ABSTRACT
There exists a demand for production of metal nanoparticles for today's emerging nanotechnology. Aerosol-generated metal nanoparticles can oxidize during particle formation due to impurities in the carrier gas. One method to produce unoxidized metal nanoparticles is to first generate metal oxides and then reduce them during sintering. Here, we propose to instead prevent oxidation by introducing the reducing agent already at particle formation. We show that by mixing 5% hydrogen into the nitrogen carrier gas, we can generate single crystalline metal nanoparticles by spark discharge from gold, cobalt, bismuth, and tin electrodes. The non-noble nanoparticles exhibit signs of surface oxidation likely formed post-deposition when exposed to air. Nanoparticles generated without hydrogen are found to be primarily polycrystalline and oxidized. To demonstrate the advantages of supplying the reducing agent at generation, we compare to nanoparticles that are generated in nitrogen and sintered in a hydrogen mixture. For bismuth and tin, the crystal quality of the particles after sintering is considerably higher when hydrogen is introduced at particle generation compared to at sintering, whereas for cobalt it is equally effective to only add hydrogen at sintering. We propose that hydrogen present at particle generation prevents the formation of oxide primary particles, thus improving the ability to sinter the nanoparticles to compact and single crystals of metal. This method is general and can be applied to other aerosol generation systems, to improve the generation of size-controlled nanoparticles of non-noble metals with a suitable reducing agent.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
Introduction
Production of nanoparticles with controlled shape and composition is beneficial for many applications, such as semiconductor nanostructures (Messing et al. Citation2009; Magnusson et al. Citation2014), catalysis (Messing et al. Citation2010; Ramsurn and Gupta Citation2013), biomedical applications (Gupta and Gupta Citation2004; Fan et al. Citation2012), textiles (Yetisen et al. Citation2016), and hydrogen storage (Vons et al. Citation2011a). A promising production method is electric ablation in a spark discharge generator (SDG), which can generate nanoparticles of basically any non-insulating material that can be shaped into a set of electrodes (Meuller et al. Citation2012) and is not limited to unary material systems (Kim and Chang Citation2005; Byeon et al. Citation2008; Tabrizi et al. Citation2009; Blomberg et al. Citation2013; Muntean et al. Citation2016; Kala et al. Citation2016). In SDG, the particle purity is mainly limited by the electrode material and the carrier gas, and is even comparable to the particle purity for laser ablation (Itina and Voloshko Citation2013, Pfeiffer et al. Citation2014). Furthermore, SDG has the potential to be up-scaled for industrial nanoparticle production by using, for example, several energy-efficient switched SDG units (Pfeiffer et al. Citation2014) in parallel (Feng et al. Citation2016a) at kHz discharge frequencies (Noh et al. Citation2017). However, similar to most aerosol generation routes, it is challenging to produce unoxidized particles of non-noble metals.
An SDG generates nanoparticles by electric ablation via the local heating and evaporation of electrode material. Some control of the initial particle size can be achieved by tuning the environment during condensation, e.g., by carrier flow rate, spark discharge frequency, and energy per spark (Ludvigsson et al. Citation2015; Feng et al. Citation2016b). However, when the particle concentration is high, the particles collide frequently and form larger clusters of nanoparticles referred to as agglomerates (Kruis et al. Citation1993), that consist of similarly sized primary particles. If the agglomerates are sintered, they may coalesce and form more compact and denser nanoparticles due to an increase in the solid state diffusion (Schmidt-Ott Citation1988; Nanda et al. Citation2011). We will for consistency use the term sintering also when nanoparticles are heated above the bulk melting point of the electrode material and use the term compaction to describe changes in the particle morphology or mobility diameter.
The compaction of aerosol nanoparticles during sintering has been proposed to occur via an increase in surface and grain boundary diffusion (Weber and Friedlander Citation1997). Diffusion increases with temperature and a sintering temperature in the range of 1/3 to 2/3 (in Kelvin) of the bulk melting temperature is often sufficient for complete compaction (Karlsson et al. Citation2005). At higher temperatures, new crystal structures and facets form, and the particles may even evaporate (Schmidt-Ott Citation1988) or be thermally charged (Magnusson et al. Citation1999). Oxides are known to be more difficult to compact than pure metals because of their higher melting temperatures and lower solid-state diffusivities (Karlsson et al. Citation2005), and even adsorbed oxygen or surface oxides can impede compaction (Weber and Friedlander Citation1997).
Most metal surfaces are prone to oxidize in air, and it is common to detect oxide phases in generated metal aerosol nanoparticles post-deposition. Vons et al. (Citation2011a) demonstrated the production of mainly unoxidized silicon and magnesium (Vons et al. Citation2011b) nanoparticles in an SDG with a combination of gas purification, outgassing, and using the initial particles as a getter to further deplete the remaining oxygen. Unfortunately, such procedures are associated with high costs and are not always readily accessible, and so nanoparticles of non-noble metals produced by aerosol methods are typically oxidized. Seipenbusch et al. (Citation2003) produced nickel and platinum nanoparticles with an SDG in a nitrogen atmosphere, and found that the nickel nanoparticles were oxidized. By introducing hydrogen at sintering, they observed a more rapid compaction of the nickel nanoparticles, associated with a reduction of the nickel oxide, whereas the platinum nanoparticles studied for comparison were unaffected.
We show that one route toward production of pure metal particles is to supply hydrogen already at generation to prevent and suppress oxidation. We compare the compaction behavior as well as composition and morphology of the produced nanoparticles for four different elements: gold, bismuth, cobalt, and tin. Gold is a well-known noble metal and is resistant to bulk oxidation, whereas the non-noble metals readily form oxides: Bi3O4, SnO, SnO2, CoO, and Co3O4 that are thermodynamically stable even below ppb levels of oxygen at temperatures below 700°C. The oxides are thus expected to form even in a high purity nitrogen atmosphere, but can be reduced with hydrogen (Luidold and Antrekowitsch Citation2007) over a wide temperature range. Only SnO2 requires a temperature above 550°C to be reduced with hydrogen (Otsuka et al. Citation1983). The addition of a hydrogen atmosphere to a system with low water and oxygen levels will consume oxygen to form water. Hence, with hydrogen present at generation the oxidation of the metal nanoparticles is prevented, as is attributed to a combination of metal oxide reduction and lower oxygen levels due to water formation. Hydrogen-assisted generation of metal nanoparticles is applicable to other aerosol generation systems, and enables the production of unoxidized nanoparticles of non-noble metals, that may be of use in nanoparticle-based applications.
Method
Particle generation set-up
Nanoparticles were generated from bismuth, tin, cobalt, and gold electrodes using an SDG, see . The electrodes were provided by Goodfellow and the melting points of the metals and identified metal oxides are presented in .
Table 1. Melting temperature TM of the produced metals and metal oxides.
Figure 1. The SDG produces nanoparticles in a carrier gas that are neutralized by a radioactive 63Ni source, and size-selected in two DMAs separated by a tube furnace. Finally, an ESP is used for particle deposition and an electrometer to measure the particle concentration. Hydrogen is supplied either at generation or between the first DMA and the tube furnace.
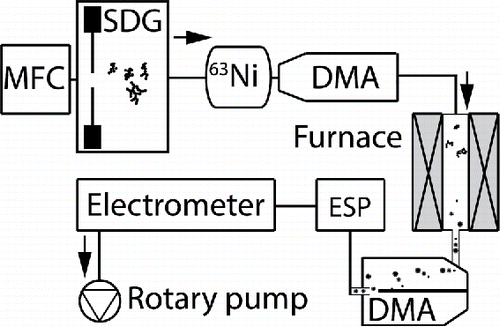
Particle generation was performed by spark discharge between two electrodes set 2 mm apart. The discharge frequency and energy were indirectly controlled by connecting the electrodes to a capacitance of 38 nF charged with a current of 10 mA. The discharge is triggered when the bias over the capacitor exceeds the breakdown voltage of the electrode gap (Borra Citation2006; Meuller et al. Citation2012). A radioactive 63Ni foil was used to neutralize the particle charge distribution, forming a known charge distribution with a low fraction of nanoparticles with multiple charges (Wiedensohler Citation1988). Two differential mobility analyzers (DMAs) were used in tandem, surrounding a tube furnace (Lenton LTF). A dedicated aluminium oxide tube (Haldenwanger) was used for each electrode material. The first DMA (TSI 3081 Long) was calibrated in the range 10–210 nm and the second smaller DMA (custom built Vienna type, Knutson and Whitby Citation1975) was calibrated in the range 5–110 nm. Finally, the particle concentration was measured with an electrometer (TSI 3086B) and particle depositions were performed with a custom built electrostatic precipitator (ESP, Deppert et al. Citation1996; Dixkens and Fissan Citation1999).
Two different carrier gases were used and controlled by a mass flow controller (MFC), either 99.9999% N2, referred to as nitrogen, or 95% N2 and 5% H2, referred to as hydrogen mixture. The carrier gas flow was set to 1.68 lpm and the pressure of the system to 1004 kPa. For experiments where hydrogen was present during sintering but not at generation, a 100% H2 flow of 0.1 lpm was added to the gas-mixture between the first DMA and the furnace, corresponding to 5% H2. Before initiating an experiment, the system was pumped down to below 10 mbar and purged with nitrogen twice. Oxygen measurements on the system done at a date separate from the experiments show that the oxygen partial pressure is below ppm levels when in operation both with and without the addition of hydrogen.
Temperature-dependent compaction
The compaction of nanoparticles was investigated with the tandem DMA set-up, set to measure negatively charged nanoparticles. Particles with a mobility diameter of 80 nm were selected with the first DMA and transported through the tube furnace. The temperature of the furnace was adjusted in steps of 25 or 50°C, up to 1200°C or until no further compaction was observed. The size of the nanoparticles after sintering was measured by scanning the second DMA and measuring the particle concentration with the electrometer. Each temperature and gas composition was scanned twice with nearly identical results before increasing the temperature. The measured particle distribution was fitted to a log-normal distribution, and the mode of the distribution was used to describe the size of the sintered nanoparticles. When the log-normal fit was poor, the mode(s) of the particle distribution was estimated manually from the peak positions. When presenting the mobility diameter as a function of temperature, the data points are connected with a line as a visual aid.
The compaction temperature was identified as the temperature above which no further decrease in mobility diameter was observed to occur. At high sintering temperatures (>700°C), thermal charging becomes significant, but was accounted for by identifying peaks associated with multiple charges (Intra and Tippayawong Citation2008). The mobility diameter calibrations for the two DMAs differed by 5% and the measurements of the second DMA have been normalized to the first DMA by a factor of 0.95.
Characterization
Depositions of bismuth, tin, cobalt, and gold nanoparticles were performed for both carrier gas compositions at selected temperatures. To further support the reproducibility of the results, the depositions were performed at a separate date and the mobility diameter for each temperature was measured and compared to the reference data, with identical trends and similar particle sizes, see Figure S1. For transmission electron microscopy (TEM) analysis, the first DMA was set to 80 nm whereas the second DMA was bypassed. The nanoparticles were deposited on copper lacey carbon TEM grids with the ESP set to a voltage of 6.5 keV. The samples were briefly exposed to an ambient atmosphere, then stored in a high purity nitrogen glove box until the day of TEM analysis. High-resolution transmission electron microscopy (HRTEM) analysis of the nanoparticle morphology and crystal structure was performed with a 300 keV JEOL 3000F operated mainly in bright field. Fast Fourier transforms (FFT) were used to compare the reciprocal structure with known crystal structures of the metal and likely metal oxides.
The oxygen concentration in the nanoparticles was acquired with energy dispersive X-ray spectroscopy (XEDS) in scanning transmission electron microscopy (STEM) mode. The compositional analysis was performed for between eight and twenty nanoparticles per deposition. To limit artifacts from post-deposition oxidation, the particles generated in nitrogen were analyzed immediately before the particles generated in hydrogen. The crystal structure results from TEM are complemented with X-ray powder diffraction (XRD) analysis of compacted nanoparticles. Deposition was performed onto quartz glass covers, with the second DMA bypassed to ensure a high particle density. The XRD data were acquired on a diffractometer (STOE Stadi MP) controlled by software (WinXPow) in reflection mode using Cu Kα1 radiation and a Mythen detector. The data were acquired in steps of 0.2° between 2° and 90° (2θ) and each step was acquired for 20 s. A reference sample without deposited particles was measured and compared with the samples containing particles, and the full spectra are included in Figure S2.
Hydrogen added at generation or sintering
To investigate whether introducing hydrogen solely during sintering would have the same effect as introducing it at generation, a set of experiments was designed for tin, bismuth, and cobalt. For each metal, a temperature that corresponded to a significant difference in mobility diameter for the two carrier gases was selected. At that temperature, the mobility diameter after sintering was measured consecutively when switching between different gas compositions at particle generation. Moreover, hydrogen was also introduced solely at sintering with the same methodology.
Results and discussion
Bismuth
The mobility diameter of nanoparticles generated from bismuth electrodes as a function of temperature for the two types of carrier gases is shown in . The mobility diameter is almost constant up to 150°C for both carrier gas compositions. Nanoparticles generated in nitrogen compact significantly in the temperature range 300–450°C, apparent from the decrease in mobility diameter from 80 to 40 nm. Further compaction is more gradual and the mobility diameter decreases to 32 nm in the temperature range 450–600°C. Finally, above 800°C the mobility diameter of particles generated in nitrogen decreases further to below 20 nm. We speculate that bismuth may evaporate due to the relatively high vapor pressure (10−5 atm) above 800°C. The compaction temperature of 450°C is above the melting temperature of bismuth but below that of Bi3O4, which indicates the presence of the oxide.
Figure 2. (a) Mobility diameter of 80 nm bismuth nanoparticles after sintering, for particles generated in either a hydrogen mixture or pure nitrogen. TEM images of typical nanoparticles sintered at different temperatures and generated in (b–f) nitrogen or (g–k) a hydrogen mixture.
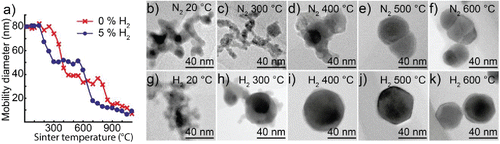
TEM images of typical nanoparticles generated in nitrogen are shown in . The nanoparticles are clearly agglomerated for sintering temperatures up to 300°C, see and . The sharp decrease in mobility diameter between 300 and 400°C is associated with the formation of a much denser nanoparticle that consists of a symmetric darker phase surrounded by an irregular brighter phase, see . At higher temperatures of 500–600°C, the particles are almost fully compact but two phases are still distinguishable, see and .
Bismuth nanoparticles generated in a hydrogen mixture compact differently than particles generated in nitrogen, see . The first sharp decrease in mobility diameter occurs at a lower temperature (150–300°C). In addition, a clear compaction temperature of 300°C is apparent as the mobility diameter is stable at 50 nm for a wide temperature range 300–550°C. When sintered above 550°C the mobility diameter drops below 50 nm and decreases below 20 nm by 700°C. Similarly, to the nanoparticles generated in nitrogen, we believe that bismuth evaporates. The compaction temperature of 300°C is still above the melting temperature of bismuth, but is significantly lower than for nanoparticles generated in nitrogen, which suggests that there is less oxidation.
TEM images of typical nanoparticles generated in the hydrogen mixture are shown in . The nanoparticles sintered at 300°C are rather compact but share a similar irregular outer phase with the nanoparticles generated in nitrogen, see . Particles sintered at 400–500°C are fully compact and have the symmetric facets associated with a single crystalline nanoparticle, see and . Some of the nanoparticles have a visible shell covering one or several facets. Finally, sintering above 600°C results in a decrease in particle size, consistent with evaporation.
High-resolution TEM analysis of fully compacted nanoparticles for each carrier gas is presented in . Bismuth nanoparticles generated in the hydrogen mixture and sintered at 400°C are shown in . The particles are faceted, single crystalline, and sometimes have a thin non-epitaxial partial shell, see and . The FFT of the particle is shown in , and matches that of bismuth viewed along a zone axis. The d-spacing of 3.28 Å are in perfect agreement with Bi
. Particles that are kept in an inert 99.9999% N2 atmosphere for a few days only have a thin and partial shell, whereas the shell on particles stored in air for an additional 9 months is more complete and thicker, see . The bismuth nanoparticles are expected to oxidize in air and the thicker shells are interpreted as further oxidation. Finally, the XRD data support the conclusion that most of the particles are unoxidized as the average crystal structure matches that of bismuth. There is also a small contribution from Bi2O3 that may originate from the observed shell, but it is not possible to determine to what extent the oxide layer had formed before deposition. The average oxygen concentration estimated by XEDS is 34 ± 13 at%. However, we believe that our XEDS measurements overestimate the oxygen concentration, as is discussed later in the manuscript.
Figure 3. (a) TEM image of a bismuth nanoparticle generated in a hydrogen mixture and compacted at 400°C, (b) with facets and a non-epitaxial shell. (c) An FFT of the particle in (a) matching the reciprocal structure of bismuth viewed along a zone axis. (d) TEM image of different particles acquired the same day and (e) 9 months later. (f) Nanoparticles generated in nitrogen and compacted at 500°C with, (g) a darker, (h) and brighter phase. (i) FFT of particle in (f) with d-spacings corresponding to Bi2O3 (220) in the bright phase and Bi
) for the dark phase. (j) XRD data of nanoparticles sintered at 500°C generated in the two carrier gases, matching mostly Bi for the hydrogen mixture and Bi + Bi2O3 for the nitrogen gas.
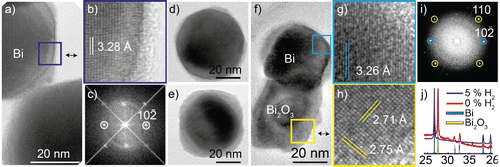
Nanoparticles generated in nitrogen and sintered at 500°C consist of two phases, one dark and one brighter phase, see . In this nanoparticle, we identify the dark phase to be single crystalline bismuth, see and . The dark phase exhibits a d-spacing of 3.26 Å, consistent with Bi. The brighter phase visible in is identified to be polycrystalline Bi2O3 with a d-spacing of 2.73 Å that matches the Bi2O3 (220). Extra spots not marked in the FFT in are simply a rotation of the oxide phase. The apparent segregation into an oxidized and unoxidized phase is also supported by the compositional analysis with XEDS, where the oxygen content of the dark phase was significantly lower (
7 at% oxygen) compared to the bright phase (
70 at% oxygen). The average oxygen concentration of particles generated in nitrogen was high (60 ± 7 at%). Finally, the XRD support the conclusion that the average crystal structure of the deposited nanoparticles is a combination of Bi and Bi2O3, see .
Tin
The mobility diameter of nanoparticles generated from tin electrodes as a function of temperature for the two types of carrier gases is shown in . Typical nanoparticles generated in a nitrogen atmosphere are shown in and nanoparticles generated in hydrogen mixture in . The compaction is negligible for both carrier gas types below 300°C, after which particles generated in a hydrogen mixture compact gradually with increasing temperature. Nanoparticles generated in nitrogen begin to compact rapidly for sintering temperatures above 750°C, where a large decrease in mobility diameter is observed from 70 to 20 nm. Neither carrier gas exhibits a compaction curve with a clear compaction temperature. The absence of a plateau in the compaction curve indicates that the nanoparticles evaporate as they compact, and is supported by the TEM analysis shown later. Nevertheless, for both carrier gases the compaction temperature is much higher than expected for unoxidized tin, with a melting point of only 234°C. On the other hand, the melting points of the oxides are much higher: SnO, 1080°C and SnO2, 1630°C. As for bismuth, we suspect that the presence of oxides is responsible for the resistance to compaction.
Figure 4. (a) Mobility diameter of 80 nm tin nanoparticles after sintering, for nanoparticles generated in nitrogen or a hydrogen mixture. TEM images of nanoparticles sintered at different temperatures: (b–e) generated in nitrogen; (f–i) generated in a hydrogen mixture.
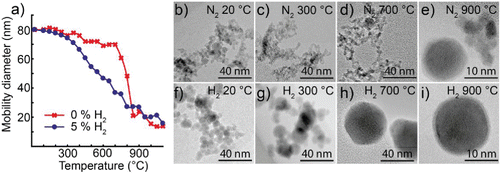
Nanoparticles generated in a nitrogen atmosphere from tin electrodes are agglomerates for sintering temperatures below 700°C, see . When compacted at 900°C, the particles are much smaller (10–20 nm in diameter), and some of them are spherical in shape, see . Particles generated in a hydrogen mixture form larger primary particles, see , that merge and grow as the temperature increases, see . Eventually they form single spherical particles that diminish in size with increasing sintering temperature, see and . When sintered at 700°C, we have also observed polycrystalline particles with a strained lattice, as well as single crystals, see Figure S3.
In , a typical tin nanoparticle generated in a hydrogen mixture and sintered at 900°C is displayed. The core has a crystal structure with d-spacings of 2.02 Å matching β-Sn and 1.44 Å matching β-Sn
, see and . There is a thin shell covering the nanoparticles that we assume is a surface oxide. Moreover, the conclusion that the nanoparticles are mainly unoxidized tin is supported by the XRD data, see , whereas the XEDS quantification suggests a higher oxygen content of 34 ± 11 at%.
Figure 5. (a) TEM image of a tin nanoparticle generated in a hydrogen mixture and compacted at 900°C, (b) with a single crystalline structure. (c) The FFT of the particle match the reciprocal structure of Sn viewed along a [110] zone axis. (d) XRD of tin nanoparticles generated in a hydrogen mixture and compacted at 900°C. (e) TEM image of spherical nanoparticle generated in nitrogen and compacted at 900°C, with a polycrystalline structure. (f) The FFT of the particle with two different d-spacings that match both Sn+SnO and SnO+SnO2 planes. (g) TEM image of agglomerate generated in nitrogen and compacted at 900°C and (h) FFT with d-spacings matching SnO2 (110).
![Figure 5. (a) TEM image of a tin nanoparticle generated in a hydrogen mixture and compacted at 900°C, (b) with a single crystalline structure. (c) The FFT of the particle match the reciprocal structure of Sn viewed along a [110] zone axis. (d) XRD of tin nanoparticles generated in a hydrogen mixture and compacted at 900°C. (e) TEM image of spherical nanoparticle generated in nitrogen and compacted at 900°C, with a polycrystalline structure. (f) The FFT of the particle with two different d-spacings that match both Sn+SnO and SnO+SnO2 planes. (g) TEM image of agglomerate generated in nitrogen and compacted at 900°C and (h) FFT with d-spacings matching SnO2 (110).](/cms/asset/cb289b29-66c9-4767-9296-10dbac46bc64/uast_a_1411580_f0005_oc.gif)
Nanoparticles generated in nitrogen from tin electrodes were as previously mentioned heterogenous, consisting of both spherical nanoparticles and agglomerates. An example of a spherical particle is displayed in . The polycrystalline structure is apparent from the FFT in , with a d-spacing of 2.96 Å that within 0.1 Å matches planes of Sn and SnO, whereas the 2.63 Å d-spacing matches planes of SnO and SnO2. The nanoparticle is at least partially oxidized, but we are unable to determine conclusively which oxides or the extent of oxidation. For the agglomerates, there is a d-spacing of 3.28 Å that agree with the SnO2 (110) with an expected d-spacing of 3.35 Å, see and . The particle density was too low for XRD, but XEDS quantification of both morphologies confirms a consistently high average oxygen content (72 ± 7 at%).
Cobalt
The mobility diameter of nanoparticles generated from cobalt electrodes as a function of temperature for the two types of carrier gases is shown in . TEM images of typical nanoparticles generated in a nitrogen atmosphere are shown in and nanoparticles generated in hydrogen in . The nanoparticles generated from cobalt electrodes exhibit a weak compaction behavior up to 250°C for both carrier gas types, see . Particles generated in nitrogen compact gradually over the entire studied temperature range, stabilizing at a final diameter of 36 nm when heated above the compaction temperature of 850°C. In contrast, nanoparticles generated in a hydrogen mixture compact at lower temperatures and reach a final mobility diameter around 30 nm when heated above the compaction temperature of 650°C. The shape of the curves is as expected, with a gradual decrease in mobility diameter until the compaction temperature is achieved. Furthermore, the compaction temperatures fit well with what is expected based on the melting point of Co and CoO. For cobalt, we do not observe any apparent evaporation during sintering, explained from the higher melting point and lower vapor pressure of cobalt.
Figure 6. (a) Mobility diameter of 80 nm cobalt nanoparticles after sintering for nanoparticles generated in a hydrogen mixture and nanoparticles generated in nitrogen. TEM images of nanoparticles sintered at different temperatures and generated in nitrogen (b–e) or a hydrogen mixture (f–i).
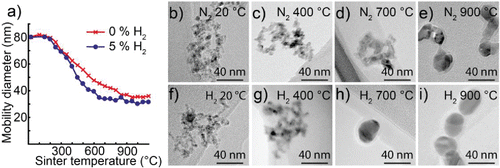
The morphology of the compacted cobalt nanoparticles is presented in . Below 400°C, the nanoparticles generated in nitrogen are agglomerates, see and and so are nanoparticles generated in a hydrogen mixture, see and . In agreement with the compaction series in , the nanoparticles generated in a hydrogen mixture are fully compacted at 700°C and only minor reshaping occurs up to 900°C, see and . However, nanoparticles generated in nitrogen are still not fully compact after sintering at 900°C, see and .
High-resolution TEM images of fully compacted nanoparticles from cobalt electrodes are presented in . Nanoparticles generated in hydrogen are single crystalline when compacted above 700°C, see and . The observed symmetry of the structure matches face-centered cubic cobalt viewed along a zone axis, see the FFT in . The d-spacings do not match perfectly, but are within 0.1 Å from what is expected for Co, where Co
should be 2.05 Å and Co
should be 1.78 Å. Furthermore, the cobalt phase is representative of the average nanoparticle, as is supported from the XRD spectra in . On the other hand, the XEDS quantification indicates that the oxygen content varies between particles, with an average oxygen concentration of 40 ± 16 at%.
Figure 7. (a) TEM images of cobalt nanoparticle generated in a hydrogen mixture and sintered at 700°C. (b) High-resolution magnification of particle and (c) FFT of compared to face centered cubic Co viewed along a [110] zone axis. (d) TEM images of oxidized Co nanoparticle generated in nitrogen and sintered at 700°C. (e) High-resolution inset with d-spacings matching CoO (in yellow), and either CoO or Co3O4 in white. (f) FFT of the particle from (d). (g) XRD spectra of nanoparticles from (a), (d) with reference spectra for Co and CoO.
![Figure 7. (a) TEM images of cobalt nanoparticle generated in a hydrogen mixture and sintered at 700°C. (b) High-resolution magnification of particle and (c) FFT of compared to face centered cubic Co viewed along a [110] zone axis. (d) TEM images of oxidized Co nanoparticle generated in nitrogen and sintered at 700°C. (e) High-resolution inset with d-spacings matching CoO (in yellow), and either CoO or Co3O4 in white. (f) FFT of the particle from (d). (g) XRD spectra of nanoparticles from (a), (d) with reference spectra for Co and CoO.](/cms/asset/ae4b3174-eb87-44d6-900a-7a9d7c2363d7/uast_a_1411580_f0007_oc.gif)
When generated in nitrogen and compacted at 900°C, see the nanoparticles are polycrystalline. The CoO phase is identified from the symmetry and d-spacings in the high-resolution image and the FFT, see and . The d-spacings of 2.41 Å and 2.22 Å match the 2.43 Å d-spacing of CoO and 2.13 Å d-spacing of CoO
. Due to the polycrystalline structure, there are also rotations of these planes visible in the FFT. The d-spacing of 1.60 Å potentially matches either CoO or Co3O4 due to numerous similar d-spacings of high index planes. Unfortunately, the XRD signal is too weak to identify the predominant phases, but a small peak suggests that there is some unoxidized cobalt, see . On the other hand, the XEDS quantification indicates that all particles are oxidized, with an average oxygen content of 61 ± 5 at%.
Gold
Gold nanoparticles show an almost identical compaction behavior for the two gases, see . Compaction occurs gradually in the temperature range 20–375°C with a decrease in mobility diameter from 80 to 45 nm. The compaction temperature is determined to be roughly 375°C. It is well known that gold nanoparticles in this size range are stable for a wide span of temperatures, but will for extreme temperatures decrease in size as they evaporate (Magnusson et al. Citation1999; Nanda et al. Citation2008).
Figure 8. (a) Mobility diameter of 80 nm gold nanoparticles after sintering at different temperatures for the two carrier gases. Nanoparticles generated in nitrogen and sintered at (b) room temperature or (c) 500°C. (d) A magnification of the crystal structure with (e) the FFT matching Au viewed along [110]. Gold nanoparticles generated in hydrogen mixture and sintered at (f) room temperature or (g) 500°C. (h) Magnification of particle with (i) the FFT matching Au viewed along [100].
![Figure 8. (a) Mobility diameter of 80 nm gold nanoparticles after sintering at different temperatures for the two carrier gases. Nanoparticles generated in nitrogen and sintered at (b) room temperature or (c) 500°C. (d) A magnification of the crystal structure with (e) the FFT matching Au viewed along [110]. Gold nanoparticles generated in hydrogen mixture and sintered at (f) room temperature or (g) 500°C. (h) Magnification of particle with (i) the FFT matching Au viewed along [100].](/cms/asset/309b037e-aeeb-486b-89a7-0180c3764b53/uast_a_1411580_f0008_oc.gif)
shows TEM images of typical gold nanoparticles compacted at different temperatures and verifies that hydrogen has a negligible effect on the compaction behavior for 80 nm gold agglomerates. The size and morphology of the agglomerates are similar for unsintered nanoparticles, see and . The compacted particle size (40 nm) and shape are also similar when compacted at a temperature of 500°C, see and . The crystal structure matches that of Au for both carrier gases. In and , we identify a 2.35 Å d-spacing that matches Au
, and in and we identify a 2.05 Å d-spacing that matches Au
. Like the other materials, the XEDS signal is higher than expected, with an average of 27 ± 20 at% oxygen for nanoparticles generated in nitrogen, and 25 ± 10 at% oxygen for nanoparticles generated in a hydrogen mixture. With gold being a noble metal, we establish that our quantification of oxygen is systematically overestimated with XEDS.
Phase of the nanoparticles
A summary of the observed particle phases and oxygen concentration from the different characterization methods is presented in .
Table 2. Composition and identified phases of compacted nanoparticles generated in a hydrogen mixture or in nitrogen.
Nanoparticles generated in a hydrogen mixture from bismuth, tin, cobalt, and gold electrodes are mostly non-oxidized metal nanoparticles. The absolute oxygen concentration from XEDS should be interpreted with caution as the technique is less accurate in the quantification of light elements such as oxygen. The XEDS quantification indicated oxygen concentrations on the order of 30–40 at% for bismuth, tin, and cobalt nanoparticles generated in a hydrogen mixture. The actual oxygen concentration is likely much lower, as even gold nanoparticles had an oxygen concentration of around 25 at% estimated by XEDS. Since most metals adsorb oxygen almost immediately in air (Fehlner and Mott Citation1970), a surface oxide is expected on most of the nanoparticles, although it can be thin as oxidation slows down and may stop after only a few monolayers if the oxide forms a self-passivated layer (Brennan et al. Citation1960; Cabrera and Mott Citation2002). One crude method to predict whether a self-passivated oxide film is likely to form on a bulk metal surface is to determine whether the Pilling-Bedworth ratio RPB of the metal and its oxide is between 1 and 2. The RPB of Co:CoO is 1.9, and for Sn:SnO it is 1.3. To a first approximation, we expect fully compacted cobalt and tin nanoparticles to self-passivate, forming only thin oxide shells. For Bi:Bi3O4, the RPB is 3.7 and it is predicted that the oxide will consume the nanoparticle, as was indicated by the growing oxide shell. To protect metal nanoparticles that lack a self-passivated oxide layer, we believe it will be important to engineer passivated shells that can be applied to different metal nanoparticles after compaction.
Nanoparticles generated in nitrogen from bismuth, tin, and cobalt electrodes are at least partially oxidized. For tin and cobalt, the XRD signal was too low to determine the dominating crystal phase, whereas for bismuth it was clear that there exists a considerable amount of both unoxidized and oxidized bismuth. The bismuth nanoparticles were partially oxidized, with a Bi and Bi2O3 phase, identified from the crystal structure and supported by XEDS. It is possible that the nanoparticles generated from cobalt and tin electrodes were also only partially oxidized, but we were unable to identify metallic phases and the oxygen content with XEDS was consistently high (60–70 at% oxygen) and did not vary significantly between nanoparticles. The consistent high oxygen signal together with identification of only oxidized crystals indicates that most nanoparticles from tin and cobalt electrodes were oxidized when generated in a nitrogen atmosphere.
The compaction temperature
The compaction temperature of a nanoparticle is the lowest temperature required to form a completely compact nanoparticle, with no voids to fill and is often associated with a nearly spherical shape. However, different experimental set-ups will have slight variations in the shape and purity of the initial nanoparticles as well as different temperature and flow profiles of the tube furnace, and some variations in the compaction temperature are expected. As mentioned in the introduction, the compaction temperature often lies in the interval of 1/3–2/3 (in Kelvin) of the bulk melting temperature, as reported by Karlsson et al. (Citation2005) when reviewing the compaction behavior of Ag, Au, PbS, Fe, SnO2, and TiO2 aerosol nanoparticles. This agrees with our results for nanoparticles of Au (0.50), Co (0.49), and CoO (0.50). However, it does not agree with the results for the low melting point metals, Bi (1.05) and Sn (1.93). For bismuth and tin nanoparticles generated in a hydrogen mixture, the compaction temperature corresponds better to that expected of Bi3O4 (0.53) and for SnO2 (0.51). We speculate that the nanoparticles have adsorbed oxygen when they transition from the hydrogen mixture to a nitrogen atmosphere at the first size selection. Even adsorbed oxygen may be sufficient to impede compaction, but would have a negligible effect on the final composition and crystal structure of a compacted nanoparticle. This would explain why the particles appear to be nearly free of oxides but have a higher than expected compaction temperature. Thus, for future work it would be interesting to also investigate whether a hydrogen atmosphere should be maintained until after sintering.
Hydrogen at generation or at sintering
compares the compaction of bismuth, tin, and cobalt nanoparticles sintered at a temperature selected to correspond to a significant difference in mobility diameter for the two carrier gas compositions. We know that the change in mobility diameter is associated with a more compact and unoxidized nanoparticle, which we apply to indirectly investigate changes in oxidation. At this temperature, the mobility diameter after sintering was investigated as the hydrogen content in the carrier gas was switched on and off, see . For bismuth, the larger mobility diameter is expected to correspond to a more compact nanoparticle. This may appear unintuitive, but from and we have deduced that the larger nanoparticles are associated with a fully compact bismuth phase.
Figure 9. The mode of nanoparticles after sintering for two series of gas modifications, for (a) bismuth, (b) tin, and (c) cobalt. Hydrogen is introduced either at generation or at sintering. The reference refers to the expected mobility diameter. TEM images of typical nanoparticles generated in nitrogen but sintered in a hydrogen mixture for (d) bismuth, (e) tin and (f) cobalt.
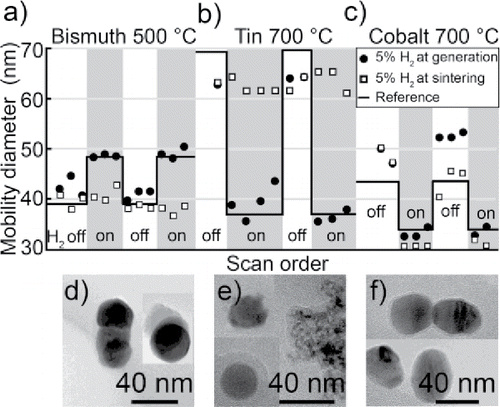
The mobility diameters of nanoparticles produced in a hydrogen mixture is consistently close to the reference mobility diameters from , , and . The mobility diameter of bismuth and cobalt is consistently within 3 nm from the reference, whereas it for tin fluctuates more. As the sintering of bismuth and cobalt nanoparticles is performed at a temperature above the compaction temperature, the mobility diameter is expected to be more stable than for the tin nanoparticles, that is destabilized by the competition of compaction and evaporation. Whenever the carrier gas mixture is switched between a hydrogen mixture and nitrogen, there is a significant and consistent change in mobility diameter for the three metals. Nanoparticles that are not exposed to any hydrogen (i.e., H2 off in figures) sometimes compact differently to the reference, such as for tin with 7 nm smaller nanoparticles and the hysteresis observed for cobalt after hydrogen has been present at generation. The difference for tin is not surprising due to the high temperature sensitivity at the selected sintering temperature, which is just on the on-set of a sharp decrease in mobility diameter. However, for cobalt the compaction behavior changes with the history of the system as would be expected if the compaction is affected by changes in the impurity levels of the system when the carrier gas is switched.
For bismuth and tin, it was not as effective to supply hydrogen at sintering. Most of the nanoparticles have the same mobility diameter as for reference nanoparticles not exposed to hydrogen, see , but there is a small widening of the particle distribution (not shown). The widening is a result of a fraction of nanoparticles becoming smaller and more similar in morphology to nanoparticles generated in a hydrogen mixture, see and . However, the morphology of most nanoparticles is the same as nanoparticles not exposed to hydrogen. For cobalt, adding hydrogen at sintering resulted in a change in nanoparticle morphology, with nanoparticles very similar in shape and morphology to those generated in the hydrogen mixture, see . We believe that the change in morphology is associated with a reduced metal oxide as shown to be the case for particles generated in hydrogen.
From the presented results, we see that tin, cobalt, and bismuth nanoparticles generated in an inert nitrogen atmosphere will oxidize even for sub ppm levels of oxygen. As oxidation is more rapid at elevated temperatures, it is likely that this occurs during particle formation, when the vapor is quenched as it exits a very high temperature (Reinmann and Akram Citation1999; Kohut et al. Citation2017) plasma. To heat a metal oxide under the presence of a reducing agent such as hydrogen is a general method to prevent formation of oxides. However, to reduce an oxide takes time and is only energetically favorable above a certain temperature. We believe that the bismuth and tin nanoparticles sintered in hydrogen were partially reduced, and that a complete reduction of the oxides would be possible if the reduction reaction would have more time or be sped up by, e.g., a higher sintering temperature, a higher hydrogen concentration, or use of a stronger reducing agent. Although hydrogen is a relatively harmless reducing agent, it may pose a potential hazard if metal hydrides are formed during, e.g., the spark discharge. Metal hydrides are in general very volatile and are prone to thermal decomposition even at room temperature. Therefore, we believe that if any toxic hydrides are formed, they will decompose in the tube furnace. However, for up-scaled production it may be necessary to further investigate risks associated with a potential hydride formation at the spark.
Conclusions
The addition of 5% hydrogen in the nitrogen carrier gas during generation has clear benefits when generating and compacting nanoparticles from non-noble metal electrodes but negligible effects when generating nanoparticles from gold electrodes. The nanoparticles compact to single crystalline metal nanoparticles and some indications of surface oxidation after compaction are apparent. However, it is likely that the oxidation occurs mainly after deposition and this can in principle be prevented via a self-passivated layer. This is in stark contrast to nanoparticles generated in nitrogen, where the oxide is an integral part of polycrystalline nanoparticles. For bismuth, the nanoparticles segregate into Bi and Bi2O3 when sintered, and applying hydrogen at sintering results in some reduction of the oxide phase. For tin and cobalt, the oxidized nanoparticles compact very poorly and are found to be at least partially oxidized. The confirmed oxide phases are SnO2 and CoO, but other phases such as Sn, SnO, Co, and Co3O4 may also be present. When the oxidized tin nanoparticles are sintered in a hydrogen mixture, only some of the nanoparticles are fully compacted, whereas all cobalt nanoparticles are. We attribute the resistance to compaction to be a sign of incomplete oxide reduction or partial oxidation occurring after generation.
We have demonstrated the method for four different metals, one reference metal and three metals known to be reducible by hydrogen. If the metal does not oxidize in the inert atmosphere, hydrogen has a negligible effect on the particle generation. If the metal is readily reduced by hydrogen, the nanoparticles are unoxidized and compact at lower sintering temperatures. Further research is required to confirm whether the produced particles are free of oxides at generation and to clarify how metals and oxides interact during particle generation and compaction. Our work has clearly demonstrated that adding hydrogen during generation is beneficial to produce unoxidized nanoparticles, with clear and consistent support that this is due to the prevention of initial oxidation at particle generation.
UAST_1411580_Supplemental_File.zip
Download Zip (336.8 KB)Acknowledgments
The authors wish to thank Crispin Hetherington, Sebastian Lehmann, and Martin Magnusson for valuable discussions and Stephanie Smith for assistance in data collection.
Additional information
Funding
References
- Blomberg, S., Gustafson, J., Martin, N. M., Messing, M. E., Deppert, K., Liu, Z., Chang, R., Fernandes, V. R., Borg, A., Grönbeck, H., and Lundgren, E. (2013). Generation and Oxidation of Aerosol Deposited PdAg Nanoparticles. Surf. Sci., 616:186–191. doi:10.1016/J.SUSC.2013.06.005.
- Borra, J.-P. (2006). Nucleation and Aerosol Processing in Atmospheric Pressure Electrical Discharges: Powders Production, Coatings and Filtration. J. Phys. D: Appl. Phys., 39(2):R19–R54. doi:10.1088/0022-3727/39/2/R01.
- Brennan, D., Hayward, D. O., and Trapnell, B. M. W. (1960). The Calorimetric Determination of the Heats of Adsorption of Oxygen on Evaporated Metal Films. Proc. R. Soc. Lond. A, 256(May):81–105. doi:10.1098/rspa.1960.0094.
- Byeon, J. H., Park, J. H., and Hwang, J. (2008). Spark Generation of Monometallic and Bimetallic Aerosol Nanoparticles. J. Aerosol Sci., 39(10):888–896. doi:10.1016/j.jaerosci.2008.05.006.
- Cabrera, N., and Mott, N. F. (2002). Theory of the Oxidation of Metals. Rep. Prog. Phys., 12(1):163–184. doi:10.1088/0034-4885/12/1/308.
- Deppert, K., Schmidt, F., Krinke, T., Dixkens, J., and Fissan, H. (1996). Electrostatic Precipitator for Homogeneous Deposition of Ultrafine Particles to Create Quantum-Dot Structures. J. Aerosol Sci., 27:S151–S152. doi:10.1016/0021-8502(96)00148-6.
- Dixkens, J., and Fissan, H. (1999). Development of an Electrostatic Precipitator for Off-Line Particle Analysis. Aerosol Sci. Technol., 30(5):438–453. doi:10.1080/027868299304480.
- Fan, K., Cao, C., Pan, Y., Lu, D., Yang, D., Feng, Y., Song, L, Liang, M., and Yan, X. (2012). Magnetoferritin Nanoparticles for Targeting and Visualizing Tumour Tissues. Nature Nanotechnol., 7(July):459–464. doi:10.1038/NNANO.2012.90.
- Fehlner, F. P., and Mott, N. F. (1970). Low-Temperature Oxidation. Oxid. Met., 2(1):59–99. doi:10.1007/BF00603582.
- Feng, J., Hontañón, E., Blanes, M., Meyer, J., Guo, X., Santos, L., Paltrinieri, L., Ramlawi, N., Smet, L. C. P. M., Nirschl, H., Kruis, F. E., Schmidt-Ott, A., and Biskos, G. (2016a). Scalable and Environmentally Benign Process for Smart Textile Nanofinishing. ACS Appl. Mater. Interf., 8(23):14756–14765. doi:10.1021/acsami.6b03632.
- Feng, J., Huang, L., Ludvigsson, L., Messing, M. E., Maisser, A., Biskos, G., and Schmidt-Ott, A. (2016b). General Approach to the Evolution of Singlet Nanoparticles from a Rapidly Quenched Point Source. J. Phys. Chem. C, 120(1):621–630. doi:10.1021/acs.jpcc.5b06503.
- Gupta, A. K., and Gupta, M. (2004). Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials, 26:3995–4021. doi:10.1016/j.biomaterials.2004.10.012.
- Intra, P., and Tippayawong, N. (2008). An Overview of Differential Mobility Analyzers for Size Classification of Nanometer-Sized Aerosol Particles. Songklanakarin J. Sci. Technol., 30(2):243–256.
- Itina, T. E., and Voloshko, A. (2013). Nanoparticle Formation by Laser Ablation in Air and by Spark Discharges at Atmospheric Pressure. Appl. Phys. B, 113:473–478. doi:10.1007/s00340-013-5490-6.
- Kala, S., Theissmann, R., Rouenhoff, M., and Kruis, F. E. (2016). Metal-Semiconductor Pair Nanoparticles by a Physical Route Based on Bipolar Mixing. Nanotechnology, 27(12):125604. doi:10.1088/0957-4484/27/12/125604.
- Karlsson, M. N. A., Deppert, K., Karlsson, L. S., Magnusson, M. H., Malm, J-O., and Srinivasan, N. S. (2005). Compaction of Agglomerates of Aerosol Nanoparticles: A Compilation of Experimental Data. J. Nanopart. Res., 7(1):43–49. doi:10.1007/s11051-004-7218-3.
- Kim, J.-T., and Chang, J.-S. (2005). Generation of Metal Oxide Aerosol Particles by a Pulsed Spark Discharge Technique. J. Electrost., 63(6–10):911–916. doi:10.1016/j.elstat.2005.03.066.
- Knutson, E. O., and Whitby, K. T. (1975). Aerosol Classification by Electric Mobility: Apparatus, Theory, and Applications. J. Aerosol Sci., 6(6):443–451. doi:10.1016/0021-8502(75)90060-9.
- Kohut, A., Ludvigsson, L., Meuller, B. O., Deppert, K., Messing, M. E., Gábor, G., Geretovszky, G., and Geretovszky, Z. (2017). From Plasma to Nanoparticles: Optical and Particle Emission of a Spark Discharge Generator. Nanotechnology. Advance Online Publication, https://doi.org/10.1088/1361-6528/aa8f84
- Kruis, F. E., Kusters, K. A., Pratsinis, S. E., and Scarlett, B. (1993). A Simple Model for the Evolution of the Characteristics of Aggregate Particles Undergoing Coagulation and Sintering. Aerosol Sci. Technol., 19(4):514–526. doi:10.1080/02786829308959656.
- Ludvigsson, L., Meuller, B. M., and Messing, M. E. (2015). Investigations of Initial Particle Stages during Spark Discharge. J. Phys. D: Appl. Phys., 48(31):314012. doi:10.1088/0022-3727/48/31/314012.
- Luidold, S., and Antrekowitsch, H. (2007). Hydrogen as a Reducing Agent: Thermodynamic Possibilities. JOM, 59(10):58–62. doi:10.1007/s11837-007-0133-1.
- Magnusson, M. H., Deppert, K., Malm, J-O., Bovin, J-O., and Samuelson, L. (1999). Gold Nanoparticles: Production, Reshaping, and Thermal Charging. J. Nanopart. Res., 1:243–251. doi:10.1023/A:1010012802415.
- Magnusson, M. H., Ohlsson, B. J., Björk, M. T., Dick, K. A., Borgström, M. T., Deppert, K., and Samuelson, L. (2014). Semiconductor Nanostructures Enabled by Aerosol Technology. Front. Phys., 9(3):398–418. doi:10.1007/s11467-013-0405-x.
- Messing, M. E., Hillerich, K., Johansson, J., Deppert, K., and Dick, K. D. (2009). The Use of Gold for Fabrication of Nanowire Structures. Gold Bull., 42(3):172–181. doi:10.1007/BF03214931.
- Messing, M. E., Westerström, R., Meuller, B. O., Blomberg, S., Gustafson, J., Andersen, J. N., Lundgren, E., Rijin, R., Balmes, O., Bluhm, H., and Deppert, K. (2010). Generation of Pd Model Catalyst Nanoparticles by Spark Discharge. J. Phys. Chem. C, 114(20):9257–9263. doi:10.1021/jp101390a.
- Meuller, B. O., Messing, M. E., Engberg, D. L. J., Jansson, A. M., Johansson, L. I. M., Norlén, S. M., Tureson, N., and Deppert, D. (2012). Review of Spark Discharge Generators for Production of Nanoparticle Aerosols. Aerosol Sci. Technol., 46(11):1256–1270. doi:10.1080/02786826.2012.705448.
- Muntean, A., Wagner, M., Meyer, J., and Seipenbusch, M. (2016). Generation of Copper, Nickel, and CuNi Alloy Nanoparticles by Spark Discharge. J. Nanopart. Res., 18 229. doi:10.1007/s11051-016-3547-2.
- Nanda, K. K., Maisels, A., and Kruis, F. E. (2008). Surface Tension and Sintering of Free Gold Nanoparticles. J. Phys. Chem. C, 112(35):13488–13491. doi:10.1021/jp803934n.
- Nanda, K. K., Maisels, A., and Kruis, F. E. (2011). Evolution of Crystallinity of Free Gold Agglomerates and Shape Transformation. RSC Adv., 1(4):568–572. doi:10.1039/C1RA00208B.
- Noh, S. R., Lee, D., Park, S. J., Kim, D. S., and Choi, M. (2017). High Throughput Nanoparticle Generation Utilizing High-Frequency Spark Discharges via Rapid Spark Plasma Removal. Aerosol Sci. Technol., 51(1):116–122. doi:10.1080/02786826.2016.1239814.
- Otsuka, K., Murakoshi, S., and Morikawa, A. (1983). Hydrogen Production from Water by Reduced Tin Oxide. Fuel Process. Technol., 7(3):213–223. doi:10.1016/0378-3820(83)90003-6.
- Pfeiffer, T. V., Feng, J., and Schmidt-Ott, A. (2014). New Developments in Spark Production of Nanoparticles. Adv. Powder Technol., 25(1):56–70. doi:10.1016/j.apt.2013.12.005.
- Ramsurn, H., and Gupta, R. B. (2013). Hydrogenation by Nanoparticle Catalysts, in New and Future Developments in Catalysis, S. L. Suib, ed., Elsevier, Amsterdam, pp. 347–374. doi:10.1016/B978-0-444-53874-1.00016-0.
- Reinmann, R., and Akram, M. (1999). Temporal Investigation of a Fast Spark Discharge in Chemically Inert Gases. J. Phys. D: Appl. Phys., 30(7):1125–1134. doi:10.1088/0022-3727/30/7/010.
- Schmidt-Ott, A. (1988). New Approaches to in Situ Characterization of Ultrafine Agglomerates. J. Aerosol Sci., 19(5):553–563. doi:10.1016/0021-8502(88)90207-8.
- Seipenbusch, M., Weber, A. P., Schiel, A., and Kasper, G. (2003). Influence of the Gas Atmosphere on Restructuring and Sintering Kinetics of Nickel and Platinum Aerosol Nanoparticle Agglomerates. J. Aerosol Sci, 34(12):1699–1709. doi:10.1016/S0021-8502(03)00355-0.
- Tabrizi, N. S., Xu, Q., Van Der Pers, N. M., Lafont, U., and Schmidt-Ott, A. (2009). Synthesis of Mixed Metallic Nanoparticles by Spark Discharge. J. Nanopart. Res., 11:1209–1218. doi:10.1007/s11051-008-9568-8.
- Weber, A. P., and Friedlander, S. K. (1997). In Situ Determination of the Activation Energy for Restructuring of Nanometer Aerosol Agglomerates. J. Aerosol Sci., 28(2):179–192. doi:10.1016/S0021-8502(96)00062-6.
- Wiedensohler, A. (1988). An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range. J. Aerosol Sci., 19(3):387–389. doi:10.1016/0021-8502(88)90278-9.
- Vons, V. A., Anastasopol, A., Legerstee, W. J., Mulder, F. M., Eijt, S. W. H., and Schmidt-Ott, A. (2011a). Low-Temperature Hydrogen Desorption and the Structural Properties of Spark Discharge Generated Mg Nanoparticles. Acta Mater., 59(8):3070–3080. doi:10.1016/j.actamat.2011.01.047.
- Vons, V. A., Smet, L. C. P. M., Munao, D., Evirgen, A., Kelder, E. M., and Schmidt-Ott, A. (2011b). Silicon Nanoparticles Produced by Spark Discharge. J. Nanopart. Res., 13(10):4867–4879. doi:10.1007/s11051-011-0466-0.
- Yetisen, A. K., Qu, H., Manbachi, A., Butt, H., Dokmeci, M. R., Hinestroza, J. P, Skorobogatiy, M., Khademhosseini, A., and Yun, S. Y. (2016). Nanotechnology in Textiles. ACS Nano., 10(3):3042–3068. doi:10.1021/acsnano.5b08176.
