer) or vapor concentration (in the case of vapor transport) is represented by u at the tube inlet (u0). Further, um is the mean quantity at a given distance L from the tube inlet and uw is the quantity at the boundary (i.e. the tube wall). Q is the volume flow per time and α either stands for the diffusion coefficient D in the case of diffusive vapor transport or for κ /(cpρ) in the case of conductive heat transport (where ρ is the gas density, κ is the heat conductivity and cp is the specific heat capacity of the gas). Constants An are 0.820; 0.0972; 0.0135 and βn are 2.705; 6.66; 10.3, for n = 1,2,3.
In order to be able to consider sources and sinks of heat and vapor which is crucial for the present experiments, each of the exponential terms of Equationeq. 6[6] was developed into a Taylor series:
[7] where dtube is the inner tube diameter. Now the mean quantity um,i at time i is a function of the quantity at time i-1 which allows for numerical calculation. Further, parameters (u, α) may not be constant anymore. Replacing the exponential terms by Taylor series causes inaccuracies. However, since time steps and thus the exponential term
<< 1 (we observed relative deviation from the analytical solution not larger than 10−4 in the present work), the Taylor series can safely be stopped after the second term (i.e. m = 1).
Deposition
For the determination of particle deposition in the vertically arranged glass tube featuring a laminar gas flow mechanisms such as sedimentation or impaction can be neglected. Also the settling velocity of larger particles that reduces their residence time in the tube compared to smaller particles is negligible. Thus the only condensed phase deposition mechanism taken into account in this work is deposition by diffusion. The diffusion coefficient for the particles can be calculated by using (Hinds Citation1999):[8] where kB is the Boltzmann constant, η is the gas viscosity and Cc is the Cunningham correction factor. Note that cigarette smoke particles are spherical and of almost unity density (Johnson et al. Citation2014) thus making Equation Equation(8)
[8] a good estimate.
Deposition based on the diffusion coefficient was determined using the same equations as applied for diffusive vapor deposition.
Particle phase chemistry
The particle phase chemistry considers conjugate acid-base reactions. Reaction rates are neglected since we assume equilibrium chemistry (i.e. forward and reverse reactions of reactants and products are equal). The acid donates a proton or hydronium ion while the base accepts protons:[9] where [HA] is the molar concentration of the acid HA; H+, A−, B, BH+ are hydronium ion, conjugate form of acid, base and conjugate form of the base, respectively. Equilibrium constants for acids ka and bases kb are given as:
[10]
Total concentrations of acids [HAtot] and bases [Btot] (i.e. the sum of the substance plus its conjugate form) can be given as:[11]
Combined with the assumption of charge equilibrium:[12] where n-m is the number of bases and m is the number of acids. Combining Equations (Equation9
[9] ) to (Equation12
[12] ) results in a polynomial of H+ (i.e. the only unknown in the equation) which can be solved using numerical methods (i.e. zero root finder). Equation (Equation10
[10] ) can then be used to determine the charged fraction of each substance. The general derivation of the polynomial can be found in the supplementary material. This inorganic equilibrium phase chemistry model was validated against several test cases obtained from literature (Seinfeld and Pandis Citation1998).
The higher the charged fraction of the species, the lower its vapor pressure gets as we assume that only uncharged molecules show a considerable vapor pressure.
Chemical species and reactions considered by the model are listed in . Water dissociates to form a hydroxyl ion and a hydronium ion. Nicotine accepts a proton and is converted to its single protonated form. Ammonia accepts a proton to form an ammonium ion. Acetic acid donates a proton and is converted to its conjugate base. Dissolved carbon dioxide donates a proton and becomes a carbonate ion. Higher order reactions (formation of double protonated nicotine or the bicarbonate ion is neglected as their concentrations are very low within the experimentally observed pH range: pH ∼ 5.2 to 8; Lauterbach et al. (Citation2010); Pankow et al. (Citation2003); Wayne et al. (Citation2006)).
Table 1. Chemical species, reactions considered and equilibrium constants. Subscript “dis” stands for dissolved.
Results and discussion
Similar to the work by Pichelstorfer and Hofmann (Citation2015), four different sets of data on nicotine deposition are used for the model comparison. Lewis et al. (Citation1994), Lewis et al. (Citation1995), Lipowicz and Piade (Citation2004) determined nicotine retention in the denuder tube, while Ingebrethsen et al. (Citation2001) additionally investigated the deposition of ammonia within the tubes. All experimental setups are similar with some minor differences which are discussed elsewhere (Pichelstorfer and Hofmann Citation2015).
The goal of this study was to replicate the experiments by means of computer simulation. Details on the inputs used for the model are listed in the supplementary material. Results comprise the evolution of the particle size distribution, the size dependent composition of the particles, the particle's chemistry (i.e. pH and ionization of molecules), the vapor phase composition and temperature, respectively. Further, the deposition of substances in particulate and vapor phase is recorded (note that in the present work we assume only alkaline vapor molecules to be captured by the acid covered tube walls).
In the experiment the aerosol enters the denuder tube carrying nicotine and ammonia almost solely in the particle phase. Some fraction of the two substances remain in the tube while the relative amount of non-volatile mass depositing in the tube (described by Equations Equation(7)[7] and Equation(8)
[8] ) is much smaller (by roughly 1 order of magnitude in the case of nicotine and by about two orders of magnitude in the case of ammonia). Accordingly, the two volatiles evaporate from the particles (described by Equations Equation(3)
[3] and Equation(4)
[4] ) and deposit as vapor molecules within the tube (described by Equation Equation(7)
[7] ). Further the aerosol size distribution undergoes continuous transformation due to interaction of the individual particles (described by Equation Equation(2)
[2] ). Note that the vapor pressure und thus the phase transition process depends on the uncharged fraction of a substance which can be calculated solving Equations Equation(9)
[9] to Equation(12)
[12] . Currently, a quantification of those processes can only be provided by means of computer simulation. A basic description of the evolution of the aerosol within the tube based on the processes described can be found in the supplementary material.
Simulation results predict a very low particle phase deposition (the calculated deposition is around 0.5% of the initial condensed mass). Further, the rate of condensed phase deposition shows a quasi-exponential decay. This can be explained by the effect of coagulation removing the smallest particles. Since diffusion is the only relevant deposition mechanism it gets less and less pronounced as the distribution is moved towards higher particle diameters by the collisions of particles (the concentration is reduced by more than 90%, median number and median mass diameter increase by roughly 250%). Such a low deposition of condensed matter has also been found in the experiments (Lewis et al. Citation1994).
Deposition fractions for nicotine and ammonia found experimentally are much higher than 0.5% which was found for the condensed phase (nicotine: a few percent in all experiments; ammonia: > 40%) suggesting that the substances deposit mostly in vaporous form. However, the observed rates of deposition (see and ) are much lower than expected diffusive deposition for vapor molecules in a laminar flow field. Thus most of the deposited alkaline substances are in the particle phase initially and, on release to the vapor phase by evaporation, diffuse to the tube walls where they are captured.
Figure 3. Results from literature and modeled nicotine deposition in [% cm2/s]Footnote2 as a function of tube length over volume flow depicted by symbols and lines, respectively.
![Figure 3. Results from literature and modeled nicotine deposition in [% cm2/s]Footnote2 as a function of tube length over volume flow depicted by symbols and lines, respectively.](/cms/asset/d7b77dc3-848a-4a58-b250-ffd06ca2a392/uast_a_1363867_f0003_b.gif)
Figure 4. Measured (Ingebrethsen et al., Citation2001) and simulated ammonia deposition in [% cm2/s] as a function of tube length over volume flow for different initial pH values.
![Figure 4. Measured (Ingebrethsen et al., Citation2001) and simulated ammonia deposition in [% cm2/s] as a function of tube length over volume flow for different initial pH values.](/cms/asset/57bc68bc-bb7e-46d3-9de4-a8ddad71511c/uast_a_1363867_f0004_b.gif)
A comparison of experimental and simulated nicotine deposition as a function of tube length over volume flow is shown by . Note that the ordinate depicts deposited nicotine [%] times volume flow [cm3/s] over tube length [cm]. This representation is chosen in order to be able to include experiments featuring different volume flows. The only parameter in this simulation is the amount of acetic acid which is used to regulate the particle pH. An initial pH of 6.25 fits the experimental data measured in the lower tube sections by Ingebrethsen et al. (Citation2001) and Lipowicz and Piade (Citation2004). Similarly, an initial pH of 5.9 fits the experimental data determined by Lewis et al. (Citation1994 and Citation1995). These two simulations are referred to as the baseline scenarios “baseline pH 6.25” and “baseline pH 5.9”. Note that considers both, nicotine deposited in vaporous as well as nicotine deposited in condensed form as part of a particle. We assumed that, no matter in which from nicotine deposits, the wall is a perfect sink. Further, the fraction of nicotine being deposited in vaporous form was above 98% for all simulations.
Surprisingly, the experimental curves show a rather strong initial decay in nicotine deposition throughout the tube. This is surprising since the deposition of nicotine is closely related to the release of nicotine from the particle to the vapor phase. Nicotine once released to the vapor phase diffuses to the tube walls quickly. Thus a decay of nicotine deposition rate is caused most probably by changes to the particle phase. Interestingly, the model does not suggest such significant changes. Coagulation continuously reshapes the particle size distribution. Its effect on nicotine vapor pressure at the particle surface is quite small (Pichelstorfer and Hofmann, Citation2015). Further the model does not suggest significant changes in the composition of the particle by means of phase transition. Of course, ammonia is significantly reduced due to evaporation and subsequent deposition but has, due to its low contribution to the particle mass, low influence on the nicotine vapor pressure (with respect to the Roult term). Particle phase deposition is almost negligible. Finally, chemical reactions do suggest a drop in pH due to ammonia evaporation and thus reduces the volatile fraction of nicotine. However, the effect on the evaporation of nicotine and its subsequent deposition is not large enough.
Various parameters can potentially explain the discrepancy between simulation and experimental findings, e.g.:
| • | Lewis et al. (Citation1994) suggested that vapor phase nicotine depositing in the early sections of the tube might explain the findings. However, he found that apparent nicotine diffusion coefficients required to support the theory are 50% to 85% less than values found in literature. | ||||
| • | The strong decay may also suggest that nicotine is being exploited in the particle phase. Nevertheless, this does not seem to be the case since only a few percent of nicotine are deposited in the tube. | ||||
| • | A limited (with respect to time) conversion rate from protonated nicotine to unprotonated nicotine may explain the reduction in nicotine vapor deposition by reducing the nicotine partial vapor pressure at the particle surface. However, no experimental studies are known to the authors suggesting such a behavior. | ||||
| • | The measurement of NH3 deposition within denuder tubes rather suggests a drop in particle pH which shifts the ratio of volatile (i.e. unprotonated) to non-volatile (i.e. protonated) nicotine towards smaller numbers. This also reduces the partial nicotine vapor pressure at the particle surface and, as a consequence, reduces nicotine evaporation. | ||||
Experimentally determined (Ingebrethsen et al. Citation2001) and simulated NH3 deposition is shown by . The two simulated curves refer to the baseline scenarios. The simulation “baseline pH 6.25” overestimates the ammonia deposition by roughly 25% in the first section in the tube and up to 300% in the later sections of the tube. The second baseline scenario underestimates the deposition by roughly 75% in the first tube section of the tube and nicely predicts the deposition in the last two thirds of the tube. Regarding the nicotine deposition in the first half of the denuder tube we hypothesize that partial vapor pressure of volatile nicotine is initially higher than suggested by our model. This might be caused by a) a significant change of the particles' composition (this option is not supported by the model) or b) by chemical reactions the model presently does not capture. Since the model only considers simple acid-base reactions in equilibrium and, in the current simulations, takes into account no more than 5 substances plus their conjugate acids/bases, we hypothesize that the chemistry model does not capture all relevant reactions.
However, increasing the amount of alkaline substances initially present in the system potentially explains the differences between experiment and simulation (see ). This was done by adding another alkaline substance featuring similar properties as ammonia. Thus we effectively raised the amount of NH3 by a factor of 2 (from 7* 10−9 g/cm3 to 1.4*10−8 g/cm3). Accordingly, the initial pH changes from 6.25 and 5.9 in the baseline scenarios to 6.8 and 6.20, respectively. While the increase in initial pH reduces the gap between experimental and simulated nicotine deposition rates it also lowers the ammonium to ammonia ratio. This results in higher ammonia evaporation and consequently a higher ammonia deposition at the tube wall. As depicted by , the simulation featuring a lower initial pH approaches the experimentally determined deposition rates. The related total simulated ammonia retention in the denuder tube is 44% which is similar to the experimental findings by Ingebrethsen et al. (Citation2001). They found roughly 40% ammonia deposition in an experiment aiming to replicate the studies by Lewis et al. (Citation1995). In contrast, the second simulation featuring an initial pH of 6.8 results in an overestimation of the deposition rate compared to experimental findings as it suggests almost total ammonia retention at the tube walls (∼94%).
Figure 5. Results from literature and modeled nicotine deposition in [% cm2/s] as a function of tube length over volume flow depicted by symbols and lines, respectively. Note that the initial amount of alkaline substances is increased for this simulation.
![Figure 5. Results from literature and modeled nicotine deposition in [% cm2/s] as a function of tube length over volume flow depicted by symbols and lines, respectively. Note that the initial amount of alkaline substances is increased for this simulation.](/cms/asset/9410f755-9dbc-4f70-931a-53383b1b8467/uast_a_1363867_f0005_b.gif)
Figure 6. Measured (Ingebrethsen et al., Citation2001) and simulated ammonia deposition in [% cm2/s] as a function of tube length over volume flow for different initial pH values. Note that the initial amount of alkaline substances is increased for this simulation.
![Figure 6. Measured (Ingebrethsen et al., Citation2001) and simulated ammonia deposition in [% cm2/s] as a function of tube length over volume flow for different initial pH values. Note that the initial amount of alkaline substances is increased for this simulation.](/cms/asset/7129fe53-e77f-4e71-aa70-be7dff8817da/uast_a_1363867_f0006_b.gif)
Additional information on the modeling results can be found in the supplementary material. It provides deeper insight in how the individual processes described affect the outcome of the simulation.
Summary and conclusions
The present study aims to reproduce findings from denuder tube studies reported in the literature by means of computer simulation. This, in principle, is not a new attempt. However, the novelty is that the present model does not apply any parametrizations regarding to processes described or to physico-chemical properties of the substances considered. The only parameters applied are the amount of acetic substance initially in the system (it was applied to all simulations) and the amount of additional alkaline substance (it was not applied in the baseline scenarios). This independence of experiment-related parameters allows the model to be applied in other setups as well.
We found that at an initial average particle phase pH of 6.25 and 5.9, the nicotine deposition rate in the later tube sections found experimentally could be reproduced (i.e. the baseline scenario). Further, the ammonia deposition rates are of the same order of magnitude as the experimental findings by Ingebrethsen et al. Total simulated deposition ranged from 3% to 7% (experimental: 3% – 8%) for nicotine and from 30% to 80% (experimental: ∼ 40%) for ammonia. By far the largest part of nicotine and ammonia are deposited in vaporous form. The fraction of condensed nicotine (i.e. nicotine being part of a particle) being deposited is less than 2% for all simulations. For ammonia this fraction is even smaller (below 0.5%).
In contrast to the experimental findings, the simulated deposition rates show a much weaker reduction with increasing L/Q. This may be subject to several reasons (Pichelstorfer and Hofmann Citation2015). The probably simplest solution to reduce this discrepancy is to increase the amount of alkaline molality by roughly 20%. This was achieved by increasing the alkaline substance mass by 2% by adding additional ammonia. As a result, the gap between simulated and measured nicotine deposition rate reduced significantly. The total nicotine deposition increased to a range from 4% to 8%. On the other hand, also the ammonia deposition rate increased. The simulation featuring a lower initial pH shows similar deposition rates and total deposition (44%) compared to the experiment, while the second simulation with increased alkaline content and an initial average particle pH of 6.8 overestimates the ammonia deposition rate as well as total NH3 deposition (∼ 94%).
Of course the present inorganic chemistry model represents an oversimplification of the processes in the cigarette smoke particles that contain thousands of organic and many inorganic chemicals. However, note that the deposition rates would be 2 to 3 orders of magnitude higher in case the chemistry model was not applied. Potentially organic chemical reactions, which are not described by the model, are changing ammonia's partial vapor pressure by converting it to less volatile substances while the nicotine is not affected. Aldehydes are considered as possible reaction candidates (Nielsen et al. Citation1973; Nielsen et al. Citation1979). Roemer et al. (Citation2012) report several aldehydes (formaldehyde, acetaldehyde, acrolein, propionaldehyde and crotonaldehyde) which are present in sufficient amounts in cigarette smoke. Exemplary, the main reaction path of ammonia with formaldehyde is outlined: Formaldehyde and ammonia condense to form methyleneimine which trimerizes to trimethylolcyclotrimethylenetriamine. The latter forms hexamine by condensation with another ammonia molecule (Richmond et al. Citation1948). Unfortunately, the current version of the chemistry module is not capable of considering such rather complex reaction paths.
Other processes not reported in the experimental studies, such as water loss to the tube walls, acetic substance exchange with the tube walls or diffusion limited evaporation may have considerable influence on the deposition rate of nicotine and ammonia. Note that there is no experimentally determined complete description of the initial aerosol. This is due to the fact that one always either suffers from coagulation (in the case the aerosol is not diluted right after being generated) or from phase transition (in the case the aerosol is diluted). Further, some parameters such as the activity coefficients for mixtures have to be estimated due to a lack of experimental data. Additionally, the parameters (e.g. surface tension) that are reported in literature typically have been determined using macroscopic amounts of substance while those aerosol particles are orders of magnitude smaller and may feature different properties. Accordingly, it is currently not possible to accurately describe the dynamics of cigarette smoke. This stresses the importance for comparison of modeled results and experimental data.
The findings of this work suggest that the evaporation of nicotine and ammonia and the subsequent deposition strongly depend on properties of the mixture, such as the specific vapor pressure of the substance at the particle surface. An accurate replication of the denuder tube experiments requires detailed knowledge of these quantities. This is currently not possible due to the high complexity of tobacco smoke. However, the present work demonstrates that, nevertheless, a process based calculation is already possible. It allows, due to independence from experiment specific parameters, its use in various environments.
The combined use of the full ADiC model and the lung deposition code IDEAL is planned. This will provide new insight in the deposition mechanism of (semi-) volatile substances (e.g. cigarette smoke, e-cigarette aerosol or pharmaceuticals). Knowledge created that way may bridge in vitro studies to in vitro predictions.
Finally note that nicotine as well as ammonia is, at the entrance of the denuder tube, mainly in the particle phase though it deposits almost solely in vaporous form. Thus the particle phase serves as a vector for the volatiles through the tube while releasing some of them. Accordingly, the transport of those volatiles is governed by aspects of the particles while deposition is governed by aspects of molecular dynamics. This is also true for the inhalation of (semi-)volatiles into the human lung, although condensed phase deposition is higher. As a consequence, the use of an aerosol dynamics model is strongly recommended when simulating such experiments.
UAST_1363867_Supplemental_File.zip
Download Zip (360.1 KB)Funding
This research was funded in part by British American Tobacco (Investments) Limited, Southampton, UK (Grantno. A10012363SV).
Notes
References
- Alderman, S. L., and Ingebrethsen, B. J. (2011). Characterization of Mainstream Cigarette Smoke Particle Size Distributions from Commercial Cigarettes Using a DMS500 Fast Particulate Spectrometer and Smoking Cycle Simulator. Aerosol Sci. Technol., 45(12):1409–1421. doi: 10.1080/02786826.2011.596862
- Chang, R. (2010). Chemistry (10th ed.). McGraw Hill, New York.
- Goldberg, R. N., Kishore, N., and Lennen, R. M. (2002). Thermodynamic Quantities for the Ionization Reactions of Buffers. J. Phys. Chem. Ref. Data, 31(2):231–370. doi: 10.1063/1.1416902
- Hidy, G. (1984). Aerosols, an Industrial and Environmental Science. Academic Press, London.
- Hinds, W. C. (1999). Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Wiley, New York.
- Ingebrethsen, B. J., Alderman, S. L., and Ademe, B. (2011). Coagulation of Mainstream Cigarette Smoke in the Mouth During Puffing and Inhalation. Aerosol Sci. Technol., 45(12):1422–1428. doi: 10.1080/02786826.2011.596863
- Ingebrethsen, B. J., Lyman, C. S., Risner, C. H., Martin, P., and Gordon, B. M. (2001). Particle-Gas Equilibria of Ammonia and Nicotine in Mainstream Cigarette Smoke. Aerosol Sci. Technol., 35(5):874–886. doi: 10.1080/02786820126850
- Ingham, D. B. (1975). Diffusion of Aerosols from a Stream Flowing Through a Cylindrical Tube. J. Aerosol Sci., 6(2):125–132. doi: https://doi.org/10.1016/0021-8502(75)90005-1
- Jacob, M. (1950). Heat Transfer. Wiley, Ney York.
- Johnson, T. J., Olfert, J. S., Cabot, R., Treacy, C., Yurteri, C. U., Dickens, C., … Symonds, J. P. R. (2014). Steady-State Measurement of the Effective Particle Density of Cigarette Smoke. J. Aerosol Sci., 75:9–16. doi: https://doi.org/10.1016/j.jaerosci.2014.04.006
- Lauterbach, J. H., Bao, M., Joza, P. J., and Rickert, W. S. (2010). Free-Base Nicotine in Tobacco Products. Part I. Determination of Free-Base Nicotine in the Particulate Phase of Mainstream Cigarette Smoke and the Relevance of these Findings to Product Design Parameters. Regul. Toxicol. Pharm., 58(1):45–63. doi: 10.1016/j.yrtph.2010.05.007
- Lewis, D. A., Colbeck, I., & Mariner, D. C. (1994). Diffusion Denuder Method for Sampling Vapor-Phase Nicotine in Mainstream Tobacco Smoke. Anal. Chem., 66(20):3525–3527. doi: 10.1021/ac00092a037
- Lewis, D. A., Colbeck, I., and Mariner, D. C. (1995). Dilution of Mainstream Tobacco Smoke and Its Effects Upon the Evaporation and Diffusion of Nicotine. J. Aerosol Sci., 26(5):841–846. doi: https://doi.org/10.1016/0021-8502(95)00005-W
- Lipowicz, P. J., and Piadé, J. J. (2004). Evaporation and Subsequent Deposition of Nicotine from Mainstream Cigarette Smoke in A Denuder Tube. J. Aerosol Sci., 35(1):33–45. doi: https://doi.org/10.1016/S0021-8502(03)00385-9
- Mattila, T., Kulmala, M., & Vesala, T. (1997). On the Condensational Growth of a Multicomponent Droplet. J. Aerosol Sci., 28(4):553–564. doi: https://doi.org/10.1016/S0021-8502(96)00458-2
- Nielsen, A. T., Atkins, R. L., Moore, D. W., Scott, R., Mallory, D., and LaBerge, J. M. (1973). Structure and chemistry of the aldehyde ammonias. 1-Amino-1-alkanols, 2,4,6-trialkyl-1,3,5-hexahydrotriazines, and N,N-dialkylidene-1,1-diaminoalkanes. J. Org. Chem., 38(19):3288–3295. doi: 10.1021/jo00959a010
- Nielsen, A. T., Moore, D. W., Ogan, M. D., and Atkins, R. L. (1979). Structure and Chemistry of the Aldehyde Ammonias. 3. Formaldehyde-Ammonia Reaction. 1,3,5-Hexahydrotriazine. J. Org. Chem., 44(10):1678–1684. doi: 10.1021/jo01324a021
- Pankow, J. F., Tavakoli, A. D., Luo, W., and Isabelle, L. M. (2003). Percent Free Base Nicotine in the Tobacco Smoke Particulate Matter of Selected Commercial and Reference Cigarettes. Chem. Res. Toxicol., 16(8):1014–1018. doi: 10.1021/tx0340596
- Perfetti, T. A., and Rodgman, A. (2014). The Complexity of Tobacco and Tobacco Smoke. Beiträge zur Tabakforschung / Contrib. Tob. Res., 24(5):215. doi: 10.2478/cttr-2013-0902
- Pichelstorfer, L., and Hofmann, W. (2015). Modeling Aerosol Dynamics of Cigarette Smoke in a Denuder Tube. J. Aerosol Sci., 88:72–89. doi: https://doi.org/10.1016/j.jaerosci.2015.05.009
- Pichelstorfer, L., Hofmann, W., Winkler-Heil, R., Yurteri, C. U., and McAughey, J. (2016). Simulation of Aerosol Dynamics and Deposition of Combustible and Electronic Cigarette Aerosols in the Human Respiratory Tract. J. Aerosol Sci., 99:125–132. doi: https://doi.org/10.1016/j.jaerosci.2016.01.017
- Pichelstorfer, L., Winkler-Heil, R., and Hofmann, W. (2013). Lagrangian/Eulerian Model of Coagulation and Deposition of Inhaled Particles in the Human Lung. J. Aerosol Sci., 64(0):125–142. doi: https://doi.org/10.1016/j.jaerosci.2013.05.007
- Richmond, H. H., Myers, G. S., and Wright, G. F. (1948). The Reaction Between Formaldehyde and Ammonia. J. Am. Chem. Soc., 70(11):3659–3664. doi: 10.1021/ja01191a034
- Roemer, E., Schramke, H., Weiler, H., Buettner, A., Kausche, S., Weber, S., … Wittke, S. (2012). Mainstream Smoke Chemistry and in Vitro and In Vivo Toxicity of the Reference Cigarettes 3R4F and 2R4F Beiträge zur Tabakforschung / Contributions to Tobacco Research. (Vol. 25, p. 316).
- Seinfeld, J. H., and Pandis, N. P. (1998). Atmospheric Chemistry and Physics of Air Pollution. J. Wiley and Sons, New York.
- Seinfeld, J. H., and Pandis, N. P. (1998). Atmospheric Chemistry and Physics of Air Pollution. J. Wiley and Sons, New York.
- Smoluchowski, M. V. (1917). Versuch Einer Mathematischen Theorie Der Koagulationskinetik kolloider Lösungen. Zeitschrift für Phys. Chemie, 92:129–168.
- Vesala, T., Kulmala, M., Rudolf, R., Vrtala, A., & Wagner, P. E. (1997). Models for Condensational Growth and Evaporation of Binary Aerosol Particles. J. Aerosol Sci., 28(4):565–598. doi: 10.1016/S0021-8502(96)00461-2
- Wayne, G. F., Connolly, G. N., and Henningfield, J. E. (2006). Brand Differences of Free‐Base Nicotine Delivery in Cigarette Smoke: The View of the Tobacco Industry Documents. Tob. Control, 15(3):189–198. doi: 10.1136/tc.2005.013805
- Weast, R. (1972). Handbook of Chemistry and Physics (53rd ed.). The Chemical Rubber Company, Cleveland, Ohio.
- Wright, C. (2015). Standardized Methods for the Regulation of Cigarette-Smoke Constituents. TrAC Trends Anal. Chem., 66:118–127. doi: https://doi.org/10.1016/j.trac.2014.11.011
