ABSTRACT
The nasal aerosol filtration properties of infants 0–3 months old have been quantified through in vitro measurements. Computed tomography (CT) scan data was obtained of seven individuals with ages of 5–79 days. Nasal airway replicas based on these images were manufactured using rapid prototyping. Deposition in the replicas was measured using an electrical low pressure impactor (ELPI) to measure the concentration of aerosol particles in the inertial regime. Comparing the difference in concentration when sampling through the model versus sampling through a blank line gave the deposition fraction. Deposition was measured for particles with aerodynamic diameters between 0.53 and 5.54 μm. Nonlinear least squares curve fitting was performed to collapse intersubject variability and represent the data with a single curve. To achieve satisfactory intersubject variability collapse, a non-dimensional pressure drop, the Euler number (Eu), was required in addition to the Reynolds number (Re) and the particle Stokes number (Stk) where the dimensionless parameters are evaluated with a length scale, D, defined as the airway volume divided by the airway surface area. The equation describing the deposition fraction, η, is η = 1- (14590 / (14590 + Stk1.2201Re1.7742Eu1.5772))0.3687. An analysis of the expected intersubject variability in in vivo deposition was also performed, yielding a method for predicting variance in neonatal nasal airway deposition.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
Introduction
Aerosolized medications are an indispensable tool for the treatment of respiratory ailments, allowing targeted delivery of therapeutic agents to the lungs and delivery of drugs with low oral bioavailability (Everard Citation2003). Conversely, aerosols in the form of airborne pollutants can be hazardous to health with effects ranging from mild irritation to cancer (Kampa and Castanas Citation2008). While the extrathoracic airways act to filter ambient particulate matter, particles making up therapeutic aerosols must successfully traverse these airways to achieve their desired effect in the lungs. In adults, the oral airway has long been known to filter less particulate matter than the nasal airway (Heyder et al. Citation1975) so has been preferred for the delivery of therapeutic aerosols. However, nasal delivery is relevant to populations such as infants, who are obligate nasal breathers, and young children who cannot learn to use an oral inhaler. Furthermore, since nasal breathing is common at rest at all ages (Bennett et al. Citation2008) lung exposure to atmospheric aerosols depends on nasal filtration. Therefore, the filtration properties of a target population's nasal airways must be well understood to aid in the design of effective pharmaceutical aerosolization devices and the development of air quality standards.
Deposition in adult nasal airways has been well investigated through in vivo and in vitro measurements and in silico simulations, e.g., Hsu and Chuang (Citation2012) and Walenga et al. (Citation2014) and references therein. Far fewer studies have been carried out to quantify nasal deposition in children and have compared filtration in children to adults (Becquemin et al. Citation1991; Bennett et al. Citation2008; Golshahi et al. Citation2011; Phalen et al. Citation1989) but with varying results. Despite reports of increased infant mortality with exposure to increased levels of ambient particulate matter (Son et al. Citation2008; Woodruff et al. Citation1997), even fewer have studied the filtration properties of infant nasal airways. Storey-Bishoff et al. (Citation2008) comprehensively measured nasal deposition in 11 different nasal models of infants aged 3–18 months old and identified a correlation describing the nasal filtration of these airways as a function of particle size, inhalation flow rate, and airway characteristic diameter. It is unclear if their correlation can be extrapolated to neonates or infants younger than 3 months old. Swift (Citation1991) and Zhou et al. (Citation2014) each present isolated in vitro measurements of nasal deposition in an infant younger than 3 months old but do not investigate enough subjects to account for the intersubject variability expected within this population. The filtration, and variability therein, of the nasal passage of neonatal infants remains to be fully described. We have designed the present in vitro study to expand current understanding of neonatal nasal particulate filtration. This study presents the deposition characteristics of seven anatomically realistic nasal replicas of neonatal infants along with a correlation that provides average nasal filtration in this population. In addition, an estimate of the variability in deposition due to geometric dissimilarity, that is, variation in airway shape, in the population is provided.
Methods
Nasal replica models
Nasal replicas were constructed based on computed tomography (CT) scan data acquired under anonymity from the University of Alberta Diagnostic Imaging Archives with approval from the University of Alberta Research Ethics Office. Infants underwent imaging for health reasons unrelated to this study and in all cases the nasal airway was considered normal. The images were imported and segmented using Mimics software (Materialise, Leuven, Belgium). In-plane resolution of the scan images ranged from 260 to 434 μm and axial slice thicknesses ranged from 0.6 to 1.5 mm producing voxel sizes of 0.113 ± 0.063 mm3. Airways were identified based on an upper density limit of about −240 Hounsfield units. Stereolithography files of the face and airway were smoothed in 3-Matic (Materialise) to eliminate roughness introduced by the discretization of the naturally smooth surfaces and then exported to the Magics software package (Materialise) where the model was created.
Each model consisted of three parts; the first part included the entire face and the airway just distal to the nasal valve, the second included the airway up to the nasopharynx and the final part extended from the nasopharynx through the larynx into the trachea. The outlet of the airway model was made such that it ended orthogonal to the axis of the trachea and the expected direction of flow. This multi-piece construction was used to accommodate removal of support material after rapid prototyping. The models were fastened together with machine screws and sealed externally with silicone sealant (737, Dow Corning, Midland, MI, USA). Models were printed with an Eden 350 V (Stratasys, Eden Prairie, MN, USA) printer using VeroGray and VeroBlack photopolymer (Stratasys). The two materials have the same properties except color and were used based on availability. The resolution of the build is 42 μm in the x- and y-axis (in plane resolution) and 16 μm in z-axis (build direction). After printing and cleaning, the pieces were CT scanned to ensure support material had been adequately removed.
Select dimensions of the airways, including the airway volume and surface area, were obtained in the 3-Matic work environment for evaluation of dimensionless parameters. Storey-Bishoff et al. (Citation2008) found airway volume divided by surface area provided a length scale, DV/As, which collapsed intersubject variability, motivating consideration of this dimension here. Additionally, Garcia et al. (Citation2009) suggest a dimension, referred to herein as DGarcia, based on nasal resistance which has also been found to fit inertial deposition data well by others (Golshahi et al. Citation2011; Zhou et al. Citation2014). Briefly, the procedure to measure this dimension involves taking pressure drop measurements across the nasal airway and obtaining the nasal resistance (Rnose) by fitting the resulting data to:[1] where ΔP is the pressure drop across the model at corresponding flow rate, Q, through the model. The dimension is then obtained by evaluating
[2] where Lnose is the length of the nose defined as the distance from the nostrils to the end of the septum and k is a constant depending on fluid properties (0.0181 kg/[(m10/4)·(sec1/4)] for air at standard conditions). The resulting dimension is the diameter of a circular pipe that has the same length and resistance as the nasal airway for turbulent internal air flow.
Pressure drop across the nasal airways was measured with a low range digital manometer (HHP-103; OMEGA Engineering, Stamford, CT, USA) at constant flow rates between 2 and 18 standard L/min. Least squares fitting was used to fit the pressure drop data as suggested by Garcia et al. (Citation2009). The data fit this equation well with r2 values greater than 0.99 for each subject. Demographic information and dimensions for each subject are given in .
Table 1. Subject demographics and airway characteristics.
Breathing patterns
Deposition was measured under simulated tidal breathing conditions; a sinusoidal wave form was used. Breath parameters were selected based on values observed in the literature and such that physiologically realistic inhalation flow rates would be achieved. Haddad et al. (Citation1979) found that the duty cycle does not change significantly with age in the first four months of life. Thus, a duty cycle of 0.32 was selected based on the mean observed value. A realistic range of average inhalation flow rates were identified based on minute ventilation data including a minimum of 1.6 L/min based on 10th centile minute ventilation of 142.9 mL/(min·kg) for an infant with mass 3.5 kg and a maximum of 14.6 L/min based on 90th centile minute ventilation of 707.7 mL/(min·kg) and an infant with a mass of 6.6 kg (Estol et al. Citation1988). It should be noted that a 6.6 kg infant is out of the range studied by Estol et al. (Citation1988) however this only represents a 19% increase from a maximum based on 613 mL/(min·kg) minute ventilation and a 6.4 kg infant (giving 12.3 L/min) which are values observed by Fuchs et al. (Citation2011) for unsedated infants during quite sleep. Respiratory rates in the range of 40–60 breaths per minute are common (Rusconi and Castagneto Citation1994) and values as extreme as 20.7 and 117.5 breaths per minute have been reported (Richards et al. Citation1984). Estol et al. (Citation1988) give 10th and 90th centiles for body mass normalized tidal volume as 2.21 and 10.34 mL/kg and Fuchs et al. (Citation2011) observed a maximum of 11.8 mL/kg giving a tidal volume range of 7.7 to 77.9 mL. After identifying acceptable ranges for each breath defining parameter, seven different breath profiles were selected. In the present work, the tidal volume range was extended up to 100 mL for comparison with deposition in infants reported by Storey-Bishoff et al. (Citation2008). The defining parameters are listed in .
Table 2. Breath defining parameters.
Experimental apparatus
Deposition measurements were carried out in an aerosol exposure plenum built to the specifications presented by Golshahi et al. (Citation2011). In brief, the plenum is cubic with side length of 0.6 m; the top section is a mixing region 10 cm tall where two opposing fans create a well-mixed aerosol which is introduced to the exposure region (45 cm tall). The two regions are separated by a flow straightener to minimize secondary flow patterns in the supply air. The flow straightener is a 2 inch (5 cm) thick aluminum honeycomb with 0.25 inch hexagonal cells (HoneyCommCore, Jupiter, FL, USA).
A rigid piping system of 3/8″ NPT stainless steel pipe and 3/4″ (.0675″ wall) aluminum tubing carried the sampled aerosol to an electrical low pressure impactor (ELPI) (Dekati Ltd., Kangasala, Finland) for classification. Aerosol of jojoba oil (ρ = 870 kg/m3) was generated using a 1-jet Collison nebulizer (MesaLabs, Butler, NJ, USA) operated at approximately 5 psi connected to the building compressed air source; the resulting aerosol had a mass median aerodynamic diameter of 2.4 μm and a geometric standard deviation of approximately 2 (estimated by interpolating the discrete cumulative distribution measured by the ELPI).
The ELPI draws a constant flow of 30 L/min; this flow was matched by flow control on a tank of extra dry compressed air (Praxair, Danbury, CT, USA) such that there was no flow through the model while only the ELPI was operating. This flow rate was monitored with a mass flowmeter (4043, TSI Inc., Shoreview, MN, USA) throughout the experiments. Flow profiles were created by an ASL 5000 Breathing Simulator (IngMar Medical, Pittsburgh, PA, USA). As was done by Storey-Bishoff et al. (Citation2008), to eliminate the issue of large dead space in the experimental apparatus, the exhale portion of the breath was directed through a check valve near the ASL 5000; in this way the airway replicas were tested under inhalation only and were stagnant during the exhalation phase of the breath. Flow profiles were recorded with another TSI 4043 flowmeter at a 10 ms sampling rate.
Two sampling lines extended into the aerosol plenum, one a blank line for characterizing the aerosol within the plenum, the other the model line which aerosol first traversed an airway replica before being routed to the ELPI. Rigid piping was used to eliminate any differences in particle deposition occurring within the two sampling lines; identical fittings and lengths were used in each line. Aerosol flow was switched between the two lines and directed to the ELPI with a three way, quarter turn ball valve. A schematic of the deposition measurement system is shown in .
Experimental design
Each measurement consisted of two minutes of sampling through the blank line, two minutes through the model line, and another two minutes through the blank line. Sampling through the blank line twice was done to ensure aerosol concentrations were constant for the duration of the measurement. Deposition was measured by comparing the aerosol sampled through the blank line to that sampled through the model line on a bin-by-bin basis. The ELPI provides concentration data for 12 particle size bins with aerodynamic diameter geometric centers between 45 nm and 9 μm. Only data for the range of particles dominated by inertial deposition was analyzed. The largest bin had too few counts to provide meaningful data. Data for bins with geometric centers of 0.53, 0.83, 1.34, 2.12, 3.34, and 5.54 μm were thus used. The fraction of particles depositing in the model is given by:[3] Here cblank is the average concentration measured during both periods of sampling through the blank line (representative of the aerosol entering the model) and cmodel is the average concentration measured after passing through a nasal replica. Note that due to added resistance in the model line, a slightly smaller tidal volume is generated by the ASL 5000 and thus the concentration measured must be corrected for the reduced number of particles entering the model simply due to a smaller volume of aerosol entering. This correction is achieved by multiplying cmodel by the ratio of tidal volume measured through the blank line to the tidal volume measured through the model. The correction factor was calculated for each subject and each breathing pattern independently.
To ensure the validity of deposition measurements, several validations were made prior to carrying out the experiment. Sampling through two blank lines of identical length showed no difference in aerosol concentration which verifies that deposition within the piping was equal in each branch and thus not skewing deposition measurements systematically higher or lower. Sampling through blank lines of different lengths and/or different orientations allowed the spatial dependence of the aerosol distribution to be checked. It was found that the aerosol distribution within the exposure plenum did not depend on spatial coordinate as equal concentrations were measured through both sampling lines when sampling at different points within the chamber. This justifies the assumption that the aerosol concentration measured by the sampling line is indicative of the concentration entering the nostrils of the nasal replicas. Finally, deposition measurements were performed in the two youngest nasal replicas made by Storey-Bishoff et al. (Citation2008) using the breath profiles reported in that study. Good agreement between the new measurements and the reported values provided further confidence in the experimental apparatus.
Deposition was measured in each nasal replica for each breath profile; each measurement was repeated three times. The standard deviation of repeated deposition measurements was on average 0.45% indicating repeatability was good. Statistical analysis and numerical methods were performed in MATLAB (R2014a, MathWorks, Natick, MA, USA), a significance level of α = 0.05 was used for statistical analysis. The Levenberg-Marquardt algorithm with unit weighting was used for non-linear least squares fitting.
Results and discussion
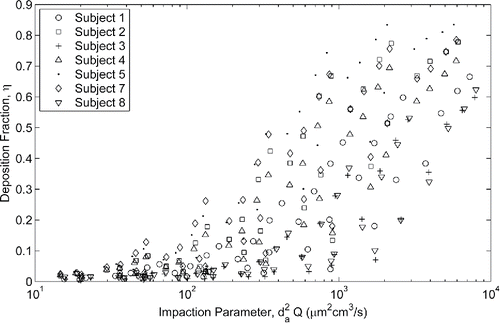
Although it is expected there will be large spread in the data points when plotted against the well-known impaction parameter, it provides a starting point for comparison of the deposition measured in the neonatal models to that of the infant models. uses the impaction parameter to compare deposition in the younger neonate models and the older infant models studied by Storey-Bishoff et al. (Citation2008) and additionally to the deposition measured in a nasal replica of a 10 day old infant under constant flow reported by Zhou et al. (Citation2014).
Figure 2. Comparison of deposition in population of neonate models (this study) to deposition in the population of infant nasal airways (Storey-Bishoff et al. Citation2008) and to deposition in a 10 day old infant (Zhou et al. Citation2014).
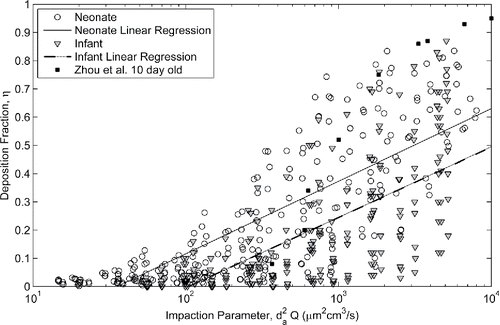
While there is substantial overlap in the data sets due to intersubject variability, significantly more deposition is measured in the neonatal population than the infant population for a given impaction parameter (p < 0.001, t-test against regression coefficients). The slopes of the neonate and infant linear regressions are not statistically different, but for any given impaction parameter there is an average of about 12% (absolute) more deposition in a neonate versus an infant.
Agreement is seen in the deposition measured by Zhou et al. (Citation2014) and the neonatal population studied here although it appears that there may be higher deposition measured compared to our neonate models at the higher impaction parameter range. This could be due to roughness associated with construction of their model; Zhou et al. (Citation2014) used rapid prototyping with a layer thickness of approximately 0.25 mm, whereas our models were built with a layer thickness of 16 µm. Kelly et al. (Citation2004) and Schroeter et al. (Citation2011) have shown that excessive surface roughness of the nasal model can affect deposition, exhibiting higher deposition that asymptotes with decreasing roughness to that measured in a smooth model. For this reason neither the Zhou et al. (Citation2014) nor Swift (Citation1991) data (3 mm surface roughness) will be considered further here. With a layer thickness that is 3 times thinner than the smoothest models tested by Kelly et al. (Citation2004), we believe that wall roughness effects are negligible in our models.
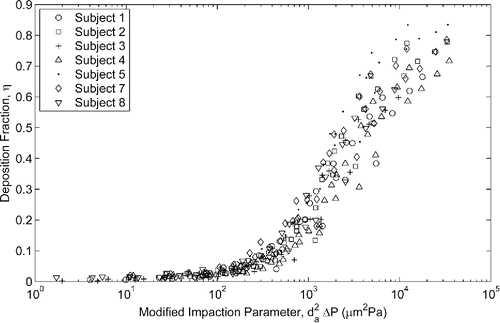
The impaction parameter does not include any subject specific factor and thus shows how significant the variation in deposition can be within a population. This is evident in which compares the deposition between each neonate subject as a function of the impaction parameter.
The inclusion of subject specific factors to account for intersubject variability is desirable to provide a better estimate of the average deposition in the population. Hounam et al. (Citation1971) first identified transnasal pressure drop as one such factor and modified the impaction parameter by replacing flow rate with the resulting pressure drop. Plotting deposition against this pressure-drop-based impaction parameter () reduces the scatter of the data considerably.
This parameter does not explicitly include any characteristic length scale, which particle dynamics suggest should be included in the form of the particle Stokes number, but the pressure drop across a duct is known to be strongly correlated with duct diameter. This pressure based impaction parameter was further varied by Garcia et al. (Citation2009) who found adult nasal deposition data was better collapsed by a parameter of da2ΔP2/3; however, use of this parameter did not improve the description of our neonatal nasal deposition data compared to the use of da2ΔP.
Nondimensional analysis
Following previous work (Cheng Citation2003; Golshahi et al. Citation2011; Storey-Bishoff et al. Citation2008; Zhou et al. Citation2014), further non-dimensional analysis of the deposition data should allow extension of these deposition results to other carrier gases, larger particle sizes, and other sizes of similarly shaped geometries. Using deposition results in this way is subject to the non-dimensional parameters remaining within the range studied and that impaction remains the dominating mechanism of deposition. Extension to non-similar geometries is a violation of scaling laws (Çengel and Cimbala Citation2010) (different subjects are not exact scales of one another) and Storey-Bishoff et al. (Citation2008) attribute the discrepancies in measured deposition and their non-dimensional equation to this fact. Nevertheless, it is interesting to explore the ability of the correlation developed by Storey-Bishoff et al. (Citation2008) for nasal filtration in 3- to 18-month-old infants to describe deposition measured in the neonatal models. With a trivial modification to account for our use of the standard definitions of Reynolds number (Re) and Stokes number (Stk) the correlation of Storey-Bishoff et al. is:[4] where Davg is the average volume over surface area diameter reported by Storey-Bishoff et al. Citation2008 as 1.20 mm. The definitions of Stk and Re are
[5]
[6] where Q is the average flow rate during the inhalation period of the breath, ρ0 is the reference density (1000 kg/m3), da is the particle aerodynamic diameter, μf is the fluid dynamic viscosity (1.8 × 10−5 kg/m·s for air), D is the characteristic diameter of the geometry, ρf is the fluid density (1.10 kg/m3 for air at 93 kPa, normal ambient pressure at the location of the experiment), and Cc is the Cunningham correction factor that accounts for non-continuum effects given by
[7] where λ is the mean free path of air (74 nm for air at 21°C and 93 kPa). Using these definitions, measured deposition can be plotted with an abscissa of the combination of dimensionless parameters from EquationEquation (4)
[4] as is done in .
Figure 5. Deposition measured in neonatal nasal airways compared to the correlation developed by Storey-Bishoff et al. (Citation2008) for deposition measured in infants (r2 = 0.47).
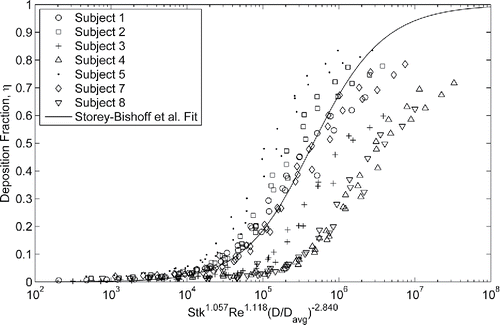
While this deposition parameter does not collapse the intersubject variability in this population, the correlation does a fair job predicting the average deposition. Recently, Yang et al. (Citation2017) examined the ability of several correlations to predict oral extrathoracic aerosol deposition in adults by comparing to in vivo measurements. They found that while correlations can predict average deposition in a population, they fail to accurately predict subject specific deposition. This failure was attributed to extrapolation of the predictive correlations to conditions they were not developed under such as different average inhalation flow rates, tidal volumes, and respiratory rates. Errors were also attributed to the violation of geometric similarity in the use of dimensional analysis. These sources of error are present in this study when attempting to use the correlation developed by Storey-Bishoff et al. (Citation2008) to collapse deposition data in smaller models of different subjects tested under different breathing conditions.
To further collapse intersubject variability in the presentation of the data, other combinations of the dimensionless parameters evaluated using the different characteristic diameters were explored. The Euler number (Eu), a dimensionless pressure drop, was also explored based on the results of plotting deposition vs. the pressure based impaction parameter () and is defined as:[8] where ΔP is the transnasal pressure drop at corresponding average inhalation flow rate, Q. Different products of dimensionless parameters (always including the Stokes number since aerosol theory describes the value of the Stokes number as being the extent to which a particle's path deviates from streamlines and thus may undergo inertial deposition) were used as the abscissa; the powers of each parameter were allowed to vary and found through non-linear least squares regression. The highest coefficient of determination value, r2, found for each deposition parameter evaluated with each characteristic diameter is listed in .
Table 3. Summary of r2 values for each characteristic diameter. Values of α, β, γ are different in each case found using non-linear least squares fitting.
While optimizing the constants of EquationEquation (4)[4] improved the r2 value for the neonate data, this characteristic diameter (DV/As) and deposition parameter combination (Re, Stk, and D/Davg) does not fully collapse the data, contrary to observations by Storey-Bishoff et al. (Citation2008). Interestingly, the collapse is essentially the same for the two characteristic diameters studied when the Euler number is used despite fits excluding Eu being better when using DGarcia. This is likely due to the inherent inclusion of the transnasal pressure drop in this characteristic diameter via the nasal resistance factor in the calculation. Since pressure drop is not only sensitive to the size of the airway, but also to the shape, inclusion of this measurement can be thought of as including information about some of the geometric dissimilarities between models. For example, a large but convoluted airway may have similar filtration properties as a smaller, simpler airway which would not be immediately clear based on purely geometric measurements such as DV/As, whereas comparing the nasal resistance provides some insight to the shape of the airway. That is to say, DV/As is purely a measure of airway size, while DGarcia includes not only the airway size but also some degree of measure of its shape. Further, including the Euler number in the deposition parameter introduces this measure of shape to the correlation when evaluating with DV/As. Since the shape cannot be fully defined with only these two measures, the remaining dissimilarity remains in the data, to the same extent, regardless of choice of characteristic diameter.
Here, the Euler number is found to significantly improve the fit, in contrast to the observation by Golshahi et al. (Citation2011) in older children. The best correlation found is[9] where DV/As is used in the evaluation. demonstrates the ability of this deposition parameter and resulting correlation to describe the deposition in these models.
Figure 6. Deposition in neonatal nasal airways vs. non-dimensional deposition parameter, characteristic diameter defined D = V/AS, fit defined in EquationEquation (9)[9] , r2 = 0.97.
![Figure 6. Deposition in neonatal nasal airways vs. non-dimensional deposition parameter, characteristic diameter defined D = V/AS, fit defined in EquationEquation (9)[9] , r2 = 0.97.](/cms/asset/c1a7e1a1-997b-4c2c-b222-a1c8f678bf52/uast_a_1413489_f0006_b.gif)
The rate at which infants develop in the first years of life may account for the fact that inclusion of the Euler number was not required to fit deposition in children (Golshahi et al. Citation2011) as intersubject variability due to the degree of development of the nasal passage, and thus shape, may be less in that population. This is corroborated by the observations of Golshahi et al. (Citation2011) who found that infant nasal deposition was higher than in children, with considerable scatter remaining in the data when applying the infant nasal deposition equation to child deposition data, and that a single correlation to describe deposition in infants and children could not be developed. They also note that nasal deposition in children and adults as a function of impaction parameter showed considerable overlap, while for a given impaction parameter deposition for infants is generally higher. This is attributed to the similar magnitude of child and adult characteristic diameters opposed to much smaller diameters of infants.
EquationEquation (9)[9] can be evaluated with population average values to represent the average deposition expected in the neonatal subjects studied here. Using the average DV/As = 0.906 mm, an inhalation flow rate of 4.3 L/min and thus a pressure drop of 152 Pa (obtained using the average nasal resistance), a 2 µm particle has a 14.1% deposition fraction. Storey-Bishoff et al. (Citation2008) report a filtration of only 2.0% for the same particle diameter and flow rate for their population average DV/As = 1.20 mm, further exhibiting the increased deposition measured in the younger population.
Estimating total in vivo deposition variability
While the result of non-dimensional analysis on the in vitro data shows a strong ability to account for intersubject variability and may suggest the potential for EquationEquation (9)[9] to be used in a predictive capacity, the accuracy of predictive correlations on a subject specific basis has recently been called into question. Alternatively, these equations have been found to well predict the average deposition of a population (Yang et al. Citation2017). The errors associated with using deposition correlations in a predictive capacity were further analyzed by Ruzycki et al. (Citation2017) who proposed a novel approach using the deposition equation to provide an estimate of the variance of deposition expected in a population. Their predictions accurately mirrored the in vivo results of Yang et al. (Citation2017) and thus present an approach for a deposition correlation to be used to predict an average and standard deviation of in vivo deposition in a population, as opposed to expected values in individual subjects.
The details of this analysis are described by Ruzycki et al. (Citation2017), so will not be fully described here. In summary, the total deposited fraction on a mass basis is calculated by integrating the deposition of particles over a defined aerosol distribution. The average deposition is calculated using population average values for inhalation flow rate and characteristic diameter. Then, using a method based on the concept of propagation of uncertainty, an estimate for the variance in total deposition is obtained by combining the variance in deposition caused by the variance of each constitutive parameter using the root-sum-of-squares.
To use this method, the variance of each parameter must be known and thus data for each parameter must follow a known distribution. The nasal resistance data fails the Anderson-Darling normality test (p = 0.04 using adtest function in MATLAB) and thus the variance in deposition due to nasal resistance cannot be identified and the method cannot be performed using EquationEquation (9)[9] . A simplified equation which does not depend on transnasal pressure drop is required for this method. Deposition as a function of only the Stokes number in our subjects is given by
[10] where Stk is evaluated with DV/As. The simplicity of this equation reduces its accuracy but permits the estimation of deposition variance. The fit of this deposition correlation is shown in .
Figure 7. Neonatal nasal deposition as a function of Stokes number, characteristic diameter defined D = V/AS, fit defined in EquationEquation (10)[10] , r2 = 0.69.
![Figure 7. Neonatal nasal deposition as a function of Stokes number, characteristic diameter defined D = V/AS, fit defined in EquationEquation (10)[10] , r2 = 0.69.](/cms/asset/8722061c-49a6-4b96-9ca8-c4c282d1a8df/uast_a_1413489_f0007_b.gif)
Using this simplified equation allows direct application of the method as developed by Ruzycki et al. (Citation2017) where variation in deposition,, is due to variation in flow rate,
; characteristic diameter,
; and geometric dissimilarity,
. The exclusion of the Euler number in the deposition parameter can be thought of as returning shape differences to the data, these differences being then accounted for by the variance in deposition due to geometric dissimilarity.
The distribution of input parameter values is required to evaluate and
. The characteristic dimensions of our nasal models are known and pass the Anderson-Darling normality test (P > 0.05) so the average and standard deviation of this sample (D = 0.906 ± 0.123 mm) can be used. An expected distribution of inhalation flow rates for these subjects is also required. Haddad et al. (Citation1979) report average inhalation flow rate during quiet sleep throughout the first four months of life; their reported results for birth to three months of age have been pooled to give an estimate of the expected distribution of flow rates of 2.90 ± 1.04 L/min.
Examining the result of individually varying each parameter to values one standard deviation above and below the average value () leads to estimates of the variability due to flow rate and characteristic diameter. For example, variation due to inhalation flow rate is found by calculating deposition at a flow rate one standard deviation below the average (Q1) and one standard deviation above the average (Q2) yielding values for and
, respectively. Note that total deposition is calculated by integrating deposition across the aerosol distribution as was done by Ruzycki et al. (Citation2017). This requires a specified aerosol distribution; an MMAD of 3.7 µm and GSD of 2 were used for illustrative purposes to define the aerosol here, which are the values used by Ruzycki et al. (Citation2017). The magnitude of the difference in calculated deposition is two times the variation due to flow rate (i.e.,
) since flow rate has been varied plus and minus one standard deviation from the mean. The same procedure is followed for variation due to characteristic diameter. The resulting estimate of variation in deposition due to flow rate is
, and variation in deposition due to characteristic diameter is
.
Table 4. Total deposition (on a mass basis) of aerosol with MMAD of 3.7 µm and GSD of 2.
The estimate for variation due to geometric dissimilarity is obtained by examining the errors associated with using EquationEquation (10)[10] to describe deposition in the nasal replicas. If the characteristic diameter fully defined the shape of the airway, the data points would fall on a single curve (which is the intent of non-dimensional analysis). Since the characteristic diameter of the nasal replicas and inhalation flow rates during deposition testing are well quantified, presumably the residuals associated with the non-dimensional correlation are due to shape differences in the nasal replicas not captured by the characteristic diameter, that is, geometric dissimilarity. Plotting the deposition prediction obtained using EquationEquation (10)
[10] versus the measured in vitro deposition allows these errors to be inspected (). Using the fact that 95% of the data lie within 2 standard deviations of the mean leads to an estimate of the variation due to geometric dissimilarity. In this case, 95% of the data are bounded by linear bands 32% above and below the line of identity (where the model perfectly describes the in vitro data) yielding a variation due to geometric dissimilarity of
.
Figure 8. Predicted deposition (using EquationEquation (10)[10] ) versus in-vitro measured deposition. Solid line indicates line of identity; dashed lines bound 95% of the data.
![Figure 8. Predicted deposition (using EquationEquation (10)[10] ) versus in-vitro measured deposition. Solid line indicates line of identity; dashed lines bound 95% of the data.](/cms/asset/34ccf553-5f6e-47a9-b89a-ac8183ee576d/uast_a_1413489_f0008_b.gif)
The estimates of the three identified sources of variation yield a first-order estimate of the total variation in nasal deposition through the application of the -norm:
[11] Evaluating EquationEquation (11)
[11] gives a standard deviation of
. Thus, for the population of infants aged zero to three months, breathing nasally, during sleep, and receiving an aerosol with an MMAD of 3.7 µm and GSD of 2, one can expect 29.8 ± 18.1% of the inhaled aerosol to deposit in the nasal passage. Repeating the above calculations using instead MMADs of 2.7 and 4.7 µm with the same conditions and a constant GSD of 2, we find average and standard deviations of deposition of 21.1 ± 17.5% and 37.2 ± 18.6%, respectively. Similar analysis can be performed for other aerosol distributions and inhalation flowrates to provide average nasal deposition and expected variance for the given conditions.
Although this method was developed based on a combination of in vivo and in vitro measurements and for oral inhalation in adults, the underlying method is valid. Estimates obtained in this way are thus the best currently available estimates of nasal deposition and expected variance in a population of neonatal infants. Comparison to in vivo measurements to validate these estimates is desirable. However, due to the risks associated with making these measurements using current technology (radiation exposure to a developing infant) and the degree of cooperation which would be required of the infants, this remains a topic for future research.
Comparing these results to those reported by Ruzycki et al. (Citation2017) for oral, tidal breathing in adults is of some interest (). As expected, total deposition is much higher in the neonatal population despite the significantly lower inhalation flow rates. This is due to the route of inhalation and the size of the airways. It has long been known that, for adults, oral delivery of aerosols is favorable over nasal delivery since the nasal airway filters particles more effectively than the oral airway (Heyder et al. Citation1975). Coincidentally, the variations due to flow rate and due to characteristic diameter are comparable between the two cases. Thus, the increased variation in deposition in neonates is primarily due to increased variation due to geometric dissimilarity; in adults this variation is only 5%. This major difference in the two models is likely due to the inhalation route and the previously mentioned rate at which infants develop. Storey-Bishoff et al. (Citation2008) compared the collapse of deposition data in oral and nasal geometries and attributed the additional dependence on characteristic diameter to describe infant nasal deposition partly to the increased extent that the nasal cross sectional area departs from a circular shape. The same observation is made here where transnasal pressure drop (additional information about the airway shape) was required to fully collapse the deposition data in neonates. The oral cross sectional area is much more circular and thus a given characteristic diameter better defines the oral airway reducing variation due to geometric dissimilarity. Further, geometric dissimilarity in the population of neonatal infants is likely higher due to the developing nature of infants. Since the airways of neonatal infants are growing and developing it is expected there would be larger shape variations within the population when compared to the fully developed oral airways of adults. Finally, in a relative sense, the variability in deposition is not vastly different between populations. The expected variation in neonatal nasal deposition is 60% of the average value whereas the variation in adults is 53% of the average.
Table 5. Comparison of average and variability in total deposition in adults (Ruzycki et al. Citation2017) and neonatal infants (in vitro) when normally inhaling an aerosol with MMAD 3.7 µm and GSD of 2.
Conclusions
Inertial nasal filtration in infants between the ages of 5 and 79 days has been quantified via in vitro measurements. Increased deposition was measured in this population compared to existing in vitro data (Storey-Bishoff et al. Citation2008) in infants aged 3–18 months. An empirical equation describing the deposition in our neonatal models was identified, with the best fit obtained by including non-dimensional pressure drop in the form of the Euler number in addition to the Stokes and Reynolds numbers. A simplified equation depending only on Stokes number was also identified; using this Stokes-number-only equation an estimate of the variance in nasal filtration expected within the population of neonatal infants is provided. Using this method, the Stokes-number-only equation can be used to predict an expected intersubject variation of total nasal aerosol deposition within a neonatal population. This work may be helpful to those designing aerosol treatments for infant respiratory ailments and to those studying the risks associated with the exposure of infants to ambient particulate matter.
Acknowledgments
The authors gratefully thank the professionals at the Institute for Reconstructive Sciences in Medicine, especially Andrew Grosvenor and Heather Logan, for their guidance in interpreting CT images and assistance in designing the airway replica models for additive manufacturing.
References
- Becquemin, M. H., Swift, D. L., Bouchikhi, A., Roy, M., Teillac, A. (1991), Particle Deposition and Resistance in the Noses of Adults and Children. Eur. Respir. J., 4(6):694–702.
- Bennett, W. D., Zeman, K. L., Jarabek, A. M. (2008), Nasal Contribution to Breathing and Fine Particle Deposition in Children versus Adults. J. Toxicol. Environ. Health, 71(3):227–237. doi.org/10.1080/15287390701598200.
- Cheng, Y. S. (2003), Aerosol Deposition in the Extrathoracic Region. Aerosol Sci. Technol., 37(8):659–671.
- Çengel, Y. A., Cimbala, J. M. (2010). Fluid Mechanics: Fundamentals and Applications. McGraw-Hill Higher Education, New York, NY.
- Estol, P., Priz, H., Pintos, L., Nieto, F., Simini, F. (1988), Assessment of Pulmonary Dynamics in Normal Newborns: A Pneumotachographic Method. J. Perinat. Med., 16(3):183–192.
- Everard, M. L. (2003), Inhalation Therapy for Infants. Adv. Drug. Deliv. Rev., 55(7):869–878.
- Fuchs, O., Latzin, P., Thamrin, C., Stern, G., Frischknecht, P., Singer, F., Kieninger, E., Proietti, E., Riedel, T., Frey, U. (2011), Normative Data for Lung Function and Exhaled Nitric Oxide in Unsedated Healthy Infants. Eur. Respir. J., 37(5):1208–1216.
- Garcia, G. J., Tewksbury, E. W., Wong, B. A., Kimbell, J. S. (2009), Interindividual Variability in Nasal Filtration as a Function of Nasal Cavity Geometry. J Aerosol Med. Pulm. Drug. Deliv., 22(2):139–155.
- Golshahi, L., Noga, M. L., Thompson, R. B., Finlay, W. H. (2011), In Vitro Deposition Measurement of Inhaled Micrometer-Sized Particles in Extrathoracic Airways of Children and Adolescents during Nose Breathing. J. Aerosol Sci., 42(7):474–488.
- Haddad, G. G., Epstein, R. A., Epstein, M. A., Leistner, H. L., Marino, P. A., Mellins, R. B. (1979), Maturation of Ventilation and Ventilatory Pattern in Normal Sleeping Infants. J. Appl. Physiol., 46(5):998–1002.
- Heyder, J., Armbruster, L., Gebhart, J., Grein, E., Stahlhofen, W. (1975), Total Deposition of Aerosol Particles in the Human Respiratory Tract for Nose and Mouth Breathing. J. Aerosol Sci., 6(5):311–328.
- Hounam, R. F., Black, A., Walsh, M. (1971), The Deposition of Aerosol Particles in the Nasopharyngeal Region of the Human Respiratory Tract. J. Aerosol Sci., 2(1):47–61.
- Hsu, D. J., Chuang, M. H. (2012), In-Vivo Measurements of Micrometer-Sized Particle Deposition in the Nasal Cavities of Taiwanese Adults. Aerosol Sci. Technol., 46(6):631–638.
- Kampa, M., Castanas, E. (2008), Human Health Effects of Air Pollution. Environ. Pollut., 151(2):362–367.
- Kelly, J., Asgharian, B., Kimbell, J., Wong, B. (2004), Particle Deposition in Human Nasal Airway Replicas Manufactured by Different Methods. Part I: Inertial Regime Particles. Aerosol Sci. Technol., 38(11):1063–1071.
- Phalen, R. F., Oldham, M. J., Mautz, W. J. (1989), Aerosol Deposition in the Nose as a Function of Body Size. Health Phys., 57:299–305.
- Richards, J. M., Alexander, J. R., Shinebourne, E. A., de Swiet, M., Wilson, A. J., Southall, D. P. (1984), Sequential 22-Hour Profiles of Breathing Patterns and Heart Rate in 110 Full-Term Infants During Their First 6 Months of Life. Pediatrics, 74(5):763–777.
- Rusconi, F., Castagneto, M. (1994), Reference Values for Respiratory Rate in the First 3 Years of Life. Pediatrics, 94(3):350.
- Ruzycki, C. A., Yang, M., Chan, H., Finlay, W. H. (2017), Improved Prediction of Intersubject Variability in Extrathoracic Aerosol Deposition using Algebraic Correlations. Aerosol Sci. Technol., 51(6):667–673.
- Schroeter, J. D., Garcia, G. J. M., Kimbell, J. S. (2011), Effects of Surface Smoothness on Inertial Particle Deposition in Human Nasal Models. J. Aerosol Sci., 42(1):52–63.
- Son, J., Cho, Y., Lee, J. (2008), Effects of Air Pollution on Postneonatal Infant Mortality among Firstborn Infants in Seoul, Korea: Case-Crossover and Time-Series Analyses. Arch. Environ. Occup. Health, 63(3):108–113.
- Storey-Bishoff, J., Noga, M., Finlay, W. H. (2008), Deposition of Micrometer-sized Aerosol Particles in Infant Nasal Airway Replicas. J. Aerosol Sci., 39(12):1055–1065.
- Swift, D. L. (1991), Inspiratory Inertial Deposition of Aerosols in Human Nasal Airway Replicate Casts: Implication for the Proposed NCRP Lung Model. Radiat. Prot. Dosim., 38(1–3):29–34. doi:10.1093/rpd/38.1-3.29.
- Walenga, R. L., Tian, G., Hindle, M., Yelverton, J., Dodson, K., Longest, P. W. (2014), Variability in Nose-to-lung Aerosol Delivery. J. Aerosol Sci., 78:11–29.
- Woodruff, T. J., Grillo, J., Schoendorf, K. C. (1997), The Relationship between Selected Causes of Postneonatal Infant Mortality and Particulate Air Pollution in the United States. Environ. Health Perspect., 105(6):608–612.
- Yang, M. Y., Ruzycki, C., Verschuer, J., Katsifis, A., Eberl, S., Wong, K., Golshahi, L., Brannan, J. D., Finlay, W. H., Chan, H. (2017), Examining the Ability of Empirical Correlations to Predict Subject Specific In-Vivo Extrathoracic Aerosol Deposition during Tidal Breathing. Aerosol Sci. Technol., 51(3):363–376.
- Zhou, Y., Guo, M., Xi, J., Irshad, H., Cheng, Y. (2014), Nasal Deposition in Infants and Children. J. Aerosol Med. Pulm. Drug. Deliv., 27(2):110–116.