ABSTRACT
The increasing need for engineered alloy nanoparticles (NPs) in diverse fields has spurred efforts to explore efficient/green synthesis methods. In this respect, spark ablation provides a scalable and viable way for producing widely different types of mixed NPs. Most importantly, implementation of the spark has the great advantage to combine a wider range of materials, thereby allowing the synthesis of mixed NPs with virtually unlimited combinations. Here we show that polarity reversal of spark discharges between two electrodes consisting of different materials enables synthesis of alloy NPs, while having a good potential to control the broadness of their composition distribution. A model developed in this work provides a tool for tuning the ablation ratio between the electrodes by adjusting the electric characteristics of the spark circuit. The ablation ratio is equal to the mean composition of the resulting NPs. The model predictions are in accordance with measurements obtained here and in earlier works. The unique way of producing alloy NPs by spark ablation shown in this work becomes especially useful when the starting electrode materials are immiscible at macroscopic scale.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
1. Introduction
Efficient/green production of mixed nanoparticles (NPs) is essential for the development of future industrial scale applications (Naqshbandi et al. Citation2012; Charitidis et al. Citation2014; Sebastian et al. Citation2014; Madhu et al. Citation2016; Tiwari et al. Citation2016). At the same time, alloy NPs can potentially replace non-abundant or unavailable combinations of some critical elements. Gas-phase spark ablation enables the manufacturing of a range of NPs with different properties (Feng Citation2016). Its unique feature lies in the capability of single-step manufacturing NPs with virtually unlimited mixing combinations (Byeon et al. Citation2008; Tabrizi et al. Citation2009b; Maisser et al. Citation2015; Feng et al. Citation2015, Citation2016a, Citation2016c). The resulting NPs cover any form of nanoparticulate mixtures, mixed at nanometer and/or atomic scale. The former, also called externally mixed NPs, can be achieved by close proximity of the sparks and turbulent mixing or by putting two or more spark generator units in series (Pfeiffer et al. Citation2014), whereas the latter are generally referred to as alloy NPs. Spark ablation has also been used to create crystalline phases that only exist in the nanoparticulate state (Tabrizi et al. Citation2010). This phenomenon has been pointed out (Lahiri et al. Citation2005; Xiong et al. Citation2011), and related to the fact that a large fraction of the atoms occupies the material interface that influences their miscibility. Overall, producing singlet mixed NPs for which one can have great control over their size (Feng et al. Citation2015, Citation2016c) can enormously extends the capabilities and scope of resulting functional materials (Kim et al. Citation2006; Byeon and Roberts Citation2012; Anastasopol et al. Citation2013; Byeon and Kim Citation2014; Isaac et al. Citation2015; Feng et al. Citation2016b; Jang et al. Citation2016; Kang et al. Citation2016).
In a spark-discharge NP generator, a pair of electrodes is connected to a pulse-forming electrical circuit that periodically initiates a spark discharge in the interelectrode gap, where high-purity inert gas flows (Schwyn et al. Citation1988; Feng et al. Citation2016a). The sparks evaporate electrode materials, and the resulting vapors subsequently form NPs that retain the electrode (bulk) composition. Following this principle, alloy NPs can be produced by using electrode materials that consist of alloys or sintered powders (mixture of (sub)micron sized powders that are sintered by magnetic compaction [Pfeiffer et al. Citation2014]), which, however, may not exist (nonalloy) or be difficult to obtain. To overcome this problem, here we use two electrodes correspondingly consisting of materials A and B as illustrated in (Vons Citation2008; Tabrizi et al. Citation2009b). Upon sparking, the vapors of different materials resulting from the two electrodes are rapidly condensed into internally mixed NPs (e.g., alloyed or atomically mixed; Tabrizi et al. Citation2009b, Citation2010; Pfeiffer et al. Citation2014; Sebastian et al. Citation2014; Feng et al. Citation2015).
Figure 1. Schematic illustration of internal NP mixing by using two different electrodes. Variable x indicates the fraction of one (here is material A) electrode material in the resulting NPs, which in turn have a composition distribution. Spatial distribution of vapors produced by the two different electrodes can lead to NPs composed of a single element (i.e., x = 0 or 1).
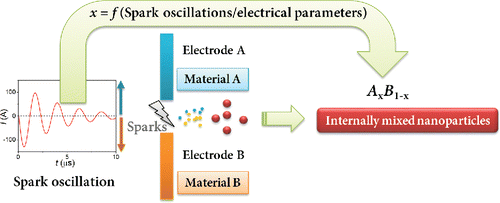
Although internal NP mixing was demonstrated by using two different electrodes in the past (Vons Citation2008; Tabrizi et al. Citation2009b), explaining and controlling the NP composition has remained unexplored until now. This article unravels the mechanism of controlling mixed NP composition by changing the oscillatory behavior of spark discharges (). The discharge current (denoted as current oscillation hereafter) oscillates between its positive and negative polarity with a natural frequency of approximately 1 MHz, thereby vaporizing the electrodes of different materials. The current oscillation is related to electrical properties (e.g., capacitance, resistance, and inductance) of the spark circuit. To understand their relation to the NP mixing ratio, we develop a model that links the current oscillation to the relative ablation of the two electrodes. The ablation ratio is essentially the same as the mean particle composition. We also experimentally show that this ratio can be controlled by the electrical parameters. Model predictions are validated with gravimetric measurements (i.e., weighing the electrodes before and after sparking). We also compare the predictions with chemical composition analysis already available in the literature that used energy-dispersive X-ray spectroscopy (EDX) and inductively coupled plasma mass spectrometry (ICP-MS) (Tabrizi et al. Citation2009a,Citationb). Since the vapors generated from the two electrodes may not be homogeneously mixed, we further investigate the composition distribution of the resulting NPs. We use example combinations Pd–Ag and Pd–Au from literature to show that their composition distribution can be controlled.
2. Understanding internal NP mixing
This section revisits results from previous works (Tabrizi et al. Citation2009b, Citation2010) to obtain the mean composition and variations of the composition of a small number of NPs selected randomly. We further explain that the mean composition, predicted by the ablation ratio of the electrodes consisting of different materials, can be expressed by the product of a material independent factor k and a material dependent term CA/CB. The latter captures the “ablatabilities” of materials A and B (). We also show that the empirical data are consistent with our theoretical considerations below.
2.1. Composition variations among the NPs
The concept of mixing NPs by spark ablation, using different materials for the anode and the cathode, leads to vapor clouds that are initially not homogenously mixed. Such vapors go through the first steps of homogeneous nucleation independently before mixing, thereby resulting in a composition distribution of the final particles. The average composition of the NPs can be determined by EDX, ICP-MS analysis on collected NP samples, and by gravimetric measurements of the electrodes before and after sparking.
In this work, the subscript “ = C” denotes that the material is used as the cathode, while “ = A” signifies that the material is used as the anode. For instance, is the mass portion of Pd in a particle when the cathode electrode is Pd.
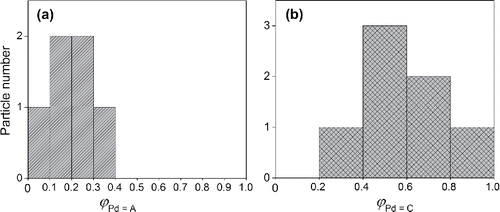
shows the composition of a number of randomly selected NPs, produced by spark ablation using electrodes of different materials (i.e., Pd and Au), as estimated by EDX in a scanning transmission electron microscope (Tabrizi et al. Citation2009b). Because of the limited particle number (6 and 7 randomly selected particles), the NP composition distribution can only be considered as an approximation and the histograms are only used to reflect how the particle compositions varied. It should be noted that their mean compositions were comparable to that determined by ICP-MS (e.g., ,
).
Figure 2. Distribution of the particle composition reported in the literature (Tabrizi et al. Citation2009b), determined by EDX on a random selection of (a) six particles produced using Pd and Au electrodes, and (b) seven particles produced using Pd and Ag electrodes. The compositions of the particles are defined with respect to Pd as and
. Sample standard deviations
are 0.10 for Pd–Au (a) and 0.20 for Pd–Ag (b).
2.2. Separation of the material-independent factor and the material constant
The following part explains the assumption that the particle composition is given by a material independent factor k, which is constant for a given spark circuit and carrier gas. A material dependent term CA/CB also captures the “ablatabilities” of electrodes A and B. Following this notion, the model (Section 3) will predict k using the electrical discharge characteristics in the configuration of two different electrodes.
The prior work (Tabrizi et al. Citation2009b) showed that the mean particle composition depends on which material is used for the cathode and the anode. The corresponding measurements obtained by ICP-MS are provided in . Based on the energy balance of the process (Llewellyn Jones Citation1950), the total mass of both electrodes ablated per spark at a given spark energy E can be estimated as (Feng et al. Citation2015):
[1]
Table 1. Mass ratios ablated from the cathodes and the anodes of different materials (determined by ICP-MS) (Tabrizi et al. Citation2009b). The fraction mAu = C/mPd = A denotes the mass ratio of the cathode and the anode materials in the NPs. These values are equal to the ratio of ∆mC/∆mA (mass ablated per spark from the cathode to that of the anode), as the mass ablated from the electrodes is essentially converted to NPs (Feng et al. Citation2016a).
Here is a material-independent parameter representing the fraction of spark energy consumed for material evaporation, whereas Cm is a material constant representing the mass evaporated per unit of energy absorbed by the electrodes, estimated as (Llewellyn Jones Citation1950; Feng et al. Citation2015):
[2] where cps (J K−1 kg−1) is the heat capacity of the solid material, Tc and Tb (K) are the temperature of the carrier gas and the boiling point of the electrode materials, whereas Hm and He (J kg−1) are their enthalpies of melting and vaporization. All these constants for the materials investigated in this work are provided in Table S1 in the online supplemental information (SI).
A complication of using two different electrodes to mix NPs lies in the fact that in inert gases the cathode is always ablated more strongly than the anode (Tabrizi et al. Citation2009a). We attribute this to the fact that the ablation is, at least in part, caused by the collisions of charge carriers with the electrodes. These charge carriers include the ionized gas atoms/molecules and free electrons. Positive ions with greater mass/energy are attracted to collide with the negative electrode, causing stronger ablation than on the positive electrode (the negatively charged species in the gas are mainly electrons). Below we show that the sparks obtained by the discharge of a capacitor are generally oscillatory, so that the electrodes take turns in experiencing strong ablation. Modifying the current oscillation by changing electrical parameters, therefore, changes the NP mixing ratio. For further analysis, we define the cathode as the electrode being negative during the period that most of the spark energy is dissipated.
The mass ablated from the cathode or the anode can be determined separately using EquationEquation (1)[1] , if α is correspondingly replaced by the fraction of spark energy consumed for cathode ablation αC or anode ablation αA. The products of these fractions and spark energy (i.e., αAE and αCE) are then the energies dissipated by evaporating each electrode. We express the ratio of energies absorbed by the cathode and the anode as:
[3]
EquationEquation (3)[3] shows that k is solely dependent on the dissipated spark energies on each electrode. This treatment also implies that k is independent of electrode materials. However, k certainly depends on the mass of the ions formed in the discharge due to their effect of sputtering the electrodes, and thus on the type of the carrier gas used. Besides the characteristics of the oscillating discharge, the gas composition thus has an influence on k. Importantly, we see no physical reason for k to vary for any given gas and discharge characteristics. This expectation is consistent with the experimental data reported previously (Tabrizi et al. Citation2009b), as discussed further below.
Substituting EquationEquation (3)[3] into EquationEquation (1)
[1] , the ratio of mass ablated per spark between the cathode (ΔmC) and the anode (ΔmA) can be calculated as:
[4]
EquationEquation (4)[4] relates a mass ratio of the ablated materials to k and material constants. This ratio is equal to the mean mixing ratio of NPs, and it can also be experimentally determined from either gravimetric measurements of the electrodes, or EDX and ICP-MS measurements of the resulting NPs. Evidently, k equals to the mass ablation ratio when using the same electrode materials (i.e., CC = CA).
Applying EquationEquation (4)[4] to the Au–Pd combination, as shown in , yields:
[5]
Substituting the values from into EquationEquation (5)[5] , we obtain k = 1.3 for Au–Pd, and k = 1.1 for Ag–Pd. This discrepancy in k can be attributed to the approximation of mass ablated per spark (
) using EquationEquation (1)
[1] . The empirical outcome suggests that k can be considered as being material independent. This is additionally supported by the results for the case of the identical electrodes in the same circuit: Gravimetric measurement gave similar values for k = mC/mA: i.e., 1.3, 1.1, and 1.2 for Pd, Au, and Ag, respectively (Tabrizi et al. Citation2009a), with an average value being 1.2. We can thus state that our expectation of a constant k from physical considerations is consistent with empirical data. In the discussion below, we will derive a formula for k based on the current signal of the discharge.
EquationEquation (4)[4] approximates the material constant ratios (CC/CA) when using two different electrodes. In the case of identical electrodes, the associated material constant ratios can be estimated from two subsequent gravimetric measurements of two identical electrodes’ combinations (e.g., Pd–Pd and Au–Au; see details in the SI). The associated results for these coupled measurements are shown in and are also compared with predictions using EquationEquation (2)
[2] .
Table 2. Comparison of the material constant ratios estimated when using different electrodes (the second and the third columns; EquationEquation (4)[4] ) with those of using the identical electrodes (the forth column; calculations are based on a number of gravimetric measurements for Au–Au and Pd–Pd combinations as well as Ag–Ag and Pd–Pd combinations).
shows that the material constant ratios have similarities regardless whether different or identical electrodes are employed, which implies the material independence of k. This supports the above assumption that the value of k (EquationEquation (3)[3] ) for the configuration using electrodes of the same material is representative for the case of different electrodes. Therefore, we can use identical electrodes to investigate the relation between k and the electrical characteristics of the spark circuit in the following. Note that the measurements for CPd/CAg deviate substantially from predictions using EquationEquation (2)
[2] . This is probably because EquationEquation (1)
[1] and therewith EquationEquation (4)
[4] does not capture the energy loss by thermal conduction of the electrodes (Ag conductivity is approximately six times higher than that of Pd) (Tabrizi et al. Citation2009a; Feng et al. Citation2015, Citation2016c).
3. Predicting the mean composition of the mixed NPs
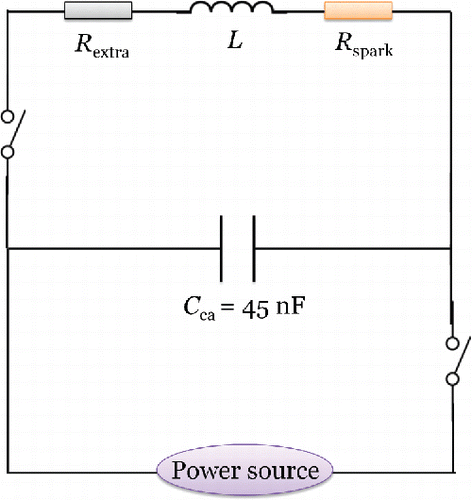
shows a simplification of the circuit of the high frequency sparks (HFS) used in this work (Feng et al. Citation2016a). Here we develop a model for linking the electrical parameters to the current oscillations, which in turn determines the ablation ratio of the electrodes. The spark discharge can be considered as a constant resistance Rspark in the order of 1 Ω. Additional cables and electrode resistances can be neglected when using metal electrodes (Vons et al. Citation2011). According to Kirchhoff's rule and the voltage–current relations, for the inductance Li, the time t, the total resistance of the circuit Rtot = Rspark + Rextra, and its capacitance Cca, one can write:[6]
Figure 3. Simplified drawing of the switching circuit used in the HFS. The initial potential difference UC applied over the electrodes can be set independently of the breakdown voltage due to the decoupling of the charge cycle from that of discharge (Feng et al. Citation2016a). Such separation is achieved by adding fast switches in the classical spark circuit (Pfeiffer et al. Citation2014; Feng et al. Citation2016a). Besides keeping a constant Uc, a low-power source provides a continuous low current in the discharge gap. This low current reduces the breakdown voltage of the carrier gas, guaranteeing that Uc is always high enough to ignite the spark for the gap distances used here. Although the frequency of the HFS can go up to 25 kHz, we used the spark repetition frequency of 1 kHz, at which there is no substantial electrode heating at the flow rate used in the experiment.
Oscillations occur for the case of underdamping (), where EquationEquation (6)
[6] is solved to:
[7]
Here Uc is the igniting voltage across the gap, is the damping factor, and
is the natural frequency of the oscillation.
The unknown parameters Rspark and Li are derived from matching EquationEquation (7)[7] with the current oscillations measured in this work. If Rextra = 0, the spark energy (
) can also be expressed as (with an oscillatory duration of τ):
[8]
In the oscillatory discharge, the electrodes take turns in having a negative and positive polarity. The electrode momentarily having the negative polarity is bombarded with positive ions, thereby emitting a vapor. The positive ions have larger mass and carry more energy to the associated electrode than electrons. This brings about the assumption that the ablation only occurs on the electrode acting momentarily as the negative electrode. As a result, one electrode emits vapors during the initial negative-wave, whereas the other one takes over when the measured current becomes positive (). We define the cathode as the electrode being negative during the time that most of the energy is dissipated. For an underdamped oscillation, the cathode is the electrode that starts with the negative polarity.
As the ablated mass is roughly proportional to the spark energy (EquationEquation (1)[1] ), the ablated mass of the cathode corresponds to the dissipated energy when I < 0, while the ablated mass of the anode is given by the dissipated energy when I > 0. According to EquationEquation (3)
[3] , the ratio of the dissipated energies at the cathode and the anode is given by:
[9]
For electrodes of the same material (CA = CC), k can be determined by measuring the weight losses of both electrodes, as the mass losses all go to the NPs (Feng et al. Citation2016a).
In accordance with mass conservation, the average particle composition can be determined gravimetrically (weight losses of the anode and the cathode), which are equal to the mass ablated per spark from the corresponding electrode. The mean NP composition is expressed as (with respect to the cathode material):
[10]
Alternatively, can be formulated by substituting EquationEquation (4)
[4] to EquationEquation (10)
[10] , which yields:
[11] where the material term
can be estimated by EquationEquation (2)
[2] , while k is determined by EquationEquation (9)
[9] .
4. Experimental section
In order to determine k by EquationEquation (9)[9] , a pair of identical Au electrodes (each rod having diameters of 3 mm and a length of 30 mm; 99.99% purity) was used in the HFS with a fixed capacitance of 45 nF. The gap distance between the electrodes was varied from 0.5 to 2 mm by a fine micrometer screw with a precision of 0.01 mm, while a He carrier gas (99.999% purity) was perpendicularly passed through this gap at a flow rate of 12.4 standard liters per min (slm). The potential difference between the electrodes Uc was manually set between 1.1 and 1.2 kV for the entire range of gap distances, so that we can change the spark energy. The spark repetition frequency was kept at 1 kHz throughout the measurements.
The amount of NP production was derived from weighing the electrodes before and after sparking, and this was measured by an electric balance (AT201 Mettler Toledo) with a precision of 0.01 mg. In addition, the current oscillations were recorded by a Pearson Current Monitor (model 110), and an oscilloscope (Hameg HMO 1024) connected to the HFS using 1:100 High Voltage probes (Testec HV250).
5. Results and discussion
The electric circuit () used for determining k assumes a constant spark resistance Rspark, and this is justified by a perfect fit of the experimental current oscillation with the model predictions from EquationEquation (7)[7] as shown in . For Cca = 45 nF, which leads to a fixed spark energy (
), the damping behavior is associated with Rspark and the damping frequency mainly depends on Li, so that both values can be acquired accurately. shows that Rspark increases as the electrode gap distance increases, and the corresponding values are pointed out by the arrows in the figure (provided Li = 2.9 µH). It is also evident that the total resistance influences the mixing ratio, as it follows from EquationEquations (4)
[4] , (Equation7
[7] ), and (Equation9
[9] ). Because adding resistors to the circuit increases heat losses, it is in principle more efficient to change only the spark gap for varying the ablation ratio. The parameter
embedded in EquationEquation (1)
[1] is constant as discussed previously (Tabrizi et al. Citation2009a; Feng et al. Citation2015). The results in also validate this claim, showing that
is insensitive to both the electrode gap and spark energy. Mass production rates of the NPs at different spark energies and electrode gaps are shown in Table S2 of the SI.
Figure 4. Current oscillations during a single spark between two electrodes at different gap distances. (a) Current oscillation at a spark energy of 26.7 mJ and a gap distance of 1.5 mm. The symbols represent the measurements, while the curve is a fit of EquationEquation (7)[7] . (b) Fits of EquationEquation (7)
[7] to measurements at different gap distances, yielding the corresponding values for Li (2.9 µH) and Rspark (i.e., 0.5 mm, 1.29 Ω; 1.0 mm, 1.45 Ω; 2.0 mm, 1.67 Ω).
![Figure 4. Current oscillations during a single spark between two electrodes at different gap distances. (a) Current oscillation at a spark energy of 26.7 mJ and a gap distance of 1.5 mm. The symbols represent the measurements, while the curve is a fit of EquationEquation (7)[7] . (b) Fits of EquationEquation (7)[7] to measurements at different gap distances, yielding the corresponding values for Li (2.9 µH) and Rspark (i.e., 0.5 mm, 1.29 Ω; 1.0 mm, 1.45 Ω; 2.0 mm, 1.67 Ω).](/cms/asset/08049260-25b7-4daf-a94c-3f32a2a46e16/uast_a_1427852_f0004_oc.gif)
Table 3. Dependence of on gap distance and energy per spark.
The parameter k represents the energy ratio going into the ablation of the cathode and anode electrodes. EquationEquation (9)[9] links the model to the electrical characteristics of the spark circuit (). compares the values of k and
determined by EquationEquations (9)
[9] and (Equation11
[11] ) with those estimated by the gravimetric measurements at three different gaps. The results are in line with each other and reflect the same trend for increasing the gap distance. Most importantly, this correspondence verifies the assumption made in the model that material ablation is strongly dominated by the temporarily negative electrode within the spark oscillation.
Table 4. Comparison of k and the corresponding (EquationEquation (11)
[11] with CA = CC) determined by the gravimetric measurements (using EquationEquation (10)
[10] ) and the model predictions using EquationEquation (9)
[9] (E = 31.7 mJ, Rextra = 0).
EquationEquations (7)[7] and (Equation9
[9] ) imply that k increases (more ablation from the cathode with respect to the anode) as Li or Cca decreases. Since Cca also influences the spark energy, changing it becomes impractical. Li could be decreased by careful design of the circuit (e.g., coaxial cables) and increased by adding an inductor. The most simple way for increasing k is to add a resistance Rextra to the circuit. However, its drawback lies in the reduction of energy efficiency, because Rextra causes the energy efficiency reduced from
to
. When Rtot (i.e., Rspark + Rextra) reaches the point of critical damping (
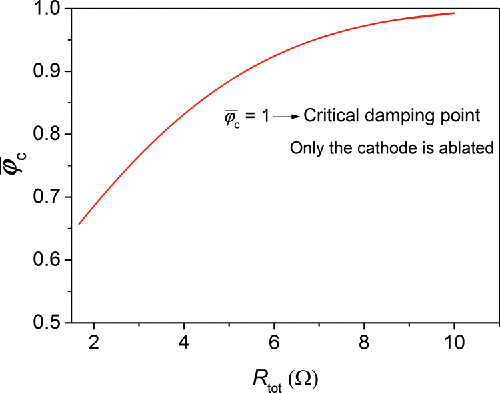
), only the cathode is ablated due to the absence of polarity reversal ().
As briefly mentioned above, another measure of controlling the particle composition is to adjust the spark gap. This is because the spark gap directly relates to the length of the spark plasma, which influences the spark resistance. As confirmed by the data shown in , the energy efficiency α remains almost unchanged regardless of the gap distances. shows that the electrode gap distance indeed has an influence on . This influence, however, is minor and the discharges become unstable with the gaps larger than 2 mm.
shows the change in the mean composition as derived from EquationEquations (9)
[9] and (Equation11
[11] ) for the special case of CA = CC, which are representative for other material combinations as validated experimentally (cf. Section 2 and the data corresponding to Al–Mg combinations shown in Figure S1 of the SI). The NP composition (i.e.,
) covers a considerable range from 0.6 to 1.0, but one can get the composition range between 0.0 and 0.4 by exchanging the anode with the cathode.
6. Explanation of the broadness of NP composition distribution
This section develops a general approach to predicting the composition broadness of NPs produced by spark discharges between electrodes of different materials. According to the model described above and its consistency with the experiments, internal NP mixing can be controlled through the oscillatory behavior of the discharge that makes electrodes take turns in emitting vapor clouds. The broadness of the particle composition distribution shown in is attributed to partially mixed vapors of the two materials at the initial stage and/or their emissions at different times. The latter is negligible with respect to the spatial separation, since the oscillatory frequency of the discharge is approximately 1 MHz. The dominance of the spatial separation of the two material sources implies that the spark gap should have an influence on
. Mixing is incomplete in the initial phase of particle growth, but it is subsequently completed by diffusion and turbulence during the subsequent stages of NP growth. Particle formation can thus be modeled by a simplified process in which:
| i. | Particles of one electrode material (‘single component particles’) with diameter | ||||
| ii. | The resulting aerosols are completely mixed, and | ||||
| iii. | Particle–particle collisions cause their further growth. | ||||
The colliding particles fully coalesce below a critical size (i.e., primary particle size within agglomerates) or their growth is interrupted by rapid dilution or deposition onto a substrate (Feng et al. Citation2015). Note that due to the initially unmixed growth, the final particles exhibit a spread of compositions, even if subsequent growth takes place under well-mixed conditions. The fraction of one component in these final particles having diameter Dpf can be described by Poisson statistics. Considering that the number of initial particles per final particle is
, the composition distribution can be approximated by Stirling's formula given by (Bronstein and Semendjajew Citation1998):
[12]
The standard deviation in EquationEquation (12)[12] is given by (Bronstein and Semendjajew Citation1998):
[13]
To determine using EquationEquation (13)
[13] , the composition
can be estimated by gravimetric measurements or predicted by EquationEquation (11)
[11] using EquationEquation (9)
[9] for k and EquationEquation (2)
[2] for CA/CC. As mentioned in Section 2, k is influenced by the type of the carrier gas (mainly the mass of the ions) and the electrical properties of the circuit, both of which also determine the composition distribution and the standard deviation. The influence of the electrode materials captured by
also determines the predictions by EquationEquation (12)
[12] and (Equation13
[13] ).
This mixing model is simplistic, because in reality an overlap appears between formation of the ‘single-component particles’ and the mixing phase, and because the particles have a distribution in size. Nevertheless, this analysis provides an estimation on how the process parameters influence the broadness of the NP composition distribution. On the experimental side, provides a rough estimate of . The primary particle diameters are estimated from the corresponding micrographs (Tabrizi et al. Citation2009b) as
for the Pd–Au combination and
for that of Pd–Ag. Agreement between experimental measurements and model predictions can be tested by inserting
from into EquationEquation (13)
[13] , as well as these diameters and the corresponding mean compositions
. We then get the initial diameters of the ‘single-component particles’ Dpi. It should be noted here that both values of Dpi arrive at approximately
, which is expected considering the similar conditions in both cases. This implies a similar particle growth history for these two electrode combinations (Pd–Ag and Pd–Au), in line with the description of the particle growth (Feng et al. Citation2016c). Supported by this qualitative consistency, EquationEquations (12)
[12] and (Equation13
[13] ) are key to predicting the influence of spark operating parameters on the broadness of the composition distributions in a qualitative sense. Quantitative predictions would require an appropriate value for Dpi. However, EquationEquation (12)
[12] correctly predicts that the Pd–Ag distribution () is broader than that of Pd–Au (), because of its smaller
(5 nm) and its
(0.375) closer to 0.5 (where
has its maximum).
In general, more turbulent mixing in the vicinity of the sparks should decrease Dpi. According to the particle growth model (Feng et al. Citation2016c), a decrease in spark energy or an increase in the gas flow rate also decreases Dpi, and therefore reduces . Another alternative for reducing
is to increase
, which is, in turn, closely associated with the temperature history and the surface state of the particles (Feng et al. Citation2015, Citation2016a). Finally, the dominance of the spatial separation of the two material sources over time effects explained above implies that a larger distance between the electrodes is expected to extend the non-mixed period, thereby increasing Dpi and
.
7. Conclusions
We have shown the influence of the oscillatory behavior of a single spark on the relative ablation of the two electrodes, which can be used to change the mean mixing ratio of the resulting NPs. Separation of the material-dependent and circuit-dependent contributions enables us to develop a model for predicting an electrical factor k. The model assumes that discharges only ablate the electrode momentarily having negative polarity. Tuning k through the electrical parameters can be used to vary the mean particle composition (EquationEquations (7)
[7] , (Equation9
[9] ), and (Equation11
[11] )). Analysis of the measured mixing ratios reported in the literature (Tabrizi et al. Citation2009a,Citationb) is consistent with the assumption that the electrical factor k, representing the energy ratio transferred to the electrodes, is material independent and can thus also be applied to identical electrodes. The mean compositions predicted by EquationEquation (11)
[11] correlates well with the gravimetric measurements in this work and experimental results in the literature (Vons Citation2008; Tabrizi et al. Citation2009b). Stirling's formula is used to correlate the broadness of NP composition distribution with the operating conditions in a qualitative sense.
The findings from this work shed light into the mechanism of internal NP mixing by using electrodes of different materials in spark ablation. We have shown that the mixing of the NPs can be well controlled by only changing the electrical properties of the circuit. This highlights the attractiveness of spark ablation to produce unique alloy NPs consisting of raw materials that may be immiscible at macroscopic scale. Furthermore, the models can be used as a tool for controlling the mean composition and broadness of the NP composition distribution in the configuration of two different electrodes in spark ablation.
UAST_1427852_Supplemental_Files.zip
Download Zip (119 KB)Additional information
Funding
References
- Anastasopol, A., Pfeiffer, T. V., Middelkoop, J., Lafont, U., Canales-Perez, R. J., Schmidt-Ott, A., Mulder, F. M., and Eijt, S. W. H. (2013). Reduced enthalpy of metal hydride formation for Mg-Ti nanocomposites produced by spark discharge generation. J. Am. Chem. Soc., 135(21):7891–7900. doi:10.1021/ja3123416.
- Bronstein, I. N. and Semendjajew, K. A. (1998). Taschenbuch der Mathematik. Deutsch Harri GmbH, Frankfurt.
- Byeon, J. H. and Kim, J.-W. (2014). Ambient plasma synthesis of TiO2@graphite oxide nanocomposites for efficient photocatalytic hydrogenation. J. Mater. Chem. A, 2(19):6939–6944. doi:10.1039/c4ta00287c.
- Byeon, J. H., Park, J. H., and Hwang, J. (2008). Spark generation of monometallic and bimetallic aerosol nanoparticles. J. Aerosol Sci., 39(10):888–896. doi:10.1016/j.jaerosci.2008.05.006.
- Byeon, J. H. and Roberts, J. T. (2012). Silver deposition on a polymer substrate catalyzed by singly charged monodisperse copper nanoparticles. ACS Appl. Mater. Interfaces, 4(5):2515–2520. doi:10.1021/am300217n.
- Charitidis, C. A., Georgiou, P., Koklioti, M. A., Trompeta, A.-F., and Markakis, V. (2014). Manufacturing nanomaterials: from research to industry. Manuf. Rev., 1(11):1–19. doi:10.1051/mfreview/2014009.
- Feng, J. (2016). Scalable Spark Ablation Synthesis of Nanoparticles: Fundamental Considerations and Application in Textile Nanofinishing. Delft University of Technology, Delft. doi:10.4233/uuid:fb6c0122-587b-471d-8009-b52ef9b69b07
- Feng, J., Biskos, G., and Schmidt-Ott, A. (2015). Toward industrial scale synthesis of ultrapure singlet nanoparticles with controllable sizes in a continuous gas-phase process. Sci. Rep., 5:15788. doi:10.1038/srep15788.
- Feng, J., Guo, X., Ramlawi, N., Pfeiffer, T. V., Geutjens, R., Basak, S., Nirschl, H., Biskos, G., Zandbergen, H. W., and Schmidt-ott, A. (2016a). Green manufacturing of metallic nanoparticles: a facile and universal approach to scaling up. J. Mater. Chem. A, 4:11222–11227. doi:10.1039/C6TA03221D.
- Feng, J., Hontañón, E., Blanes, M., Meyer, J., Guo, X., Santos, L., Paltrinier, L., Ramlawi, N., de Smet, L. C. P. M., Nirschl, H., Kruis, F. E., Schmidt-Ott, A., and Biskos, G. (2016b). Scalable and environmentally benign process for smart textile nanofinishing. ACS Appl. Mater. Interfaces, 8(23):14756–14765. doi:10.1021/acsami.6b03632.
- Feng, J., Huang, L., Ludvigsson, L., Messing, M. E., Maisser, A., Biskos, G., and Schmidt-Ott, A. (2016c). General approach to the evolution of singlet nanoparticles from a rapidly quenched point source. J. Phys. Chem. C, 120(1):621–630. doi:10.1021/acs.jpcc.5b06503.
- Isaac, N. A., Ngene, P., Westerwaal, R. J., Gaury, J., Dam, B., Schmidt-Ott, A., and Biskos, G. (2015). Optical hydrogen sensing with nanoparticulate Pd–Au films produced by spark ablation. Sensors Actuators, B, 221:290–296. doi:10.1016/j.snb.2015.05.095.
- Jang, S., Yoon, J., Ha, K., Kim, M., Kim, D. H., Kim, S. M., Kang, S. M., Park, S. J., Jung, H. S., and Choi, M. (2016). Facile fabrication of three-dimensional TiO2 structures for highly efficient perovskite solar cells. Nano Energy, 22:499–506. doi:10.1016/j.nanoen.2016.02.050.
- Kang, S. M., Jang, S., Lee, J.-K., Yoon, J., Yoo, D.-E., Lee, J.-W., Choi, M., and Park, N.-G. (2016). Moth-eye TiO2 layer for improving light harvesting efficiency in Perovskite solar cells. Small, 12(18):2443–2449. doi:10.1002/smll.201600428.
- Kim, H., Kim, J., Yang, H., Suh, J., Kim, T., Han, B., Kim, S., Kim, D. S., Pikhitsa, P. V., and Choi, M. (2006). Parallel patterning of nanoparticles via electrodynamic focusing of charged aerosols. Nat. Nanotechnol., 1(2):117–121. doi:10.1038/nnano.2006.94.
- Lahiri, D., Bunker, B., Mishra, B., Zhang, Z., Meisel, D., Doudna, C. M., Bertino, M. F., Blum, F. D., Tokuhiro, A. T., Chattopadhyay, S., Shibata, T., and Terry, J. (2005). Bimetallic Pt–Ag and Pd–Ag nanoparticles. J. Appl. Phys., 97(9):94304. doi:10.1063/1.1888043.
- Llewellyn Jones, F. (1950). Electrode erosion by spark discharges. Br. J. Appl. Phys., 1(3):60–65. doi:10.1088/0508-3443/1/3/302.
- Madhu, R., Veeramani, V., Chen, S.-M., Veerakumar, P., Liu, S.-B., and Miyamoto, N. (2016). Functional porous carbon–ZnO nanocomposites for high-performance biosensors and energy storage applications. Phys. Chem. Chem. Phys., 236:369–374. doi:10.1039/c6cp01285j.
- Maisser, A., Barmpounis, K., Attoui, M. B., Biskos, G., and Schmidt-Ott, A. (2015). Atomic cluster generation with an atmospheric pressure spark discharge generator. Aerosol Sci. Technol., 49(10):886–894. doi:10.1080/02786826.2015.1080812.
- Naqshbandi, M., Canning, J., Gibson, B. C., Nash, M. M., and Crossley, M. J. (2012). Room temperature self-assembly of mixed nanoparticles into photonic structures. Nat. Commun., 3:1188. doi:10.1038/ncomms2182.
- Pfeiffer, T. V., Feng, J., and Schmidt-Ott, A. (2014). New developments in spark production of nanoparticles. Adv. Powder Technol., 25(1):56–70. doi:10.1016/j.apt.2013.12.005.
- Schwyn, S., Garwin, E., and Schmidt-Ott, A. (1988). Aerosol generation by spark discharge. J. Aerosol Sci., 19(5):639–642. doi:10.1016/0021-8502(88)90215-7.
- Sebastian, V., Arruebo, M., and Santamaria, J. (2014). Reaction engineering strategies for the production of inorganic nanomaterials. Small, 10(5):835–853. doi:10.1002/smll.201301641.
- Tabrizi, N. S., Ullmann, M., Vons, V. A., Lafont, U., and Schmidt-Ott, A. (2009a). Generation of nanoparticles by spark discharge. J. Nanoparticle Res., 11:315–332. doi:10.1007/s11051-008-9407-y.
- Tabrizi, N. S., Xu, Q., Pers, N. M., and Schmidt-Ott, A. (2010). Generation of mixed metallic nanoparticles from immiscible metals by spark discharge. J. Nanoparticle Res., 12(1):247–259. doi:10.1007/s11051-009-9603-4.
- Tabrizi, N. S., Xu, Q., van der Pers, N. M., Lafont, U., and Schmidt-Ott, A. (2009b). Synthesis of mixed metallic nanoparticles by spark discharge. J. Nanoparticle Res., 11:1209–1218. doi:10.1007/s11051-008-9568-8.
- Tiwari, A., Mondal, I., Ghosh, S., Chattopadhyay, N., and Pal, U. (2016). Fabrication of mixed phase TiO 2 heterojunction nanorods and their enhanced photoactivities. Phys. Chem. Chem. Phys., 18(22):15260–15268. doi:10.1039/C6CP00486E.
- Vons, V. (2008). Spark Discharge Generated Nanoparticles for Hydrogen Storage Applications. Delft University of Technology, Delft. uuid:c7c61f73-1552-4892-8676-a09012c7af53.
- Vons, V. A., de Smet, L. C. P. M., Munao, D., Evirgen, A., Kelder, E. M., and Schmidt-Ott, A. (2011). Silicon nanoparticles produced by spark discharge. J. Nanoparticle Res., 13(10):4867–4879. doi:10.1007/s11051-011-0466-0.
- Xiong, S., Qi, W., Huang, B., and Wang, M. (2011). Size-, shape- and composition-dependent alloying ability of bimetallic nanoparticles. ChemPhysChem, 12(7):1317–1324. doi:10.1002/cphc.201100001.