 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Along with greenhouse gases, worldwide biomass-burning events add substantial amounts of a complex optical system consisting of elemental carbon and organic particulate matter to the atmosphere. These particulate matters significantly affect the Earth’s radiation balance as well as perturb the precipitation pattern. Considerable attention has been given to the impact of physicochemical transformation or aging on the optical properties of aqueous solution (a homogeneous medium) of light-absorbing fractions of the organic particulate matter. However, their heterogeneous photochemical aging remains largely unexplored. This experimental study explores heterogeneous absorbance photo-bleaching kinetics of the water-soluble organic-carbon (WSOC) intrinsic to from rice straw smoldering smoke. In the wavelength region of 280–400 nm, the absorption ability decreased consistently with aging while a characteristic initial increase of absorption was observed in the 400–500 nm intervals, which again decreased with further aging. The dynamic light absorptivity of the WSOC may not be related to the corresponding mass concentrations. The relative quantum efficiency of the heterogeneous aging of these WSOC was estimated to be (0.01‒0.3) %. The kinetic results suggest that their half-life could be nearly a week under ambient solar radiation.
Copyright © 2019 American Association for Aerosol Research
EDITOR:
1. Introduction
The black carbon (BC) fraction of the atmospheric carbonaceous aerosol was thought to be the sole absorber of solar radiation, whereas the organic carbon (OC) fraction being responsible for the scattering of sunlight (Bond et al. Citation2013; Jacobson Citation2001; Menon et al. Citation2002). Although, BC is the major absorber across the entire solar spectrum, it is now well known that a considerable OC fraction substantially absorbs in the lower visible to the ultraviolet region (Bahadur et al. Citation2012; Feng et al. Citation2013). Their contribution was estimated up to , which is around 19% of the global climate forcing (Feng et al. Citation2013). Moreover, a striking observation of reduced surface UVB actinic flux in the Amazon basin was attributed to the combined absorptive effects of OCs and BC resulting in the slower rate of ROX formation and consequently longer lifetimes of
and its precursors (Mok et al. Citation2016). Compositionally atmospheric OC is a complex mixture of several thousands of organic compounds. Their complexity arises due to the unknown number and nature of organics yielded by various anthropogenic and biogenic sources as well as continuous (photo)chemical transformations or “aging,” yielding new products (Laskin, Laskin, and Nizkorodov Citation2015). Biomass burning is a prominent source of atmospheric OC (Di Lorenzo et al. Citation2017; Jacobson Citation2014) and very frequent in the south and southeast Asian countries (Taylor Citation2010). Consequent impacts on regional lower atmospheric warming trends, affecting the Asian monsoon and accelerated melting of the Himalayan glacier are not unexpected (Huang et al. Citation2013; Zhang, Liao, and Li Citation2010).
Nevertheless, research on climate response of the light-absorbing OC is only in its early stage. In fact, dynamic response to photochemical aging due to varying chemical compositions of OC introduces uncertainties in estimating its contribution to the radiative forcing (Laskin, Laskin, and Nizkorodov Citation2015; Laskin et al. Citation2014; Saleh et al. Citation2013; Zhong and Jang Citation2014). Earlier studies have addressed the photochemical aging of laboratory-generated proxies of OC (Chan et al. Citation2014; Lee et al. Citation2014; Liu et al. Citation2016; Sareen, Moussa, and McNeill Citation2013), OC in ambient measurements (Forrister et al. Citation2015; Lin et al. Citation2016) as well as primary OC from wood smoke emissions (Wong, Nenes, and Weber Citation2017), rice, wheat, and corn straw burning (Fang et al. Citation2017) and very recently from the smoldering smoke of ponderosa pine needle litters (Browne et al. Citation2019). Prior studies have described some interesting findings like photo-enhancement (enhanced light absorption) followed by photo-bleaching (reduced light absorption) of light-absorbing OC during the aging process (Zhong and Jang Citation2014) as well as greater climate response of larger chromophores due to their persistence over the smaller chromophores (Liu et al. Citation2014). However, the mechanisms leading to these observations remain speculative.
Unlike commonly used proxy organic compounds and/or ambient samples that are already aged up to an unknown degree (Ervens, Turpin, and Weber Citation2011; Sumlin et al. Citation2017), we studied the aging of water-soluble organic-carbon (WSOC), freshly generated by smoldering of rice-straw in the laboratory. The atmospheric organics majorly undergo homogeneous oxidation in gas or aqueous phase as well as heterogeneous oxidation at the gas-particle interface by means of atmospheric gaseous oxidants, such as ozone or OH radicals (Ervens, Turpin, and Weber Citation2011; Kroll et al. Citation2015). Heterogeneous oxidation may generate highly oxygenated species (Smith et al. Citation2009) and/or more volatile species through fragmentation (Slade and Knopf Citation2013), which could alter the aerosol properties like size, density, thermal, and optical properties as well as hygroscopicity (Cappa et al. Citation2011; Emanuelsson et al. Citation2014; Kroll et al. Citation2015; Li, Smith, and Cappa Citation2018). Recently, OH-initiated oxidation of squalane coated onto ammonium sulfate particles was found to be faster by an order of magnitude than that of the pure compound (Lim et al. Citation2017). The objective of this laboratory-based work was to explore the influence of photochemical aging on the kinetics of light absorption by WSOC as well as to assess the efficiency of heterogeneous photoreactions. The atmospheric implications of those results have been discussed in this article.
2. Experimental Section
2.1. Rice-straw smoldering & PM2.5 sampling
Rice straw was chosen as the biomass fuel source of the OC because it is cheap and easily available; smoldering of rice-straw is easy and most importantly, in recent years postharvest crop residue burning (Fang et al. Citation2017), mainly in Asia and South America, has become a serious environmental issue. For example, favored by high relative humidity, large scale crop residue burning in the Indo-Gangetic Plain from December to March is believed to create heavy fog and haze which has serious effects on poor visibility and public health (Shamjad et al. Citation2015).
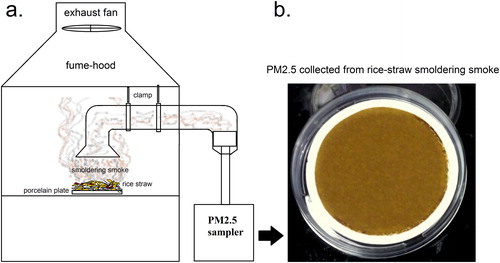
Considering higher implications due to a longer lifetime of fine mode PM (aerodynamic diameter ≤2.5 µm) in ambient air as well as accessibility as far as to the bloodstream (Xing et al. Citation2016), we were to study the photochemical transformations of WSOC intrinsic to . shows the experimental setup for collecting
from rice straw smoldering smoke. Approximately 0.2 kg rice straw (moisture content 9.83
0.61%) cut in small pieces was smoldered (smoldering rate ∼0.5
) on a porcelain plate inside a fume-hood keeping the exhaust fan turned off. The aerosols were collected on pre-baked Whatman quartz filters (QMA, 47 mm diameter) inside a fine dust sampler (Environtech APM 550, flow rate 1
) placed close to the fume-hood. One end of an aluminum pipe was tied with the sampling head of the sampler (to avoid indoor aerosol interference) and another end of the pipe was inserted into the fume-hood. Before each smoldering event, “control” sampling was performed to check the background interference which showed a collection of <0.01 mg after 1 h. To avoid interference of existing particulate matter from the preceding sampling, one sampling event was performed in one day and an exhaust fan of the fume-hood was kept turned on overnight.
Figure 1. Schematic diagram of the (a) setup for collecting from rice-straw smoldering smoke inside a fume-hood and (b) collected
on 47 mm QMA filter paper. The detail of the setup is given in subsection 2.1.
2.2. Irradiation of PM2.5
A detailed description of the irradiation setup was demonstrated in our recently published work (Ray, Ghosh, and Raha Citation2019). In brief, each filter paper was divided into eight sectors using a custom-built cutter. To minimize the potential artifact of inhomogeneity in the aerosol generation, we kept the smoldering conditions almost the same. We also performed a pilot experiment to check the homogeneity of the aerosol in each sector in terms of the mass absorption coefficients (OC mass normalized absorbance) of the corresponding aqueous extracts. The variation of absorbance was <5%. Each sector with fresh OC was kept inside a standard quartz cuvette (1 cm path length and ∼3.5 mL volume) and its mouth was closed with a rubber septum. This cuvette was placed inside a custom-built holder that was fixed on an optical bench. The fresh OC on the filter was then irradiated through a rectangular (area = 2 cm × 1 cm) opening on one side of the holder for different time intervals by lensing the light from an Osram 300 W ULTRA-VITALUX® lamp. At the same time, high purity synthetic air (99.999%, Bengal Gases, India) was flown through the cuvette at the flow rate of 2 . Moreover, insignificant change (<1% after 24 h) in OC mass concentrations were estimated after repeating the irradiation experiments by wrapping the cuvette with aluminum foil. At the same time, ∼9% change was estimated in the corresponding mass absorption coefficient at a representative wavelength of 365 nm (Figure S1). However, this “blank” experiment was performed only to get an idea of the heating effect and we have reported the experimental data without any modification. Furthermore, the photon flux of the lamp (
) was estimated as
using 2-nitrobenzaldehyde (2NB) as the chemical actinometer (Galbavy, Ram, and Anastasio Citation2010) (see the online supplementary infromation [SI]).
2.3. Extraction of water-soluble organic carbon
We explored the water-soluble fraction because of its considerable presence in the ambient atmosphere. For example, water-soluble fraction of the total OC aerosol is commonly 50–80% over South Asia (Dasari et al. Citation2019). Nevertheless, each of the eight filter paper sector (fresh and irradiated) was put inside a 10 mL precleaned test tube. The WSOC was extracted first with 5 mL milliQ water for 1 h in a sonic bath. The temperature of water in the bath was kept constant at 18 °C. The resulting solution was filtered through 0.22 µm PTFE filters. These steps were repeated two more times with another 10 mL milliQ water. The total extracted volume which was ∼15 mL, carefully reduced to ∼2 mL by lyophilization. This ∼2 mL solution was then transferred to a 5 mL volumetric flask and the volume was made up to the mark. This solution was then wrapped with aluminum foil and kept at −4 °C until analysis.
2.4. Determination of OC mass concentration (
 )
)
The Walkley–Black method has been extensively used to determine soil organic carbon (Bahadori and Tofighi Citation2016; Lettens et al. Citation2007; Mikhailova, Noble, and Post Citation2003). Instead of the classical titrimetric method (Walkley and Black Citation1934), we spectrophotometrically determined the OC mass concentration. First, 2 mL of the freshly prepared 0.8 N solution was added into 1 mL WSOC solution. Then, 1 mL 98%
was slowly added into the test tube and the solution was left overnight for maximum digestion to occur. We preferred not to heat the solution to avoid any loss of acidic
. In other words,
was reduced by OC (EquationEquation (1)
(1)
(1) ) so that visually we observe a color change from bright orange to the bluish-green of
(Bahadori and Tofighi Citation2016).
(1)
(1)
The absorbance of the remaining ions in solution were measured by a Multiscan GO UV Vis spectrophotometer and the corresponding OC mass concentration (
) was evaluated from a standard curve. The absorbance at 350 nm versus
(
) plot was prepared by varying the concentrations of anhydrous dextrose as described in Figure S3. The inset of Figure S3 shows the spectra of the remaining
after reduction with different anhydrous dextrose concentrations in terms of
. The dotted black line is the spectrum of a typical OC solution of “unknown” concentration. Calculation of this “unknown” concentration is given in Figure S3 using the corresponding absorbance value from the inset.
2.5. Measurement of absorbance of the WSOC solutions
Absorbance (A) of each aqueous WSOC solution inside a standard 1 cm quartz cuvette was scanned from 280 to 700 nm by using a Multiscan GO UV Vis spectrophotometer. The baseline was obtained by using MilliQ water as a blank reference. The instrument detection limit was A = 0.03. Dilution tests ranging from 10:1 to 2:1 confirmed that the Beer–Lambert law was valid for these samples in the range A < 3.2.
2.6. FT-IR measurements
Vibrational spectra were recorded on a single-beam Perkin Elmer Spectrum 100 Fourier-transform infrared (FT-IR) spectrometer in the spectral range of 1000–4000 . For FT-IR measurements, a film of the dried organic carbons (OC) was deposited onto the surface of 3 mm thick zinc-selenide (ZnSe) optical windows (Cradley Crystals Corp.). To make the OC film, we first dried of water from aqueous BrC solutions by careful lyophilization. Then, few drops of methanol were added to the dried OC to transfer it onto the optical window. Methanol was then dried off with a gentle flow of nitrogen gas.
3. Results and discussion
3.1. Mass absorption coefficients of fresh & aged WSOC
Absorptive characteristics of the WSOC in water extracts of fresh and irradiated filter paper sectors were measured by UV–visible spectroscopy. The base-10 absorbance () of these aqueous solutions along 0.01 m path length (l) of a standard quartz cuvette, were normalized by corresponding WSOC concentrations (in units of
, vide supra) to derive a wavelength-dependent parameter, mass absorption coefficient (MAC(λ), in units of
) using EquationEquation (2)
(2)
(2) (Lee et al. Citation2014):
(2)
(2)
Similar to prior studies (Chen and Bond Citation2010; Hecobian et al. Citation2010), the MAC(λ) gradually declined with increasing wavelengths as shown in . A power law (EquationEquation (3)(3)
(3) ) can describe the wavelength-dependent decrease of MAC:
(3)
(3)
where AAE (absorption Ångström exponent) quantifies the spectral dependence of light absorption by the WSOC solutions. To define AAE, the EquationEquation (3)
(3)
(3) can be written as,
(4)
(4)
Figure 2. Changes in light absorptivity of water-soluble organic-carbon (WSOC) from rice straw smoldering due to heterogeneous photochemical aging. (a) Absorption spectra of fresh and irradiated WSOC. Wavelength dependent mass absorption coefficients (MAC) are plotted along the y-axis and corresponding wavelengths are plotted along the x-axis. The inset summarizes the absorption Ångström exponents (AAE) and OC mass concentrations () of the fresh and each of the irradiated samples. The AAE values were calculated by using EquationEquation (4)
(4)
(4) . (b) Linear fits of lnMAC(λ) in the 280–400 nm and 400–500 nm intervals and at 365 nm are plotted against corresponding irradiation times to determine the photo-bleaching rate constant. Error bars represent standard deviations of three individual experimental values.
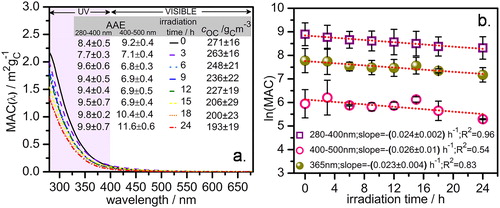
The AAE values were determined for = 280 nm and
= 400 nm as well as for
= 400 nm and
= 500 nm using the corresponding MAC values. The calculated AAE values for the fresh and irradiated samples are summarized inside . The AAE values of the fresh samples are typical for WSOC and comparable to biomass burning samples from other studies, for example, Yu et al. (Citation2017). In addition, given that, AAE of pure black carbon is unity (Kirchstetter, Novakov, and Hobbs Citation2004), the effective light absorption by the fresh samples was greater in the 280–400 nm interval relative to that in the 400–500 nm interval. Relative to their initial values, the lower values of both
and
shows a temporary absorbance photo-enhancement. Also, the absorbance photo-bleaching predominated in the 280–400 nm intervals after the first 3 h of irradiation, whereas this phenomenon occurred in the 400–500 nm intervals after 15 h of irradiation. This observation is in quantitative agreement with several earlier studies reporting initial photo-enhancement followed by photo-bleaching (Wong, Nenes, and Weber Citation2017; Zhong and Jang Citation2014). Absorbance photo-enhancement may occur if reaction products are stronger light absorbers and/or mass concentrations of organic carbon (
) are reduced due to the escape of volatile reaction products from the reactor. However, the former seems to be more crucial in this case because the
values declined consistently with irradiation time (inside . Indeed, despite ∼30% reduction in
after 24 h of irradiation, the corresponding AAE values show absorbance photo-bleaching. Lin et al. (Citation2016) recently reported that a limited number of strong chromophores with complex and varying compositions are responsible for the major fraction of the light absorption. Mainly the π–π* and n–π* electronic transitions in the organic chromophores lead to their light absorption in the UV and near-visible regime. Similar to Wong, Nenes, and Weber (Citation2017), our experimental data in are also indicative of volatile photolysis product formation, which might be evaporating to the gas phase. At the same time, the remaining fraction on the filter paper might contain stronger light absorbers in the visible region. Earlier studies reported that majorly aromatics with multiple hydroxyl, carbonyls, and nitro groups as well as possible charge transfer complexes formed due to intermolecular interactions are responsible for the light absorption by biomass burning aerosols (Chang and Thompson Citation2010; Duarte, Freire, and Duarte Citation2015; Phillips and Smith Citation2014). In fact, the aromatic carbonyls, as well as in-situ produced N-derivatized imidazoles, may act as photosensitizers to produce light-absorbing secondary organics (Fu et al. Citation2015; Rossignol et al. Citation2014). Such photosensitizers can also be the indirect photochemical radical source (e.g., singlet oxygen 1O2) to cause aerosol aging (Fu et al. Citation2015; Glover and Rosario-Ortiz Citation2013).
3.2. Observed photo-bleaching rate
The average MAC values in the 280–400 nm and 400–500 nm intervals were fit in EquationEquation (5)(5)
(5) against corresponding irradiation times to evaluate their decay or photo-bleaching kinetics.
(5)
(5)
where
and
are the MAC values of fresh and aged solutions. Since the number and nature of WSOC components are mostly unknown, the
represents an effective or observed absorbance-decay or photo-bleaching rate constant. The absorbance photo-bleaching is reasonably demonstrated by first-order kinetics given the linearity of the lnMAC(λ) vs irradiation time plots fit with an uncertainty weighted-least-squares fit (. The
value for the 400–500 nm interval must be taken with caution because absorbance values above ∼490 nm fell below the instrumental detection limit. Also, it is noteworthy that the experimental
cannot directly be compared with that measured in the atmosphere, for example, (Dasari et al. Citation2019) reported an ambient first-order absorbance photo-bleaching rate of ∼0.008
during over-ocean transit of light-absorbing organic aerosols. Therefore, we converted the experimental
≈ 0.02
at 365 nm to an equivalent ambient value of ∼0.005
(see SI), which is in excellent agreement with the rate constant value reported by Dasari et al.
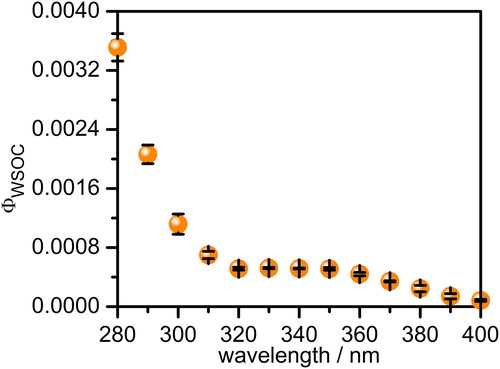
3.3. Relative quantum efficiency
To assess the efficiency of the heterogeneous photo-reactions, the relative quantum efficiency of WSOC phototransformations () was estimated based on their light absorption and consequent spectral changes. The
was determined by using EquationEquation (6)
(6)
(6) (Schwarzenbach, Gschwend, and Imboden Citation2003):
(6)
(6)
where
is the quantum efficiency of a reference compound, 2-nitrobenzaldehyde (2NB), which is a convenient, photochemically sensitive, and thermally robust actinometer. Galbavy, Ram, and Anastasio recommended the value of
for the wavelength interval of 280–405 nm to be 0.42 (2010);
and
are the absorbance decay rate of WSOC and 2NB, respectively;
and
are the molar absorption coefficients of WSOC and 2NB, respectively. The wavelength dependence of
in the 280–400 nm interval is shown in . The wavelength-dependent values of
,
,
, and
were determined experimentally and shown in Figure S4. The
decreases consistently with increasing wavelength, however, 280–320 nm interval showed a much faster decrease relative to that of the 320–400 nm region. The
values reveal that (0.01–0.3)% of the incident light (280–400 nm) was responsible for phototransformations of the present kind of WSOC under our experimental conditions.
Figure 3. Wavelength dependent variation of the relative quantum efficiency (, in the y-axis) of the water-soluble organics in
from rice-straw smoldering smoke. The
values were calculated using EquationEquation (6)
(6)
(6) and data from Figure S4. The error bars represent standard deviations calculated from three individual experiments.
3.4. Imaginary refractive index (κ)
Recently, Moosmüller and Sorensen showed that aerosol single scattering albedo (SSA), which is the measure of aerosol “whiteness” is an important parameter in determining the radiative forcing (Moosmüller and Sorensen Citation2018). The SSA of homogeneous and spherical aerosols can be determined using Mie theory (Mie Citation1908) calculations as SSA depends on particle size as well as its complex refractive index, , where n and κ are the real and imaginary parts of the refractive index, respectively. In fact, κ is the manifestation of light absorption by aerosols and intrinsic organics whereas n is responsible for scattering (Chakrabarty et al. Citation2016). Unfortunately, experimental determination of SSA, for example, using the measurements of scattering coefficients (Sumlin et al. Citation2017), was beyond the scope of this work. However, we calculated the wavelength-dependent values of κ using EquationEquation (7)
(7)
(7) (Bohren and Huffman Citation1998),
(7)
(7)
where
is the density of WSOC solution for which we used the value as 1.1
(Schkolnik et al. Citation2007),
is the wavelength in meter, and MAC(λ) is the mass absorption coefficient in
. In agreement with the wavelength dependence of MAC(λ), shows that κ is declining with increasing wavelengths, which is a typical observation for organic carbons reported by earlier works (Kirchstetter, Novakov, and Hobbs Citation2004; Liu, Zhang, and Martin Citation2013; Liu et al. Citation2015). The κ of black carbon was reported to be independent of wavelength (Kirchstetter, Novakov, and Hobbs Citation2004). In prior studies, similar κ values were reported for fulvic acids (Liu et al. Citation2015), boreal forest peat smoldering (Sumlin et al. Citation2017), and water extracts of wood and Florida peat burning (Sengupta et al. Citation2018). In contrast, around three times higher κ values were reported for wood and savanna burning aerosols (Kirchstetter, Novakov, and Hobbs Citation2004), while more than 1 order of magnitude lower values of κ were reported for urban aerosols (Cappa et al. Citation2012).
Figure 4. (a) Plot of the imaginary part of refractive index (κ) along the y-axis against corresponding wavelengths along the x-axis. The downward arrows indicate the representative wavelengths of which the κ values are plotted in the inset figure; Inset: Decay of κ (in log scale) with irradiation time. Error bars represent standard deviations of three individual experimental values. (b) FTIR spectra of fresh and aged water-soluble organic-carbon.
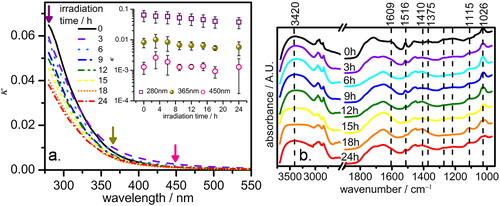
In general, the κ of the fresh WSOC samples () is greater than the aged samples below ∼450 nm except those aged for 3 h (
) and 6 h (
). Both
and
started to override the
from ∼350 nm. This observation is illustrated in the inset of , showing the influence of photochemical aging on κ at three representative wavelengths such as 280 nm, 365 nm, and 450 nm. The κ values consistently declined with irradiation time at 280 nm but a temporary photo-enhancement was observed after 3 h at 365 nm, which is more prominent at 450 nm. However, photo-bleaching becomes typical at both 365 nm and 450 nm as the samples underwent further aging after 3 h irradiation. Similar observations of
of toluene or m-xylene reacted with
overrode the
values in the UV region were reported by Liu et al. (Citation2015). The authors argued for the formation of nitroaromatics that are majorly strong UV absorbers. In addition, Sengupta et al. (Citation2018), recently reported smaller κ values for aged biomass burning organic aerosols relative to that of fresh samples between 400 and 550 nm wavelength regions.
3.5. Functional group analysis by FTIR spectroscopy
The general characteristics as well as discriminating features in terms of characteristic IR absorption by different molecular structures having different stretching and bending vibrations (Fu et al. Citation2015) of the fresh and aged WSOC are qualitatively compared in . We assigned the IR absorbance peaks to corresponding functional groups by comparing with previously recorded reference spectra (Lin-Vien et al. Citation1991). The strong band centered at around 3420 can be attributed to O–H stretching of alcohols. This broadened band in the aged samples suggests the formation of COOH groups during photo-oxidation. Also, the gradually enhancing peak at 1410
(due to H–OCO stretching) suggests the formation of carboxylic acid in the aged samples. Recently, Hems and Abbatt (Citation2018), showed the formation of smaller organic acids as the products of nitrophenol (significantly present in biomass burning aerosols) photo-oxidation. Although, the carboxyl group absorbs only at wavelengths well below 250 nm, however, the temporary photo-enhancement in the 400–500 nm interval () can be attributed to the enhanced electron drift from electron-withdrawing groups, for example –OH to electron-donating groups like –COOH through π-conjugation of aromatic rings leading to a red-shift (Klan and Wirz Citation2009). However, the reduced broadness of this peak in
samples can be attributed to the photolytic decarboxylation of the COOH, which is in agreement with earlier observations (Chiu et al. Citation2017; Takahashi et al. Citation2008). The photo-enhancement, especially in the 400–500 nm intervals ( and ) may also occur through the formation of phenoxy radicals, which undergo oxidative polymerization generating colored coupling derivatives (Gelencsér et al. Citation2003). Indeed, the absorption band at 1375
, which can be attributed to phenolic O–H stretching, decreased with irradiation time. Photodeconjugation reactions occur in α,β-unsaturated esters or ketones (Piva Citation2004) which have been significantly found in biomass burning aerosols (Graham et al. Citation2002). The photoproducts do not absorb in the visible wavelengths as the C=C bond is no longer in the conjugation with C=O leading to photo-bleaching. The IR absorption band at 1609
can be attributed to C=C stretching of α,β-unsaturated esters or ketones which shows a gradual decrease with prolonged irradiation. In our previous work, using similar experimental setup we showed 10–15% and 40–70% degradation of the 5–6-ring and 3–4-ring polycyclic aromatic hydrocarbons (PAHs) (Ray, Ghosh, and Raha Citation2019). Indeed, the aromatic rings are indicated by their stretching vibration bands around 1516
, which decreased with irradiation time. In addition to the band at 1516
, the band at 1115
can be due to aromatic C-O stretching are suggestive of the presence of lignin (a typical biomass burning marker)-like structures (Bui et al. Citation2015). The intense peak around 1026
due to the C–O bond stretching of polysaccharides (Fan et al. Citation2016) gradually reduced with aging time.
4. Conclusions
Substantial impacts of heterogeneous photochemistry on the optical properties of water-soluble biomass smoldering organic carbons were the major findings of this work. Implications of such findings can be found in atmospheric bodies like fog and cloud water, where the considerable presence of WSOC from agricultural burning has been reported earlier (Collett et al. Citation1999; Desyaterik et al. Citation2013). Half-life () of the WSOC from this work can be estimated using the experimentally derived observed absorbance photo-bleaching rate constant
0.02
. The estimated half-life of ∼35 h under our experimental conditions shows that absorbance photo-bleaching or reduction of the ability to absorb light of this WSOC occur on the time scale of days. One-hour irradiation under our lamp was estimated to be equivalent to approximately 4-h irradiation under the sun at 12:00 noon in Kolkata, India (22.5726° N, 88.3639° E, 6.6 amsl). Therefore, the half-life of our WSOC samples in ambient atmosphere can be approximately a week. In addition, the estimated relative quantum efficiency suggests that 0.01–0.3% of the incident light could be used for chemical transformations leading to absorbance photo-bleaching of the WSOC. This result further suggests that not all the solar energy absorbed by WSOC is transformed into heat leading to atmospheric warming but a fraction is transformed into chemical energy, thus, introducing uncertainties in radiative forcing calculation. Therefore, it is essential to carry out experiments for precise quantification of light-to-heat conversion efficiency of WSOC, which are currently undergoing in our laboratory.
Supplemental Material
Download MS Word (1.9 MB)Acknowledgments
The authors thank Mr. Dipak Chandra Konar for FTIR measurements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bahadori, M., and H. Tofighi. 2016. A modified Walkley–Black method based on spectrophotometric procedure. Commun. Soil Sci. Plan. 47 (2):213–220. doi: 10.1080/00103624.2015.1118118.
- Bahadur, R., P. S. Praveen, Y. Xu, and V. Ramanathan. 2012. Solar absorption by elemental and brown carbon determined from spectral observations. Proc. Natl. Acad. Sci. USA 109 (43):17366–17371. doi: 10.1073/pnas.1205910109.
- Bohren, C. F., and D. R. Huffman. 1998. Absorption and scattering of light by small particles. New York, NY: Wiley.
- Bond, T. C., S. J. Doherty, D. W. Fahey, P. M. Forster, T. Berntsen, B. J. DeAngelo, M. G. Flanner, S. Ghan, B. Kärcher, D. Koch, et al. 2013. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 118 (11):5380–5552. doi: 10.1002/jgrd.50171.
- Browne, E. C., X. Zhang, J. P. Franklin, K. J. Ridley, T. W. Kirchstetter, K. R. Wilson, C. D. Cappa, and J. H. Kroll. 2019. Effect of heterogeneous oxidative aging on light absorption by biomass burning organic aerosol. Aerosol Sci. Technol. 53 (6):663–674. doi: 10.1080/02786826.2019.1599321.
- Bui, N. Q., P. Fongarland, F. Rataboul, C. Dartiguelongue, N. Charon, C. Vallée, and N. Essayem. 2015. FTIR as a simple tool to quantify unconverted lignin from chars in biomass liquefaction process: Application to SC ethanol liquefaction of pine wood. Fuel Process. Technol. 134:378–386. doi: 10.1016/j.fuproc.2015.02.020..
- Cappa, C. D., D. L. Che, S. H. Kessler, J. H. Kroll, and K. R. Wilson. 2011. Variations in organic aerosol optical and hygroscopic properties upon heterogeneous OH oxidation. J. Geophys. Res. Atmos. 116:D15204. doi: 10.1029/2011jd015918.
- Cappa, C. D., T. B. Onasch, P. Massoli, D. R. Worsnop, T. S. Bates, E. S. Cross, P. Davidovits, J. Hakala, K. L. Hayden, B. T. Jobson, et al. 2012. Radiative absorption enhancements due to the mixing state of atmospheric black carbon. Science 337 (6098):1078–1081. doi: 10.1126/science.1223447.
- Chakrabarty, R. K., M. Gyawali, R. L. N. Yatavelli, A. Pandey, A. C. Watts, J. Knue, L. W. A. Chen, R. R. Pattison, A. Tsibart, V. Samburova, et al. 2016. Brown carbon aerosols from burning of boreal peatlands: Microphysical properties, emission factors, and implications for direct radiative forcing. Atmos. Chem. Phys. 16 (5):3033–3040. doi: 10.5194/acp-16-3033-2016.
- Chan, M. N., H. Zhang, A. H. Goldstein, and K. R. Wilson. 2014. Role of water and phase in the heterogeneous oxidation of solid and aqueous succinic acid aerosol by hydroxyl radicals. J. Phys. Chem. C 118 (50):28978–28992. doi: 10.1021/jp5012022.
- Chang, J. L., and J. E. Thompson. 2010. Characterization of colored products formed during irradiation of aqueous solutions containing H2O2 and phenolic compounds. Atmos. Environ. 44 (4):541–551. doi: 10.1016/j.atmosenv.2009.10.042.
- Chen, Y., and T. C. Bond. 2010. Light absorption by organic carbon from wood combustion. Atmos. Chem. Phys. 10 (4):1773–1783. doi: 10.5194/acp-10-1773-2010.
- Chiu, R., L. Tinel, L. Gonzalez, R. Ciuraru, F. Bernard, C. George, and R. Volkamer. 2017. UV photochemistry of carboxylic acids at the air-sea boundary: A relevant source of glyoxal and other oxygenated VOC in the marine atmosphere. Geophys. Res. Lett. 44 (2):1079–1087. doi: 10.1002/2016GL071240.
- Collett, J. L., K. J. Hoag, X. Rao, and S. N. Pandis. 1999. Internal acid buffering in San Joaquin Valley fog drops and its influence on aerosol processing. Atmos. Environ. 33 (29):4833–4847. doi: 10.1016/S1352-2310(99)00221-6.
- Dasari, S., A. Andersson, S. Bikkina, H. Holmstrand, K. Budhavant, S. Satheesh, E. Asmi, J. Kesti, J. Backman, A. Salam, et al. 2019. Photochemical degradation affects the light absorption of water-soluble brown carbon in the South Asian outflow. Sci. Adv. 5 (1):eaau8066. doi: 10.1126/sciadv.aau8066.
- Desyaterik, Y., Y. Sun, X. Shen, T. Lee, X. Wang, T. Wang, and J. L. Collett. Jr.2013. Speciation of “brown” carbon in cloud water impacted by agricultural biomass burning in Eastern China. J. Geophys. Res. Atmos. 118 (13):7389–7399. doi: 10.1002/jgrd.50561.
- Di Lorenzo, R. A., R. A. Washenfelder, A. R. Attwood, H. Guo, L. Xu, N. L. Ng, R. J. Weber, K. Baumann, E. Edgerton, and C. J. Young. 2017. Molecular-size-separated brown carbon absorption for biomass-burning aerosol at multiple field sites. Environ. Sci. Technol. 51 (6):3128–3137. doi: 10.1021/acs.est.6b06160.
- Duarte, R. M. B. O., S. M. S. C. Freire, and A. C. Duarte. 2015. Investigating the water-soluble organic functionality of urban aerosols using two-dimensional correlation of solid-state 13C NMR and FTIR spectral data. Atmos. Environ. 116:245–252. doi: 10.1016/j.atmosenv.2015.06.043.
- Emanuelsson, E. U., T. F. Mentel, A. K. Watne, C. Spindler, B. Bohn, T. Brauers, H. P. Dorn, A. M. Hallquist, R. Haseler, A. Kiendler-Scharr, et al. 2014. Parameterization of thermal properties of aging secondary organic aerosol produced by Photo-Oxidation of selected terpene mixtures. Environ. Sci. Technol. 48 (11):6168–6176. doi: 10.1021/es405412p.
- Ervens, B., B. J. Turpin, and R. J. Weber. 2011. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos. Chem. Phys. 11 (21):11069–11102. doi: 10.5194/acp-11-11069-2011.
- Fan, X., S. Wei, M. Zhu, J. Song, and P. Peng. 2016. Comprehensive characterization of humic-like substances in smoke PM2.5 emitted from the combustion of biomass materials and fossil fuels. Atmos. Chem. Phys. 16 (20):13321–13340. doi: 10.5194/acp-16-13321-2016.
- Fang, Z., W. Deng, Y. Zhang, X. Ding, M. Tang, T. Liu, Q. Hu, M. Zhu, Z. Wang, W. Yang, et al. 2017. Open burning of rice, corn and wheat straws: Primary emissions, photochemical aging, and secondary organic aerosol formation. Atmos. Chem. Phys. 17 (24):14821–14839. doi: 10.5194/acp-17-14821-2017.
- Feng, Y., V. Ramanathan, and V. R. Kotamarthi. 2013. Brown carbon: A significant atmospheric absorber of solar radiation? Atmos. Chem. Phys. 13 (17):8607–8621. doi: 10.5194/acp-13-8607-2013.
- Forrister, H., J. Liu, E. Scheuer, J. Dibb, L. Ziemba, K. L. Thornhill, B. Anderson, G. Diskin, A. E. Perring, J. P. Schwarz, et al. 2015. Evolution of brown carbon in wildfire plumes. Geophys. Res. Lett. 42 (11):4623–4630. doi: 10.1002/2015GL063897.
- Fu, H., R. Ciuraru, Y. Dupart, M. Passananti, L. Tinel, S. Rossignol, S. Perrier, D. J. Donaldson, J. Chen, and C. George. 2015. Photosensitized production of atmospherically reactive organic compounds at the air/aqueous interface. J. Am. Chem. Soc. 137 (26):8348–8351. doi: 10.1021/jacs.5b04051.
- Galbavy, E. S., K. Ram, and C. Anastasio. 2010. 2-Nitrobenzaldehyde as a chemical actinometer for solution and ice photochemistry. J. Photochem. Photobiol. A Chem. 209 (2–3):186–192. doi: 10.1016/j.jphotochem.2009.11.013.
- Gelencsér, A., A. Hoffer, G. Kiss, E. Tombácz, R. Kurdi, and L. Bencze. 2003. In-situ formation of Light-Absorbing organic matter in cloud water. J. Atmos. Chem. 45 (1):25–33. doi: 10.1023/a:1024060428172.
- Glover, C. M., and F. L. Rosario-Ortiz. 2013. Impact of halides on the photoproduction of reactive intermediates from organic matter. Environ. Sci. Technol. 47 (24):13949–13956. doi: 10.1021/es4026886.
- Graham, B., O. L. Mayol-Bracero, P. Guyon, G. C. Roberts, S. Decesari, M. C. Facchini, P. Artaxo, W. Maenhaut, P. Köll, and M. O. Andreae. 2002. Water-soluble organic compounds in biomass burning aerosols over Amazonia 1. Characterization by NMR and GC-MS. J. Geophys. Res. 107 (D20):LBA 14-11-LBA 14-16. doi: 10.1029/2001JD000336.
- Hecobian, A., X. Zhang, M. Zheng, N. Frank, E. S. Edgerton, and R. J. Weber. 2010. Water-soluble organic aerosol material and the light-absorption characteristics of aqueous extracts measured over the southeastern United States. Atmos. Chem. Phys. 10 (13):5965–5977. doi: 10.5194/acp-10-5965-2010.
- Hems, R. F., and J. P. D. Abbatt. 2018. Aqueous phase photo-oxidation of brown carbon nitrophenols: Reaction kinetics, mechanism, and evolution of light absorption. ACS Earth Space Chem. 2 (3):225–234. doi: 10.1021/acsearthspacechem.7b00123.
- Huang, K., J. S. Fu, N. C. Hsu, Y. Gao, X. Dong, S. C. Tsay, and Y. F. Lam. 2013. Impact assessment of biomass burning on air quality in Southeast and East Asia during BASE-ASIA. Atmos. Environ. 78:291–302. doi: 10.1016/j.atmosenv.2012.03.048.
- Jacobson, M. Z. 2001. Strong radiative heating due to the mixing state of black carbon in atmospheric aerosols. Nature 409 (6821):695. doi: 10.1038/35055518.
- Jacobson, M. Z. 2014. Effects of biomass burning on climate, accounting for heat and moisture fluxes, black and brown carbon, and cloud absorption effects. J. Geophys. Res. Atmos. 119 (14):8980–9002. doi: 10.1002/2014JD021861.
- Kirchstetter, T. W., T. Novakov, and P. V. Hobbs. 2004. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. 109 (D21). doi: 10.1029/2004JD004999.
- Klan, O., and J. Wirz. 2009. Photochemistry of organic compounds: From concepts to practice. 1st ed., 296–338. Hoboken: Wiley.
- Kroll, J. H., C. Y. Lim, S. H. Kessler, and K. R. Wilson. 2015. Heterogeneous oxidation of atmospheric organic aerosol: Kinetics of changes to the amount and oxidation state of particle-phase organic carbon. J. Phys. Chem. A 119 (44):10767–10783. doi: 10.1021/acs.jpca.5b06946.
- Laskin, A., J. Laskin, and S. A. Nizkorodov. 2015. Chemistry of atmospheric brown carbon. Chem. Rev 115 (10):4335–4382. doi: 10.1021/cr5006167.
- Laskin, J., A. Laskin, S. A. Nizkorodov, P. Roach, P. Eckert, M. K. Gilles, B. Wang, H. J. Lee, and Q. Hu. 2014. Molecular selectivity of brown carbon chromophores. Environ. Sci. Technol. 48 (20):12047–12055. doi: 10.1021/es503432r.
- Lee, H. J., P. K. Aiona, A. Laskin, J. Laskin, and S. A. Nizkorodov. 2014. Effect of solar radiation on the optical properties and molecular composition of laboratory proxies of atmospheric brown carbon. Environ. Sci. Technol. 48 (17):10217–10226. doi: 10.1021/es502515r.
- Lettens, S., B. De Vos, P. Quataert, B. Van Wesemael, B. Muys, and J. Van Orshoven. 2007. Variable carbon recovery of Walkley–Black analysis and implications for national soil organic carbon accounting. Eur. J. Soil Sci. 58 (6):1244–1253. doi: 10.1111/j.1365-2389.2007.00916.x.
- Li, Z., A. K. Smith, and C. D. Cappa. 2018. Influence of relative humidity on the heterogeneous oxidation of secondary organic aerosol. Atmos. Chem. Phys 18 (19):14585–14608. doi: 10.5194/acp-18-14585-2018.
- Lim, C. Y., E. C. Browne, R. A. Sugrue, and J. H. Kroll. 2017. Rapid heterogeneous oxidation of organic coatings on submicron aerosols. Geophys. Res. Lett. 44 (6):2949–2957. doi: 10.1002/2017GL072585.
- Lin, P., P. K. Aiona, Y. Li, M. Shiraiwa, J. Laskin, J. S. A. Nizkorodov, and A. Laskin. 2016. Molecular characterization of brown carbon in biomass burning aerosol particles. Environ. Sci. Technol. 50 (21):11815–11824. doi: 10.1021/acs.est.6b03024.
- Lin-Vien, D., N. B. Colthup, W. G. Fateley, and J. G. Grasselli. 1991. Alcohols and phenols. In The handbook of infrared and Raman characteristic frequencies of organic molecules, eds. D. Lin-Vien, N. B. Colthup, W. G. Fateley, and J. G. Grasselli, 45–60. San Diego: Academic Press.
- Liu, J., P. Lin, A. Laskin, J. Laskin, S. M. Kathmann, M. Wise, R. Caylor, F. Imholt, V. Selimovic, and J. E. Shilling. 2016. Optical properties and aging of light-absorbing secondary organic aerosol. Atmos. Chem. Phys. 16 (19):12815–12827. doi: 10.5194/acp-16-12815-2016.
- Liu, J., E. Scheuer, J. Dibb, L. D. Ziemba, K. L. Thornhill, B. E. Anderson, A. Wisthaler, T. Mikoviny, J. J. Devi, M. Bergin, et al. 2014. Brown carbon in the continental troposphere. Geophys. Res. Lett. 41 (6):2191–2195. doi: 10.1002/2013GL058976.
- Liu, P., Y. Zhang, and S. T. Martin. 2013. Complex refractive indices of thin films of secondary organic materials by spectroscopic ellipsometry from 220 to 1200 nm. Environ. Sci. Technol. 47 (23):13594–13601. doi: 10.1021/es403411e.
- Liu, P. F., N. Abdelmalki, H. M. Hung, Y. Wang, W. H. Brune, and S. T. Martin. 2015. Ultraviolet and visible complex refractive indices of secondary organic material produced by photooxidation of the aromatic compounds toluene and m-xylene. Atmos. Chem. Phys. 15 (3):1435–1446. doi: 10.5194/acp-15-1435-2015.
- Menon, S., J. Hansen, L. Nazarenko, and Y. Luo. 2002. Climate effects of black carbon aerosols in China and India. Science 297 (5590):2250–2253. doi: 10.1126/science.1075159.
- Mie, G. 1908. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys-Berlin, 330 (3):377–445. doi: 10.1002/andp.19083300302.
- Mikhailova, E. A., R. R. P. Noble, and C. J. Post. 2003. Comparison of soil organic carbon recovery by Walkley–Black and dry combustion methods in the russian chernozem. Commun. Soil Sci. Plan. 34 (13–14):1853–1860. doi: 10.1081/CSS-120023220.
- Mok, J., N. A. Krotkov, A. Arola, O. Torres, H. Jethva, M. Andrade, G. Labow, T. F. Eck, Z. Li, R. R. Dickerson, et al. 2016. Impacts of brown carbon from biomass burning on surface UV and ozone photochemistry in the amazon basin. Sci. Rep. 6:36940. doi: 10.1038/srep36940.
- Moosmüller, H., and C. M. Sorensen. 2018. Small and large particle limits of single scattering albedo for homogeneous, spherical particles. J. Quant. Spectrosc. Radiat. Transf. 204:250–255. doi: 10.1016/j.jqsrt.2017.09.029.
- Phillips, S. M., and G. D. Smith. 2014. Light absorption by charge transfer complexes in brown carbon aerosols. Environ. Sci. Technol. Lett. 1 (10):382–386. doi: 10.1021/ez500263j.
- Piva, O. 2004. Chapter 70: Photodeconjugation of enones and carboxylic acid derivatives. In CRC handbook of organic photochemistry and photobiology, eds. W. M. Horspool and F. Lenci, 1–18. Boca Raton, FL: CRC Press LLC.
- Ray, D., S. K. Ghosh, and S. Raha. 2019. Impacts of photochemical ageing on the half-lives and diagnostic ratio of polycyclic aromatic hydrocarbons intrinsic to PM2.5 collected from ‘real-world’ like combustion events of wood and rice straw burning. J. Hazard. Mater. 366:10–15. doi: 10.1016/j.jhazmat.2018.11.079.
- Rossignol, S., K. Z. Aregahegn, L. Tinel, L. Fine, B. Nozière, and C. George. 2014. Glyoxal induced atmospheric photosensitized chemistry leading to organic aerosol growth. Environ. Sci. Technol. 48 (6):3218–3227. doi: 10.1021/es405581g.
- Saleh, R., C. J. Hennigan, G. R. McMeeking, W. K. Chuang, E. S. Robinson, H. Coe, N. M. Donahue, and A. L. Robinson. 2013. Absorptivity of brown carbon in fresh and photo-chemically aged biomass-burning emissions. Atmos. Chem. Phys. 13 (15):7683–7693. doi: 10.5194/acp-13-7683-2013.
- Sareen, N., S. G. Moussa, and V. F. McNeill. 2013. Photochemical aging of Light-Absorbing secondary organic aerosol material. J. Phys. Chem. A 117 (14):2987–2996. doi: 10.1021/jp309413j.
- Schkolnik, G., D. Chand, A. Hoffer, M. O. Andreae, C. Erlick, E. Swietlicki, and Y. Rudich. 2007. Constraining the density and complex refractive index of elemental and organic carbon in biomass burning aerosol using optical and chemical measurements. Atmos. Environ. 41 (5):1107–1118. doi: 10.1016/j.atmosenv.2006.09.035.
- Schwarzenbach, R. P., P. M. Gschwend, and D. M. Imboden. 2003. Environmental organic chemistry. 2nd ed. Hoboken: Wiley.
- Sengupta, D., V. Samburova, C. Bhattarai, E. Kirillova, L. Mazzoleni, M. Iaukea-Lum, A. Watts, H. Moosmüller, and A. Khlystov. 2018. Light absorption by polar and non-polar aerosol compounds from laboratory biomass combustion. Atmos. Chem. Phys. 18 (15):10849–10867. doi: 10.5194/acp-18-10849-2018.
- Shamjad, P. M., S. N. Tripathi, R. Pathak, M. Hallquist, A. Arola, and M. H. Bergin. 2015. Contribution of brown carbon to direct radiative forcing over the Indo-Gangetic plain. Environ. Sci. Technol. 49 (17):10474–10481. doi: 10.1021/acs.est.5b03368.
- Slade, J. H., and D. A. Knopf. 2013. Heterogeneous OH oxidation of biomass burning organic aerosol surrogate compounds: Assessment of volatilisation products and the role of OH concentration on the reactive uptake kinetics. Phys. Chem. Chem. Phys. 15 (16):5898–5915. doi: 10.1039/c3cp44695f.
- Smith, J. D., J. H. Kroll, C. D. Cappa, D. L. Che, C. L. Liu, M. Ahmed, S. R. Leone, D. R. Worsnop, and K. R. Wilson. 2009. The heterogeneous reaction of hydroxyl radicals with submicron squalane particles: A model system for understanding the oxidative aging of ambient aerosols. Atmos. Chem. Phys. 9 (9):3209–3222. doi: 10.5194/acp-9-3209-2009.
- Sumlin, B. J., A. Pandey, M. J. Walker, R. S. Pattison, B. J. Williams, and R. K. Chakrabarty. 2017. Atmospheric photooxidation diminishes light absorption by primary brown carbon aerosol from biomass burning. Environ. Sci. Technol. Lett. 4 (12):540–545. doi: 10.1021/acs.estlett.7b00393.
- Takahashi, K., K. L. Plath, R. T. Skodje, and V. Vaida. 2008. Dynamics of vibrational overtone excited pyruvic acid in the gas phase: Line broadening through Hydrogen-Atom chattering. J. Phys. Chem. A 112 (32):7321–7331. doi: 10.1021/jp803225c.
- Taylor, D. 2010. Biomass burning, humans and climate change in southeast asia. Biodiversity Conserv. 19 (4):1025–1042. doi: 10.1007/s10531-009-9756-6.
- Walkley, A., and I. A. Black. 1934. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37 (1):29–38. doi: 10.1097/00010694-193401000-00003.
- Wong, J. P. S., A. Nenes, and R. J. Weber. 2017. Changes in light absorptivity of molecular weight separated brown carbon due to photolytic aging. Environ. Sci. Technol. 51 (15):8414–8421. doi: 10.1021/acs.est.7b01739.
- Xing, Y. F., Y. H. Xu, M. H. Shi, and Y. X. Lian. 2016. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 8 (1):E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19.
- Yu, J., G. H. Yu, S. Park, and M. S. Bae. 2017. Chemical and absorption characteristics of water-soluble organic carbon and humic-like substances in size-segregated particles from biomass burning emissions. Asian J. Atmos. Environ. 11 (2):96–106. doi: 10.5572/ajae.2017.11.2.096.
- Zhang, L., H. Liao, and J. Li. 2010. Impact of the Southeast Asian summer monsoon strength on the outflow of aerosols from South Asia. Ann. Geophys. 28 (1):277–287. doi: 10.5194/angeo-28-277-2010.
- Zhong, M., and M. Jang. 2014. Dynamic light absorption of biomass-burning organic carbon photochemically aged under natural sunlight. Atmos. Chem. Phys. 14 (3):1517–1523. doi: 10.5194/acp-14-1517-2014.
