ABSTRACT
Understanding the links between aerosol and cloud and radiative properties remains a large uncertainty in predicting Earth's changing energy budget. Surfactants are observed in ambient atmospheric aerosol particles, and their effect on cloud droplet growth is a mechanism that was, until recently, neglected in model calculations of particle activation and droplet growth. In this study, coarse mode aqueous aerosol particles were created containing the surfactant Igepal CA-630 and NaCl. The evaporation and condensation of these individual aqueous particles were investigated using an aerosol optical trap combined with Raman spectroscopy. For a relative humidity (RH) change from 70% to 80%, droplets containing both Igepal and NaCl at atmospheric concentrations exhibited on average more than 4% larger changes in droplet radii, compared to droplets containing NaCl only. This indicates enhanced water uptake in the presence of surfactants, but this result is unexpected based on the standard calculation of the effect of surfactants, using surface tension reduction and/or hygroscopicity changes, for particles of this size. One implication of these results is that in periods with increasing RH, surfactant-containing aqueous particles may grow larger than similarly sized aqueous NaCl particles without surfactants, thus shifting atmospheric particle size distributions, influencing particle growth, and affecting aerosol loading, visibility, and radiative forcing.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
1. Introduction
Aerosol particles vary in composition based on their sources and reaction pathways in the atmosphere (Poschl Citation2005; Jimenez et al. Citation2009). The variability in particle composition can affect their climate and air quality properties, such as influencing activation into cloud droplets and the scattering and absorption of radiation (Poschl Citation2005). Cloud droplet formation on atmospheric aerosols has been widely studied. The activation of aerosol particles into cloud droplets is generally represented by an adaptation of Köhler theory (Köhler Citation1936), in which the composition, or hygroscopicity, and the size of the initial, dry particle are the primary terms affecting whether a particle grows to become a cloud droplet. The hygroscopic growth of particles containing inorganic compounds such as salt is well established, while the effect of organics, especially those with surface affinity, is recently gaining more attention (Petters and Petters Citation2016; Ovadnevaite et al. Citation2017). In current models, the surface tension of the dry particle as it is growing is generally considered to be the same as that of water, which excludes any influence from surface-active compounds on the surface tension (Prisle et al. Citation2012). In contrast, if all other parameters remain unchanged for a given particle type and dry particle size, incorporating a surface tension depression into Köhler theory results in predictions of a decrease in the critical supersaturation (Sc) and an increase in the critical wet diameter (Dp,c) of particles, reducing the barrier to cloud formation. Such a trend was calculated by Sorjamaa et al. (Citation2004) when an assumed partitioning of surface-active organics between the bulk and surface of the particles was taken into account.
Aqueous particles in sub-saturated conditions can affect visibility and radiative forcing through scattering and absorption of visible and infrared radiation. A study of the radiative effect of aerosol particles in central California found that under clean conditions, neglecting coarse mode particles and calculating direct aerosol radiative forcing using submicron particles only resulted in an up to 45% overestimation of direct aerosol radiative forcing (Kassianov et al. Citation2012). This highlights the necessity of making accurate predictions of the growth of coarse mode particles in the atmosphere. Coarse mode particles (greater than 1 µm) are less efficient at scattering solar radiation than smaller, accumulation mode particles (0.1–1 µm) (Bohren and Huffman Citation1983). Additionally, particle composition affects light scattering and visibility through the interaction of the compounds with visible light. For example, carbon is highly absorbing (imaginary part of the complex refractive index (k) of −0.79 at 550 nm; Bond and Bergstrom Citation2006), while mineral dust has little to no absorption (k of −0.006 at 550 nm; Tegen et al. Citation1996). Liquid water is non-absorbing in the visible region of solar radiation due to its negligibly small value of k but is highly absorbing in the infrared (Hale and Querry Citation1973; Seinfeld and Pandis Citation2006). Changing the water content of aerosol particles through hygroscopic growth changes their composition and size, thus affecting both their absorption and scattering properties.
Surfactants are compounds that reduce the free energy of surfaces and interfaces, often referred to as reducing the surface or interfacial tension. In particles, this acts to decrease the energetic barrier to growth. The ability of surfactants or surface-active compounds to reduce the surface tension of water has been widely studied in macroscopic systems (Petters and Petters Citation2016). In a solution, before reaching the critical micelle concentration (CMC), the surface tension varies strongly with the concentration of surfactant. At the CMC, micelle formation occurs, and further increasing the concentration of surfactant in the system does not further reduce the surface tension.
A largely unconstrained aspect of aerosol properties is the presence and effect of surfactants in individual aerosol particles. Few studies have measured the properties of surfactants in micron-sized particles. Recent work using a single droplet technique demonstrated that the surface tension depression due to surfactants within a droplet (radius 5–10 µm) is the same as that within a bulk system (Bzdek et al. Citation2016). Additionally, Lee et al. (2017) measured the surface tension of aqueous salt and dicarboxylic acid mixtures to be the same in submicron particles as in bulk solutions. Together with our work, this shows that the field is moving toward directly measuring the effects of surfactants in micron-sized particles. The effects of surfactants in particles have not been constrained but may be based on the properties of the surfactants. Surfactants can contribute to surface tension depression depending on their concentrations and the properties of the solution, they may create a monolayer surface coverage given high enough concentrations and specific surface-active properties (Garrett Citation1971; Davies et al. Citation2013), and they may contribute to differences in hygroscopicity given their organic content (Sorjamaa et al. Citation2004).
The presence of surfactants in aerosol particles is mainly inferred from measurements of surface tension depression and organic composition. However, one recent study presents a method to extract and quantify the surfactant fraction of particle mass (Gérard et al. Citation2016). Briefly, aerosol particles less than 2.5 µm in diameter were collected on filters. The particle mass was extracted in water, and the surfactant fraction was separated using a solid-phase extraction. The surfactant fraction was quantified using dye complexes for three surfactant ionic types (anionic, cationic, and nonionic) and UV-Vis spectroscopy. The CMCs of these surfactant samples were then measured using pendant drop tensiometry at a range of dilutions. The concentrations of the extracted surfactants were found to be more than 100 times the measured CMC concentrations, indicating that these surfactants were at high enough concentrations to exert a significant surface tension depression, reducing the surface tension to below 35 mN m−1 (a significant decrease compared to that of pure water at 72 mN m−1) (Gérard et al. Citation2016).
Other studies indicate that surface tension depression is a common feature of organic compounds sampled in ambient particles, using it as a measure of the presence of surfactants. Surface tension depression in fog and cloud water samples from the Po Valley in Italy and from Zurich, Switzerland was found to be correlated with increasing water-soluble organic carbon (WSOC) concentrations (Capel et al. Citation1990; Facchini et al. Citation2000). Marine aerosol particles collected at Mace Head, Ireland also contained WSOC, a large fraction of which was aliphatic, and the measured surface tension of the samples was inversely correlated to the WSOC concentrations, indicating the presence of surface-active compounds (Cavalli et al. Citation2004). The hydrophobic fraction of WSOC in biomass burning aerosol particles was determined to contain strong surfactants, based on contact angle surface tension measurements, while the hydrophilic fraction contributed little to no surface tension depression (Asa-Awuku et al. Citation2008). Dry, atmospheric particles collected in marine conditions were found to contain organic coatings enhanced in carboxylic acid groups, predicted to be surface-active, measured in collected single particles using soft X-ray spectromicroscopy (Russell et al. Citation2002). Using modified Köhler theory (Roberts et al. Citation2002), similar coatings, modeled as glutaric acid, were calculated to reduce the critical supersaturation of black carbon particles by up to 0.1%, mainly due to the difference in particle solubility (Takahama et al. Citation2010).
Using simpler systems than ambient particles, recent laboratory work has investigated the role of surfactants. Ruehl et al. (Citation2016) suggest that surface-active organics in aerosol particles will partition to the outside of growing particles and reduce the surface tension allowing the particles to grow to a larger size before activation. They measured the diameters of particles containing different atmospherically abundant dicarboxylic acids as the humidity was increased to determine the diameters at activation (Ruehl et al. Citation2016). Using CCN measurements of laboratory-generated particles with surfactant-salt mixtures, Prisle et al. (Citation2010) determined that partitioning of surfactants to the surface of the particles reduced the effect of surface tension depression and explained the observed critical supersaturations. The evaporation rate of supermicron aqueous NaCl particles with the surfactant sodium dodecyl sulfate (SDS) at relative humidity (RH) values between 90% and 98% exhibited no surfactant effect for concentrations of 10 mM (Buajarern et al. Citation2007c), while the evaporation rate decreased at higher concentrations (∼3 M) of SDS in a different study (Davies et al. Citation2012). Organics, such as decane, have been observed to form a layer on the surface of an aqueous supermicron particle that reduces water evaporation proportionally to the layer thickness (Buajarern et al. Citation2007b). However, to inhibit evaporation, a full monolayer or condensed film of the organic (fatty alcohols with chain lengths from 12 to 17, in the referenced study) must surround the aqueous particle (5–20 µm in an electrodynamic balance); particles with patches of organics on the surface have the same evaporation rates as those containing no organics (Davies et al. Citation2013).
In this study, we use a surfactant with a similar CMC value and comparable concentrations to those extracted from atmospheric aerosol particles (Gérard et al. Citation2016) to create three component model aqueous particles with NaCl, water, and surfactant. We study the effect of this surfactant on the condensation and evaporation of individual aqueous particles using an aerosol optical trap combined with Raman spectroscopy and bright-field microscopy.
2. Experimental
An aerosol optical trap was used to trap and study individual aqueous particles, following the outline of Hopkins et al. (Citation2004). Briefly, a 514 nm laser (Genesis MX514-STM; Coherent, Santa Clara, CA, USA) is used for both trapping and excitation. The complex refractive index of water is near a minimum at this wavelength (Seinfeld and Pandis Citation2006), minimizing aqueous particle heating due to absorption. The 1 mm diameter beam passes through lenses (40 mm and 300 mm) that expand the beam to 7.5 mm, which just overfills the back aperture of the microscope objective (60x, water immersion, NA 1.2; Olympus, Center Valley, PA, USA). The objective focuses the beam through the window (15 mm diameter, 0.25 mm thick; Sapphire Glass, Guild Optical Associates, Inc., Amherst, NH, USA) in the bottom of the stainless steel chamber to a point above the glass (objective working distance 0.28 mm) to create the trap inside the chamber (∼0.03 mm above the glass). A blue LED (455 nm) illuminates the aqueous particle through the top window (50 mm diameter, 1.1 mm thick; Gorilla Glass, Edmund Optics, Barrington, NJ, USA) for imaging with bright-field microscopy using a CMOS camera (Monochrome, 1.2 MP, 60 fps; Imaging Source, Charlotte, NC, USA). A dichroic mirror (Chroma Technology, Bellows Falls, VT, USA) separates the trapping beam from the Raman backscattered light. The Raman backscattered light is collimated and focused into a 0.5 m monochromator (Spectra Pro 500i; Acton Research Corporation, Acton, MA, USA, diffraction grating blazed at 500 nm with 1200 g mm−1) equipped with a CCD detector (Princeton Instruments, Trenton, NJ, USA, PIXIS 100 F, 0.03 nm spectral resolution).
Aqueous particles are introduced into the stainless steel chamber using an atomizer (Single Jet 9302; TSI Inc., Shoreview, MN, USA) attached to one of the four inlet ports. After an aqueous particle is trapped, the other ports are used to introduce a ∼200 sccm flow of dry or humid air to control the RH. The RH (±2%) and temperature (±0.3°C) are monitored and recorded (SHT15; SparkFun, Niwot, CO, USA) within 1 cm of the trapped aqueous particle. A small reservoir of water on the inside edge of the chamber allows the initial RH to stabilize at ∼80% and replenishes gas phase water after the drying step (Knox Citation2011). The outer height of the chamber is 3 cm, and the inner volume is ∼15 cm3.
Individual trapped aqueous particles are sized by comparing the structure of the stimulated Raman scattering (SRS) bands, also referred to as whispering gallery modes (WGMs), to those predicted by Mie scattering, as described in Hopkins et al. (Citation2004) and Knox (Citation2011). The Bohren and Huffman (Citation1983) Fortran code was adapted to calculate Mie scattering spectra, with 0.01 nm wavelength resolution (from 600 to 642 nm). The spectra were calculated for aqueous particle radii ranging from 1500 to 4000 nm with 1 nm resolution and for refractive indices from 1.33 to 1.40 (resolution of 0.001 to 0.0025). Peak locations were assembled in a three-dimensional lookup table (aqueous particle radii, refractive indices, and number of peaks). The WGM peak locations in the aqueous particle spectra were then matched with the Mie scattering peaks to determine the aqueous particle radius and refractive index. More details are in the online supplemental information (SI; Text S1 and Figures S1, S2, and S3). Aqueous particles with radii between 2 µm and 4 µm at 70% to 80% RH were analyzed in this experiment. Measurements of surface tension were not made in this experiment.
The median standard deviation of the calculated radii averaged over a given RH in these measurements is ±35 nm (see the SI Text S1). This is less than 1.8% of the smaller radii (2000 nm) and less than 1% of the larger radii (4000 nm) and is less than the observed effect of adding surfactant measured in this study, as shown in Section 3.2.
Individual aqueous particles were also sized using the images from bright-field microscopy. The number of pixels in each aqueous particle image was quantified (ImageJ64) and converted to aqueous particle radii. While the sizes measured by the two techniques are strongly correlated (r = 0.98), the size from the WGMs is considered to be more accurate (Buajarern et al. Citation2006) and is used in this study. If the aqueous particles were too small to size with the Raman method (i.e., right before evaporating completely, less than 1.5 µm radius), the normalized radius from the image sizing was used.
The nonionic surfactant Igepal was used in this study as a proxy for atmospheric surfactants. Igepal is a large alcohol with a repeating ether chain connected to a benzene with an attached branched alkane (C14H21(C2H4O)9OH), and it has a CMC value of 0.08 mM (mols surfactant per L water, binary mixture of Igepal and water) (Mohanty and Mukherji Citation2012). This is in the range of CMC values (0.05 to 0.25 mM) measured for surfactants extracted from sub-2.5 µm atmospheric aerosol particles by Gérard et al. (Citation2016), as described earlier.
Solutions of NaCl and varying concentrations of Igepal were made for atomization into aqueous particles, as listed in . To determine the effect of surfactants on the evaporation and condensation of water, individual aqueous particles (radii of 2–4 µm) from the solutions of NaCl and Igepal () were trapped. The five initial concentrations of Igepal used in the experiments are: 0, 0.04, 0.35, 1.76, and 3.87 mM, which correspond to 0, 0.4, 4, 22, and 48 times the CMC value of Igepal, respectively. These are referred to as NaCl only and Cases A, B, C, and D, respectively. All cases have NaCl concentrations of 611 mM. The NaCl in the solution reduces the vapor pressure of the aqueous particle and allows it to remain trapped in subsaturated conditions (Hopkins et al. Citation2004; Buajarern et al. Citation2007a). The aqueous particle size was measured while the RH was changed in three steps and held as the aqueous particle came to equilibrium: (i) RH at 80% initially, (ii) RH reduced to 70%, and (iii) RH returned to 80%. Each experiment lasted ∼20 min, and the average of 10 or more experiments () from each case is presented in the discussion.
Table 1. Concentrations of Igepal and NaCl in initial, atomized solutions and measured corresponding r70/r80 values (mean ± standard deviation).
3. Results and discussion
3.1. Equilibrium within the chamber
During evaporation and condensation, the total mass of NaCl remains the same, so given the same set of conditions within the chamber at 80% RH, the initial and final aqueous particle sizes should be the same. As a test of equilibrium in the chamber, the radii of the aqueous particles were compared when the RH was initially held at 80% and when the RH was returned to 80% after the step at 70% RH (). These radii were found to be unchanged (, r = 0.99, slope = 1.01) for both the NaCl only and Cases A and B aqueous particles (the radii at 80% RH finally were not measured for Cases C and D, as discussed later). demonstrates the well-mixed nature of the chamber and the ability of the experimental system to reach equilibrium when varying RH, even with the presence of surfactants.
Figure 1. (a) Comparison of aqueous particle radii at 80% RH (r80) initially and finally for all of the aqueous particles in the evaporation and condensation experiment. (b) Comparison of NaCl aqueous particle radii at 80% RH (r80) and 70% RH (r70) calculated using Köhler theory (gray squares) and measured in the experiment (circles). The black line represents the r70/r80 ratio (0.91) for similar aqueous particles (Clegg et al. Citation1998). The gray shading shows the uncertainty associated with the RH measurements (r70/r80 of 0.86 to 0.94). In both panels, error bars represent the standard deviation of the averaged values.
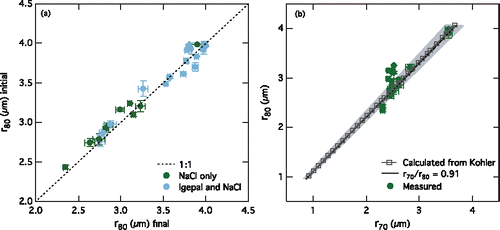
This single particle technique has the ability to hold aqueous particles indefinitely and thus allows time for the surfactants in the particles to come to equilibrium. The properties of surfactants, such as their effect on surface tension, may be dependent on their time in a system and may change as they reach equilibrium, so it is important to make measurements of particle effects after they reach equilibrium. The time required for surfactants to approach equilibrium and have a steady surface tension depression may be variable based on surfactant type. The equilibrium time measured using dynamic surface tension measurements on 0.8 mm diameter droplets was found to be immediate for SDS, about 9 s for surfactin, and more than 8 min for rhamnolipids (Nozière et al. Citation2014). The similarity of the initial and final aqueous particle radii at 80% RH () shows that the aqueous particle radii achieve equilibrium in this experiment.
In this study, we use the ratio of the particle radius at 70% RH to the particle radius at 80% RH (r70/r80) as a measure of the change in particle size with a change in RH. We calculated the r70/r80 of aqueous NaCl particles (radii of 2–4 µm) using Köhler theory (van't Hoff factor of 2 and surface tension of 75 mN m−1) to be 0.87. Instead, allowing the van't Hoff factor to vary (Low Citation1969) with calculated changes in NaCl concentration as the particle size changes gives an r70/r80 ratio of 0.90 (). When the surface tension was changed in these calculations to 40 mN m−1, there was no difference in the output r70/r80.
The trapped NaCl only aqueous particles in this experiment have a median r70/r80 of 0.88 ± 0.06 (mean of 0.86). The measured r70/r80 of the aqueous NaCl only particles is similar to that calculated from Köhler theory at the same concentrations and sizes, as shown in . This is also consistent with a previous experimental study of supermicron sea salt particles (dry diameters 6–8 µm) with r70/r80 of 0.91 (Tang et al. Citation1997; Lewis and Schwartz Citation2004) and with hygroscopic growth of NaCl as modeled by AIM Model III which predicts r70/r80 of 0.91 (Clegg et al. Citation1998). Previous measurements of aqueous NaCl particle evaporation in a similar optical trap also show good agreement between measured particle radii with varying RH and calculated radii from Köhler theory (Mitchem et al. Citation2006). While higher concentrations of NaCl in the atomizer and thus in the aqueous particles result in larger initial and dry diameters, the r70/r80 is the same.
The uncertainty introduced by the RH measurements (±2% RH) may contribute to the observed spread in the measured r70/r80 values for NaCl only aqueous particles (). For NaCl only aqueous particles, the upper (r72/r78 = 0.94) and lower (r68/r82 = 0.86) bounds of r70/r80 with this uncertainty in RH, calculated from the growth factors of NaCl (Topping and McFiggans Citation2012), are shown in and as gray shading. The median measured r70/r80 value for NaCl only falls within this range, as shown in .
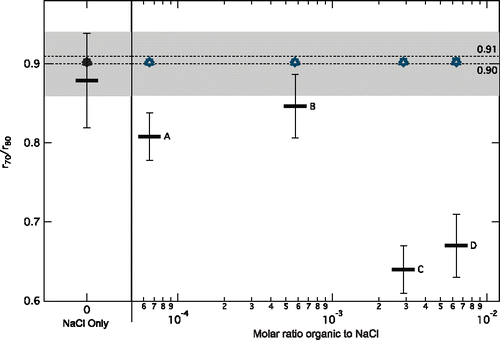
Figure 2. The median ratio of the experimental equilibrium radius at 70% RH (r70) to the equilibrium radius at 80% RH (r80) for aqueous particles containing NaCl only and NaCl with 0.4 and 4 times the CMC concentration of Igepal (Cases A and B, respectively). For aqueous particles with NaCl and 22 and 48 times the CMC concentration of Igepal (Cases C and D, respectively), the equilibrium radius at the point the aqueous particle was lost (>70% RH) is used instead. Error bars represent the standard deviation for each case. The dashed horizontal lines are at r70/r80 = 0.90 (Köhler theory) and 0.91 (Tang et al. Citation1997; Clegg et al. Citation1998). The gray shading represents the range of uncertainty around the predicted r70/r80 of 0.91 for NaCl only aqueous particles (from 0.86 to 0.94) arising from the uncertainty in the RH measurements. The overlapping open markers represent model calculated r70/r80 (Topping and McFiggans Citation2012) at the experimental molar ratios for oxalic acid (circles), succinic acid (triangles), malonic acid (diamonds), and pinolenic acid (squares).
3.2. Measured effect of surfactants
We observe in this experiment that the particles containing surfactant always have an r70/r80 ratio smaller than that of the NaCl only particles. This indicates that the size change of particles with a change in RH is larger for particles containing surfactant than those without. This further implies the presence of an increase in water uptake with an increase in RH and an increase in evaporation with a decrease in RH. This trend is not predicted by Köhler theory calculations or hygroscopic uptake models for particles of these sizes. Here, we discuss the observed effects at different concentrations of surfactant and discuss the potential factors contributing to this observation.
3.2.1. Effect of low concentrations of surfactants
We observe that aqueous particles containing the surfactant Igepal at a concentration of 0.4 times the CMC value (0.04 mM, Case A) on average have a smaller r70/r80 ratio (0.81 ± 0.03) compared to the NaCl only aqueous particles (). This smaller ratio is representative of a larger change in aqueous particle radii with a change in RH for those containing Igepal and indicates a deviation from the equilibrium evaporation and condensation observed in NaCl only aqueous particles. The same trend was true for Igepal concentrations of about four times greater than the CMC (0.35 mM, Case B) that had an average r70/r80 ratio of 0.84 ± 0.04. For these low concentrations of Igepal, the r70/r80 ratio was overall similar (0.82 ± 0.04 together), which is more than 4% lower than that of the NaCl only aqueous particles and outside of the range of uncertainty, as shown in . This lower r70/r80 ratio indicates that low concentrations of the surfactant Igepal may influence aqueous particle growth and evaporation.
Based on the properties of surfactants, two initial hypotheses may be suggested for the behavior of the particles with the low concentration of surfactants: (i) a reduction in surface tension caused by the presence of surfactants in the particles and/or (ii) a difference in the potential for hygroscopic uptake with the addition of an organic. Previous studies have looked at the effect of organic components on the uptake of water by particles and have mainly focused on the effect of the organics on the hygroscopicity (Petters and Kreidenweis Citation2007). While some organic compounds have been found to significantly change the hygroscopicity of the particles, other studies found no change in the efflorescence or deliquescence behavior of single aqueous NaCl particles (3.5–6 µm in radii) with the addition of the surface-active organic compound oleic acid, measured using Raman spectroscopy (Dennis-Smither et al. Citation2012).
To investigate the effect of a change in hygroscopicity with the addition of surfactants, we calculated the hygroscopic growth factors of similarly sized particles with the addition of proxy model organics. In this study, the Igepal concentrations relative to the concentrations of NaCl (see ) are low enough to not significantly affect the calculated r70/r80 of the aqueous particle using a hygroscopic growth model (Topping and McFiggans Citation2012, dry diameter 2 µm, non-ideal interactions calculated with the AIOMFAC model). We calculated the growth factors and r70/r80 ratios (Topping and McFiggans Citation2012) for four organic acid and NaCl mixtures at the molar ratios in this experiment (Cases A through D). With 611.2 mmol of NaCl without an organic, the calculated r70/r80 is 0.90 (same as calculated from Köhler theory). Adding oxalic, succinic, malonic, or pinoleic acid to the NaCl in the model (concentrations of Cases A through D) did not change the calculated r70/r80 ratio from 0.90 in the model (). While oxalic, malonic, and succinic acids are small acids, pinoleic acid is a larger fatty acid (mw of 278.4 g mol−1) and has a structure more similar to that of a surfactant with a hydrophilic acid head group on an unsaturated carbon chain. However, there is still no significant difference in the calculated r70/r80 of pinolenic acid compared to the other organic acids or NaCl only at these molar ratios in the model. This lack of change in r70/r80 calculated in the model indicates that at the measured particle sizes, the addition of organics at these concentrations do not explain the decrease in r70/r80 observed in the experiments (Figure S4).
Ruling out the influence of hygroscopicity, the changes in the particle behavior with the addition of small concentrations of Igepal may be attributed to the other hypothesis: the change in surface tension. However, calculation of r70/r80 using Köhler theory, with surface tension less than 40 mN m−1, as measured in ambient aerosol particles for surfactant concentrations less than 0.5 mM (Gérard et al. Citation2016), does not predict the result observed in the experiment (that the r70/r80 of particles with surfactants is less than the r70/r80 of particles with NaCl only). Instead, reducing the surface tension for particles in the experimental size range results in an r70/r80 the same as that of NaCl only particles, with no apparent effect of the surface tension.
In this experiment, we observe that the particles containing lower concentrations of surfactant have an r70/r80 ratio smaller than that of the NaCl only particles, demonstrating an influence of the surfactant on the particle water uptake and evaporation. However, this trend is not predicted by the current representation of hygroscopicity or surface tension in Köhler theory calculations for particles of these sizes.
3.2.2. Effect of high concentrations of surfactants
Increasing the Igepal concentration to more than 20 times the CMC value resulted in aqueous particles with different, also unexpected behavior. As the RH was decreased in the chamber, the water from these aqueous particles began rapidly evaporating at 75 ± 2% RH, resulting in the loss of the aqueous particles. With this rapid evaporation, the aqueous particles were then too small or too dry to remain trapped, and the condensation step could not be completed. Two high concentrations of Igepal were tested: 22 times the CMC (1.76 mM, Case C) and 48 times the CMC (3.87 mM, Case D). These concentrations are in the range of what has been reported in ambient aerosol samples (Gérard et al. Citation2016). In both cases, the aqueous particles were lost before reaching 70% RH, and the ratios were instead calculated as the ratio of the radius when the aqueous particle was lost (rlost) to r80 (). While evaporating, the average rlost/r80 was 0.67 ± 0.04 for the two high concentrations, which indicates a larger change in radii with the addition of larger concentrations of surfactants. The difference in r70/r80 and stronger effect compared to that of the aqueous particles at 0.4 and 4 times the CMC is not explained. This behavior may be a result of the higher concentration of surfactant causing (i) a change in the surface coverage and the formation of a monolayer, (ii) a phase separation or partitioning within the droplet, (iii) different solute interactions such as salting out, (iv) micelle structural transitions, or (v) other unknown effects.
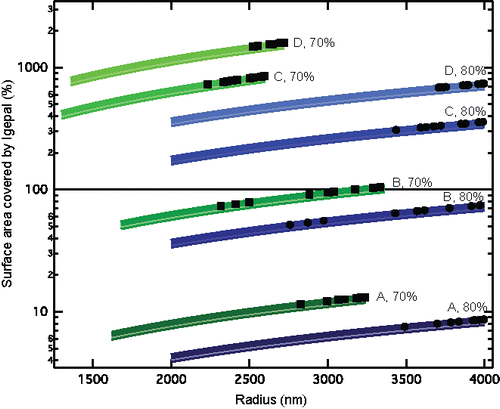
One possible explanation for the difference in this behavior compared to the lower concentrations of surfactants is the surface coverage of surfactants on the droplet. At the two lower concentrations (0.04 mM for Case A and 0.35 mM for Case B), assuming head group surface area of 0.32 nm2 (comparable to 0.357 nm2 for Triton X, which is structurally similar to Igepal; Zdziennicka et al. Citation2012), the concentration of surfactants is not enough to form a full monolayer of molecules around the aqueous particles at 80% or 70% RH, as shown in . However, at the higher concentrations (1.76 mM for Case C and 3.87 mM for Case D), the potential surface area coverage is greater than 100% ().
Figure 3. Percent of aqueous particle surface area covered by Igepal as a function of aqueous particle radii at 80% RH and 70% RH for the four concentrations of Igepal (Cases A to D). Thick lines are calculated as Igepal surface area of 0.357 nm2 per molecule, and shading connects to surface area of 0.32 nm2 per molecule. Individual markers represent calculated surface area coverage for measured aqueous particle radii at 80% RH (circles) and 70% RH (squares) with 0.357 nm2 per molecule.
With full monolayer coverage around the droplet, some theories suggest the surfactant excess may partition to the bulk of the aqueous particle and have a greater influence on the hygroscopic properties (e.g., Sorjamaa et al. Citation2004). Even though the surfactant effect on hygroscopicity was ruled out for the results of this experiment (Section 3.2.1, Cases A and B), we calculated the potential volume of Igepal in the bulk of the aqueous particles for the four cases, using the properties of the similar surfactant Triton X-100 (Zdziennicka et al. Citation2012; Petters and Petters Citation2016). In the high-concentration cases (C and D), more than 85% of the surfactant volume could have been partitioned to the bulk. With pinolenic acid as a proxy for Igepal in the Topping model (Topping and McFiggans Citation2012), only at the highest concentration (Case D) and assuming an upper bound of 100% of the Igepal dissolved and partitioned to the bulk, the growth factors were slightly reduced to 1.79 at 70% RH and 1.98 at 80% RH (Figure S4, from 1.82 and 2.01 for NaCl only, respectively). This corresponds to a calculated r70/r80 of 0.904 (compared to 0.905 of NaCl only). This small reduction compared to NaCl only further demonstrates that the surfactant concentration in the bulk had little effect on the hygroscopicity and was not enough to explain to observed r70/r80 values in this experiment.
Additionally, systems with both salt and surfactants can exhibit the effect of salting out, where the NaCl increases the surfactant at the air/water interface and may alter the CMC and decrease the surface tension of the system (Sahu et al. Citation2010). This salting out effect may be the case in the experiments with the higher concentrations of Igepal. While all of the surfactants may already be at the surface of the droplets in the lower concentration experiments, there can be a greater salting out effect at the higher concentrations due to the higher concentration of surfactant. If the main effect of salting out is to decrease the surface tension, this potential surface tension depression used in Köhler theory does not explain the observed results. Additionally, if the effect reduces the surfactant in the bulk, that would further reduce the surfactant concentration and not create the observed effect, as shown in the earlier calculations (Figure S4).
Previous studies with single coarse mode particles (5–20 µm in radius) in an electrodynamic balance have shown a barrier to water evaporation with the presence monomolecular film of long-chain fatty alcohols (of the form CnH(2n+1)OH with n ranging from 12 to 17) (Davies et al. Citation2013). As demonstrated in , this was not observed in this experiment for the aqueous particles containing low concentrations of Igepal (Cases A and B). In the case of higher concentrations of Igepal (Case C and D), the opposite was true in that the evaporation appeared to increase rather than be hindered by a barrier, even with the potential for multiple layers of surface coverage.
At concentrations higher than their CMCs, by definition, surfactants form micelles. If the surfactants that are not at the surface of the aqueous particles create micelles within the bulk, the size of the micelles with the salt solution could create the disparate behavior observed in this experiment for the two different concentration types (low: Cases A and B; and high: Cases C and D). Transitioning to higher concentrations, as observed in the experiments when the RH was decreased from 80% to 70%, the surfactant concentrations in Cases C and D may reach the second CMC where micelles undergo transitions from spherical to rod-like (Velinova et al. Citation2011). The rod-like micelles have lower volume (Velinova et al. Citation2011), and a transition to rod-like micelles thus decreases the total volume of the aqueous particle. This could create the apparent larger decrease in size observed in this experiment for aqueous particles containing high concentrations of Igepal compared to those that contain NaCl only or lower concentrations of Igepal.
3.3. Implications of surfactant effects
In this study, we observed that particles containing surfactant always had r70/r80 ratios less than those of NaCl only particles. The r70/r80 of NaCl particles in this experiment was consistent with those calculated by Köhler theory. However, the observed difference in r70/r80 for aqueous particles containing Igepal, both at high concentrations and low concentrations, compared to NaCl only particles is not predicted by Köhler theory or hygroscopic uptake models for the large aqueous particles used in this experiment (dry diameters 2–4 µm), and the results are thus novel and unusual. Similarly unexpected, Petters and Petters (Citation2016) found the CCN activity of nonionic surfactants Triton X-100 and Zonyl FS-300 to be greater than predicted by Köhler theory, even when surface tension reduction and surface partitioning were included. They suggest that solute–solute interactions, non-spherical particle shapes, or other effects may explain the differences. The change in r70/r80 observed in this experiment may be due to effects discussed earlier, including phase separations, solute interactions, or micelle structural transitions and has not been definitively identified.
Given that the observed response to surfactants in this experiment is larger than that predicted by calculations with Köhler theory and hygroscopic uptake models, their role in atmospheric particles may be to enhance particle growth with changing meteorological conditions by more than predicted in current theories. Previous measurements of surfactant concentrations were on average around 50 mM (mmol surfactant per L of particle volume), which is calculated to be 2% of dry particle mass, using the surfactant Igepal as a mimic for the ambient surfactant and an assumed particle density of 1.8 g cm−3 (Gérard et al. Citation2016). This is a 3–30 times higher concentration than in our low concentration experiments, where Igepal was 0.06% and 0.6% of the dry particle mass. Our results imply that the particles containing surfactants at 80% RH are more than 4% larger than those containing NaCl only (cartoon example is shown in Figure S5). This would favor growth for the surfactant-containing particles over the NaCl only particles and thus, atmospheric particles could grow differently than is commonly assumed.
The presence of surfactants in coarse mode particles could also have an influence on aerosol mass loading, visibility, and radiative forcing. Because aqueous particles with surfactants may grow larger than those without surfactants, this could shift the volume size distribution of particles to larger sizes and change the scattering and absorbing properties. In this experiment, low concentrations of Igepal (Cases A and B) were shown to grow more than 4% larger in radii for a change in RH from 70% to 80%, which would correspond to a greater than 13% increase in aqueous particle volume.
Surfactants are ubiquitous in atmospheric aerosols. While their specific properties and sources are still being investigated, the effects of surfactants on coarse mode particle growth and radiative properties should be considered. In this work, we observed that the growth of aqueous particles containing surfactants was greater than that of aqueous particles containing NaCl only at RH values of 70%–80%. Current representation of surfactants with surface tension depression or changes in hygroscopicity does not explain these results, and further work is needed to understand the effect of surfactants in this experiment and in atmospheric aerosol particles.
UAST_1424315_Supplementary_File.zip
Download Zip (441.7 KB)Acknowledgments
The authors gratefully acknowledge Professor Christopher Cappa at UC Davis for providing the initial Mie theory code. The data from this study are available in the SI (Tables S1 and S2).
Additional information
Funding
References
- Asa-Awuku, A., Sullivan, A. P., Hennigan, C. J., Weber, R. J., and Nenes, A. (2008). Investigation of Molar Volume and Surfactant Characteristics of Water-Soluble Organic Compounds in Biomass Burning Aerosol. Atmos. Chem. Phys., 8(4):799–812. doi:10.5194/acp-8-799-2008.
- Bohren, C. F., and Huffman, D. R. (1983). Absorption and Scattering of Light by Small Particles. Wiley, New York.
- Bond, T. C., and Bergstrom, R. W. (2006). Light Absorption by Carbonaceous Particles: An Investigative Review. Aerosol Sci. Technol., 40(1):27–67. doi:10.1080/02786820500421521.
- Buajarern, J., Mitchem, L., and Reid, J. P. (2007a). Characterizing Multiphase Organic/Inorganic/Aqueous Aerosol Droplets. J. Phys. Chem., A, 111(37):9054–9061. doi:10.1021/jp074366a.
- Buajarern, J., Mitchem, L., and Reid, J. P. (2007b). Characterizing the Formation of Organic Layers on the Surface of Inorganic/Aqueous Aerosols by Raman Spectroscopy. J. Phys. Chem. A, 111(46):11852–11859. doi:10.1021/jp075021v.
- Buajarern, J., Mitchem, L., and Reid, J. P. (2007c). Manipulation and Characterization of Aqueous Sodium Dodecyl Sulfate/Sodium Chloride Aerosol Particles. J. Phys. Chem. A, 111(50):13038–13045. doi:10.1021/jp0764034.
- Buajarern, J., Mitchem, L., Ward, A. D., Nahler, N. H., McGloin, D., and Reid, J. P. (2006). Controlling and Characterizing the Coagulation of Liquid Aerosol Droplets. J. Chem. Phys., 125(11):114506(1–10). doi:10.1063/1.2336772.
- Bzdek, B. R., Power, R. M., Simpson, S. H., Reid, J. P., and Royall, C. P. (2016). Precise, Contactless Measurements of the Surface Tension of Picolitre Aerosol Droplets. Chem. Sci., 7(1):274–285. doi:10.1039/c5sc03184b.
- Capel, P. D., Gunde, R., Zurcher, F., and Giger, W. (1990). Carbon Speciation and Surface-Tension of Fog. Environ. Sci. Technol., 24(5):722–727. doi:10.1021/es00075a017.
- Cavalli, F., Facchini, M. C., Decesari, S., Mircea, M., Emblico, L., Fuzzi, S., Ceburnis, D., Yoon, Y. J., O'Dowd, C. D., Putaud, J. P., and Dell'Acqua, A. (2004). Advances in Characterization of Size-Resolved Organic Matter in Marine Aerosol Over the North Atlantic. J. Geophys. Res.-Atmos., 109(D24). doi: D24215,10.1029/2004jd005137.
- Clegg, S. L., Brimblecombe, P., and Wexler, A. S. (1998). A Thermodynamic Model of the System H+-NH4+-Na+-SO42−−NO3−-Cl−-H2 O at 298.15 K. J. Phys. Chem. A, 102:2155–2171. Available at http://www.aim.env.uea.ac.uk/aim/model3/mod3rhw.php. doi:10.1021/jp973043j
- Davies, J. F., Haddrell, A. E., Miles, R. E. H., Bull, C. R., and Reid, J. P. (2012). Bulk, Surface, and Gas-Phase Limited Water Transport in Aerosol. J. Phys. Chem. A, 116(45):10987–10998. doi:10.1021/jp3086667.
- Davies, J. F., Miles, R. E. H., Haddrell, A. E., and Reid, J. P. (2013). Influence of Organic Films on the Evaporation and Condensation of Water in Aerosol. Proc. Natl. Acad. Sci. USA, 110(22):8807–8812. doi:10.1073/pnas.1305277110.
- Dennis-Smither, B. J., Hanford, K. L., Kwamena, N.-O. A., Miles, R. E. H., and Reid, J. P. (2012). Phase, Morphology, and Hygroscopicity of Mixed Oleic Acid/Sodium Chloride/Water Aerosol Particles Before and After Ozonolysis. J. Phys. Chem. A, 116(24):6159–6168. doi:10.1021/jp211429f.
- Facchini, M. C., Decesari, S., Mircea, M., Fuzzi, S., and Loglio, G. (2000). Surface Tension of Atmospheric Wet Aerosol and Cloud/Fog Droplets in Relation to Their Organic Carbon Content and Chemical Composition. Atmos. Environ., 34(28):4853–4857.
- Garrett, W. (1971). Retardation of Water Drop Evaporation with Monomolecular Surface Films. J. Atmos. Sci., 28:816–819. doi:10.1175/1520-0469(1971)028<0816:ROWDEW>2.0.CO;2.
- Gérard, V., Noziere, B., Baduel, C., Fine, L., Frossard, A. A., and Cohen, R. C. (2016). Anionic, Cationic, and Nonionic Surfactants in Atmospheric Aerosols from the Baltic Coast at Asko, Sweden: Implications for Cloud Droplet Activation. Environ. Sci. Technol., 50(6):2974–2982. doi:10.1021/acs.est.5b05809.
- Hale, G. M., and Querry, M. R. (1973). Optical-Constants of Water in 200-nm to 200-um Wavelength Region. Appl. Opt., 12(3):555–563. doi:10.1364/ao.12.000555.
- Hopkins, R. J., Mitchem, L., Ward, A. D., and Reid, J. P. (2004). Control and Characterisation of a Single Aerosol Droplet in a Single-Beam Gradient-Force Optical Trap. Phys. Chem. Chem. Phys., 6(21):4924–4927. doi:10.1039/b414459g.
- Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S. H., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., Ng, N. L., Aiken, A. C., Docherty, K. S., Ulbrich, I. M., Grieshop, A. P., Robinson, A. L., Duplissy, J., Smith, J. D., Wilson, K. R., Lanz, V. A., Hueglin, C., Sun, Y. L., Tian, J., Laaksonen, A., Raatikainen, T., Rautiainen, J., Vaattovaara, P., Ehn, M., Kulmala, M., Tomlinson, J. M., Collins, D. R., Cubison, M. J., Dunlea, E. J., Huffman, J. A., Onasch, T. B., Alfarra, M. R., Williams, P. I., Bower, K., Kondo, Y., Schneider, J., Drewnick, F., Borrmann, S., Weimer, S., Demerjian, K., Salcedo, D., Cottrell, L., Griffin, R., Takami, A., Miyoshi, T., Hatakeyama, S., Shimono, A., Sun, J. Y., Zhang, Y. M., Dzepina, K., Kimmel, J. R., Sueper, D., Jayne, J. T., Herndon, S. C., Trimborn, A. M., Williams, L. R., Wood, E. C., Middlebrook, A. M., Kolb, C. E., Baltensperger, U., and Worsnop, D. R. (2009). Evolution of Organic Aerosols in the Atmosphere. Science, 326(5959):1525–1529. doi:10.1126/science.1180353.
- Kassianov, E., Pekour, M., and Barnard, J. (2012). Aerosols in Central California: Unexpectedly Large Contribution of Coarse Mode to Aerosol Radiative Forcing. Geophys. Res. Lett., 39. doi:10.1029/2012gl053469.
- Knox, K. J. (2011). Thesis: Light-Induced Processes in Optically-Tweezed Aerosol Droplets. Springer-Verlag, Berlin Heidelberg, pp. 1–204. doi:10.1007/978-3-642-16348-7.
- Köhler, H. (1936). The Nucleus in and the Growth of Hygroscopic Droplets. Trans. Faraday Soc., 32(2):1152–1161. doi:10.1039/tf9363201152.
- Lee, H. D., Estillore, A. D., Morris, H. S., Ray, K. K., Alejandro, A., Grassian, V. H., and Tivanski, A. V. (2017). Direct Surface Tension Measurements of Individual Sub-Micrometer Particles Using Atomic Force Microscopy. J. Phys. Chem. A, 121:8296–8305. doi:10.1021/acs.jpca.7b04041.
- Lewis, E. R., and Schwartz, S. E. (2004). Sea Salt Aerosol Production: Mechanisms, Methods, Measurements, and Models – A Critical Review (Geophysical Monograph Series). Vol. 152. American Geophysical Union, Washington.
- Low, R. D. H. (1969). A Generalized Equation for the Solution Effect in Droplet Growth. J. Atmos. Sci., (26):608–611. doi.org/10.1175/1520-0469(1969)026<0608:AGEFTS>2.0.CO;2.
- Mitchem, L., Buajarern, J., Hopkins, R. J., Ward, A. D., Gilham, R. J. J., Johnston, R. L., and Reid, J. P. (2006). Spectroscopy of Growing and Evaporating Water Droplets: Exploring the Variation in Equilibrium Droplet Size with Relative Humidity. J. Phys. Chem. A, 110(26):8116–8125. doi:10.1021/jp061135f.
- Mohanty, S., and Mukherji, S. (2012). Alteration in Cell Surface Properties of Burkholderia spp. During Surfactant-Aided Biodegradation of Petroleum Hydrocarbons. Appl. Microbiol. Biotechnol., 94(1):193–204. doi:10.1007/s00253-011-3703-7.
- Nozière, B., Baduel, C., and Jaffrezo, J.-L. (2014). The Dynamic Surface Tension of Atmospheric Aerosol Surfactants Reveals New Aspects of Cloud Activation. Nature Commun., 5:3335–3335. doi:10.1038/ncomms4335.
- Ovadnevaite, J., Zuend, A., Laaksonen, A., Sanchez, K. J., Roberts, G., Ceburnis, D., Decesari, S., Rinaldi, M., Hodas, N., Facchini, M. C., Seinfeld, J. H., and Dowd, C. O. (2017). Surface Tension Prevails Over Solute Effect in Organic-Influenced Cloud Droplet Activation. Nature, 546(7660):637–641, doi:10.1038/nature22806.
- Petters, M. D., and Kreidenweis, S. M. (2007). A Single Parameter Representation of Hygroscopic Growth and Cloud Condensation Nucleus Activity. Atmos. Chem. Phys., 7(8):1961–1971.
- Petters, S. S., and Petters, M. D. (2016). Surfactant Effect on Cloud Condensation Nuclei for Two-Component Internally Mixed Aerosols. J. Geophys. Res.-Atmos., 121(4):1878–1895. doi:10.1002/2015jd024090.
- Poschl, U. (2005). Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem.-Int. Ed., 44(46):7520–7540. doi:10.1002/anie.200501122.
- Prisle, N. L., Asmi, A., Topping, D., Partanen, A. I., Romakkaniemi, S., Dal Maso, M., Kulmala, M., Laaksonen, A., Lehtinen, K. E. J., McFiggans, G., and Kokkola, H. (2012). Surfactant Effects in Global Simulations of Cloud Droplet Activation. Geophys. Res. Lett., 39. doi:10.1029/2011gl050467.
- Prisle, N. L., Raatikainen, T., Laaksonen, A., and Bilde, M. (2010). Surfactants in Cloud Droplet Activation: Mixed Organic-Inorganic. Atmos. Chem. Phys., 10:5663–5683. doi:10.5194/acp-10-5663-2010.
- Roberts, G. C., Artaxo, P., Zhou, J. C., Swietlicki, E., and Andreae, M. O. (2002). Sensitivity of CCN Spectra on Chemical and Physical Properties of Aerosol: A Case Study from the Amazon Basin. J. Geophys. Res., 107(D20):8070. doi:10.1029/2001JD000583.
- Ruehl, C. R., Davies, J. F., and Wilson, K. R. (2016). An Interfacial Mechanism for Cloud Droplet Formation on Organic Aerosols. Science, 351(6280):1447–1450. doi:10.1126/science.aad4889.
- Russell, L. M., Maria, S. F., and Myneni, S. C. B. (2002). Mapping Organic Coatings on Atmospheric Particles. Geophys. Res. Lett., 29(16). doi:10.1029/2002GL014874.
- Sahu, K., McNeill, V. F., and Eisenthal, K. B. (2010). Effect of Salt on the Adsorption Affinity of an Aromatic Carbonyl Molecule to the Air-Aqueous Interface: Insight for Aqueous Environmental Interfaces. J. Phys. Chem. C, 114(42):18258–18262. doi:10.1021/jp1071742.
- Seinfeld, J. H., and Pandis, S. N. (2006). Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. 2nd edition. J. Wiley, New York.
- Sorjamaa, R., Svenningsson, B., Raatikainen, T., Henning, S., Bilde, M., and Laaksonen, A. (2004). The Role of Surfactants in Kohler Theory Reconsidered. Atmos. Chem. Phys., 4:2107–2117. doi:10.5194/acp-4-2107-2004.
- Takahama, S., Liu, S., and Russell, L. M. (2010). Coatings and Clusters of Carboxylic Acids in Carbon-Containing Atmospheric Particles from Spectromicroscopy and Their Implications for Cloud-Nucleating and Optical Properties. J. Geophys. Res.-Atmos., 115:21. doi:10.1029/2009jd012622.
- Tang, I. N., Tridicio, A. C., and Fung, K. H. (1997). Thermodynamic and Optical Properties of Mixed-Salt Aerosols of Atmospheric Importance. J. Geophys. Res.-Atmos., 102(D2):1883–1893.
- Tegen, I., Lacis, A. A., and Fung, I. (1996). The Influence on Climate Forcing of Mineral Aerosols from Disturbed Soils. Nature, 380(6573):419–422. doi:10.1038/380419a0.
- Topping, D. O., and McFiggans, G. (2012). Tight Coupling of Particle Size, Number, and Composition in Atmospheric Cloud Droplet Activation. Atmos. Chem. Phys., 12:3253–3260. Available at http://umansysprop.seaes.manchester.ac.uk/tool/hygroscopic_growth_factor_inorg_org. doi:10.5194/acp-12-3253-2012.
- Velinova, M., Sengupta, D., Tadjer, A. V., and Marrink, S. J. (2011). Sphere-To-Rod Transitions of Nonionic Surfactant Micelles in Aqueous Solution Modeled by Molecular Dynamics Simulations. Langmuir, 27(23):14071–14077. doi:10.1021/la203055t.
- Zdziennicka, A., Szymczyk, K., Krawczyk, J., and Janczuk, B. (2012). Activity and Thermodynamic Parameters of Some Surfactants Adsorption at the Water-Air Interface. Fluid Phase Equilib., 318:25–33. doi:10.1016/j.fluid.2012.01.014.
