ABSTRACT
A novel inversion method is presented, which derives the two-variable number distribution for black carbon aerosol, using a coupled centrifugal particle mass analyzer (CPMA) and single particle soot photometer (SP2). The CPMA classifies all particles by their mass-to-charge ratio, and the SP2 detects the mass of refractive black carbon (rBC) in each individual particle. The results of the inversion are the simultaneous number distributions of both rBC mass and total particle mass. Using the distribution, the coating distribution on a population of rBC particles can be identified visually. Furthermore, the distribution can be integrated to find one-variable mass and number concentration distributions as a function of total or rBC particle mass. These capabilities were demonstrated via smog chamber experiments, where an organic (non-rBC) coating was grown onto uncoated rBC aerosol over several hours via photo-oxidation of p-xylene. The particle distributions were constructed using the inversion over a range of 1–60 fg of total particle mass. As the non-rBC coating thickness increased over time, a shift in the number distribution toward higher total mass was observed. At the end of the experiment, uncoated rBC was injected into the chamber, and the distribution was clearly resolved using the inversion. The CPMA-SP2 method offers several advantages over “SP-2 only” methods, namely, (i) coating mass information can be obtained over a wider range of total particle mass, (ii) total particle mass is measured directly, and (iii) it does not make core–shell morphology assumptions.
Copyright © 2018 American Association for Aerosol Research
EDITOR:
1. Introduction
Aerosols containing refractory black carbon (rBC) particles in the atmosphere have important climate impacts due to their ability to absorb and scatter light. Refractive black carbon aerosols are the second most influential anthropogenic climate changing compound; however, the magnitude of the impact of rBC on climate change is highly uncertain (Bond et al. Citation2013). A major source of this uncertainty is due to coating of non-rBC compounds onto rBC, changing the particles absorption and scattering characteristics (aerosol direct effects on radiative forcing) and influences on cloud life-cycle (semi-direct and indirect effects on radiative forcing) (Menon et al. Citation2002; Ramanathan and Carmichael Citation2008). Acquisition of non-rBC coating can occur at the source of emission (e.g., from unburned fuel or engine oil condensed on soot emitted from engines [Maricq Citation2007], or from secondary organic aerosol condensing or coagulating with the rBC [Riemer et al. Citation2004; Schnaiter et al. Citation2005; Moosmüller et al. Citation2009]). On a single particle, the mass ratio of non-rBC to rBC may vary, and this mass ratio is referred to as mass fraction. Measuring the mass distribution on atmospheric rBC is important for understanding the effect of coating on climate. To reduce uncertainty on the effect of rBC on climate forcing, it is desirable to quantify mass and number distribution characteristics of an aerosol population containing rBC particles.
The most common methodology to determine coating characteristics of atmospheric rBC particles is to infer them using a single particle soot photometer (SP2; Droplet Measurement Technologies, Boulder, CO, USA). SP2 methodologies are explained in detail elsewhere (Moteki and Kondo Citation2007; Shiraiwa et al. Citation2007; Schwarz et al. Citation2008; McMeeking et al. Citation2010, Citation2011; Sedlacek et al. Citation2012), and briefly in Section 2.1. Bare rBC particles passing through an SP2 Nd:YAG laser produce incandescence and scattering signals with near-simultaneous peak intensities. Particles with a non-rBC coating undergo a delay on the onset of the LII peak signal as organic particle coating must be vaporized before the rBC core begins to incandesce. Therefore, the non-rBC coating mass can be inferred by correlating the difference in time of the scattering and incandescence signal peaks to coating mass. Another approach relies on sizing rBC-containing particles optically and estimating the coating amount using assumed morphology and refractive indices (Gao et al. Citation2007). Inferring the coating mass using peak time differencing can be difficult because the time to vaporize organic coating depends on physical and thermodynamic characteristics (Moteki and Kondo Citation2007) which may be challenging to quantify for atmospheric rBC. Further, these methods for estimating coating thickness are constrained by the sensitivity of the SP2’s scattering detector, which can resolve particles in the range of 200–430 nm in diameter.
We present here an improvement to an alternative method to measure the mass of non-rBC coating on populations of refractory black carbon (rBC) particles using a tandem CPMA-SP2 system (Liu et al. Citation2017). The centrifugal particle mass analyzer (CPMA; Cambustion Ltd., Cambridge, UK) classifies particles by their mass-to-charge ratio (Olfert and Collings Citation2005), and the SP2 detects the mass of rBC in each individual particle (Stephens et al. Citation2003; Slowik et al. Citation2007). In coupling the two instruments, the advantages of this method over SP2-only methods are (i) coating mass information can be obtained over a much wider range of rBC particle mass compared to SP-2 only methods (restricted in the SP2 by the sensitivity to light scattered by rBC), (ii) coating mass is measured directly: no assumptions for coating density or refractive index are needed, and (iii) it does not require assumptions of a core–shell morphology. The CPMA does not limit the ranges of particles measured by the SP2 as its classification range is 0.2 ag to 1050 fg (or a volume equivalent diameter, , of 6 to 1030 nm assuming an rBC density of 1.8 g/cm3). Regarding the SP2, only the incandescence channel is used, which has a detection range of approximately 0.3–117 fg rBC (or a volume equivalent diameter,
, of 70 to 500 nm assuming an rBC density of 1.8 g/cm3). The detection efficiency, the number concentration detected versus the actual concentration present, may be less than 100% for rBC particles below 0.9 fg (Laborde et al. Citation2012), but if the laser power is tuned the efficiency can be greater than 80% down to 0.3 fg (Liu et al. Citation2017). Furthermore, Moteki and Kondo (Citation2007) have shown that the incandescence signal is independent of coating thickness up to 650 nm (where coating thickness is defined as volume equivalent diameter of the particle minus that of the rBC core); thus, the CPMA-SP2 can measure a wide range of coating mass fractions for different rBC core masses.
In previous work, the CPMA-SP2 system was used to calculate the average coating mass on a population of rBC particles (Liu et al. Citation2017). For each CPMA setpoint, the average mass of rBC particles was determined using an SP2, and divided by the total particle mass (i.e., CPMA setpoint). This method assumes that the CPMA transfer function is very narrow, and that the proportion of multiply charged particles which cannot be removed by inspection is small. However, in the atmosphere, a population of rBC particles has a wide range of coating mass (i.e., some particles in the population may have very little coating while some particles may have considerable amounts), and may have a significant proportion of multiple charge states.
The distribution of the coating material on the rBC particles can be described using a two-variable distribution described by ; where
is the mass of an individual rBC particle and
is the mass of an individual particle. Thus, the term ∂2N represents the number concentration of particles with an rBC particle mass between
and
and total particle mass between
and
. This distribution is analogous to one-variable distributions that are often used in aerosol science to describe count or mass distributions (e.g.,
or
). The two-variable distribution derived in this article is similar to that described by Rawat et al. (Citation2016) for two-variable distributions of mobility and mass using a differential mobility analyzer–aerosol particle mass analyzer (DMA–APM) system. This work was described in further detail by Buckley et al. (Citation2017), where the two-variable distribution,
, represents the number concentration of particles with mobility diameters between
and
and masses between
and
.
A useful property of the two-variable distribution is that it can be integrated to derive one-variable distributions of the total aerosol population; for example, total mass concentration distribution as a function of total particle mass can be obtained using,[1]
Additionally, other distributions can be derived: (i) mass concentration distribution as a function of rBC particle mass , (ii) rBC mass concentration distribution as a function of total particle mass
, and (iii) rBC mass concentration distribution as a function of rBC particle mass
. Similarly, the total mass concentration, M, and total number concentration, N, can be calculated through double integration of the two-variable distribution. Note that since the SP2 only measures particles containing rBC, the total mass concentration refers to the total mass concentration of particles containing rBC, and N refers to the total number concentration of particles containing rBC. As the CPMA is an aerosol classifier, and not a single-particle instrument, a data inversion process is required to determine the two-variable distribution. The two-variable inversion is novel and different than the two-variable inversion presented by Buckley et al. (Citation2017), because the SP2 is a single particle instrument, whereas their inversion dealt with two instruments which are particle classifiers. The inversion routine is tested and validated experimentally using rBC particles coated by secondary organic aerosol in a smog chamber.
2. Experimental methods
2.1. CPMA-SP2 system
The CPMA is an aerosol instrument which classifies particles based on their mass-to-charge ratios. The principle of operation is based on balanced rotational and electrical forces between two rotating coaxial cylinders. The probability of a particle making its way through the entirety of the CPMA is described by the instrument “transfer function.” The transfer function of the CPMA is approximately triangular and can be described by its resolution, which is the inverse of the normalized full width half maximum (FWHM) of the transfer function (e.g., a resolution of 5 results in a triangular transfer function with an FWHM of 20% of the mass setpoint). During step-scan operations, the CPMA sequentially changes rotational speed and electrode voltage potential to select a chosen quantity of mass setpoints between a range of user-selected values.
The SP2 operates by drawing an aerosol stream through an Nd:YAG intra-cavity laser (λ = 1064 nm). The absorbing particles are vaporized and the scattered and incandescent lights are measured by avalanche photo-diodes and photomultiplier tubes, respectively. The scattered light signal is related to a nominal optical scattering diameter, and incandescent detectors relate the peak of the incandescent signal to rBC mass (Stephens et al. Citation2003; Moteki and Kondo Citation2010). Calibration of the SP2 was conducted by correlating peak incandescent signal to rBC mass, selected using the CPMA from the bare, uncoated particles produced by the inverted burner. Section S1 in the online supplementary information (SI) shows the calibration of rBC mass for the SP2 and efficiency of the SP2.
2.2. Experimental procedure
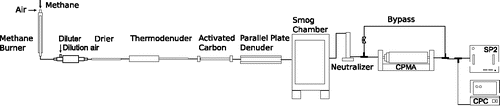
The inversion was tested using smog chamber experiments, where rBC particles with a range of secondary organic coating mass were sampled using the CPMA-SP2 system. The experimental setup is shown in . Refractory BC particles were produced from an inverted methane diffusion flame, similar to that described in Ghazi and Olfert (Citation2013). The flow rate of fuel and air was 1.6 and 17.4 standard L/min, respectively (with reference temperature and pressure of 0°C and 101.325 kPa, respectively), and this resulted in a global equivalence ratio of approximately 0.85. The particles were subsequently passed at 1 L/min through a 25 cm diffusion drier, consisting of a mesh tube surrounded by anhydrous calcium sulfate; a thermodenuder at 350°C with a heated section of 25 cm with 1/4 inches diameter tubing; a tube of activated carbon 91 cm long and diameter of 1/4 inches; and a counter-flow parallel plate denuder (similar to that described in Ruiz et al. Citation2006) prior to entering the chamber. The purpose of the diffusion drier was to remove water from the aerosol phase. The thermodenuder partitioned any organic material on the particles to the vapor phase, which either condensed onto the walls of the cooling section of the denuder or adsorbed onto the activated carbon following the denuder. The counter-flow parallel plate denuder removed trace gases which may have remained after these processes. The smog chamber has been previously described by Parsons et al. (Citation2011). It has a volume of 1.8 m3, perfluoroalkoxy film walls, and twenty-four 34 W black lights arranged on the top and opposite walls of the inside of the chamber.
The smog chamber was filled to a concentration of approximately 10,000 cm−3, verified with a Scanning Mobility Particle Sizer (SMPS; TSI Inc., Shoreview, MN, USA) connected directly to the smog chamber (not shown in experimental diagram). A precursor hydrocarbon, p-xylene (99.9%, Fisher Scientific, Hampton, NH, United States), was injected into the chamber with the lights off. Particle mass growth (i.e., coating) does not occur with the lights off due to the absence of shortwave radiation. The uncoated, bare rBC particles were sampled using the CPMA-SP2, with the SP2 recording the mass and time of CPMA-classified rBC on a single particle basis. The polydisperse particle distribution was charge-neutralized before sampling, by a Kr-85 radioactive source. A condensation particle counter (CPC, Model 3771, TSI) was set to a flow rate of 1.0 L/min and the SP2 was set to a flow rate of 0.12 L/min, resulting in a total flow through the CPMA of 1.12 L/min. When sampling with the CPMA-SP2, the CPMA was sequentially stepped over a mass range from 0.3 to 28 fg, and the SP2 measured the mass of rBC comprising individual CPMA-classified particles. Each setpoint was held for 30 s, and the CPC, in parallel with the SP2, measured total CPMA output particle concentration for reference. A valve downstream of the neutralizer and upstream of the CPMA was fitted to bypass the CPMA, so the full aerosol could also be measured by the SP2 alone. Before each CPMA scan, the CPMA was bypassed and the SP2 sampled directly from the chamber; these data are required to correct the inversion process as described in Section 3. After one scan of the bare rBC particles, hydrogen peroxide was introduced into the chamber, and the lights turned on. Photolysis of the hydrogen peroxide produced hydroxyl radicals, which reacted with the p-xylene precursor. The secondary organic material condensed onto the bare rBC over a period of 7 h, and the lights irradiated the chamber for the rest of the experiment. Over the period of coating growth, the CPMA-SP2 system sampled the aerosol from the smog chamber using the same parameters and setpoint time. After 7 h, fresh rBC was injected into the chamber resulting in co-existing populations of both bare and coated rBC particles. This mixed population was sampled using the same technique.
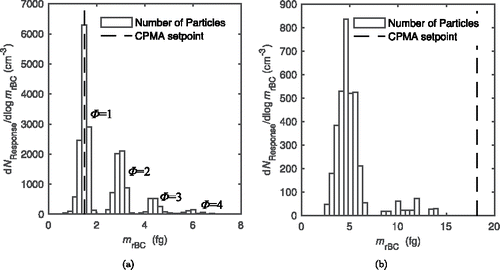
shows the output of the SP2 when a CPMA is operated upstream at a single setpoint. For a single CPMA setpoint, the output of the SP2 can be envisaged as an rBC mass distribution, hereafter referred to as the response of the system, . The response of the system is derived by the bin width normalized number concentration distribution of rBC particles measured by the SP2 for a given CPMA setpoint, and the number of bins used in the inversion is discussed in Section 5.1. For a neutralized stream of uncoated rBC particles as seen in , charge states are clearly visible—doubly charged particles of twice the mass have the same mass-to-charge ratio as singly charged particles of the setpoint mass. The first three charge states, Φ = 1, 2, 3, are clearly visible, and the CPMA setpoint is equal to the median of the singly charged particle distribution. As seen in , for coated particles, the rBC distribution blends together. The multiply charged particles are still present but they are not distinguishable from each other as particles of the same total particle mass (as classified by the CPMA) comprise a wide range of rBC mass. However, because the charge distribution and CPMA resolution is known for a neutralized stream of particles, an inversion can be used to account for the multiple charge states in order to accurately reconstruct the mass and number distributions of coated particles.
3. Inversion of CPMA-SP2 data
An inversion is the process of solving an inverse problem, where parameters which cannot be directly measured or observed are inferred. A subset of inverse problems are Fredholm integral equations which are applicable to data acquired from aerosol science instrumentation such as DMA-CPC systems or cascade impactors. The unknown quantity desired to be known for aerosol instrumentation is generally the number or mass distribution entering the instrument. Development of inversions for DMA and tandem-DMA data is analogous to the development of a CPMA-SP2 inversion and has been discussed previously (Hagen and Alofs Citation1983; Collins et al. Citation2002; Stolzenburg and McMurry Citation2008; Kulkarni et al. Citation2011). The general form of the Fredholm integral equation is[2] where R(x) is the response of the system (i.e., the observed or measured data), Γ(x, y) is the kernel function which describes the relationship between the response and the model parameters, and p(y) is an unknown quantity which cannot be directly measured. Applying Fredholm integral theory to a CPMA-SP2 system, the two-variable distribution (i.e., the unknown variable)
can be related to the response and kernel of the CPMA-SP2 system for the ith setpoint assuming the distribution and charge fraction are approximately constant over the width of the transfer function,
[3] where
,
is a correction factor,
is charge fraction, and
is the CPMA transfer function. Unlike in Rawat et al. (Citation2016), where a two-variable distribution for a DMA-APM-CPC system is calculated, the response of the CPMA-SP2 system is a number distribution with respect to
rather than the number concentration of particles. The response is calculated at the ith specific CPMA setpoint,
, as described in Section 2.
The CPMA transfer function, , describes the probability of a particle with a particular mass-to-charge ratio passing through the classifier. The transfer function is a function of the mechanical mobility, B, of a particle, and its number of charges, Φ. There are several models of transfer function available, and the diffusive function similar to that seen in Olfert and Collings (Citation2005) is used for all calculations because it is the most accurate and is not computationally intensive.
Like an SMPS, the CPMA-SP2 inversion assumes a charge distribution of neutralized aerosol (Wang and Flagan Citation1990), and the charge fraction, , describes the proportion of particles of a particular size and charge state. The charge fraction in the CPMA-SP2 inversion was calculated using the Wiedensohler equations (Wiedensohler Citation1988), which were chosen because they are commonly used. Recently, it was shown the fraction of multiply charged particles may depend on the ion composition of the neutralizer, making the Wiedensohler equations inaccurate in some cases (López-Yglesias and Flagan Citation2013; Gopalakrishnan et al. Citation2013; Maißer et al. Citation2015). Therefore, other models could be used with this inversion, but they have not been tried. The maximum number of charges,
, is specified for the inversion and typically is chosen to be 3, since a negligible number of particles exists with higher charge states for the size range investigated in this study.
Determining mobility equivalent diameter requires assumptions of particle mobility diameter and density. Both are required to calculate the charge fraction and the CPMA transfer function. The mobility diameter and density assumption affects the amplitude of the two-variable distribution, and to a lesser extent, the width. The amplitude is primarily affected, rather than the width, because these assumptions affect the shape of the CPMA transfer function, which describes how many particles pass through the CPMA. A wider transfer function results in the two-variable distribution having a higher concentration (i.e., increased amplitude). Additionally, just like all aerosol science instrumentation, diffusion and other mechanistic processes cause losses of particles within the CPMA again affecting the amplitude of the two-variable distribution. The amplitude of the two-variable distribution can be corrected by introducing the correction factor, ε. The correction factor is determined using the data from the direct SP2 sampling performed during CPMA bypass measurements following each CPMA-SP2 scan. The mass dependency of was ignored, since the SP2 can only measure rBC mass, not total particle mass, and it is not possible to determine the correction factor as a function of total particle mass. Thus, the average correction factor will be determined, represented by
. The correction factor is determined by calculating the total number concentration of the SP2 sampling alone (i.e., bypassing the CPMA), and dividing by the number concentration calculated by the two-variable CPMA-SP2 data (i.e., using the inversion),
[4] where N is the total number concentration of the two-variable distribution and
is the number concentration of particles through direct SP2 measurement (i.e., no CPMA upstream of the SP2) over the same
range as the two-variable distribution. This assumes the CPMA scan range is high enough so it covers the vast majority of total particle masses.
The entire set of CPMA setpoints can be represented by an ill-conditioned positively constrained system of equations,[5] where Q is the two-variable number distribution,
, and R is the number distribution of the response. Similar to the kernel function developed by Collins et al. (Citation2002) for scanning DMA data, the kernel function, Γi, j, represents the response of the instrument with a trapezoidal rule approximation for the ith instrument response and the jth solution element of the known number distribution of
. Ignoring the correction factor,
, for now, the kernel can be represented as,
[6] where the terms mp(j + 1/2) and mp(j − 1/2) represent the edges of the jth bin of rBC masses, where the bin edges are the geometric midpoint between adjacent bin midpoints. To solve Equation (Equation5
[5] ) for the unknown two-variable number distribution, a Twomey algorithm (Twomey Citation1975) was used. This algorithm was selected because it is easy to implement and generally stable. Briefly, the Twomey method works by first approximating the two-variable distribution with an initial guess. The initial guess is calculated by taking the response of the CPMA-SP2 system at the ith CPMA setpoint, and dividing by the charge fraction assuming all particles were singly charged and by the variable
. The variable
is the analytical solution to the integral of a triangular CPMA transfer function assuming a single charge and is described in detail in Section S4.1. Bins with an rBC mass greater than the total mass are set to zero because they are physically impossible. The initial guess is calculated using,
[7] where
is the initial guess for the ith CPMA setpoint, and
is the response of the CPMA-SP2 system at the ith CPMA setpoint.
The Twomey algorithm then iteratively calculates: (i) a theoretical response by multiplying the two-variable distribution by the Kernel function, (ii) a correction factor for each element of the two-variable distribution, and (iii) the new two-variable distribution by multiplying the old two-variable distribution by the correction factor. This sequence is iterated until the total number concentration of the two-variable solution approaches that which was originally measured by the CPMA-SP2 within 5%. Then, the amplitude is corrected by multiplying by the correction factor, . The solution may be noisy due to low rBC concentrations and oscillations induced by the Twomey algorithm. Thus, smoothing the initial guess and final solution via weighted averaging may be needed. This was implemented into the inversion as an option, and is conducted similar to that seen in Markowski (Citation1987). More details on smoothing are available in Section S3.4.
Further information on the inversion is given in the SI. Section S2 describes data pre-processing, where filters were applied to remove spurious particles and neutral particles seen at CPMA setpoints greater than 50 fg. Section S3 gives detailed information on numerical methods. Section S4 explores model validation in three ways: (i) The inversion is compared to an analytical solution under simplified conditions, (ii), comparison to direct SP2 measurement, and (iii) correction of mobility assumptions. Section S5 discusses transfer function selection.
4. Results and discussion
Results from the inversion, specifically for coated, uncoated, and a mixed population of coated and uncoated particles are presented in this section. Additional results are available in Section S5 of the SI.
4.1. Two-variable number concentration distributions
The two-variable number distributions, , which are functions of rBC particle mass and total particle mass, are shown in for populations of (a) uncoated, (b) coated, and (c) mixture of coated and uncoated particles. The color represents the magnitude of the two-variable distribution, and the units are in number of particles squared per cm6. The size of each rectangle represents the limits of the histogram bins used in the inversion. The geometric center of each bin corresponds to the ith CPMA setpoint mass in the total mass and rBC mass direction (because rBC bins were chosen to correspond to each CPMA setpoint). There were 21 setpoints for each CPMA scan, and therefore 21 bins in the total mass direction (i.e., the x-axis). The number of bins in the rBC mass direction (i.e., the y-axis) is variable; however, increasing the number of bins causes a decrease in the number of particles per bin which may lead to poor counting statistics. The white dotted line represents where particles are uncoated (i.e.,
). Particles to the left of the white dotted line of unity slope are physically impossible because the rBC particle mass exceeds that of the total particle mass of the particle, but could occur if the particle had a stronger incandescence response compared to the material used to calibrate the SP2. Smoothing was conducted for the latter two cases, but was not conducted for uncoated particles as the distribution is narrow. In , the multiple charge states are not visible for the two-variable distribution of uncoated, bare rBC particles. This is because the inversion charge-corrects the distribution by removing particles with an rBC particle mass greater than the total particle mass, then adjusting the amplitude to account for removing these particles.
Figure 3. Histogram representation of two-variable distributions, , for (a) uncoated, bare rBC particles, (b) coated rBC particles which have undergone photooxidation with p-xylene under UV lights for 7 h, and (c) mixture of coated and uncoated rBC distributions. The white dashed line represents where
.
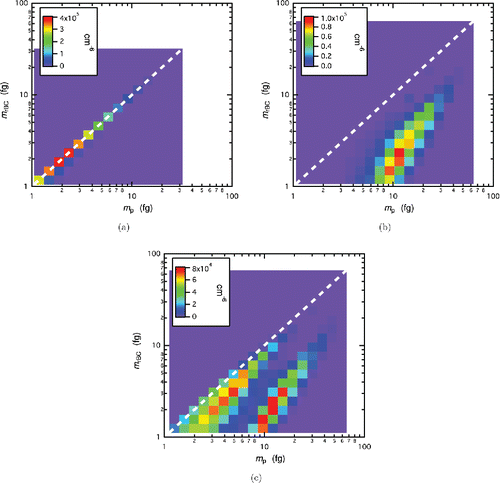
The two-variable distribution for bare, uncoated rBC particles seen in exists along a line with slope equal to one. This is because the particles are not coated and thus the total particle mass is equal to the mass of rBC. When the particles grew an organic coating as seen in , the distribution shifted toward a higher total particle mass. The distribution does not increase in the rBC particle mass direction since the rBC particle mass does not increase. The widening of the distribution is due to the particle-to-particle variation in the condensational growth rate of secondary organic aerosol, which creates a distribution of total particle mass for particles of a given rBC particle mass. When fresh uncoated rBC particles are added to the smog chamber as seen in , the two distinct populations of coated and uncoated particles can be observed. The fresh particles do not fall on the one to one line because the measurement was taken 45 min after injecting the new particles, and thus a coating had started to develop. This time delay was intentional in order to allow the particles to completely spatially mix with the coated particles.
4.2. One-variable number distribution
Direct integration of the two-variable number concentration distribution, , in the total particle mass or rBC particle mass domains (i.e., the x and y directions) yields
or
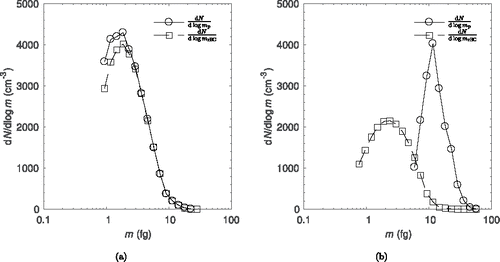
. The number distribution for uncoated and coated particles can be seen in . Note that the data are only shown for particles greater than 0.9 fg due to counting efficiency loss of the SP2 below that limit. This results in the left side of the number distribution being cut off. For uncoated particles, the distribution of
should have an amplitude equal to
; however,
has a slightly higher amplitude. This could be for two reasons. First, the distribution is very narrow, and the initial guess returns a broader distribution than expected. Since the initial guess is slightly broader than expected, the solution distribution is also broad. Second, a calibration error of the SP2 (or small amounts of coating on the rBC when the SP2 is calibrated) would result in a small shift in the distribution.
The number distributions for coated particles are seen in . For coated particles, has a median greater than
due to increased mass of the coating as expected. Comparing
for coated and uncoated particles, the distribution is similar except the median of the coated case is lower than that of the uncoated case. This is due to the particle losses in the chamber due to diffusion to the walls.
4.3. One-variable mass concentration distributions
One-variable mass distributions can be recovered by integration of along either the
or
domains and multiplying by either rBC particle mass or total particle mass (Equation (Equation1
[1] )). The mass distributions can be in terms of total particle mass concentration or rBC mass concentration. The mass concentration distribution of non-refractory material (nrBC),
or
, is given by the difference of the total particle mass distribution and the rBC mass distribution as either a function of total or rBC particle mass. As seen in , for uncoated rBC particles the rBC mass concentration distribution and total mass concentration distribution are approximately equal. This is because there is no coating on the particles and total particle mass is equivalent to rBC particle mass. As seen in for coated particles, the mass distributions as a function of rBC particle mass have approximately the same median as the uncoated case. This reflects that the organic coating growth via photo-oxidation of p-xylene does not change the rBC particle mass in the chamber. The coated one-variable mass distribution shows the difference in total particle mass (
) and rBC particle mass (
) concentration distributions, the difference in amplitude is proportional to the coating mass at a particular rBC particle mass. This figure shows a trend of smaller particles having a higher coating ratio compared to larger particles. This is expected and has been observed in particles emitted from gasoline engines (Momenimovahed and Olfert Citation2015), diesel engines (Ristimäki et al. Citation2007), diffusion flames (Dickau et al. Citation2016), and premixed flames (Ghazi and Olfert Citation2013).
Figure 5. Single variable mass distribution functions for: (a) uncoated particles as a function of rBC particle mass, (b) coated particles as a function of rBC particle mass, (c) mixture of coated and uncoated populations as a function of total particle mass, and (d) mixture of coated and uncoated populations as a function of rBC particle mass.
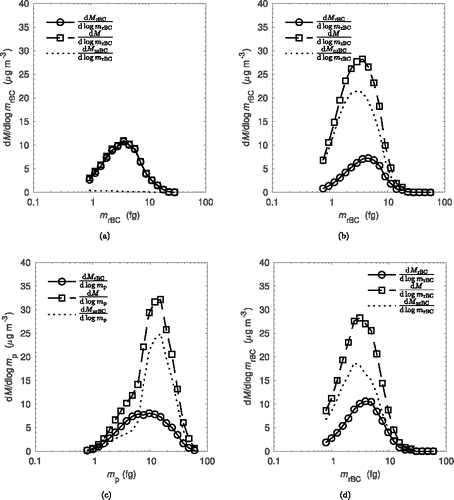
Finally, as seen in , for the case with fresh rBC particles added, the two populations of coated and lightly coated particles could be resolved when plotting mass concentration distribution versus total particle mass. In this figure, the distribution is negatively skewed, with a distinct peak at approximately 11 fg of total particle mass. From previous interpretation of the two-variable distribution in , we know that the distribution consists of two distinct populations. Therefore, the negative skew results from a bimodal distribution with each distribution having a median relatively close together. The peak at 11 fg corresponds to the distribution of coated particles, and the negative skew is from the distribution of uncoated particles, with a median total particle mass less than 11 fg. Viewing the same population as a function of rBC particle mass in , the populations can no longer be clearly distinguished because the rBC mass concentration distribution between the coated and uncoated particles have approximately the same median.
4.4. Mass concentration as a function of time
An SP2 used alone can directly resolve the mass concentration of rBC particles. However, the SP2 cannot resolve the total mass concentration of particles containing rBC. The addition of the CPMA allows determination of total mass concentration of particles containing rBC. The total mass concentration and rBC mass concentration can be calculated through integrating the one-variable mass concentration distributions,[8] and
[9]
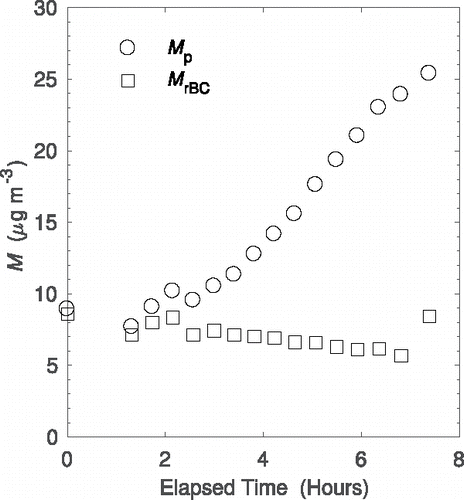
As seen in , plotting the total mass concentration and rBC mass concentration as a function of time for the smog chamber experiments shows the total mass concentration of particles is increasing. This is due to growth of organic coating due to photo-oxidation of p-xylene. The rBC mass concentration decreases as a function of time due to diffusion losses to the smog chamber walls. When fresh, uncoated rBC particles are added to the chamber for the final SP2-CPMA scan (i.e., the rightmost data point), the rBC mass concentration increases with an associated increase in total mass concentration as expected. It is expected that the total mass concentration is under-sampled due to the efficiency of the SP2 below masses of 1 fg. Therefore, the absolute values of mass concentration are not necessarily accurate, but the trends are useful to demonstrate the inversion method. In the smog chamber experiments, all particles contained rBC, and M is close to the actual total mass concentration. Used for ambient sampling, this method would return the total mass concentration of particles containing rBC, different from the total mass concentration of all particles.
5. Conclusion
A CPMA-SP2 inversion algorithm has been developed that calculates a two-variable distribution describing particle number concentrations as a function of rBC particle mass and total particle mass. The inversion method solves a Fredholm integral equation using a Twomey algorithm, resulting in a charge-corrected two-variable distribution. It was demonstrated that one-variable distribution functions, which are useful to demonstrate coating distribution properties, could be obtained simply from the two-variable distributions. The inversion was tested using smog chamber experiments where a significant coating was grown on bare rBC particles over a period of several hours via photo-oxidation of p-xylene. It was shown that in a mixed uncoated and coated aerosol the two populations could be clearly distinguished using a two-variable distribution. Similar to SMPS measurements, the CPMA-SP2 inversion makes assumptions about the charge state. Additional assumptions include assuming that efficiency correction can effectively account for errors in estimating the mass–mobility relationship and losses in the CPMA. From the two-variable distribution, one-variable mass and number distributions can be calculated through integration, which are a function of rBC or total particle mass. Total particle concentration and mass concentration can be obtained from double integration of the two-variable distribution. This method can be used for semi-continuous measurement of ambient rBC particles. As described in this article, a CPMA-SP2 scan takes approximately 20 min, so semi-continuous scanning coupled with this inversion could be used to derive the changes in rBC populations on atmospheric timescales. Because the two-variable distribution demonstrated in this article can easily identify different populations of particles (e.g., lightly or heavily coated), it could provide new insights for climate scientists.
UAST_1433812_Supplementary_File.zip
Download Zip (558.9 KB)References
- Bond, T. C., Doherty, S. J., Fahey, D. W., Forster, P. M., Berntsen, T., DeAngelo, B. J., Flanner, M. G., Ghan, S., Kärcher, B., Koch, D., Kinne, S., Kondo, Y., Quinn, P. K., Sarofim, M. C., Schultz, M. G., Schulz, M., Venkataraman, C., Zhang, H., Zhang, S., Bellouin, N., Guttikunda, S. K., Hopke, P. K., Jacobson, M. Z., Kaiser, J. W., Klimont, Z., Lohmann, U., Schwarz, J. P., Shindell, D., Storelvmo, T., Warren, S. G., and Zender, C. S. (2013). Bounding the Role of Black Carbon in the Climate System: A Scientific Assessment. J. Geophys. Res. Atmos., 118(11):5380–5552.
- Buckley, D. T., Kimoto, S., Lee, M.-H., Fukushima, N., and Hogan Jr, C. J. (2017). A Corrected Two Dimensional Data Inversion Routine for Tandem Mobility-Mass Measurements. J. Aerosol Sci., 114:157–168.
- Collins, D. R., Flagan, R. C., and Seinfeld, J. H. (2002). Improved Inversion of Scanning DMA Data. Aerosol Sci. Technol., 36(1):1–9.
- Dickau, M., Olfert, J., Stettler, M. E. J., Boies, A., Momenimovahed, A., Thomson, K., Smallwood, G., and Johnson, M. (2016). Methodology for Quantifying the Volatile Mixing State of an Aerosol. Aerosol Sci. Technol., 50(8):759–772.
- Gao, R. S., Schwarz, J. P., Kelly, K. K., Fahey, D. W., Watts, L. A., Thompson, T. L., Spackman, J. R., Slowik, J. G., Cross, E. S., Han, J.-H., Davidovits, P., Onasch, T. B., and Worsnop, D. R. (2007). A Novel Method for Estimating Light-Scattering Properties of Soot Aerosols Using a Modified Single-Particle Soot Photometer. Aerosol Sci. Technol., 41(2):125–135.
- Ghazi, R., and Olfert, J. S. (2013). Coating Mass Dependence of Soot Aggregate Restructuring Due to Coatings of Oleic Acid and Dioctyl Sebacate. Aerosol Sci. Technol., 47(2):192–200.
- Gopalakrishnan, R., Meredith, M. J., Larriba-Andaluz, C., and Hogan, C. J. (2013). Brownian Dynamics Determination of the Bipolar Steady State Charge Distribution on Spheres and Non-Spheres in the Transition Regime. J. Aerosol Sci., 63:126–145.
- Hagen, D. E., and Alofs, D. J. (1983). Linear Inversion Method to Obtain Aerosol Size Distributions from Measurements with a Differential Mobility Analyzer. Aerosol Sci. Technol., 2(4):465–475.
- Kulkarni, P., Baron, P. A., and Willeke, K. (2011). Aerosol Measurement: Principles, Techniques, and Applications. John Wiley & Sons, Hoboken, New Jersey, United States.
- Laborde, M., Schnaiter, M., Linke, C., Saathoff, H., Naumann, K.-H., Möhler, O., Berlenz, S., Wagner, U., Taylor, J. W., Liu, D., Flynn, M., Allan, J. D., Coe, H., Heimerl, K., Dahlkötter, F., Weinzierl, B., Wollny, A. G., Zanatta, M., Cozic, J., Laj, P., Hitzenberger, R., Schwarz, J. P., and Gysel, M. (2012). Single Particle Soot Photometer Intercomparison at the Aida Chamber. Atmos. Meas. Tech., 5(12):3077–3097.
- Liu, D., Whitehead, J., Alfarra, M. R., Reyes-Villegas, E., Spracklen, D. V., Reddington, C. L., Kong, S., Williams, P. I., Ting, Y.-C., Haslett, S., Taylor, J. W., Flynn, M. J., Morgan, W. T., McFiggans, G., Coe, H., and Allan, J. D. (2017). Black-Carbon Absorption Enhancement in the Atmosphere Determined by Particle Mixing State. Nature Geosci., 10(3):184–188.
- López-Yglesias, X., and Flagan, R. C. (2013). Ion–Aerosol Flux Coefficients and the Steady-State Charge Distribution of Aerosols in a Bipolar Ion Environment. Aerosol Sci. Technol., 47(6):688–704.
- Maißer, A., Thomas, J. M., Larriba-Andaluz, C., He, S., and Hogan, C. J. (2015). The Mass–Mobility Distributions of Ions Produced by a po-210 Source in Air. J. Aerosol Sci., 90:36–50.
- Maricq, M. M. (2007). Chemical Characterization of Particulate Emissions from Diesel Engines: A Review. J. Aerosol Sci., 38(11):1079–1118.
- Markowski, G. R. (1987). Improving Twomey’s Algorithm for Inversion of Aerosol Measurement Data. Aerosol Sci. Technol., 7(2):127–141.
- McMeeking, G. R., Hamburger, T., Liu, D., Flynn, M., Morgan, W. T., Northway, M., Highwood, E. J., Krejci, R., Allan, J. D., Minikin, A., and Coe, H. (2010). Black Carbon Measurements in the Boundary Layer Over Western and Northern Europe. Atmos. Chem. Phys., 10(19):9393–9414.
- McMeeking, G. R., Morgan, W. T., Flynn, M., Highwood, E. J., Turnbull, K., Haywood, J., and Coe, H. (2011). Black Carbon Aerosol Mixing State, Organic Aerosols and Aerosol Optical Properties Over the United Kingdom. Atmos. Chem. Phys., 11(17):9037–9052.
- Menon, S., Hansen, J., Nazarenko, L., and Luo, Y. (2002). Climate Effects of Black Carbon Aerosols in China and India. Science, 297(5590):2250–2253.
- Momenimovahed, A., and Olfert, J. (2015). Effective Density and Volatility of Particles Emitted from Gasoline Direct Injection Vehicles and Implications for Particle Mass Measurement. Aerosol Sci. Technol., 49(11):1051–1062.
- Moosmüller, H., Chakrabarty, R., and Arnott, W. (2009). Aerosol Light Absorption and Its Measurement: A Review. J. Quantitat. Spectr. Radiat. Trans., 110(11):844–878.
- Moteki, N., and Kondo, Y. (2007). Effects of Mixing State on Black Carbon Measurements by Laser-Induced Incandescence. Aerosol Sci. Technol., 41(4):398–417.
- Moteki, N., and Kondo, Y. (2010). Dependence of Laser-Induced Incandescence on Physical Properties of Black Carbon Aerosols: Measurements and Theoretical Interpretation. Aerosol Sci. Technol., 44(8):663–675.
- Olfert, J., and Collings, N. (2005). New Method for Particle Mass Classification–The Couette Centrifugal Particle Mass Analyzer. J. Aerosol Sci., 36(11):1338–1352.
- Parsons, M. T., Sydoryk, I., Lim, A., McIntyre, T. J., Tulip, J., Jäger, W., and McDonald, K. (2011). Real-Time Monitoring of Benzene, Toluene, and p-Xylene in a Photoreaction Chamber with a Tunable mid-Infrared Laser and Ultraviolet Differential Optical Absorption Spectroscopy. Appl. Opt., 50(4):A90–A99.
- Ramanathan, V., and Carmichael, G. (2008). Global and Regional Climate Changes Due to Black Carbon. Nature Geosci., 1(4):221–227.
- Rawat, V. K., Buckley, D. T., Kimoto, S., Lee, M.-H., Fukushima, N., and Jr, C. J. H. (2016). Two Dimensional Size–mass Distribution Function Inversion from Differential Mobility Analyzer–Aerosol Particle Mass Analyzer (DMA–APM) Measurements. J. Aerosol Sci., 92:70–82.
- Riemer, N., Vogel, H., and Vogel, B. (2004). Soot Aging Time Scales in Polluted Regions During Day and Night. Atmos. Chem. Phys., 4(7):1885–1893.
- Ristimäki, J., Vaaraslahti, K., Lappi, M., and Keskinen, J. (2007). Hydrocarbon condensation in heavy-duty diesel exhaust. Environ. Sci. Technol., 41(18):6397–6402.
- Ruiz, P. A., Lawrence, J. E., Ferguson, S. T., Wolfson, J. M., and Koutrakis, P. (2006). A Counter-Current Parallel-Plate Membrane Denuder for the Non-Specific Removal of Trace Gases. Environ. Sci. Technol., 40(16):5058–5063.
- Schnaiter, M., Linke, C., Möhler, O., Naumann, K.-H., Saathoff, H., Wagner, R., Schurath, U., and Wehner, B. (2005). Absorption Amplification of Black Carbon Internally Mixed with Secondary Organic Aerosol. J. Geophys. Res. Atmos., 110(D19):n/a–n/a.
- Schwarz, J. P., Spackman, J. R., Fahey, D. W., Gao, R. S., Lohmann, U., Stier, P., Watts, L. A., Thomson, D. S., Lack, D. A., Pfister, L., Mahoney, M. J., Baumgardner, D., Wilson, J. C., and Reeves, J. M. (2008). Coatings and Their Enhancement of Black Carbon Light Absorption in the Tropical Atmosphere. J. Geophys. Res. Atmos., 113(D3):n/a–n/a.
- Sedlacek, A. J., Lewis, E. R., Kleinman, L., Xu, J., and Zhang, Q. (2012). Determination of and Evidence for Non-Core-Shell Structure of Particles Containing Black Carbon Using the Single-Particle Soot Photometer (sp2). Geophys. Res. Lett., 39(6):n/a–n/a.
- Shiraiwa, M., Kondo, Y., Moteki, N., Takegawa, N., Miyazaki, Y., and Blake, D. R. (2007). Evolution of Mixing State of Black Carbon in Polluted Air from Tokyo. Geophys. Res. Lett., 34(16):n/a–n/a.
- Slowik, J. G., Cross, E. S., Han, J.-H., Davidovits, P., Onasch, T. B., Jayne, J. T., Williams, L. R., Canagaratna, M. R., Worsnop, D. R., Chakrabarty, R. K., Moosmüller, H., Arnott, W. P., Schwarz, J. P., Gao, R.-S., Fahey, D. W., Kok, G. L., and Petzold, A. (2007). An Inter-Comparison of Instruments Measuring Black Carbon Content of Soot Particles. Aerosol Sci. Technol., 41(3):295–314.
- Stephens, M., Turner, N., and Sandberg, J. (2003). Particle Identification by Laser-Induced Incandescence in a Solid-State Laser Cavity. Appl. Opt., 42(19):3726–3736.
- Stolzenburg, M. R., and McMurry, P. H. (2008). Equations Governing Single and Tandem DMA Configurations and a New Lognormal Approximation to the Transfer Function. Aerosol Sci. Technol., 42(6):421–432.
- Twomey, S. (1975). Comparison of Constrained Linear Inversion and an Iterative Nonlinear Algorithm Applied to the Indirect Estimation of Particle Size Distributions. J. Comput. Phys., 18(2):188–200.
- Wang, S. C., and Flagan, R. C. (1990). Scanning Electrical Mobility Spectrometer. Aerosol Sci. Technol., 13(2):230–240.
- Wiedensohler, A. (1988). An Approximation of the Bipolar Charge Distribution for Particles in the Submicron Size Range. J. Aerosol Sci., 19(3):387–389.