Abstract
Outdoor field measurements of bioaerosols are performed within a wide range of basic and applied scientific disciplines, each with its own goals, assumptions, and terminology. This article contains brief reviews of outdoor field bioaerosol research from these diverse interests, with emphasis on perspectives from the atmospheric sciences. The focus is on a high-level discussion of pressing scientific questions, grand challenges, and needs for cross-disciplinary collaboration. The research topics, in which bioaerosol field measurement is important, include (i) atmospheric physics, clouds, climate, and hydrological cycle; (ii) atmospheric chemistry; (iii) airborne allergen-containing particles; (iv) airborne human pathogens and national security; (v) airborne livestock and crop pathogens; and (vi) biogeography and biodiversity. We concisely review bioaerosol impacts and discuss properties that distinguish bioaerosols from abiological aerosols. We give extra focus to regions of specific interest, i.e., forests, polar regions, marine and coastal environments, deserts, urban and rural areas, and summarize key considerations related to bioaerosol measurements, such as of fluxes, of long-range transport, and of sampling from both stationary and vessel-driven platforms. Keeping in mind a series of key scientific questions posed within the diverse communities, we suggest that pressing scientific questions include the following: (i) emission sources and flux estimates; (ii) spatial distribution; (iii) changes in distribution; (iv) atmospheric aging; (v) metabolic activity; (vi) urbanization of allergies; (vii) transport of human pathogens; and (viii) climate-relevant properties.
EDITOR:
1. Introduction
The term bioaerosol encompasses a broad range of primary atmospheric organic particles associated with and emitted from both living and dead organisms, as defined rigorously by Després et al. (Citation2012). Atmospheric bioaerosols can originate from sources in every terrestrial and marine environment, exist in air above virtually all locations on the globe, and exhibit a vast diversity of types, compositions, and sizes. From a functional perspective, there are three types of bioaerosols: (i) The first fraction is comprised of living organisms, such as bacteria, archaea, fungi, lichens, and microalgae, which may catalyze biochemical reactions in the atmosphere, change their own surface properties, and colonize new environments or hosts; (ii) the second fraction of bioaerosols is components such as propagules, i.e., fungal spores, bacterial spores, pollen, and viruses that are considered metabolically inactive in the atmosphere but serve as reproductive or dispersal units between plants, pathogen hosts, and environments; (iii) the last fraction of bioaerosols is microbial, plant, or animal fragments and exudates that can be aerosolized on their own or attached to non-biological particles, such as mineral or salt particles. All bioaerosols, regardless of their viability, may carry molecules that are associated with a variety of environmental processes, are toxic, or provoke allergic reactions. Bioaerosols are particles that are identified by their characteristic chemical composition (ratio of organic elements) or physical properties (shape and spectral properties), their growth in/on nutrient media, or through the presence of molecular tracers such as information molecules (e.g., DNA, RNA), energy-carrying compounds (e.g., ATP, NADH), structural compounds (e.g., ergosterol, cellulose), or functional compounds (e.g., ice nucleation-active proteins).
Bioaerosols were among the first types of atmospheric aerosols to be identified (Carnelley et al. Citation1887; De Bary Citation1887; Pasteur Citation1862; Ehrenberg Citation1847). Contemporary outdoor field measurements of bioaerosols are performed within a tremendously widespread set of basic and applied scientific disciplines that foster research toward separate scientific goals and with distinct sets of community-associated terminology and assumptions. For example, areas of scientific research with established application to outdoor bioaerosols very broadly include the following: (i) atmospheric physics, clouds, climate, and hydrological cycle, (ii) atmospheric chemistry, (iii) airborne allergen-containing particles, (iv) airborne human pathogens and national security, (v) airborne livestock and crop pathogens, and (vi) biogeography and biodiversity. The role of outdoor bioaerosol field measurements for each of these areas is discussed in Section 2.
The intention of this review is to ignite global collaborative effort across disciplines and communities in order to better understand the impacts of bioaerosols on aerosol chemistry and physics, health, climate, agriculture, and ecology. Brief, updated reviews are included, restricted to outdoor bioaerosol field measurements. Instead, we focus on a high-level discussion of pressing scientific questions, grand challenges, sampling recommendations, and needs for cross-disciplinary collaboration. Comprehensive reviews can be found elsewhere (Yao Citation2018; Buters et al. Citation2018; Delort and Amato Citation2017; Núñez et al. Citation2016a, Citation2016b; Fröhlich-Nowoisky et al. Citation2016; Sofiev and Bergmann Citation2013; Després et al. Citation2012; Xu et al. Citation2011; Morris et al. Citation2011; Womack, Bohannan, and Green Citation2010; Georgakopoulos et al. Citation2009; Jones and Harrison Citation2004; Madelin Citation1994). Contributions were compiled from a group of authors with diverse sets of backgrounds and expertise, with more emphasis added from the perspective of the atmospheric sciences than others, e.g., health sciences. This article fits within a journal special issue focused on standardizing methodology across all areas of bioaerosol measurement.
2. Research motivation
Different communities of bioaerosol researchers have widely different motivating questions and challenges. Here, we summarize comments around six broad categories of bioaerosol impacts with respect to measurements in ambient air.
2.1. Atmospheric physics, clouds, climate, and hydrological cycle
Atmospheric bioaerosols can exert direct and indirect effects on climate. Direct effects are primarily based on the capacity of bioaerosols to absorb and scatter light. The ability of aerosols to take up water at subsaturated conditions, i.e., hygroscopicity, can influence their direct climate effects (Boucher et al. Citation2013). The hygroscopic properties of bioaerosols have been only rarely assessed (Tang et al. Citation2019; Lin et al. Citation2015; Griffiths et al. Citation2012; Ghosal et al. Citation2010; Lee, Kim, and Kim Citation2002; Ko, First, and Burge Citation2000; Rubel Citation1997; Reponen et al. Citation1996), mainly due to technical challenges related to their relatively large sizes and their immense diversity. Indirect climate effects of bioaerosols are based on their interaction with clouds by acting as either cloud condensation nuclei (CCN), which nucleate liquid cloud droplets, or ice nucleating particles (INPs), which promote cloud droplet freezing. Ice particles modify cloud reflectivity and lifetime and can also affect the initiation and efficiency of precipitation (see Kanji et al. Citation2017). Ice nucleation active (INA) bioaerosols have been hypothesized to exert a much more dramatic and potentially more transformative role upon clouds, precipitation, and regional climate than bio-CCN, although the latter may play a key role as giant CCN (Fröhlich-Nowoisky et al. Citation2016; Möhler et al. Citation2007). Research priorities raised almost a decade ago (DeMott and Prenni Citation2010) remain pertinent and complement those recently outlined (Coluzza et al. Citation2017). Questions include the following: What biomolecules can act as INPs and what organisms produce them; how do emissions of INPs vary over land, freshwater, and sea, differ by ecotype (e.g., natural vs. anthropogenic), and cycle by season; over what temperature range (degree of supercooling) are bio-INPs important; and how will climate- and land use-change affect emissions? Before a worldwide map of bio-INPs can be constructed, we need to quantify their sources and understand how they are dispersed, such as, e.g., via fire and weather (precipitation, humidity, and strong winds). While we are far from fulfilling these goals, rapid progress has been made (See reviews by Després et al. Citation2012; Morris et al. Citation2014; Fröhlich-Nowoisky et al. Citation2016; Kanji et al. Citation2017; Hu et al. Citation2018; Knopf, Alpert, and Wang Citation2018). To mention a few examples, diverse and often abundant organisms producing bio-INPs have been revealed in addition to the well-known group of Gram-negative INA bacteria (Morris, Georgakopoulos, and Sands Citation2004), including Gram-positive bacteria (Failor et al. Citation2017), fungi (Fröhlich-Nowoisky et al. Citation2015; O’Sullivan et al. Citation2015; Pummer et al. Citation2015; Huffman et al. Citation2013; Morris et al. Citation2013), microalgae (Tesson and Šantl-Temkiv Citation2018), and pollen (Dreischmeier et al. Citation2017; Augustin et al. Citation2013; Pummer et al. Citation2012; von Blohn et al. Citation2005). It is also becoming clear that bio-INPs typically predominate over minerals, in terms of their numeric abundance, across a wider temperature range than previously assumed, often comprising the majority of INPs at higher temperatures and down to –23 °C (Hartmann et al. Citation2019; Suski et al. Citation2018; McCluskey et al. Citation2018; Petters and Wright Citation2015; Mason et al. Citation2015) or even colder (Tobo et al. Citation2014). There is a vital need for numerical modeling studies to investigate the impact of different types of bioaerosols on cloud and precipitation properties as well as climate (e.g., Hummel et al. Citation2018; Hoose, Kristjánsson, and Burrows Citation2010; Sahyoun et al. Citation2016), from regional to global scales, and by considering biogenic INPs beyond whole microbial cells.
2.2. Atmospheric chemistry
Chemical processes can be affected by the presence of bioaerosols, in particular metabolically active living microorganisms such as bacteria. They were indeed identified as potentially involved in the transformation of organic compounds (Šantl-Temkiv et al. Citation2013; Amato et al. Citation2007a; Ariya et al. Citation2002; Herlihy, Galloway, and Mills Citation1987) and in the scavenging and detoxification of oxidants such as free radicals and their sources and drivers (Passananti et al. Citation2016; Vinatier et al. Citation2016; Vaïtilingom et al. Citation2013). Recently, direct observations detailed the microbial activity in the atmosphere (Amato et al. Citation2019; Šantl-Temkiv et al. Citation2018; Amato et al. Citation2017; Krumins et al. Citation2014). However, we still have little understanding of the variety of microorganisms that can maintain activity under atmospheric conditions and of their metabolic rates. Overall, the extent of biological impacts on atmospheric chemical processes still needs to be evaluated quantitatively.
2.3. Airborne allergen-containing particles
The quantitative spatial and temporal understanding of airborne fungal spores and pollen is urgently needed to assist diagnosis of allergies. Seasonal allergies are driven primarily by anemophilous (i.e., wind-driven) pollen (Sofiev and Bergmann Citation2013) and monitoring networks across the globe conduct frequent measurements of airborne pollen, with the results used as inputs for forecasts that inform public health (Buters et al. Citation2018; Papadopoulos et al. Citation2012). Only recently, the focus of research also expanded to include airborne fungal spores, which are more abundant than pollen and have different occurrence patterns (Grinn-Gofroń et al. Citation2019; Damialis et al. Citation2015). Fungal spores can comprise from 5% to 80% of the coarse mode mass of atmospheric particulate matter (Fröhlich-Nowoisky et al. Citation2016; Pöschl et al. Citation2010; Elbert et al. Citation2007). Long-term continuous monitoring efforts delivered calendars of the most abundant airborne allergen-containing particles and described their spatial and temporal variability, supporting the modeling efforts (Šikoparija et al. Citation2018a; Gehrig, Maurer, and Schwierz Citation2018; D’Amato et al. Citation2016). However, to resolve peculiarities of human dose response, the capability to detect airborne allergens more specifically is needed. Recently, high-throughput DNA sequencing helped identify the diversity of airborne fungal taxa (Banchi et al. Citation2018) and grass species throughout the season (Kraaijeveld et al. Citation2015), while enzyme-linked immunosorbent assays proved that reactive pollen allergens could be monitored in the outdoor air (Grewling et al. Citation2016; Buters et al. Citation2012, Citation2015; Galan et al. Citation2013). It has been shown that a real-time monitoring of pollen with a high temporal resolution can be achieved on what is currently a limited scale (Crouzy et al. Citation2016). Much effort is also being invested in improving autonomous detection of potential allergen-containing particles in real time (Huffman et al. Citation2019; Wu et al. Citation2018; Swanson and Huffman Citation2018; Crouzy et al. Citation2016; Oteros et al. Citation2015; Kiselev, Bonacina, and Wolf Citation2013). However, there is still need for proving that new methodological approaches are robust enough for continuous long-term outdoor monitoring. In addition, the specificity of the immune response calls for a more personalized approach (Werchan et al. Citation2018; Yamamoto et al. Citation2015), which should resolve the uncertainty of the symptom thresholds.
2.4. Airborne human pathogens and national security
The rapid detection of infectious microorganisms suspended in air is of critical importance to agencies tasked with preserving public health and security. As a result, many researchers are focused solely on detecting and mitigating possible infectious viral, bacterial, or fungal threats. Metagenomic analyses and culture experiments indicate that atmospheric microbial communities can include potential pathogens hazardous to human health (Griffin Citation2007), and individual pathogens can be spread actively through acts of bioterror. The virulence-related genes, such as enterotoxin synthesis, have been also detected from atmospheric samples suggesting pathogen dispersal in air (Kobayashi et al. Citation2016). However, there are only few animal assay and epidemiological surveys that directly demonstrate the health impacts of airborne microorganisms, for example the dispersion of Kawasaki disease in humans (Rodó et al. Citation2011) and measles occurrences in western China (Ma et al. Citation2017) associated with Asian-dust events. Bioaerosol monitoring systems also play an important role in human health care and military safety, to assess the probability of exposure, predict, and reduce or prevent exposure.
2.5. Airborne livestock and crop pathogens
Monitoring of airborne plant and livestock pathogens facilitates early detection of disease because it can overcome the constraints of detecting spatially discrete or heterogeneously distributed symptoms that could be missed by field scouts (Mahaffee and Stoll Citation2016). As a consequence, commercial development of air samplers has led to a range of samplers for passive (deposition) and active (impaction) collection of airborne pathogens (Mahaffee and Stoll Citation2016). Data on aerial spread of plant pathogens have been incorporated into simulation models that help farmers strategically apply pesticides or other preventative agricultural practices. However, decision-making based solely on monitoring the presence of plant pathogens has limited direct application, and it mostly concerns decisions restricted to the scale of individual fields (Mahaffee and Stoll Citation2016). When the deposition of airborne pathogen propagules depends on washout by rainfall, collection of rain is a very useful means to monitor pathogen arrival. Rainfall collection has been deployed successfully to monitor arrival of airborne spores of soybean rust (Isard et al. Citation2011) and of downy mildew of cucurbits (Neufeld et al. Citation2018) leading to significant financial gains for the farmer and environmental benefits because of informed decisions that allow reduced pesticide applications (Isard et al. Citation2011).
2.6. Biogeography and biodiversity
Our limited knowledge of bioaerosols impedes a more comprehensive understanding of principles involved in microbial aerial dispersal and the biogeography of microorganisms. Dispersal is one of the four fundamental processes that underlie biogeographic patterns (selection, drift, dispersal, and mutation) (Hanson et al. Citation2012). The likelihood of dispersal is in some cases independent of taxon properties and relies on neutral factors, such as taxon abundance in the source community (Nemergut et al. Citation2011). Atmospheric dispersal plays a crucial role as one of the dominant types of microbial dispersion. It is thus no surprise that the atmosphere was found to be a selective boundary for dispersing microbes, enabling some taxa to grow during their dispersal, while others are killed by, e.g., UV radiation or desiccation (Šantl-Temkiv et al. Citation2013). INA microbial strains can induce their own precipitation, thereby reducing their atmospheric residence time (Amato et al. Citation2015; Burrows et al. Citation2009). It has not yet determined whether certain taxa dispersing through air might have properties that increase their chance of colonization success in already occupied habitats.
3. Current considerations related to bioaerosol field measurements
3.1. Important physical, chemical, and biological properties of bioaerosols
Bioaerosols possess immense chemical diversity: from simple or complex biological molecules (lipids, amino acids, peptides, saccharides, carboxylic acids, etc.) varying in solubility, charge, polarity, and catalytic activity, to whole dead, dormant, or metabolically active cells of various taxonomic affiliations and genetic potential. The formation of cloud droplets on biogenic CCN may be favored by the presence of biosurfactants, i.e., amphiphilic molecules responsible for a decrease of water surface tension (Noziere Citation2016; Petters and Petters Citation2016; Renard et al. Citation2016). Other molecules can nucleate ice, such as INA proteins, active from –2 to –13 °C, or structural molecules that are usually active <–15 °C (Conen et al. Citation2017; Pouzet et al. Citation2017; Hiranuma et al. Citation2015; Joly et al. Citation2014; Burrows et al. Citation2013; Hoose and Möhler Citation2012; Christner et al. Citation2008; Möhler et al. Citation2007; Morris, Georgakopoulos, and Sands Citation2004). Aside from specialized INA proteins, the rainfall effects potentially triggered by biological ice nucleators may involve multiplication through secondary ice formation (Lauber et al. Citation2018; Sullivan et al. Citation2018) and large-scale positive feedbacks (Bigg, Soubeyrand, and Morris Citation2015; Huffman et al. Citation2013). Due to their extremely complex physiology and chemical composition, the chemical and physical properties of bioaerosols remain largely unknown. For example, the sequence and structure of INA proteins remain unknown for most species of INA microorganisms (Tesson and Šantl-Temkiv Citation2018; Fröhlich-Nowoisky et al. Citation2015).
While bioaerosols include a large variety of particle classes that range in size from several nm to tens of μm, mass distributions of most bioaerosol types peak at relatively large sizes. Particle behavior is thus heavily influenced by mass and size-related effects, i.e., sedimentation and inertia. Larger particles can generally be collected with higher efficiency than smaller particles (Morris, Leyronas, and Nicot Citation2014; Reponen et al. Citation2001), though large particles (especially bacterial agglomerates, spores, and pollen) are frequently missed due to particle loss caused by poor sampling strategies. A well-designed and characterized inlet system can thus help minimize and quantify biases in the results (Mainelis Citation2019; Wiedensohler et al. Citation2014; Von Der Weiden, Drewnick, and Borrmann Citation2009).
Bioaerosols are dynamic components of the atmosphere. Similar to abiological aerosols, repeated cycles of activation/evaporation or freeze/thaw, reaction with oxidants, UV radiation, and changes in temperature can change chemical or physical surface properties. Bioaerosols may age through drying, oxidation, nitrification, or accumulation of condensable species on their surfaces (Liu et al. Citation2017; Estillore, Trueblood, and Grassian Citation2016; Santarpia et al. Citation2012). Unlike non-living aerosols, however, living microorganisms can die through these processes, but have also been hypothesized to metabolize and grow in the atmosphere (Amato et al. Citation2019; Womack, Bohannan, and Green Citation2010; Sattler, Puxbaum, and Psenner Citation2001). Additionally, atmospheric aging processes may alter the microphysical effects (Attard et al. Citation2012), allergenic potential, and toxicity of bioaerosols (Liu et al. Citation2017; Franze et al. Citation2005). The physiologically active fraction of bioaerosols can affect their own aging by modifying their physical properties, surface properties (Santl-Temkiv et al. Citation2015), gene expression, and by degrading oxidative species and atmospheric organics (Vaïtilingom et al. Citation2013). Recently, it has also been confirmed that airborne bacterial cells maintain some extent of metabolic activity: They react to the presence of substrates (Krumins et al. Citation2014), contain a high number of ribosomes (Šantl-Temkiv et al. Citation2018), and respond to oxidative stress and physiological shocks (Amato et al. Citation2019). Finally, airborne microbial assemblages are also highly complex, since they consist of mixes of cells of different taxa and physiological states. These assemblages may possess a degree of functional stability acquired via the ability to resist environmental stress (Temkiv et al. Citation2012) or even limited interactions between cells.
3.2. Study regions of special interest
[i: Forests] Tropical-rain, boreal, and temperate forests are locations of intense biological activity and are, therefore, important ecosystems for in-depth bioaerosol analysis (Andreae and Crutzen Citation1997). The number of bioaerosol studies in forested locations is still comparatively small, however, and thus, our understanding of bioaerosol-related processes in these locations has remained limited (Whitehead et al. Citation2016; Fröhlich-Nowoisky et al. Citation2016; Womack et al. Citation2015; Schumacher et al. Citation2013; Huffman et al. Citation2012; Whitehead et al. Citation2010; Pöschl et al. Citation2010; Graham et al. Citation2003). While it is acknowledged that the intense hydrological cycling involving tropical rain forests, with its large exchanges of water and energy, has major importance to the Earth climate system, the role of bioaerosol–cloud interactions driven by tropical rain forests is largely unassessed (Pöschl et al. Citation2010; Prenni et al. Citation2009). Furthermore, it is mostly unexplored as to what extent human activities (i.e., deforestation, fires, and other land use changes) have altered the abundance and properties of bioaerosol populations relative to preindustrial states of the atmosphere (Moran-Zuloaga et al. Citation2018; Morris et al. Citation2014; Andreae Citation2007).
[ii: Polar regions] Due to synoptic atmospheric patterns, polar regions are geographically isolated areas. Unconstrained feedback processes, caused by changes in albedo, sea-ice extent, ice-sheet melt, and glacial retreats, are affecting the polar radiation budgets. Melting of terrestrial ice also opens up new terrestrial surfaces for colonization. Therefore, polar regions are of special interest for aerobiology (Šantl-Temkiv et al. Citation2019; Šantl-Temkiv et al. Citation2018; Crawford et al. Citation2017; Pearce et al. Citation2016; Barbaro et al. Citation2015; Pearce et al. Citation2009). So far, a few studies proposed biogenic sources of INP in the Arctic (Wex et al. Citation2019; Tobo et al. Citation2019; Creamean et al. Citation2018a; Irish et al. Citation2017; Conen, Stopelli, and Zimmermann Citation2016; Wilson et al. Citation2015; Bigg and Leck Citation2001; Bigg Citation1996) and in Antarctica (Saxena and Weintraub Citation1988; Saxena Citation1983). Also, several studies have investigated airborne microorganisms in Antarctica and have suggested that they have an important role in colonizing Antarctic terrestrial environments (Archer et al. Citation2019; Pearce et al. Citation2009, Citation2016). There are scarce studies on Arctic bioaerosols (Šantl-Temkiv et al. Citation2018; Šantl-Temkiv et al. Citation2019; Cuthbertson et al. Citation2017; Harding et al. Citation2011) and biogenic INP (Šantl-Temkiv et al. Citation2019; Wex et al. Citation2019; Creamean et al. Citation2018a). As such, our knowledge of how biogenic INPs impact cloud dynamics in polar regions is highly fragmented and lacks a mechanistic and quantitative foundation.
[iii: Marine and coastal environments] Marine aerosols contain large amounts of inorganic salts and organic compounds as well as microbial cells, fragments, and exudates (Kuznetsova, Lee, and Aller Citation2005; Marks et al. Citation2001; Monahan et al. Citation1983). Detection of marine bacterial groups in air samples collected both at the seashores of Europe (Polymenakou et al. Citation2008) and at high altitudes in Japan (Maki et al. Citation2014) suggests inland transport of marine microorganisms. In line with this, marine bacteria carried to the free troposphere could change the airborne microbial compositions over continental regions (Cáliz et al. Citation2018; Maki et al. Citation2014; DeLeon-Rodriguez et al. Citation2013; Amato et al. Citation2007c). Microbially produced soluble and particulate organic material both in seawater and in the sea surface microlayer exhibits potent ice nucleation activity (McCluskey et al. Citation2018; Wang et al. Citation2017; Irish et al. Citation2017; Ladino et al. Citation2016; Wilson et al. Citation2015). There is, however, still very little quantitative understanding of the emission rates with which these marine INP are released to the atmosphere and which processes control these emission rates.
[iv: Deserts] The surface soils of deserts constitute the most abundant and seasonally consistent terrestrial biome on Earth (Peel, Finlayson, and McMahon Citation2007). Surface biological cover (i.e., biological crust), which stabilizes desert soil (Pointing and Belnap Citation2012), is disturbed naturally by high winds during desert storms and anthropogenically by human activities (Griffin Citation2007). Airborne bacteria over desert areas include highly diverse bacterial communities, which were found to predominately originate from terrestrial sources, such as plants and animal feces (An et al. Citation2013; Puspitasari et al. Citation2016). Microorganisms in desert habitats frequently show resistance to stressors that are shared between terrestrial and atmospheric conditions (Essoussi et al. Citation2010). During dust events, the diversity and concentration of airborne bacteria have been shown to increase compared to background conditions (Maki et al. Citation2017b; Puspitasari et al. Citation2016). After dust events, atmospheric stressors eliminate more sensitive strains of bacteria, derived from less extreme environments, while the polyresistant members arriving attached to sand particles remain unaffected (Maki et al. Citation2017b).
[v: Urban and rural areas] Urban and rural bioaerosols affect outdoor and indoor air quality and relate to human, plant and animal pathogen transmission, the distribution of allergens, and deprivation of cultural heritage (Sterflinger and Piñar Citation2013). Multiple studies have investigated the difference between urban and rural environments in affecting the atmospheric microbiome. Després et al. (Citation2012) reviewed urban vs. rural differences in the bacterial aerobiomes and showed that methods based on culturability often yield higher counts in urban environments. In contrast, methods based on genetic analysis detected a greater richness in the rural environment (Bowers et al. Citation2013; Kaarakainen et al. Citation2008; Wu et al. Citation2007) or no significant differences between the two environments (Negrin, Del Panno, and Ronco Citation2007; Negrin et al. Citation2009). There is also ambiguity regarding the concentration of fungal spores. While certain studies highlight an increase in fungal spore concentration close to rural areas (Lin et al. Citation2018; Di Filippo et al. Citation2013; Oliveira et al. Citation2009, Citation2010; Kaarakainen et al. Citation2008; Kasprzyk and Worek Citation2006), others see an enhancement in urban environments (Rathnayake et al. Citation2016; Bauer et al. Citation2008; Pei-Chih, Huey-Jen, and Chia-Yin Citation2000). Overall, it seems that the relationship between land use and bioaerosols is not well understood and the effect of meteorological factors, season, and the local environment on the airborne community assembly still needs to be determined (Liu et al. Citation2019; Wolf et al. Citation2017; Rathnayake et al. Citation2016; Morris et al. Citation2014; Bowers et al. Citation2013; Kaarakainen et al. Citation2008).
3.3. Issues related to bioaerosol measurements
[i: Choosing the temporal resolution] For a particular bioaerosol monitoring campaign, the temporal resolution should be carefully considered in connection with the type of bioaerosols of interest. Large, short-term temporal variations in bioaerosols have frequently been observed (Fierer et al. Citation2008), and the performance of bioaerosol monitoring is affected by a number of parameters such as time of the day, season, and meteorological factors (Wei et al. Citation2016; Saari et al. Citation2015; Bowers et al. Citation2011; Bowers et al. Citation2012; Jones and Harrison Citation2004). For example, humidity, atmospheric radiation, temperature, and wind have significant effects on observed bioaerosol abundance and diversity (Hu et al. Citation2018; Després et al. Citation2012; Evans, Coombes, and Dunstan Citation2006; Jones and Harrison Citation2004). Unlike inorganic dust concentrations, which are lower both during and shortly after rainfall (Issanova and Abuduwaili Citation2017), concentrations, e.g., of bioaerosols including fungal spores and bacteria, can frequently increase during and after rainfall due to both passive and active processes (Joung, Ge, and Buie Citation2017; Morris et al. Citation2017; Rathnayake et al. Citation2017; Conen et al. Citation2017; Wolf et al. Citation2017; Wang et al. Citation2016; Petters and Wright Citation2015; Hader, Wright, and Petters Citation2014; Wright et al. Citation2014; Huffman et al. Citation2013; Schumacher et al. Citation2013). Concentrations of various classes of bioaerosols, including fungal spores, bacteria, and pollen, are also known to change depending on the time of the day, influenced by a combination of biological emission processes, relative humidity, increase of turbulent kinetic energy, and also atmospheric dilution effects caused by daily changes in boundary layer height (Šikoparija et al. Citation2018b; Healy et al. Citation2014; Huffman et al. Citation2012; Fang et al. Citation2007). Finally, the speed of atmospheric dilution and microbial activity, such as growth or gene expression, occurs on a much shorter time scales than the sampling and could thus go unnoticed. Real-time sensors that detect bioaerosols continuously and relatively autonomously can help provide windows into certain emission and atmospheric processes by providing data at high time resolution (minutes or less) (Šaulienė et al. Citation2019; Huffman et al. Citation2019; Huffman and Santarpia Citation2017; Oteros et al. Citation2015; Holt and Bennett Citation2014; Huffman et al. Citation2012; Xu et al. Citation2011).
[ii: Cultivation-dependent and independent techniques] Cultivation-dependent techniques were long the reference methods for investigating microbial communities in the environment, including aerosols and atmospheric waters (e.g., Lighthart Citation1997). Most environmental microorganisms cannot be cultivated efficiently, despite the fact that they are viable (Amann, Ludwig, and Schleifer Citation1995). Proportions of viable bacteria culturable after collection from the atmosphere range widely, e.g., from <1% (Amato et al. Citation2007b) to >10% (Temkiv et al. Citation2012; Tong and Lighthart Citation2000). This is either due to most cells being damaged, or because suitable cultivation techniques or appropriate growth media were not used. Therefore, cultivation-dependent techniques significantly underestimate both the number and the diversity of microorganisms (Šantl-Temkiv et al. Citation2013; Temkiv et al. Citation2012). Recently, molecular and single-cell approaches have been applied and provided insights into the true microbial diversity of the atmosphere (e.g., Bowers et al. Citation2009; DeLeon-Rodriguez et al. Citation2013; Temkiv et al. Citation2012).
[iii: The problem of low biomass] In the atmosphere, microbial cells are found at concentrations varying from ∼102 to ∼106 cells m−3 of air. Hence, a representative sample with sufficient biomass for downstream analyses should comprise collecting bioaerosols from >1 to several 100 m3 of air. For microbial aerosols, the high-volume filter samplers (Dommergue et al. Citation2019) and high-flow-rate impingers (Šantl-Temkiv et al. Citation2017) are currently the most appropriate instruments for sampling in areas, characterized with low biomass, and can be coupled with molecular, microbial, and INP analysis. Aerosol concentrators of different design, e.g., virtual impactors, also allow overcoming low biomass issues (Kim et al. Citation2001). Another implication of the low biomass is the high susceptibility to contamination. Aside from high sample volumes, good practices include stringent cleaning, sterilization, or baking of the sampling equipment (Dommergue et al. Citation2019; Šantl-Temkiv et al. Citation2017; Šantl-Temkiv et al. Citation2018; Lever et al. Citation2015), sterile techniques when handling and analyzing the samples (Šantl-Temkiv et al. Citation2013; Temkiv et al. Citation2012) as well as performing periodical handling—and operational blanks to verify that measurements are not influenced by contamination (Dommergue et al. Citation2019; Šantl-Temkiv et al. Citation2017; Temkiv et al. Citation2012). Finally, using real-time samplers (e.g., fluorescence or mass spectrometric analyzers) as a component of the study can complement other work by providing qualitative or semi-quantitative results at a much higher time resolution (Huffman et al. Citation2019). These methods can also offer real-time windows into atmospheric processes that can help inform the sampling strategy for lower resolution devices, though all techniques that rely primarily on light scattering or chemical information suffer from lower specificity.
[iv: Preserving the in situ state during sampling, storage, and transportation] For understanding the in situ state of bioaerosols, sampling and storage processes should preserve sample integrity, including viability, physiological state, ice nucleation activity, or mixing state as much as possible, and allow relevant downstream applications, such as DNA/RNA-based analyses, viability assays, cultivation, or microscopy. Long sampling times affect the physiological state and viability of organisms, for example by passing large volumes of desiccated air across filter-bound particles. The use of impingers allows sampling in physiological liquid or a fixative, thus preserving bioaerosols in their in situ states (Šantl-Temkiv et al. Citation2017). For example, for some INA microorganisms, there is evidence that the ice nucleation activity increases due to starvation and low temperature (Fall and Fall Citation1998; Nemecek-Marshall, LaDuca, and Fall Citation1993). Thus, when quantifying biogenic INP, storing environmental samples at 4 °C, which is a common short storage strategy during sampling campaigns, is problematic. In addition, numerous microorganisms are known to grow at 4 °C, therefore altering the quantitative assessment. Cryopreservation (<–20 °C or –80 °C) might be an adequate procedure for many of the analyses, but might affect future culturability of microbial aerosols (Donegan et al. Citation1991). Shipment with frozen ice packs can keep samples cool and shipment on dry ice (solid CO2) can keep samples frozen for up to several days. Consideration should always be made, and reported, for possible effects of additional freezing/thawing of samples.
An additional challenge to characterizing bioaerosols is their complex mixing state. Bacterial aerosols for example are rarely free floating, but often clumped together or attached to other particles (Turnbull et al. Citation1998; Lighthart et al. Citation1993); thus, breaking up of aggregates can introduce biases in the quantification of bacterial aerosols. Microscopic investigations of the bioaerosol mixing states (e.g., biological particle attached to mineral dust grains) thus rely on “soft” sampling techniques (e.g., electrostatic precipitation) that preserve their authentic state (Zavala et al. Citation2014; Mainelis et al. Citation2002).
3.4. Emission/deposition fluxes and long-range transport
[i: Wind speed] Originating from diverse sources, bioaerosol populations are often heterogeneous and represent a mixture of local and long-range transported biological particles (Šantl-Temkiv et al. Citation2018; Weil et al. Citation2017; Mazar et al. Citation2016). While the emission of inorganic dust increases with wind speed, microbial aerosol release has a more complex relationship with wind (Waggoner Citation1973). For example, minimum wind speeds of approximately 0.4 m s−1 are required for microbial aerosol emission from plant canopies (Jones and Harrison Citation2004), whereas 4 m s−1 is required for emission via sea spray (O’Dowd and de Leeuw Citation2007). After aerosolization, microbial cells and other bioaerosols with a diameter of ∼1 µm have been modeled to spend on average 3–4 days aloft in the atmosphere (Burrows et al. Citation2009), but are also observed and modeled to be airborne over longer periods (Barberán et al. Citation2015; Wilkinson et al. Citation2012; Kellogg and Griffin Citation2006). Active mixing processes at the boundary layer, such as turbulence and wind, transport microbial populations into the free atmosphere and induce the long-range transport by winds (Iwasaka et al. Citation2009; Maki et al. Citation2008).
[ii: Long-range transport and mixing processes] Microorganisms are known to disperse on long-range scales (≫1,000 km), e.g., with dust events (Weil et al. Citation2017; Mazar et al. Citation2016). In seeking to identify long-range sources of bioaerosols, including bacteria, sampling is frequently performed at sites that can at least occasionally sample free tropospheric air, such as mountaintop observatories (Weil et al. Citation2017; Smith et al. Citation2013; Vaitilingom et al. Citation2013; Bowers et al. Citation2012), tall towers (Uetake et al. Citation2019; Moran-Zuloaga et al. Citation2018; Jeon et al. Citation2011), and networks of roof-level stations distributed over the continent (de Weger et al. Citation2016; Šikoparija et al. Citation2013). During long-range transport, microbial aerosols change dynamically due to mixing with new air masses, microbial activity, and aging. The long-range transported microorganisms at high altitudes are often mixed with anthropogenic particles originating from agricultural (Suski et al. Citation2018) and industrial processes (Maki et al. Citation2017a), with sea-spray particles over marine environments (Maki et al. Citation2019; Mazar et al. Citation2016; Maki et al. Citation2014; Yamaguchi et al. Citation2012; Polymenakou et al. Citation2008) or particles derived from freshwater and plant surfaces. Dust events have been shown to have increased abundance of airborne microorganisms by 10- to 100-fold (Hara and Zhang Citation2012; Lim et al. Citation2011; Prospero et al. Citation2005). In areas downwind of dust events, regional airborne microbial communities are changed after mixing with bioaerosols originating from the dust event (Weil et al. Citation2017; Maki et al. Citation2014). In many cases, microorganisms are damaged by atmospheric stressors during their long-range transport with dust, e.g., leaving only ∼20% of microorganisms viable despite the fact that agglomeration with dust can provide protection from heat or UV flux (Hara and Zhang Citation2012). Experiments in atmospheric chambers allowed estimation of a half-life of ∼4 h for Pseudomonas syringae bacteria (Amato et al. Citation2015). In the atmosphere, many environmental factors will have additional adverse effects on bacterial cells, resulting in even shorter half-lives (Wirgot et al. Citation2017; Joly et al. Citation2015; Smith et al. Citation2011).
[iii: Assessing fluxes] Models that try to simulate the environmental impacts of bioaerosols suffer from several uncertainties. Studies have estimated concentrations of various classes of bioaerosols, but large uncertainties (e.g., 80%–870%) in emission estimates arise (Burrows et al. Citation2009) from the fact that very few direct measurements of bioaerosol emission or deposition have been performed (Sesartic, Lohmann, and Storelvmo Citation2012; Elbert et al. Citation2007). The exchange of aerosols between a surface and the atmosphere is quantified by measuring its flux, taking into account both emission and deposition rates. Past attempts to estimate bioaerosol fluxes relied on the gradient method, which scales a gradient of concentrations measured at different heights to a flux through the so-called eddy-diffusivity coefficient (Baldocchi, Hincks, and Meyers Citation1988). These are not direct measurements, as the estimation depends on the parameterization of the diffusivity coefficient. Varying results were obtained by this technique, which may in many cases be associated with the choice of specific parameterization and the errors associated with the cultivation-dependent techniques used to quantify concentrations (Carotenuto et al. Citation2017; Mayol et al. Citation2014; Crawford et al. Citation2014; Huffman et al. Citation2013; Lighthart and Shaffer Citation1994; Lindemann and Upper Citation1985; Lindemann et al. Citation1982). The gradient method also tends to fail in specific cases such as under canopies, as in forests, due to the formation of counter gradients (Baldocchi, Hincks, and Meyers Citation1988). In contrast, the eddy-covariance method (Baldocchi, Hincks, and Meyers Citation1988), which is the best available measure for fluxes, is based on direct measurements. To apply the eddy-covariance method, bioaerosol concentration should, however, be measured at a frequency of at least ten times per second. This may be possible in specific cases with high bioaerosol concentrations by using real-time bioaerosol sensors, but these sensors most often sample with insufficient flow rates to enable meaningful statistics at normally low atmospheric bioaerosol concentrations (<100 L−1). Thus, no currently available sensors can enable bioaerosol flux measurements by the eddy-covariance method for general use at different bioaerosol concentrations. Further improvements that would allow direct flux measurements may be possible using real-time optical methods that are under constant improvement, such as UV-laser-induced fluorescence (Huffman et al. Citation2019). While the detected particle concentrations are likely not sufficient for direct measurement by the eddy-covariance method, the real-time bioaerosol sensors are suitable for flux measurements following the disjunct eddy-covariance approach (Crawford et al. Citation2014; Whitehead et al. Citation2010; Haugen Citation1978). As such, real-time sensors are capable of providing environmental concentration values and therefore could potentially provide the opportunity to directly measure fluxes, e.g., of fluorescent aerosols. An associated challenge is the analytical uncertainty linking fluorescent aerosol with classes of bioaerosols, but advancements in instrumentation and analysis strategy continue to reduce these uncertainties (Huffman et al. Citation2019; Savage and Huffman Citation2018; Ruske et al. Citation2017).
3.5. Stationary bioaerosol research
[i: Choosing the location] Bioaerosol sources can be hyperlocal in nature. For example, individual plants can emit pollen, spores, or bacteria that could overwhelm a sampler and mislead interpretation of results meant to characterize the region more generally. The amount of the upwind area that contributes to the collected sample (i.e., the concentration footprint) will depend on the height of sampling (Rojo et al. Citation2019), wind speed, and atmospheric stability (Vesala et al. Citation2008). Due to this strong dependence on environmental conditions, there is not a single “recipe” for correct sampling, though certain sampling practices are encouraged. The ideal sampling location should be located above the roughness sublayer to minimize influence from local turbulent disruptions, i.e., 2–5 times the height of the roughness elements (Raupach, Antonia, and Rajagopalan Citation1991) and with as much homogeneous surface area upwind as possible (Vesala et al. Citation2008). Aside from choosing an appropriate sampling location, the spatial heterogeneity of the atmosphere can be assessed through replicate sampling (Temkiv et al. Citation2012, see also Mainelis Citation2019).
[ii: Monitoring networks] Providing data for health management requires a widespread network of measurements, which must ensure comparability and reproducibility. Both the sampling and analysis could introduce errors, and notable efforts are made to ensure high quality regarding identification of bioaerosols, which is commonly done manually (Sikoparija et al. Citation2017; Galán et al. Citation2014). The achieved level of standardization (British Standards Institution Citation2019) is now challenged by introduction of new methods that involve a notable portion of automatization and where identification of bioaerosols is achieved by computational tools (Šaulienė et al. Citation2019). It is becoming increasingly apparent that what is measured is often not the same as what we breathe. For example, while aiming to provide data representative of a relatively large geographical region, measurements are often performed at roof level resulting in notable underestimation of bioaerosol concentrations at the street (i.e., breathing) level (Rojo et al. Citation2019). As a result, personal and portable samplers are important to link health effects to exposure. Also, it seems that exposure to allergen-containing particles alone does not have to result in health impacts, as was seen with respect to mugwort pollen, where sensitization occurs only from pollen contaminated with bacterial endotoxins (Oteros et al. Citation2019). In order to adequately support health impact assessments, outdoor field measurements should provide data on all important cofactors. This strategy goes beyond identification of allergen-containing particles (i.e., pollen grain and fungal spore) and requires simultaneous identification and quantification of modulators of immunological reaction. In addition, the allergen modeling community requires bioaerosol measurements in real time to support model assimilation. At the same time, however, new measurement approaches should be compatible with historical datasets in order to integrate with long-term climatological studies (Ziska et al. Citation2019).
3.6. Vessel-driven bioaerosol research
[i: Marine sampling] Ships, both powered and sailing, are excellent platforms for sampling marine bioaerosols. Ship-borne sampling has been used to measure intact marine entities in aerosols (Könemann et al. Citation2018; Mayol et al. Citation2014; Fröhlich-Nowoisky et al. Citation2012; Leck and Bigg Citation2008; Aller et al. Citation2005), their excretions (e.g., exopolymer gels, carbohydrates) and decomposition products (Chance et al. Citation2018; Aller et al. Citation2017; Gantt and Meskhidze Citation2013; Orellana et al. Citation2011; Russell et al. Citation2010; Leck and Bigg Citation2008), CCN (Gantt and Meskhidze Citation2013; Orellana et al. Citation2011), and INPs (Irish et al. Citation2019; McCluskey et al. Citation2018; DeMott et al. Citation2016; Wilson et al. Citation2015; Burrows et al. Citation2013; Schnell Citation1977; Bigg Citation1973). Recently, a number of scientific consortia have begun contributing to large-scale transoceanic research programs designed to better understand ocean–atmosphere interactions, and so significant advances in these areas are expected in coming years (Behrenfeld et al. Citation2019). INP and microbial concentrations over remote oceans are often extremely low compared with terrestrial values, and thus, different sampling strategies are frequently required to achieve detectable concentrations. Under such pristine conditions, preferably >50 m3 of air should be sampled to allow molecular, microbial, or INP analyses, and hence, high-flow-rate samplers should be employed (Šantl-Temkiv et al. Citation2017). Care should also be taken to avoid exhaust plume contamination (Thomson et al. Citation2018).
[ii: Aerial sampling] Aerial vessels such as planes, drones, and balloons facilitate the exploration of spatial dynamics in both horizontal (i.e., transects) and vertical (i.e., profiles) dimensions. While moving through the air, they also allow for collection of volumes of air that represent a wide spectrum of spatial variability in a single sample. Bioaerosol profiles, particularly spanning across the lower troposphere, provide crucial information on the amount and identity of bioaerosols being transported to altitudes where they could influence aerosol-cloud processes. Bioaerosol samples have been collected by aircraft (Smith et al. Citation2018; Ziemba et al. Citation2016; Maki et al. Citation2015; DeLeon-Rodriguez et al. Citation2013; Yamaguchi et al. Citation2012; Kourtev et al. Citation2011), helicopters (Maki et al. Citation2017b), blimps (Perring et al. Citation2015), and altitude-controlled balloons (Creamean et al. Citation2018b) to investigate their long-range transport, while avoiding the ground surface contamination. Drones and other unpiloted aerial vessels can also be used to explore hazardous areas. When rotary wings are employed, the sensors’ inlets have to be placed in disturbance-free areas to ensure representative measurements (Villa et al. Citation2016). In some cases, new samplers have been developed specifically for use on drones, from simple impaction on Petri dishes, spore collectors (Jimenez-Sanchez et al. Citation2018; Powers et al. Citation2018; Lin et al. Citation2013; Schmale Iii, Dingus, and Reinholtz Citation2008; Schmale et al. Citation2012; Aylor et al. Citation2011; Gonzalez et al. Citation2011), and filters (Crazzolara et al. Citation2019; Lateran et al. Citation2016; Smith et al. Citation2015), to more complex and specialized methods paired with immunoassays (Anderson et al. Citation1999; Ligler et al. Citation1998), surface plasmon resonance (Palframan et al. Citation2014), and cryopumps (Harris et al. Citation2002). Simultaneous recovery of bioaerosols from different altitudes has been attempted with drones (Powers et al. Citation2018; Lateran et al. Citation2016; Techy, Schmale, and Woolsey Citation2010; Yang et al. Citation2008). While physical collectors can only integrate bioaerosols information over a given sampling time, optical methods would be able to give real-time information on airborne bioaerosols (Huffman et al. Citation2019). Drones able to carry real-time sensors for bioaerosols would represent a game changer in airborne outdoor measurements, allowing the retrieval of information at high-spatial resolution in three dimensions. While scientific drones generally have short endurance and are able to explore only the lower troposphere, balloon-carried devices can explore all the way to the stratosphere (Bryan et al. Citation2014; Yang et al. Citation2008; Harris et al. Citation2002; Rogers and Meier Citation1937) and have frequently been used to collect bioaerosols on filters (Maki et al. Citation2017a; Maki et al. Citation2015; Maki et al. Citation2013; Chen et al. Citation2011; Iwasaka et al. Citation2009; Maki et al. Citation2008; Kakikawa et al. Citation2008). Other kinds of airships such as blimps or zeppelins could be viable airborne laboratories since they are able to perform long flights at low speeds. In one such example, the behavior of airborne biological fluorescent particles was characterized in a transcontinental flight across the United States (Perring et al. Citation2015).
4. Research needs and future concerning outdoor bioaerosol measurements
4.1. Pressing scientific questions
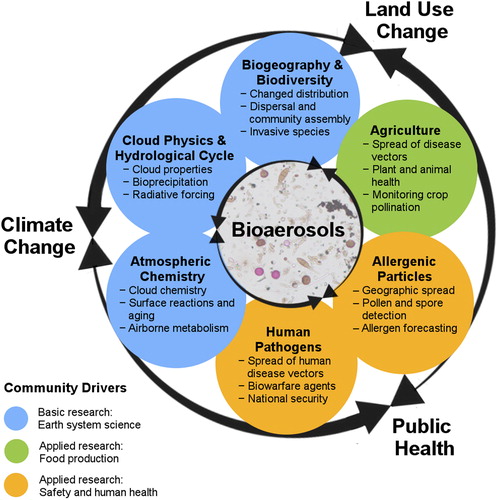
The increased interest in bioaerosols has been motivated by their health, agricultural, environmental, and climate effects, which are related to several U.N. Sustainable Development Goals (United Nations Citation2015). The inter-related communities that conduct outdoor bioaerosol field measurements (see Section 2) are guided by a variety of motivations, broadly summarized as colored circles in , which are further driven primarily by some combination of scientific, economic, societal, and safety interests. These motivations fall within inter-related scientific and social grand challenges, including climate change, land cover/use change, and public health. Below, we summarize eight areas of pressing scientific needs. Many ideas concern several of these topic areas, and each topic can have relevance to multiple scientific disciplines.
Figure 1. Motivating topics of outdoor bioaerosol field measurements (circles) from the perspective of different research communities, with bulleted examples of application. These are embedded into the context of current scientific and social grand challenges, i.e., climate change, land use change, and public health, and categorized by the primary community drivers. Community drivers are listed separately to highlight differences in communities, although there is also substantial overlap. The drivers primarily imply differences in scientific motivation (e.g., basic vs. applied questions), funding source (e.g., research agencies, governmental, industrial), and result dissemination strategy (e.g., peer-reviewed publications, reports, proprietary information). Dual-directional arrows visually represent overlap and interaction between grand challenges and between fields of bioaerosol research. All topics are influenced in some measure by each of the three grand challenges listed. Similarly, each of the topic circles can have some influence from each of the community drivers (color), but in each case, the weight of influence is different. Central image shows a mixture of bioaerosols analyzed via optical microscopy.
Emission sources and flux estimates: The upward vertical transport of bioaerosols from sources at the surface to the lowest layers of the atmosphere, i.e., emission flux, is technically challenging to assess. This has led to a significant lack of quantitative estimates of surface-atmosphere bioaerosol exchange. Direct assessment of fluxes is important to resolve major questions concerning the mechanisms and strength of bioaerosol emissions from the diverse marine and terrestrial sources, including natural emissions (e.g., due to the active release of propagules or passive release of bioaerosols due to wind), and anthropogenic emissions (e.g., caused by wheat harvesting or processing silage) as well as emissions caused by extreme events such as thunderstorms, strong thermals, and winds.
Spatial distribution: Another important and related question concerns how bioaerosols are distributed vertically and over different habitats of interest (including urban and rural environments, pristine polar and marine environments, forests, grasslands, and deserts) and how this relates to the climate, environmental, economic, and health effects of the particles. In order to bridge the source emission strength with the bioaerosol vertical and horizontal distributions, it is necessary to (i) understand the transfer of bioaerosols to higher atmospheric layers and their long-range dispersal, (ii) obtain a mechanistic understanding of bioaerosol dynamics: their survival, aging, activity, and growth as well as interaction with water in the atmosphere, (iii) determine the rate of bioaerosol depletion through dry or wet deposition or cell death as a function of atmospheric residence time, and (iv) quantify the importance of their dynamic ‘multiplication processes’ in the atmosphere, e.g., release of smaller particles (fragments, cytosolic content) upon cell lysis or particle rupture, emission of membrane vesicles, leaching in cloud droplets, or reproduction of microorganism, each of which may alter concentration of bioaerosols that can serve as allergens and cloud nuclei (also see points [4] and [5]).
Changes in distribution: Ecosystems across the world face mounting pressure from a changing climate, and as a result, patterns of distribution are changing for all kingdoms of life. Continued studies are required to assess to what extent the natural (preindustrial) bioaerosol cycling has been altered by human activities (e.g., through climate change, large-scale land use change, alterations in the water cycle, changes in species distribution, and air pollution), by focusing on in-depth bioaerosol studies at ‘pristine’ sites. Geographic distribution and seasonal timing of pollination are changing as the climate warms, and this has important implications for airborne human allergies, ecology, agriculture, and the timing of release of pollen INPs. It has been proposed that patterns in precipitation and the hydrological cycle may be influenced by bioaerosol emission through alteration of land use driven by climate and human activity. Changes in dispersal patterns of plant pathogens, as related to both natural ecology and agriculture, are also affected. Thus, monitoring in a variety of ecosystem types is required to assess changes in bioaerosol distribution, as related to geography of airborne pollination, invasion of plant, fungal, or bacterial species, and associated changes in biodiversity.
Atmospheric aging: There is a necessity to understand how atmospheric aging affects the different bioaerosol functions, such as their hygroscopicity and nucleation, their allergenic impact, as well as their ability to colonize new surfaces or hosts. Finally, the viability half-lives of airborne microorganisms exhibit under different realistic conditions should be determined.
Metabolic activity: This unique aspect of bioaerosols should be evaluated as a function of water availability in the atmosphere. There is an urgency to understand the extent of metabolic activity in airborne microorganisms and thus their ability to change: (i) budgets of atmospheric organic and inorganic compounds, (ii) the surface properties impacting their hygroscopicity and nucleation potential, and (iii) their toxicity, pathogenicity, and ability to colonize new environments.
Urbanization of allergies: A variety of causes have been hypothesized for the increased allergies in recent decades and in urban areas, including internal mixtures of pollen, i.e., with combustion soot, atmospheric reaction with urban pollutant gases as well as alteration of physical properties and emission rates from plants exposed to pollution. Additional studies are required to understand influence of allergic diseases from factors associated with climate change, urbanization, and urban pollution. Longer-term epidemiological studies linking multiple factors are also required to study the complex relationships that influence human health.
Transport of human pathogens: The transmission of airborne pathogens to human hosts (e.g., viral and bacterial aerosols) is generally understood to take place largely in indoor spaces. The role of outdoor air for dispersal of pathogens is less well understood, including how far different pathogens can travel within a city or occupied region and the factors that influence pathogen viability. These questions relate not only to naturally dispersed aerosol (e.g., from sick individuals) but also to intentionally aerosolized harmful agents.
Climate-relevant properties: Finally, there is a pressing need to obtain a functional understanding of the environmental, climatic, health, and economical importance of bioaerosol hygroscopicity, cloud droplet activation, and ice nucleation. Aside from their climate impacts, the hygroscopic properties of bioaerosols, for example, impact deposition in the lungs and can thus have health impacts. Hygroscopicity also influences allergenic pollen deposition and thus their spatiotemporal distribution (Sofiev et al. Citation2006). Finally, liquid water droplet activation and ice nucleation activity impact atmospheric residence time and deposition rates, which leads to effects on colonization. An improved understanding of the physicochemical processes of water activation and ice nucleation on bioaerosol surfaces is required. To better understand the effects of bio-INPs on cloud formation and evolution, cloud radiative forcing, and the hydrological cycle, expanded input of measurement data to both global and regional models is needed. This necessitates close collaboration between modeling and measurement communities to streamline efforts for inclusion of the most cloud-relevant properties (i.e., simplified size distributions of INPs active at specific temperatures).
4.2. Technical challenges, recommendations, and needs
The atmosphere is dynamic, with high fluctuations in bioaerosol quantities and types. Obtaining a representative sample is a challenge in light of the diversity bioaerosols represent and the multitude of interactions they can have with aging effects. The atmosphere also has a strong diluting capability; thus, microbiological events in the air are difficult to capture. The research questions strongly orient the choice of sampling and analytical techniques. Samplers influence results due to differences in size fractionation, efficiency, and sample volume. These effects have consequences on the reliability of inter-study comparisons, especially if the studies to be compared do not have the same objectives. Thus, there is a distinct need for standardization of measurement practices (e.g., Environmental Agency Citation2018). While the diversity of research objectives implies the need for broadly diverse approaches, consistency within each approach is necessary across users and experiments. Needs include the following:
Standardization of collection strategies concerning the spatial and temporal resolution, replicated sampling, appropriate sterile practices and controls, improved intake efficiency, losses in ducts and tubes, increased sample biomass (i.e., high-volume bioaerosol sampling technique with high concentrate rate) and techniques allowing access to microbial in situ states (Cox et al. Citation2019; Mainelis Citation2019).
Improved real-time measurements: fast reliable real-time quantification and specific real-time sensing technologies which will allow high time-resolution monitoring necessary for detecting key moments that bioaerosols exhibit in the atmosphere (Huffman et al. Citation2019). Standardization of methodology and reduction in false-positive detection by real-time techniques, e.g., fluorescence sensors, will further improve the quality of bioaerosol data acquired (Savage et al. Citation2017).
Further development of new technologies is expected to improve the quality, quantity, and timescale of bioaerosol measurements. Some examples of these emerging and improving technologies include unpiloted aircraft and drones, tethersondes, additive manufacturing (i.e., 3D printing), and microfluidic detection technologies (i.e., lab-on-a-chip) which will all allow easier customization of sampling instruments.
Long-term measurements at selected sampling sites, in particular at supersites that have a broad context of complementary meteorological, ecological, trace gas, and aerosol observations to link aerobiological and physicochemical observations. This could be achieved by including bioaerosol measurements in large measurement campaigns to complement the well-documented meteorological, chemical, and physical descriptions with biological information.
A network of observatories spread at different spatial scales (e.g., low-cost detectors, distributed through citizen science or establishing bioaerosol monitoring networks for climate purpose), as was developed through FluxNet/Ameriflux for CO2 or for continuous monitoring of airborne pollen that is well established in Europe.
Development of (standardized) methods for estimating surface-to-atmosphere exchanges of bioaerosols (i.e., fluxes).
Simultaneous deployment of real-time instruments that can provide quantitative information at high time resolution (but generally with low specificity) with samplers that collect for longer periods for off-line analysis. This will allow the characterization of bioaerosols with higher specificity (i.e., organism identification via molecular biological techniques) to obtain an improved understanding of spatiotemporal variability of specific functional or taxonomic organism classes.
New solutions for specific bioaerosol detection, for example based on direct molecular biology techniques, high-throughput screening methods targeting specific groups of interest, and next-generation mass-spectra-based detectors.
Development of methods for source tracking of bioaerosols to address the questions of the contribution by local, regional, and distant sources as well as the contribution of microbial growth in air.
Pinpointing of tracers to be used for determining bioaerosol age: local and distant sources, as well as aging.
Complementing bulk measurements by ambient analyses of single bioparticle properties (i.e., morphology, surface properties, and mixing state) as well as the transformation of these properties in the atmosphere, which is crucial for an improved understanding of nucleation processes.
4.3. Needs and means for interdisciplinarity and cross-community collaboration
While all research is ultimately motivated by societal or economic factors, some questions are somewhat more basic in nature (i.e., cloud physics, atmospheric chemistry, biodiversity) and secure funding, e.g., from federal research agencies, while others are more applied in nature and funded more directly, e.g., by companies for profit investment or by national security agencies for the protection of citizens and military personnel (). These differences in motivation and funding can lead to divides in the interest and ability to share results and experience across community lines. For example, the development of improved detection technologies against agents of biowarfare is frequently hidden behind security clearances, and information related to agricultural or farming practices can be proprietary in nature. These various communities have developed somewhat independently and thus approach research questions from different perspectives. As a result, there are often discrepancies due to the lack of collaboration between communities. Due to the theoretical and technical resources needed for carrying out outdoor bioaerosol field studies, these should be approached from interdisciplinary research fields. Dedicated field measurements should be supported by chamber experiments, in order to obtain a mechanistic understanding of bioaerosol fluxes, which is crucial for forecasting their future emission strength and distribution. Therefore, there is a need to bring researchers together with expertise in microbiology, meteorology, aerosol physics, aerosol chemistry, and bioaerosol engineering to design complementary chamber, laboratory, and field experiments. In this way, the bioaerosol field could eventually introduce exciting research ideas and directions to traditional disciplines. In addition to interdisciplinary efforts, cross-community discussion and collaboration should promote exchange of ideas, approaches, and technical advances between the different disciplines studying bioaerosols. For example, the idea of monitoring networks initiated for quantifying airborne allergen-containing particles could be applied to studying climatic and pathogenic aspects of bioaerosols or advanced molecular microbiology techniques currently used in the study of microbial diversity in biogeography could be applied to fields that use more traditional techniques, such as allergen and pathogen detection. Consequently, we need to identify appropriate or establish new platforms, where researchers could meet cross disciplines and communities in order to network, exchange ideas and approaches, and identify collaborative funding opportunities.
Acknowledgments
The authors acknowledge organizers of this special issue “Bioaerosol Research: Methods, Challenges, and Perspectives,” including Shanna Ratnesar-Shumate and Alex Huffman, as well as the AAAR Bioaerosol Working Group and the Bioaerosol Standardization Workshop at the International Aerosol Conference in St Louis, Missouri in September 2018.
Additional information
Funding
References
- Aller, J. Y., M. R. Kuznetsova, C. J. Jahns, and P. F. Kemp. 2005. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 36 (5–6):801–812. doi: 10.1016/j.jaerosci.2004.10.012.
- Aller, J. Y., J. A. C. Radway, W. P. Kilthau, D. W. Bothe, T. W. Wilson, R. D. Vaillancourt, P. K. Quinn, D. J. Coffman, B. J. Murray, and D. A. Knopf. 2017. Size-resolved characterization of the polysaccharidic and proteinaceous components of sea spray aerosol. Atmos. Environ. 154:331–347. doi: 10.1016/j.atmosenv.2017.01.053.
- Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59 (1):143–169.
- Amato, P., L. Besaury, M. Joly, B. Penaud, L. Deguillaume, and A. M. Delort. 2019. Metatranscriptomic exploration of microbial functioning in clouds. Sci. Rep. 9 (1):1–12.
- Amato, P., F. Demeer, A. Melaouhi, S. Fontanella, A.-S. Martin-Biesse, M. Sancelme, P. Laj, and A. M. Delort. 2007a. A fate for organic acids, formaldehyde and methanol in cloud water: Their biotransformation by micro-organisms. Atmos. Chem. Phys. 7 (15):4159–4169. doi: 10.5194/acp-7-4159-2007.
- Amato, P., M. Joly, L. Besaury, A. Oudart, N. Taib, A. I. Moné, L. Deguillaume, A.-M. Delort, and D. Debroas. 2017. Active microorganisms thrive among extremely diverse communities in cloud water. PLoS One 12 (8):e0182869. doi: 10.1371/journal.pone.0182869.
- Amato, P., M. Joly, C. Schaupp, E. Attard, O. Möhler, C. E. Morris, Y. Brunet, and A.-M. Delort. 2015. Survival and ice nucleation activity of bacteria as aerosols in a cloud simulation chamber. Atmos. Chem. Phys. 15 (11):6455–6465. doi: 10.5194/acp-15-6455-2015.
- Amato, P., M. Parazols, M. Sancelme, P. Laj, G. Mailhot, and A. M. Delort. 2007b. Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dôme: Major groups and growth abilities at low temperatures. FEMS Microbiol. Ecol. 59 (2):242–254. doi: 10.1111/j.1574-6941.2006.00199.x.
- Amato, P., M. Parazols, M. Sancelme, G. Mailhot, P. Laj, and A.-M. Delort. 2007c. An important oceanic source of micro-organisms for cloud water at the Puy de Dôme (France). Atmos. Environ. 41 (37):8253–8263. doi: 10.1016/j.atmosenv.2007.06.022.
- An, S., C. Couteau, F. Luo, J. Neveu, and M. S. DuBow. 2013. Bacterial diversity of surface sand samples from the Gobi and Taklamaken Deserts. Microb. Ecol. 66.
- Anderson, G. P., K. D. King, D. S. Cuttino, J. P. Whelan, F. S. Ligler, J. F. MacKrell, C. S. Bovais, D. K. Indyke, and R. J. Foch. 1999. Biological agent detection with the use of an airborne biosensor. Field Anal. Chem. Technol. 3 (4–5):307–314. doi: 10.1002/(SICI)1520-6521(1999)3:4/5<307::AID-FACT9>3.0.CO;2-M.
- Andreae, M. O. 2007. Atmosphere. Aerosols before pollution. Science 315 (5808):50–51. doi: 10.1126/science.1136529.
- Andreae, M. O., and P. J. Crutzen. 1997. Atmospheric aerosols: Biogeochemical sources and role in atmospheric chemistry. Science 276 (5315):1052–1058. doi: 10.1126/science.276.5315.1052.
- Archer, S. D. J., K. C. Lee, T. Caruso, T. Maki, C. K. Lee, S. C. Cary, D. A. Cowan, F. T. Maestre, and S. B. Pointing. 2019. Airborne microbial transport limitation to isolated Antarctic soil habitats. Nat. Microbiol. 4 (6):925–932. doi: 10.1038/s41564-019-0370-4.
- Ariya, P. A., O. Nepotchatykh, O. Ignatova, and M. Amyot. 2002. Microbiological degradation of atmospheric organic compounds. Geophys. Res. Lett. 29 (22):34-1–34-4. doi: 10.1029/2002GL015637.
- Attard, E., H. Yang, A.-M. Delort, P. Amato, U. Pöschl, C. Glaux, T. Koop, and C. E. Morris. 2012. Effects of atmospheric conditions on ice nucleation activity of pseudomonas. Atmos. Chem. Phys. 12 (22):10667–10677. doi: 10.5194/acp-12-10667-2012.
- Augustin, S., H. Wex, D. Niedermeier, B. Pummer, H. Grothe, S. Hartmann, L. Tomsche, T. Clauss, J. Voigtländer, K. Ignatius, et al. 2013. Immersion freezing of birch pollen washing water. Atmos. Chem. Phys. 13 (21):10989–11003. doi: 10.5194/acp-13-10989-2013.
- Aylor, D. E., D. G. Schmale, III, E. J. Shields, M. Newcomb, and C. J. Nappo. 2011. Tracking the potato late blight pathogen in the atmosphere using unmanned aerial vehicles and Lagrangian modeling. Agric. For. Meteorol. 151 (2):251–260. doi: 10.1016/j.agrformet.2010.10.013.
- Baldocchi, D. D., B. B. Hincks, and T. P. Meyers. 1988. Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology 69 (5):1331–1340. doi: 10.2307/1941631.
- Banchi, E., C. G. Ametrano, D. Stanković, P. Verardo, O. Moretti, F. Gabrielli, S. Lazzarin, M. F. Borney, F. Tassan, M. Tretiach, et al. 2018. DNA metabarcoding uncovers fungal diversity of mixed airborne samples in Italy. PLoS One 13 (3):e0194489–20. doi: 10.1371/journal.pone.0194489.
- Barbaro, E., T. Kirchgeorg, R. Zangrando, M. Vecchiato, R. Piazza, C. Barbante, and A. Gambaro. 2015. Sugars in Antarctic aerosol. Atmos. Environ. 118:135–144. doi: 10.1016/j.atmosenv.2015.07.047.
- Barberán, A., J. Ladau, J. W. Leff, K. S. Pollard, H. L. Menninger, R. R. Dunn, and N. Fierer. 2015. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Natl. Acad. Sci. 112 (18):5756–5761. doi: 10.1073/pnas.1420815112.
- Bauer, H., E. Schueller, G. Weinke, A. Berger, R. Hitzenberger, I. L. Marr, and H. Puxbaum. 2008. Significant contributions of fungal spores to the organic carbon and to the aerosol mass balance of the urban atmospheric aerosol. Atmos. Environ. 42 (22):5542–5549. doi: 10.1016/j.atmosenv.2008.03.019.
- Behrenfeld, M. J., R. H. Moore, C. A. Hostetler, J. Graff, P. Gaube, L. M. Russell, G. Chen, S. C. Doney, S. Giovannoni, H. Liu, et al. 2019. The North Atlantic Aerosol and Marine Ecosystem Study (NAAMES): Science motive and mission overview. Front. Mar. Sci. 6:1–25. doi: 10.3389/fmars.2019.00122.
- Bigg, E. K. 1973. Ice nucleus concentrations in remote areas. J. Atmos. Sci. 30 (6):1153–1157. doi: 10.1175/1520-0469(1973)030<1153:INCIRA>2.0.CO;2.
- Bigg, E. K. 1996. Ice forming nuclei in the high Arctic. Tellus Ser. B Chem. Phys. Meteorol. 48 (2):223–233. doi: 10.1034/j.1600-0889.1996.t01-1-00007.x.
- Bigg, E. K., and C. Leck. 2001. Cloud-active particles over the central Arctic Ocean were typically in the range -3 but of IFN ranged from the Pack ice at the beginning of the expedition at the end. The differences with transport time from the ice edge were less marked. J. Geophys. Res. 106 (D23):32155–32166.
- Bigg, E. K., S. Soubeyrand, and C. E. Morris. 2015. Persistent after-effects of heavy rain on concentrations of ice nuclei and rainfall suggest a biological cause. Atmos. Chem. Phys. 15 (5):2313. doi: 10.5194/acp-15-2313-2015.
- Boucher, O., D. Randall, P. Artaxo, C. Bretherton, G. Feingold, P. Forster, V.-M. Kerminen, Y. Kondo, H. Liao, U. Lohmann, et al. 2013. Clouds and aerosols, In Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment, report of the intergovernmental panel on climate change, ed. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley, 571–657. Cambridge, UK and New York, NY: Cambridge University Press.
- Bowers, R. M., N. Clements, J. B. Emerson, C. Wiedinmyer, M. P. Hannigan, and N. Fierer. 2013. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 47 (21):12097–12106. doi: 10.1021/es402970s.
- Bowers, R. M., C. L. Lauber, C. Wiedinmyer, M. Hamady, A. G. Hallar, R. Fall, R. Knight, and N. Fierer. 2009. Characterization of airborne microbial communities at a high-elevation site and their potential to act as atmospheric ice nuclei. Appl. Environ. Microbiol. 75 (15):5121–5130. doi: 10.1128/AEM.00447-09.
- Bowers, R. M., I. B. McCubbin, A. G. Hallar, and N. Fierer. 2012. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos. Environ. 50:41–49. doi: 10.1016/j.atmosenv.2012.01.005.
- Bowers, R. M., S. McLetchie, R. Knight, and N. Fierer. 2011. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 5 (4):601–612. doi: 10.1038/ismej.2010.167.
- British Standards Institution. 2019. Ambient air - sampling and analysis of airborne pollen grains and fungal spores for networks related to allergy - volumetric Hirst method, 1–42. BS 16868.
- Bryan, N. C., M. Stewart, D. Granger, T. G. Guzik, and B. C. Christner. 2014. A method for sampling microbial aerosols using high altitude balloons. J. Microbiol. Methods 107:161–168. doi: 10.1016/j.mimet.2014.10.007.
- Burrows, S. M., T. Butler, P. Jöckel, H. Tost, A. Kerkweg, U. Pöschl, and M. G. Lawrence. 2009. Bacteria in the global atmosphere - part 2: Modeling of emissions and transport between different ecosystems. Atmos. Chem. Phys. 9 (23):9281–9297. doi: 10.5194/acp-9-9281-2009.
- Burrows, S. M., W. Elbert, M. G. Lawrence, and U. Pöschl. 2009. Bacteria in the global atmosphere – Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 9 (23):9263–9280. doi: 10.5194/acp-9-9263-2009.
- Burrows, S. M., C. Hoose, U. Pöschl, and M. G. Lawrence. 2013. Ice nuclei in marine air: Biogenic particles or dust? Atmos. Chem. Phys. 13(1):245. doi: 10.5194/acp-13-245-2013.
- Buters, J. T. M., C. Antunes, A. Galveias, K. C. Bergmann, M. Thibaudon, C. Galán, C. Schmidt-Weber, and J. Oteros. 2018. Pollen and spore monitoring in the World. Clin. Transl. Allergy 8 (1):9.
- Buters, J., M. Prank, M. Sofiev, G. Pusch, R. Albertini, I. Annesi-Maesano, C. Antunes, H. Behrendt, U. Berger, R. Brandao, et al. 2015. Variation of the group 5 grass pollen allergen content of airborne pollen in relation to geographic location and time in season the HIALINE working group. J. Allergy Clin. Immunol. 136 (1):87–95. doi: 10.1016/j.jaci.2015.01.049.
- Buters, J. T. M., M. Thibaudon, M. Smith, R. Kennedy, A. Rantio-Lehtimäki, R. Albertini, G. Reese, B. Weber, C. Galan, R. Brandao, et al. 2012. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos. Environ. 55:496–505. doi: 10.1016/j.atmosenv.2012.01.054.
- Cáliz, J., X. Triadó-Margarit, L. Camarero, and E. O. Casamayor. 2018. A long-term survey unveils strong seasonal patterns in the airborne microbiome coupled to general and regional atmospheric circulations. Proc. Natl. Acad. Sci. 115 (48):12229–12234. doi: 10.1073/pnas.1812826115.
- Carnelley, T., J. S. Haldane, and A. M. Anderson. 1887. IV. The carbonic acid, organic matter, and micro-organisms air, more especially of dwellings and schools. Philos. Trans. R. Soc. London 178:61–111.
- Carotenuto, F., T. Georgiadis, B. Gioli, C. Leyronas, C. E. Morris, M. Nardino, G. Wohlfahrt, and F. Miglietta. 2017. Measurements and modeling of surface – atmosphere exchange of microorganisms in Mediterranean grassland. Atmos. Chem. Phys. 17 (24):14919–14936. doi: 10.5194/acp-17-14919-2017.
- Chance, R. J., J. F. Hamilton, L. J. Carpenter, S. C. Hackenberg, S. J. Andrews, and T. W. Wilson. 2018. Water-soluble organic composition of the Arctic sea surface microlayer and association with ice nucleation ability. Environ. Sci. Technol. 52 (4):1817. doi: 10.1021/acs.est.7b04072.
- Chen, B., F. Kobayashi, M. Yamada, Y.-H. Kim, Y. Iwasaka, and G.-Y. Shi. 2011. Identification of culturable bioaerosols collected over dryland in northwest China: Observation using a tethered balloon. Asian J. Atmos. Environ. 5 (3):172–180. doi: 10.5572/ajae.2011.5.3.172.
- Christner, B. C., C. E. Morris, C. M. Foreman, R. Cai, and D. C. Sands. 2008. Ubiquity of biological ice nucleators in snowfall. Science 319 (5867):1214. doi: 10.1126/science.1149757.
- Coluzza, I., J. Creamean, M. J. Rossi, H. Wex, P. A. Alpert, V. Bianco, Y. Boose, C. Dellago, L. Felgitsch, J. Fröhlich-Nowoisky, et al. 2017. Perspectives on the future of ice nucleation research: Research needs and unanswered questions identified from two international workshops. Atmosphere (Basel) 8 (8):138. doi: 10.3390/atmos8080138.
- Conen, F., E. Stopelli, and L. Zimmermann. 2016. Clues that decaying leaves enrich Arctic Air with ice nucleating particles. Atmos. Environ. 129:91–94. doi: 10.1016/j.atmosenv.2016.01.027.
- Conen, F., M. V. Yakutin, K. E. Yttri, and C. Hüglin. 2017. Ice nucleating particle concentrations increase when leaves fall in autumn. Atmosphere (Basel) 8 (10):202. doi: 10.3390/atmos8100202.
- Cox, J. D., H. Mbareche, W. G. Lindsley, and C. Duchaine. 2019. Bioaerosol indoor field sampling. Aerosol Sci. Technol. In review.
- Crawford, I., M. W. Gallagher, K. N. Bower, T. W. Choularton, M. J. Flynn, S. Ruske, C. Listowski, N. Brough, T. Lachlan-Cope, Z. L. Fleming, et al. 2017. Real-time detection of airborne fluorescent bioparticles in Antarctica. Atmos. Chem. Phys. 17 (23):14291–14307.
- Crawford, I., N. H. Robinson, M. J. Flynn, V. E. Foot, M. W. Gallagher, J. A. Huffman, W. R. Stanley, and P. H. Kaye. 2014. Characterisation of bioaerosol emissions from a Colorado pine forest: Results from the BEACHON-RoMBAS experiment. Atmos. Chem. Phys. 14 (16):8559–8578. doi: 10.5194/acp-14-8559-2014.
- Crazzolara, C., M. Ebner, A. Platis, T. Miranda, J. Bange, and A. Junginger. 2019. A new multicopter-based unmanned aerial system for pollen and spores collection in the atmospheric boundary layer. Atmos. Meas. Tech. 12 (3):1581–1598. doi: 10.5194/amt-12-1581-2019.
- Creamean, J. M., R. M. Kirpes, K. A. Pratt, N. J. Spada, M. Maahn, G. de Boer, R. C. Schnell, and S. China. 2018a. Marine and terrestrial influences on ice nucleating particles during continuous springtime measurements in an Arctic oilfield location. Atmos. Chem. Phys. 18:18023–18042.
- Creamean, J. M., K. Primm, M. A. Tolbert, E. G. Hall, J. Wendell, A. Jordan, P. J. Sheridan, J. Smith, and R. C. Schnell. 2018b. HOVERCAT: A novel aerial system for evaluation of aerosol-cloud interactions. Atmos. Meas. Tech. 11 (7):3969–3985. doi: 10.5194/amt-11-3969-2018.
- Crouzy, B., M. Stella, T. Konzelmann, B. Calpini, and B. Clot. 2016. All-optical automatic pollen identification: Towards an operational system. Atmos. Environ. 140:202–212. doi: 10.1016/j.atmosenv.2016.05.062.
- Cuthbertson, L., H. Amores-Arrocha, L. Malard, N. Els, B. Sattler, and D. Pearce. 2017. Characterisation of Arctic bacterial communities in the air above Svalbard. Biology (Basel) 6 (2):29. doi: 10.3390/biology6020029.
- D’Amato, G., C. Vitale, M. Lanza, A. Molino, and M. D’Amato. 2016. Climate change, air pollution, and allergic respiratory diseases: An update. Curr. Opin. Allergy Clin. Immunol. 16 (5):434–440. doi: 10.1097/ACI.0000000000000301.
- Damialis, A., D. Vokou, D. Gioulekas, and J. M. Halley. 2015. Long-term trends in airborne fungal-spore concentrations: A comparison with pollen. Fungal Ecol. 13:150–156. doi: 10.1016/j.funeco.2014.09.010.
- De Bary, A. 1887. Comparative morphology and biology of the fungi, mycetozoa and bacteria. Oxford: Clarendon Press.
- de Weger, L. A., C. H. Pashley, B. Šikoparija, C. A. Skjøth, I. Kasprzyk, Ł. Grewling, M. Thibaudon, D. Magyar, and M. Smith. 2016. The long distance transport of airborne ambrosia pollen to the UK and The Netherlands from Central and South Europe. Int. J. Biometeorol. 60 (12):1829–1839. doi: 10.1007/s00484-016-1170-7.
- DeLeon-Rodriguez, N., T. L. Lathem, L. M. Rodriguez-R, J. M. Barazesh, B. E. Anderson, A. J. Beyersdorf, L. D. Ziemba, M. Bergin, A. Nenes, and K. T. Konstantinidis. 2013. Microbiome of the upper troposphere: Species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc. Natl. Acad. Sci. 110 (7):2575–2580. doi: 10.1073/pnas.1212089110.
- Delort, A.-M., and P. Amato. 2017. Microbiology of aerosols. New York, NY: John Wiley & Sons.
- DeMott, P. J., T. C. J. Hill, C. S. McCluskey, K. A. Prather, D. B. Collins, R. C. Sullivan, M. J. Ruppel, R. H. Mason, V. E. Irish, T. Lee, et al. 2016. Sea spray aerosol as a unique source of ice nucleating particles. Proc. Natl. Acad. Sci. 113 (21):5797–5803.
- DeMott, P. J., and A. J. Prenni. 2010. New directions: Need for defining the numbers and sources of biological aerosols acting as ice nuclei. Atmos. Environ. 44 (15):1944–1945. doi: 10.1016/j.atmosenv.2010.02.032.
- Després, V. R., Huffman, A. J. Burrows, S. M. Hoose, C. Safatov, A. S. Buryak, G. Fröhlich-Nowoisky, J. Elbert, W. Andreae, M. O. Pöschl U., et al. 2012. Primary biological aerosol particles in the atmosphere: A review. Tellus Ser. B Chem. Phys. Meteorol. 64 (1):15598. doi: 10.3402/tellusb.v64i0.15598.
- Di Filippo, P., D. Pomata, C. Riccardi, F. Buiarelli, and C. Perrino. 2013. Fungal contribution to size-segregated aerosol measured through biomarkers. Atmos. Environ. 64:132–140. doi: 10.1016/j.atmosenv.2012.10.010.
- Dommergue, A., P. Amato, R. Tignat-Perrier, O. Magand, A. Thollot, M. Joly, L. Bouvier, K. Sellegri, T. Vogel, J. E. Sonke, et al. 2019. Methods to investigate the global atmospheric microbiome. Front. Microbiol. 10:243. doi: 10.3389/fmicb.2019.00243.
- Donegan, K., C. Matyac, R. Seidler, and A. Porteous. 1991. Evaluation of methods for sampling, recovery, and enumeration of bacteria applied to the phylloplane. Appl. Environ. Microbiol. 57 (1):51–56.
- Dreischmeier, K., C. Budke, L. Wiehemeier, T. Kottke, and T. Koop. 2017. Boreal pollen contain ice-nucleating as well as ice-binding ‘antifreeze’ polysaccharides. Sci. Rep. 7:41890.
- Ehrenberg, C. G. 1847. Passat-Staub und Blut-Regen: ein großes organisches unsichtbares Wirken und Leben in der Atmosphäre. Königliche ed. Berlin, 1–192.
- Elbert, W., P. E. Taylor, M. O. Andreae, and U. Pöschl. 2007. Contribution of fungi to primary biogenic aerosols in the atmosphere: Wet and dry discharged spores, carbohydrates, and inorganic ions. Atmos. Chem. Phys. 7 (17):4569–4588. doi: 10.5194/acp-7-4569-2007.
- Environmental Agency. 2018. Technical Guidance Note (Monitoring) M9: Environmental monitoring of bioaerosols at regulated facilities.
- Essoussi, I., F. Ghodhbane-Gtari, H. Amairi, H. Sghaier, A. Jaouani, L. Brusetti, D. Daffonchio, A. Boudabous, and M. Gtari. 2010. Esterase as an enzymatic signature of geodermatophilaceae adaptability to Sahara desert stones and monuments. J. Appl. Microbiol. 108 (5):1723–1732. doi: 10.1111/j.1365-2672.2009.04580.x.
- Estillore, A. D., J. V. Trueblood, and V. H. Grassian. 2016. Atmospheric chemistry of bioaerosols: Heterogeneous and multiphase reactions with atmospheric oxidants and other trace gases. Chem. Sci. 7 (11):6604–6616. doi: 10.1039/C6SC02353C.
- Evans, C. A., P. J. Coombes, and R. H. Dunstan. 2006. Wind, rain and bacteria: The effect of weather on the microbial composition of roof-harvested rainwater. Water Res. 40 (1):37–44. doi: 10.1016/j.watres.2005.10.034.
- Failor, K. C., D. G. Schmale, B. A. Vinatzer, and C. L. Monteil. 2017. Ice nucleation active bacteria in precipitation are genetically diverse and nucleate ice by employing different mechanisms. ISME J. 11 (12):2740–2753. doi: 10.1038/ismej.2017.124.
- Fall, A. L., and R. Fall. 1998. High-Level expression of ice nuclei in Erwinia herbicola is induced by phosphate starvation and low temperature. Curr. Microbiol. 36 (6):370–376. doi: 10.1007/s002849900325.
- Fang, Z., Z. Ouyang, H. Zheng, X. Wang, and L. Hu. 2007. Culturable airborne bacteria in outdoor environments in Beijing. Microbiol. Ecol. 54 (3):487–496.
- Fierer, N., Z. Liu, M. Rodríguez-Hernández, R. Knight, M. Henn, and M. T. Hernandez. 2008. Short-term temporal variability in airborne bacterial and fungal populations. Appl. Environ. Microbiol. 74 (1):200–207. doi: 10.1128/AEM.01467-07.
- Franze, T., M. G. Weller, R. Niessner, and U. Pöschl. 2005. Protein nitration by polluted air. Environ. Sci. Technol. 39 (6):1673–1678. doi: 10.1021/es0488737.
- Fröhlich-Nowoisky, J., S. M. Burrows, Z. Xie, G. Engling, P. A. Solomon, M. P. Fraser, O. L. Mayol-Bracero, P. Artaxo, D. Begerow, R. Conrad, et al. 2012. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 9 (3):1125–1136. doi: 10.5194/bg-9-1125-2012.
- Fröhlich-Nowoisky, J., T. C. J. Hill, B. G. Pummer, P. Yordanova, G. D. Franc, and U. Pöschl. 2015. Ice nucleation activity in the widespread soil fungus Mortierella alpina. Biogeosciences 12 (4):1057–1071. doi: 10.5194/bg-12-1057-2015.
- Fröhlich-Nowoisky, J., C. J. Kampf, B. Weber, J. A. Huffman, C. Pöhlker, M. O. Andreae, N. Lang-Yona, S. M. Burrows, S. S. Gunthe, W. Elbert, et al. 2016. Bioaerosols in the earth system: Climate, health, and ecosystem interactions. Atmos. Res. 182:346–376. doi: 10.1016/j.atmosres.2016.07.018.
- Galan, C., C. Antunes, R. Brandao, C. Torres, H. Garcia-Mozo, E. Caeiro, R. Ferro, M. Prank, M. Sofiev, R. Albertini, et al. 2013. Airborne olive pollen counts are not representative of exposure to the major olive allergen Ole e 1. Allergy Eur. J. Allergy Clin. Immunol. 68 (6):809–812. doi: 10.1111/all.12144.
- Galán, C., M. Smith, M. Thibaudon, G. Frenguelli, J. Oteros, R. Gehrig, U. Berger, B. Clot, and R. Brandao. 2014. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia (Bologna) 30 (4):385–395. doi: 10.1007/s10453-014-9335-5.
- Gantt, B., and N. Meskhidze. 2013. The physical and chemical characteristics of marine primary organic aerosol: A review. Atmos. Chem. Phys. 13 (8):3979–3996. doi: 10.5194/acp-13-3979-2013.
- Gehrig, R., F. Maurer, and C. Schwierz. 2018. Designing new automatically generated pollen calendars for the public in Switzerland. Aerobiologia (Bologna) 34 (3):349–362. doi: 10.1007/s10453-018-9518-6.
- Georgakopoulos, D. G., V. Després, J. Fröhlich-Nowoisky, R. Psenner, P. A. Ariya, M. Pósfai, H. E. Ahern, B. F. Moffett, and T. C. J. Hill. 2009. Microbiology and atmospheric processes: Biological, physical and chemical characterization of aerosol particles. Biogeosciences 6 (4):721–737. doi: 10.5194/bg-6-721-2009.
- Ghosal, S., T. J. Leighton, K. E. Wheeler, I. D. Hutcheon, and P. K. Weber. 2010. Spatially resolved characterization of water and ion incorporation in Bacillus Spores. Appl. Environ. Microbiol. 76 (10):3275–3282. doi: 10.1128/AEM.02485-09.
- Gonzalez, F., M. P. G. Castro, P. Narayan, R. Walker, and L. Zeller. 2011. Development of an autonomous unmanned aerial system to collect time-stamped samples from the atmosphere and localize potential pathogen sources. J. Field Rob. 28 (6):961–976. doi: 10.1002/rob.20417.
- Graham, B., Guyon, P. Maenhaut, W. Taylor, P. E. Ebert, M. Matthias-Maser, S. Mayol-Bracero, O. L. Godoi, R. H. M. Artaxo, P. Meixner, et al. 2003. Composition and diurnal variability of the natural Amazonian aerosol. J. Geophys. Res. Atmos. 108 (D24):n/a. doi: 10.1029/2003JD004049.
- Grewling, Ł., P. Bogawski, D. Jenerowicz, M. Czarnecka-Operacz, B. Šikoparija, C. A. Skjøth, and M. Smith. 2016. Mesoscale atmospheric transport of ragweed pollen allergens from infected to uninfected areas. Int. J. Biometeorol. 60 (10):1493–1500. doi: 10.1007/s00484-016-1139-6.
- Griffin, D. W. 2007. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 20 (3):459–477. doi: 10.1128/CMR.00039-06.
- Griffiths, P. T., J. S. Borlace, P. J. Gallimore, M. Kalberer, M. Herzog, and F. D. Pope. 2012. Hygroscopic growth and cloud activation of pollen: A laboratory and modelling study. Atmos. Sci. Lett. 13 (4):289–295.
- Grinn-Gofroń, A., J. Nowosad, B. Bosiacka, I. Camacho, C. Pashley, J. Belmonte, C. De Linares, N. Ianovici, J. M. M. Manzano, M. Sadyś, et al. 2019. Airborne Alternaria and Cladosporium fungal spores in Europe: Forecasting possibilities and relationships with meteorological parameters. Sci. Total Environ. 653:938–946. doi: 10.1016/j.scitotenv.2018.10.419.
- Hader, J. D., T. P. Wright, and M. D. Petters. 2014. Contribution of pollen to atmospheric ice nuclei concentrations. Atmos. Chem. Phys. 14 (11):5433. doi: 10.5194/acp-14-5433-2014.
- Hanson, C. A., J. A. Fuhrman, M. C. Horner-Devine, and J. B. H. Martiny. 2012. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 10 (7):497.
- Hara, K., and D. Zhang. 2012. Bacterial abundance and viability in long-range transported dust. Atmos. Environ. 47:20–25. doi: 10.1016/j.atmosenv.2011.11.050.
- Harding, T., A. D. Jungblut, C. Lovejoy, and W. F. Vincent. 2011. Microbes in high arctic snow and implications for the cold biosphere. Appl. Environ. Microbiol. 77 (10):3234–3243. doi: 10.1128/AEM.02611-10.
- Harris, M. J., N. C. Wickramasinghe, D. Lloyd, J. V. Narlikar, P. Rajaratnam, M. P. Turner, S. Al-Mufti, M. K. Wallis, S. Ramadurai, and F. Hoyle. 2002. Detection of living cells in stratospheric samples. Proc. SPIE. 4495:192–198.
- Hartmann, M., T. Blunier, S. O. Brügger, J. Schmale, M. Schwikowski, A. Vogel, H. Wex, and F. Stratmann. 2019. Variation of ice nucleating particles in the European Arctic over the last centuries. Geophys. Res. Lett. 46 (7):4007–4010. doi: 10.1029/2019GL082311.
- Haugen, D. A. 1978. Effects of sampling rates and averaging periods of meteorological measurements (turbulence and wind speed data). Paper presented at Symposium on Meteorological Observations and Instrumentation, 4th, Denver, Colorado, pp. 15–18.
- Healy, D. A., J. A. Huffman, D. J. O’Connor, C. Pöhlker, U. Pöschl, and J. R. Sodeau. 2014. Ambient measurements of biological aerosol particles near Killarney, Ireland: A comparison between real-time fluorescence and microscopy techniques. Atmos. Chem. Phys. 14 (15):8055–8069. doi: 10.5194/acp-14-8055-2014.
- Herlihy, L. J., J. N. Galloway, and A. L. Mills. 1987. Bacterial utilization of formic and acetic acid in rainwater. Atmos. Environ. 21(11):2397–2402.
- Hiranuma, N., O. Möhler, K. Yamashita, T. Tajiri, A. Saito, A. Kiselev, N. Hoffmann, C. Hoose, E. Jantsch, T. Koop, et al. 2015. Ice nucleation by cellulose and its potential contribution to ice formation in clouds. Nature Geosci. 8 (4):273–277. doi: 10.1038/ngeo2374.
- Holt, K. A., and K. D. Bennett. 2014. Principles and methods for automated palynology. New Phytol. 203 (3):735. doi: 10.1111/nph.12848.
- Hoose, C., J. E. Kristjánsson, and S. M. Burrows. 2010. How important is biological ice nucleation in clouds on a global scale? Environ. Res. Lett. 5 (2):024009. doi: 10.1088/1748-9326/5/2/024009.
- Hoose, C., and O. Möhler. 2012. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 12 (20):9817. doi: 10.5194/acp-12-9817-2012.
- Hu, W., H. Niu, K. Murata, Z. Wu, M. Hu, T. Kojima, and D. Zhang. 2018. Bacteria in atmospheric waters: Detection, characteristics and implications. Atmos. Environ. 179:201–221. doi: 10.1016/j.atmosenv.2018.02.026.
- Huffman, J. A., A. E. Perring, N. J. Savage, B. Clot, B. Crouzy, F. Tummon, O. Shoshanim, B. Damit, J. Schneider, et al. 2019. Real-time sensing of bioaerosols: Review and current perspectives. Aerosol Sci. Technol. 1. doi: 10.1080/02786826.2019.1664724.
- Huffman, J. A., A. J. Prenni, P. J. Demott, C. Pöhlker, R. H. Mason, N. H. Robinson, J. Fröhlich-Nowoisky, Y. Tobo, V. R. Després, E. Garcia, et al. 2013. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 13 (13):6151–6164. doi: 10.5194/acp-13-6151-2013.
- Huffman, J. A., and J. Santarpia. 2017. Online techniques for quantification and characterization of biological aerosols. In Microbiology of aerosols, ed. A.-M. Delort and P. Amato. Hoboken, NJ: John Wiley & Sons, Inc.
- Huffman, J. A., B. Sinha, R. M. Garland, A. Snee-Pollmann, S. S. Gunthe, P. Artaxo, S. T. Martin, M. O. Andreae, and U. Pöschl. 2012. Size distributions and temporal variations of biological aerosol particles in the Amazon rainforest characterized by microscopy and real-time UV-APS fluorescence techniques during AMAZE-08. Atmos. Chem. Phys. 12 (24):11997–12019. doi: 10.5194/acp-12-11997-2012.
- Hummel, M., C. Hoose, B. Pummer, C. Schaupp, J. Fröhlich-Nowoisky, and O. Möhler. 2018. Simulating the influence of primary biological aerosol particles on clouds by heterogeneous ice nucleation. Atmos. Chem. Phys. 18 (20):15437–15450. doi: 10.5194/acp-18-15437-2018.
- Irish, V. E., P. Elizondo, J. Chen, C. Chou, J. Charette, M. Lizotte, L. A. Ladino, T. W. Wilson, M. Gosselin, B. J. Murray, et al. 2017. Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater. Atmos. Chem. Phys. 17 (17):10583–10595. doi: 10.5194/acp-17-10583-2017.
- Irish, V. E., S. J. Hanna, M. D. Willis, S. China, J. L. Thomas, J. J. B. Wentzell, A. Cirisan, M. Si, W. R. Leaitch, et al. 2019. Ice nucleating particles in the marine boundary layer in the Canadian Arctic during summer 2014. Atmos. Chem. Phys. 19 (2):1027–1039. doi: 10.5194/acp-19-1027-2019.
- Isard, S. A., C. W. Barnes, S. Hambleton, A. Ariatti, J. M. Russo, A. Tenuta, D. A. Gay, and L. J. Szabo. 2011. Predicting soybean rust incursions into the North American continental interior using crop monitoring, spore trapping, and aerobiological modeling. Plant Dis. 95 (11):1346–1357. doi: 10.1094/PDIS-01-11-0034.
- Issanova, G., and J. Abuduwaili. 2017. Natural conditions of central Asia and land-cover changes. In Aeolian proceses as dust storms in the deserts of Central Asia and Kazakhstan, ed. G. Issanova and J. Abuduwaili, 29–49. Singapore: Springer Singapore. doi: 10.1007/978-981-10-3190-8_2.
- Iwasaka, Y., G.-Y. Shi, M. Yamada, F. Kobayashi, M. Kakikawa, T. Maki, T. Naganuma, B. Chen, Y. Tobo, and C. S. Hong. 2009. Mixture of Kosa (Asian dust) and bioaerosols detected in the atmosphere over the Kosa particles source regions with balloon-borne measurements: Possibility of long-range transport. Air Qual. Atmos. Heal. 2 (1):29–38. doi: 10.1007/s11869-009-0031-5.
- Jeon, E. M., H. J. Kim, K. Jung, J. H. Kim, M. Y. Kim, Y. P. Kim, and J. O. Ka. 2011. Impact of Asian dust events on airborne bacterial community assessed by molecular analyses. Atmos. Environ. 45 (25):4313–4321. doi: 10.1016/j.atmosenv.2010.11.054.
- Jimenez-Sanchez, C., R. Hanlon, K. A. Aho, C. Powers, C. E. Morris, and D. G. Schmale, III. 2018. Diversity and ice nucleation activity of microorganisms collected with a small unmanned aircraft system (sUAS) in France and the United States. Front. Microbiol. 9:1667. doi: 10.3389/fmicb.2018.01667.
- Joly, M., P. Amato, L. Deguillaume, M. Monier, C. Hoose, and A. M. Delort. 2014. Quantification of ice nuclei active at near 0 °C temperatures in low-altitude clouds at the Puy de Dôme atmospheric station. Atmos. Chem. Phys. 14 (15):8185. doi: 10.5194/acp-14-8185-2014.
- Joly, M., P. Amato, M. Sancelme, V. Vinatier, M. Abrantes, L. Deguillaume, and A.-M. Delort. 2015. Survival of microbial isolates from clouds toward simulated atmospheric stress factors. Atmos. Environ. 117:92–98. doi: 10.1016/j.atmosenv.2015.07.009.
- Jones, A. M., and R. M. Harrison. 2004. The effects of meteorological factors on atmospheric bioaerosol concentrations - A review. Sci. Total Environ. 326 (1–3):151–180. doi: 10.1016/j.scitotenv.2003.11.021.
- Joung, Y. S., Z. Ge, and C. R. Buie. 2017. Bioaerosol generation by raindrops on soil. Nat. Commun. 8:1–10.
- Kaarakainen, P., T. Meklin, H. Rintala, A. Hyvärinen, P. Kärkkäinen, A. Vepsäläinen, M. Hirvonen, and A. Nevalainen. 2008. Seasonal variation in airborne microbial concentrations and diversity at landfill, urban and rural sites. Clean Soil Air Water 36 (7):556–563. doi: 10.1002/clen.200700179.
- Kakikawa, M., F. Kobayashi, T. Maki, M. Yamada, T. Higashi, B. Chen, G. Shi, C. Hong, Y. Tobo, and Y. Iwasaka. 2008. Dustborne microorganisms in the atmosphere over an Asian dust source region, Dunhuang. Air Qual. Atmos. Heal. 1 (4):195–202. doi: 10.1007/s11869-008-0024-9.
- Kanji, Z. A., L. A. Ladino, H. Wex, Y. Boose, M. Burkert-Kohn, D. J. Cziczo, and M. Krämer. 2017. Overview of ice nucleating particles. Meteorol. Monogr. 58:1–1-1.33. doi: 10.1175/AMSMONOGRAPHS-D-16-0006.1.
- Kasprzyk, I., and M. Worek. 2006. Airborne fungal spores in urban and rural environments in Poland. Aerobiologia (Bologna) 22 (3):169. doi: 10.1007/s10453-006-9029-8.
- Kellogg, C. A., and D. W. Griffin. 2006. Aerobiology and the global transport of desert dust. Trends Ecol. (Amst.) 21 (11):638–644. doi: 10.1016/j.tree.2006.07.004.
- Kim, S., P. A. Jaques, M. Chang, J. R. Froines, and C. Sioutas. 2001. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine and coarse ambient particles Part I: Development and laboratory characterization. J. Aerosol Sci. 32 (11):1281–1297. doi: 10.1016/S0021-8502(01)00057-X.
- Kiselev, D., L. Bonacina, and J. P. Wolf. 2013. A flash-lamp based device for fluorescence detection and identification of individual pollen grains. Rev. Sci. Instrum. 84 (3):033302.
- Knopf, D. A., P. A. Alpert, and B. Wang. 2018. The role of organic aerosol in atmospheric ice nucleation: A review. ACS Earth Sp. Chem. 2 (3):168–202.
- Ko, G., M. W. First, and H. A. Burge. 2000. Influence of relative humidity on particle size and UV sensitivity of Serratia marcescens and Mycobacterium bovis BCG Aerosols. Tuber. Lung Dis. 80 (4–5):217–228. doi: 10.1054/tuld.2000.0249.
- Kobayashi, F., K. Iwata, T. Maki, M. Kakikawa, T. Higashi, M. Yamada, T. Ichinose, and Y. Iwasaka. 2016. Evaluation of the toxicity of a Kosa (Asian duststorm) event from view of food poisoning: Observation of Kosa cloud behavior and real-time PCR analyses of Kosa bioaerosols during May 2011 in Kanazawa, Japan. Air Qual. Atmos. Heal. 9 (1):3–14.
- Könemann, T., N. Savage, C. M. Beall, E. Rodriguez-Caballero, F. Ditas, M. Dorf, H. Harder, J. Lelieveld, D. Walter, B. Weber, et al. 2018. Online bioaerosol and dust measurements during the AQABA Research Cruise around the Arabian Peninsula. Paper presented at the 10th Int. Aerosol Conf., St. Louis, Missouri, USA.
- Kourtev, P. S., K. A. Hill, P. B. Shepson, and A. Konopka. 2011. Atmospheric cloud water contains a diverse bacterial community. Atmos. Environ. 45 (30):5399–5405. doi: 10.1016/j.atmosenv.2011.06.041.
- Kraaijeveld, K., L. A. de Weger, M. Ventayol García, H. Buermans, J. Frank, P. S. Hiemstra, and J. T. den Dunnen. 2015. Efficient and sensitive identification and quantification of airborne pollen using next-generation DNA sequencing. Mol. Ecol. Resour. 15 (1):8–16. doi: 10.1111/1755-0998.12288.
- Krumins, V., G. Mainelis, L. J. Kerkhof, and D. E. Fennell. 2014. Substrate-dependent rRNA production in an airborne bacterium. Environ. Sci. Technol. Lett. 1 (9):376–381. doi: 10.1021/ez500245y.
- Kuznetsova, M., C. Lee, and J. Aller. 2005. Characterization of the proteinaceous matter in marine aerosols. Mar. Chem. 96 (3–4):359–377. doi: 10.1016/j.marchem.2005.03.007.
- Ladino, L. A., J. D. Yakobi-Hancock, W. P. Kilthau, R. H. Mason, M. Si, J. Li, L. A. Miller, C. L. Schiller, J. A. Huffman, J. Y. Aller, et al. 2016. Addressing the ice nucleating abilities of marine aerosol: A combination of deposition mode laboratory and field measurements. Atmos. Environ. 132:1–10. doi: 10.1016/j.atmosenv.2016.02.028.
- Lateran, S., M. F. Sedan, A. S. M. Harithuddin, and S. Azrad. 2016. Development of unmanned aerial vehicle (UAV) based high altitude balloon (HAB) platform for active aerosol sampling. IOP Conf. Ser. Mater. Sci. Eng. 152 (1):12018.
- Lauber, A., A. Kiselev, T. Pander, P. Handmann, and T. Leisner. 2018. Secondary ice formation during freezing of levitated droplets. J. Atmos. Sci. 75 (8):2815. doi: 10.1175/JAS-D-18-0052.1.
- Leck, C., and E. K. Bigg. 2008. Comparison of sources and nature of the tropical aerosol with the summer high arctic aerosol. Tellus Ser. B Chem. Phys. Meteorol. 60 (1):118–126. doi: 10.1111/j.1600-0889.2007.00315.x.
- Lee, B. U., S. H. Kim, and S. S. Kim. 2002. Hygroscopic growth of E. coli and B. subtilis bioaerosols. J. Aerosol Sci. 33 (12):1721–1723. doi: 10.1016/S0021-8502(02)00114-3.
- Lever, M. A., A. Torti, P. Eickenbusch, A. B. Michaud, T. Å Antl-Temkiv, and B. B. Jã¸Rgensen. 2015. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Front. Microbiol. 6:476. doi: 10.3389/fmicb.2015.00476.
- Lighthart, B. 1997. The ecology of bacteria in the alfresco atmosphere. FEMS Microbiol. Ecol. 23 (4):263–274. doi: 10.1016/S0168-6496(97)00036-6.
- Lighthart, B., and B. T. Shaffer. 1994. Bacterial flux from chaparral into the atmosphere in mid-summer at a high desert location. Atmos. Sci. 28:1267–1274. doi: 10.1016/1352-2310(94)90273-9.
- Lighthart, B., B. T. Shaffer, B. Marthi, and L. M. Ganio. 1993. Artificial wind-gust liberation of microbial bioaerosols previously deposited on plants. Aerobiologia (Bologna) 9 (2–3):189–196. doi: 10.1007/BF02066261.
- Ligler, F. S., G. P. Anderson, P. T. Davidson, R. J. Foch, J. T. Ives, K. D. King, G. Page, D. A. Stenger, and J. P. Whelan. 1998. Remote sensing using an airborne biosensor. Environ. Sci. Technol. 32 (16):2461–2466. doi: 10.1021/es970991p.
- Lim, N., C. I. Munday, G. E. Allison, T. O’Loingsigh, P. De Deckker, and N. J. Tapper. 2011. Microbiological and meteorological analysis of two Australian dust storms in April 2009. Sci. Total Environ. 412–413:223–231. doi: 10.1016/j.scitotenv.2011.10.030.
- Lin, B., A. Bozorgmagham, S. D. Ross, and D. G. Schmale Iii. 2013. Small fluctuations in the recovery of fusaria across consecutive sampling intervals with unmanned aircraft 100 m above Ground Level. Aerobiologia (Bologna) 29 (1):45–54. doi: 10.1007/s10453-012-9261-3.
- Lindemann, J., H. A. Constantinidou, W. R. Barchet, and C. D. Upper. 1982. Plants as sources of airborne bacteria, including ice nucleation-active bacteria. Appl. Environ. Microbiol. 44 (5):1059–1063.
- Lindemann, J., and C. D. Upper. 1985. Aerial dispersal of epiphytic bacteria over bean plants. Appl. Environ. Microbiol. 50 (5):1229–1232.
- Lin, H., L. Lizarraga, L. A. Bottomley, and J. Carson Meredith. 2015. Effect of water absorption on pollen adhesion. J. Colloid Interface Sci. 442:133–139. doi: 10.1016/j.jcis.2014.11.065.
- Lin, W.-R., P.-H. Wang, C.-J. Tien, W.-Y. Chen, Y.-A. Yu, and L.-Y. Hsu. 2018. Changes in airborne fungal flora along an urban to rural gradient. J. Aerosol Sci. 116:116–123. doi: 10.1016/j.jaerosci.2017.11.010.
- Liu, H., Z. Hu, M. Zhou, J. Hu, X. Yao, H. Zhang, Z. Li, L. Lou, C. Xi, H. Qian, et al. 2019. The distribution variance of airborne microorganisms in urban and rural environments. Environ. Pollut. 247:898–906.
- Liu, F., P. S. J. Lakey, T. Berkemeier, H. Tong, A. T. Kunert, H. Meusel, Y. Cheng, H. Su, J. Fröhlich-Nowoisky, S. Lai, et al. 2017. Atmospheric protein chemistry influenced by anthropogenic air pollutants: Nitration and oligomerization upon exposure to ozone and nitrogen dioxide. Faraday Discuss. 200:413–427. doi: 10.1039/C7FD00005G.
- Ma, Y., J. Zhou, S. Yang, Y. Zhao, and X. Zheng. 2017. Assessment for the impact of dust events on measles incidence in Western China. Atmos. Environ. 157:1–9. doi: 10.1016/j.atmosenv.2017.03.010.
- Madelin, T. M. 1994. Fungal aerosols: A review. J. Aerosol Sci. 25 (8):1405–1412.
- Mahaffee, W. F., and R. Stoll. 2016. The ebb and flow of airborne pathogens: monitoring and use in disease management decisions. Phytopathology 106 (5):420–431. doi: 10.1094/PHYTO-02-16-0060-RVW.
- Mainelis, G. 2019. Bioaerosol sampling: Classical approaches, advances and perspectives. Aerosol Sci. Technol 1. In review. doi: 10.1080/02786826.2019.1671950.
- Mainelis, G., K. Willeke, A. Adhikari, T. Reponen, and S. A. Grinshpun. 2002. Design and collection efficiency of a new electrostatic precipitator for bioaerosol collection. Aerosol Sci. Technol. 36 (11):1073–1085.
- Maki, T., K. Hara, A. Iwata, K. C. Lee, K. Kawai, K. Kai, F. Kobayashi, S. B. Pointing, S. Archer, H. Hasegawa, et al. 2017a. Variations in airborne bacterial communities at high altitudes over the Noto Peninsula (Japan) in response to Asian dust events. Atmos. Chem. Phys. 17 (19):11877–11897. doi: 10.5194/acp-17-11877-2017.
- Maki, T., K. Hara, F. Kobayashi, Y. Kurosaki, M. Kakikawa, A. Matsuki, B. Chen, G. Shi, H. Hasegawa, and Y. Iwasaka. 2015. Vertical distribution of airborne bacterial communities in an Asian-dust downwind area, Noto Peninsula. Atmos. Environ. 119:282–293. doi: 10.1016/j.atmosenv.2015.08.052.
- Maki, T., M. Kakikawa, F. Kobayashi, M. Yamada, A. Matsuki, H. Hasegawa, and Y. Iwasaka. 2013. Assessment of composition and origin of airborne bacteria in the free troposphere over Japan. Atmos. Environ. 74:73–82. doi: 10.1016/j.atmosenv.2013.03.029.
- Maki, T., Y. Kurosaki, K. Onishi, K. C. Lee, S. B. Pointing, D. Jugder, N. Yamanaka, H. Hasegawa, and M. Shinoda. 2017b. Variations in the structure of airborne bacterial communities in Tsogt-Ovoo of Gobi desert area during dust events. Air Qual. Atmos. Heal. 10(3):249–260. doi: 10.1007/s11869-016-0430-3.
- Maki, T., K. C. Lee, K. Kawai, K. Onishi, C. S. Hong, Y. Kurosaki, M. Shinoda, K. Kai, Y. Iwasaka, S. D. J. Archer, et al. 2019. Aeolian dispersal of bacteria associated with desert dust and anthropogenic particles over continental and oceanic surfaces. J. Geophys. Res. Atmos. 124 (10):5579–5588. doi: 10.1029/2018JD029597.
- Maki, T., F. Puspitasari, K. Hara, M. Yamada, F. Kobayashi, H. Hasegawa, and Y. Iwasaka. 2014. Variations in the structure of airborne bacterial communities in a downwind area during an Asian dust (Kosa) event. Sci. Total Environ. 488–489:75–84. doi: 10.1016/j.scitotenv.2014.04.044.
- Maki, T., S. Susuki, F. Kobayashi, M. Kakikawa, M. Yamada, T. Higashi, B. Chen, G. Shi, C. Hong, Y. Tobo, et al. 2008. Phylogenetic diversity and vertical distribution of a halobacterial community in the atmosphere of an asian dust (KOSA) source region, Dunhuang City. Air Qual. Atmos. Heal. 1 (2):81–89. doi: 10.1007/s11869-008-0016-9.
- Marks, R., K. Kruczalak, K. Jankowska, and M. Michalska. 2001. Bacteria and fungi in air over the Gulf of Gdansk and Baltic Sea. J. Aerosol Sci. 32 (2):237–250. doi: 10.1016/S0021-8502(00)00064-1.
- Mason, R. H., M. Si, J. Li, C. Chou, R. Dickie, D. Toom-Sauntry, C. Pöhlker, J. D. Yakobi-Hancock, L. A. Ladino, K. Jones, et al. 2015. Ice nucleating particles at a coastal marine boundary layer site: Correlations with aerosol type and meteorological conditions. Atmos. Chem. Phys. 15 (21):12547–12566. doi: 10.5194/acp-15-12547-2015.
- Mayol, E., M. A. Jiménez, G. J. Herndl, C. M. Duarte, and J. M. Arrieta. 2014. Resolving the abundance and air-sea fluxes of airborne microorganisms in the North Atlantic Ocean. Front. Microbiol. 5:1–9. doi: 10.3389/fmicb.2017.01971.
- Mazar, Y., E. Cytryn, Y. Erel, and Y. Rudich. 2016. Effect of dust storms on the atmospheric microbiome in the eastern Mediterranean. Environ. Sci. Technol. 50 (8):4194–4202. doi: 10.1021/acs.est.5b06348.
- McCluskey, C. S., J. Ovadnevaite, M. Rinaldi, J. Atkinson, F. Belosi, D. Ceburnis, S. Marullo, T. C. J. Hill, U. Lohmann, Z. A. Kanji, et al. 2018. Marine and terrestrial organic ice-nucleating particles in pristine marine to continentally influenced Northeast Atlantic air masses. J. Geophys. Res. Atmos. 123 (11):6196–6212. doi: 10.1029/2017JD028033.
- Möhler, O., P. J. DeMott, G. Vali, and Z. Levin. 2007. Microbiology and atmospheric processes: The role of biological particles in cloud physics. Biogeosciences 4 (6):1059–1071. doi: 10.5194/bg-4-1059-2007.
- Monahan, E. C., C. W. Fairall, K. L. Davidson, and P. J. Boyle. 1983. Observed inter‐relations between 10m winds, ocean whitecaps and marine aerosols. Q. J. R. Meteorol. Soc. 109 (460):379–392. doi: 10.1256/smsqj.46009.
- Moran-Zuloaga, D., Ditas, F. Walter, D. Saturno, J. Brito, J. Carbone, S. Chi, X. Hrabě De Angelis, I. Baars, H. H M Godoi, et al. 2018. Long-term study on coarse mode aerosols in the Amazon rain forest with the frequent intrusion of Saharan dust plumes. Atmos. Chem. Phys. 18 (13):10055–10088. doi: 10.5194/acp-18-10055-2018.
- Morris, C. E., F. Conen, J. A. Huffman, V. Phillips, U. Pöschl, and D. C. Sands. 2014. Bioprecipitation: A feedback cycle linking earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Glob Change Biol. 20 (2):341–351. doi: 10.1111/gcb.12447.
- Morris, C. E., D. G. Georgakopoulos, and D. C. Sands. 2004. Ice nucleation active bacteria and their potential role in precipitation. J. Phys. IV 121:87–103. doi: 10.1051/jp4:2004121004.
- Morris, C. E., C. Leyronas, and P. C. Nicot. 2014. Movement of bioaerosols in the atmosphere and the consequences for climate and microbial evolution. In Aerosol science, pp. 393–415.Chichester, UK: John Wiley & Sons, Ltd.
- Morris, C. E., D. C. Sands, M. Bardin, R. Jaenicke, B. Vogel, C. Leyronas, P. A. Ariya, and R. Psenner. 2011. Microbiology and atmospheric processes: research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 8 (1):17–25. doi: 10.5194/bg-8-17-2011.
- Morris, C. E., D. C. Sands, C. Glaux, J. Samsatly, S. Asaad, A. R. Moukahel, F. L. T. Gonçalves, and E. K. Bigg. 2013. Urediospores of rust fungi are ice nucleation active at >-10 °c and harbor ice nucleation active bacteria. Atmos. Chem. Phys. 13 (8):4223–4233. doi: 10.5194/acp-13-4223-2013.
- Morris, C. E., S. Soubeyrand, E. K. Bigg, J. M. Creamean, and D. C. Sands. 2017. Mapping Rainfall feedback to reveal the potential sensitivity of precipitation to biological aerosols. Bull. Amer. Meteor. Soc. 98 (6):1109–1118. doi: 10.1175/BAMS-D-15-00293.1.
- Negrin, M. M., M. T. Del Panno, and A. E. Ronco. 2007. Study of bioaerosols and site influence in, the La Plata Area (Argentina) using conventional and DNA (fingerprint) based methods. Aerobiologia (Bologna) 23 (4):249–258. doi: 10.1007/s10453-007-9069-8.
- Negrin, M., M. T. Del Panno, C. G. Terada, and A. Ronco. 2009. Characterization of indoor and outdoor bioaerosols in urban, industrial and rural sites using conventional and DNA (fingerprint) based methods. In Aerosols: Chemistry, environmental impact and health effects, ed. D. Peretz, pp. 109–125. Hauppauge, NY: Nova Science Publishers, INC.
- Nemecek-Marshall, M.,. R. LaDuca, and R. Fall. 1993. High-level expression of ice nuclei in a pseudomonas syringae strain is induced by nutrient limitation and low temperature. J. Bacteriol. 175 (13):4062–4070. doi: 10.1128/jb.175.13.4062-4070.1993.
- Nemergut, D. R., E. K. Costello, M. Hamady, C. Lozupone, L. Jiang, S. K. Schmidt, N. Fierer, A. R. Townsend, C. C. Cleveland, L. Stanish, et al. 2011. Global patterns in the biogeography of bacterial taxa. Environ. Microbiol. 13 (1):135–144. doi: 10.1111/j.1462-2920.2010.02315.x.
- Neufeld, K. N., A. P. Keinath, B. K. Gugino, M. T. McGrath, E. J. Sikora, S. A. Miller, M. L. Ivey, D. B. Langston, B. Dutta, T. Keever, et al. 2018. Predicting the risk of cucurbit downy mildew in the eastern United States using an integrated aerobiological model. Int. J. Biometeorol. 62 (4):655–668. doi: 10.1007/s00484-017-1474-2.
- Noziere, B. 2016. CLOUDS. Don't forget the surface. Science 351 (6280):1396–1397. doi: 10.1126/science.aaf3253.
- Núñez, A., G. Amo de Paz, A. Rastrojo, A. M. García, A. Alcamí, A. M. Gutiérrez-Bustillo, and D. A. Moreno. 2016a. Monitoring of airborne biological particles in outdoor atmosphere. Part 1: Importance, variability and ratios. Int. Microbiol. 19 (1):1–13.
- Núñez, A., G. Amo de Paz, A. Rastrojo, A. M. García, A. Alcamí, A. Montserrat Gutiérrez-Bustillo, and D. A. Moreno. 2016b. Monitoring of airborne biological particles in outdoor atmosphere. Part 2: Metagenomics applied to urban environments. Int. Microbiol. 19 (2):69–80.
- O’Dowd, C. D., and G. de Leeuw. 2007. Marine aerosol production: A review of the current knowledge. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 365 (1856):1753–1774. doi: 10.1098/rsta.2007.2043.
- O’Sullivan, D., B. J. Murray, J. F. Ross, T. F. Whale, H. C. Price, J. D. Atkinson, N. S. Umo, and M. E. Webb. 2015. Relevance nanoscale biological fragments ice nucleation clouds. Sci. Rep. 5:1–7.
- Oliveira, M., L. Delgado, H. Ribeiro, and I. Abreu. 2010. Fungal Spores from Pleosporales in the atmosphere of urban and rural locations in Portugal. J. Environ. Monit. 12 (5):1187–1194.
- Oliveira, M., H. Ribeiro, J. L. Delgado, and I. Abreu. 2009. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int. J. Biometeorol. 53 (1):61–73. doi: 10.1007/s00484-008-0191-2.
- Orellana, M. V., P. A. Matrai, C. Leck, C. D. Rauschenberg, A. M. Lee, and E. Coz. 2011. Marine microgels as a source of cloud condensation nuclei in the high Arctic. Proc. Natl. Acad. Sci. U.S.A. 108 (33):13612–13617. doi: 10.1073/pnas.1102457108.
- Oteros, J., E. Bartusel, F. Alessandrini, A. Núñez, D. A. Moreno, H. Behrendt, C. Schmidt-Weber, C. Traidl-Hoffmann, and J. Buters. 2019. Artemisia pollen is the main vector for airborne endotoxin. J. Allergy Clin. Immunol. 143 (1):369–377. doi: 10.1016/j.jaci.2018.05.040.
- Oteros, J., G. Pusch, I. Weichenmeier, U. Heimann, R. Möller, S. Röseler, C. Traidl-Hoffmann, C. Schmidt-Weber, and J. T. M. Buters. 2015. Automatic and online pollen monitoring. Int. Arch. Allergy Immunol. 167 (3):158–166.
- Palframan, M. C., H. A. Gruszewski, D. G. Schmale, III, and C. A. Woolsey. 2014. Detection of a surrogate biological agent with a portable surface plasmon resonance sensor onboard an unmanned aircraft system. J. Unmanned Veh. Syst. 2 (4):103–118. doi: 10.1139/juvs-2013-0019.
- Papadopoulos, N. G., I. Agache, S. Bavbek, B. M. Bilo, F. Braido, V. Cardona, A. Custovic, J. Demonchy, P. Demoly, P. Eigenmann, et al. 2012. Research needs in allergy: An EAACI Position Paper, in collaboration with EFA. Clin. Transl. Allergy 2 (1):21.
- Passananti, M., V. Vinatier, A. M. Delort, G. Mailhot, and M. Brigante. 2016. Siderophores in cloud waters and potential impact on atmospheric chemistry: Photoreactivity of iron complexes under sun-simulated conditions. Environ. Sci. Technol. 50 (17):9324–9332. doi: 10.1021/acs.est.6b02338.
- Pasteur, L. 1862. Sur les corpuscules organisés qui existent dans l’atmosphère, examen de la doctrine des générations spontanées, leçon professée à la Société clinique de Paris, le 19 mai 1861. Ann. Chim. Phys. 64:1–36.
- Pearce, D. A., I. A. Alekhina, A. Terauds, A. Wilmotte, A. Quesada, A. Edwards, A. Dommergue, B. Sattler, B. J. Adams, C. Magalhães, et al. 2016. Aerobiology over Antarctica - A new initiative for atmospheric ecology. Front. Microbiol. 7:1–7. doi: 10.3389/fmicb.2016.00016.
- Pearce, D. A., P. D. Bridge, K. A. Hughes, B. Sattler, R. Psenner, and N. J. Russell. 2009. Microorganisms in the atmosphere over Antarctica. FEMS Microbiol. Ecol. 69 (2):143–157. doi: 10.1111/j.1574-6941.2009.00706.x.
- Peel, M. C., B. L. Finlayson, and T. A. McMahon. 2007. Updated world map of the köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11 (5):1633–1644.
- Pei-Chih, W., S. Huey-Jen, and L. Chia-Yin. 2000. Characteristics of indoor and outdoor airborne fungi at suburban and urban homes in two seasons. Sci. Total Environ. 253 (1–3):111–118. doi: 10.1016/s0048-9697(00)00423-x.
- Perring, A. E., J. P. Schwarz, D. Baumgardner, M. T. Hernandez, D. V. Spracklen, C. L. Heald, R. S. Gao, G. Kok, G. R. McMeeking, J. B. McQuaid, et al. 2015. Airborne observations of regional variation in fluorescent aerosol across the United States. J. Geophys. Res. 120 (3):1153–1170. doi: 10.1002/2014JD022495.
- Petters, S. S., and M. D. Petters. 2016. Surfactant effect on cloud condensation nuclei for two-component internally mixed aerosols. J. Geophys. Res. 121 (4):1878–1895.
- Petters, M. D., and T. P. Wright. 2015. Revisiting ice nucleation from precipitation samples. Geophys. Res. Lett. 42 (20):8758–8766. doi: 10.1002/2015GL065733.
- Pointing, S. B., and J. Belnap. 2012. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 10 (8):551–562. doi: 10.1038/nrmicro2831.
- Polymenakou, P. N., M. Mandalakis, E. G. Stephanou, and A. Tselepides. 2008. Particle size distribution of airborne microorganisms and pathogens during an intense African dust event in the eastern Mediterranean. Environ. Health Perspect. 116 (3):292–296. doi: 10.1289/ehp.10684.
- Pöschl, U., S. T. Martin, B. Sinha, Q. Chen, S. S. Gunthe, J. A. Huffman, S. Borrmann, D. K. Farmer, R. M. Garland, G. Helas, et al. 2010. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science 329 (5998):1513–1516. doi: 10.1126/science.1191056.
- Pouzet, G., E. Peghaire, M. Aguès, J. L. Baray, F. Conen, and P. Amato. 2017. Atmospheric processing and variability of biological ice nucleating particles in precipitation at Opme, France. Atmosphere (Basel) 8 (11):229. doi: 10.3390/atmos8110229.
- Powers, C. W., R. Hanlon, H. Grothe, A. J. Prussin, L. C. Marr, and D. G. Schmale, III. 2018. Coordinated sampling of microorganisms over freshwater and saltwater environments using an unmanned surface vehicle (USV) and a small unmanned aircraft system (sUAS). Front. Microbiol. 9:1668. doi: 10.3389/fmicb.2018.01668.
- Prenni, A. J., M. D. Petters, S. M. Kreidenweis, C. L. Heald, S. T. Martin, P. Artaxo, R. M. Garland, A. G. Wollny, and U. Pöschl. 2009. Relative roles of biogenic emissions and Saharan dust as ice nuclei in the Amazon basin. Nat. Geosci. 2 (6):402–405. doi: 10.1038/ngeo517.
- Prospero, J. M., E. Blades, G. Mathison, and R. Naidu. 2005. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia (Bologna) 21 (1):1–19. doi: 10.1007/s10453-004-5872-7.
- Pummer, B. G., H. Bauer, J. Bernardi, S. Bleicher, and H. Grothe. 2012. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 12 (5):2541–2550. doi: 10.5194/acp-12-2541-2012.
- Pummer, B. G., C. Budke, S. Augustin-Bauditz, D. Niedermeier, L. Felgitsch, C. J. Kampf, R. G. Huber, K. R. Liedl, T. Loerting, T. Moschen, et al. 2015. Ice nucleation by water-soluble macromolecules. Atmos. Chem. Phys. 15 (8):4077–4091. doi: 10.5194/acp-15-4077-2015.
- Puspitasari, F., T. Maki, G. Shi, C. Bin, F. Kobayashi, H. Hasegawa, and Y. Iwasaka. 2016. Phylogenetic analysis of bacterial species compositions in sand dunes and dust aerosol in an Asian dust source area, the Taklimakan Desert. Air Qual. Atmos. Heal. 9 (6):631–644.
- Rathnayake, C. M., N. Metwali, Z. Baker, T. Jayarathne, P. A. Kostle, P. S. Thorne, P. T. O’Shaughnessy, and E. A. Stone. 2016. Urban enhancement of Pm10 bioaerosol tracers relative to background locations in the Midwestern United States. J. Geophys. Res. Atmos. 121 (9):5071–5089. doi: 10.1002/2015JD024538.
- Rathnayake, C. M., N. Metwali, T. Jayarathne, J. Kettler, Y. Huang, P. S. Thorne, P. T. O’Shaughnessy, and E. A. Stone. 2017. Influence of rain on the abundance of bioaerosols in fine and coarse particles. Atmos. Chem. Phys. 17 (3):2459–2475. doi: 10.5194/acp-17-2459-2017.
- Raupach, M. R., R. A. Antonia, and S. Rajagopalan. 1991. Rough-wall turbulent boundary layers. Appl. Mech. Rev. 44 (1):1–25. doi: 10.1115/1.3119492.
- Renard, P., I. Canet, M. Sancelme, N. Wirgot, L. Deguillaume, and A.-M. Delort. 2016. Screening of cloud microorganisms isolated at the Puy De Dôme (France) station for the production of biosurfactants. Atmos. Chem. Phys. 16 (18):12347–12358. doi: 10.5194/acp-16-12347-2016.
- Reponen, T., S. A. Grinshpun, K. L. Conwell, J. Wiest, and M. Anderson. 2001. Aerodynamic versus physical size of spores: Measurement and implication for respiratory deposition. Grana 40 (3):119–125. doi: 10.1080/00173130152625851.
- Reponen, T., K. Willeke, V. Ulevicius, A. Reponen, and S. A. Grinshpun. 1996. Effect of relative humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos. Environ. 30 (23):3967–3974. doi: 10.1016/1352-2310(96)00128-8.
- Rodó, X., J. Ballester, D. Cayan, M. E. Melish, Y. Nakamura, R. Uehara, and J. C. Burns. 2011. Association of Kawasaki disease with tropospheric wind patterns. Sci. Rep. 1 (152):152. doi: 10.1038/srep00152.
- Rogers, L. A., and F. C. Meier. 1937. The National Geographic Society-US Army Air Corps stratosphere flight of 1935 in the balloon “Explorer Ii.” QJR Meteorol. Soc. 63:217–218.
- Rojo, J., J. Oteros, R. Pérez-Badia, P. Cervigón, Z. Ferencova, A. M. Gutiérrez-Bustillo, K. C. Bergmann, G. Oliver, M. Thibaudon, R. Albertini, et al. 2019. Near-ground effect of height on pollen exposure. Environ. Res. 174:160–169. doi: 10.1016/j.envres.2019.04.027.
- Rubel, G. O. 1997. Measurement of water vapor sorption by single biological aerosols. Aerosol Sci. Technol. 27 (4):481–490. doi: 10.1080/02786829708965488.
- Ruske, S., D. O. Topping, V. E. Foot, P. H. Kaye, W. R. Stanley, I. Crawford, A. P. Morse, and M. W. Gallagher. 2017. Evaluation of machine learning algorithms for classification of primary biological aerosol using a new UV-LIF spectrometer. Atmos. Meas. Tech. 10 (2):695–708. doi: 10.5194/amt-10-695-2017.
- Russell, L. M., L. N. Hawkins, A. A. Frossard, P. K. Quinn, and T. S. Bates. 2010. Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting. Proc. Natl. Acad. Sci. 107 (15):6652–6657. doi: 10.1073/pnas.0908905107.
- Saari, S., J. V. Niemi, T. RöNkkö, H. Kuuluvainen, A. JäRvinen, L. Pirjola, M. Aurela, R. Hillamo, and J. Keskinen, 2015. Seasonal and diurnal variations of fluorescent bioaerosol concentration and size distribution in the urban environment. Aerosol Air Qual. Res. 15 (2):572–581. doi: 10.4209/aaqr.2014.10.0258.
- Sahyoun, M., H. Wex, U. Gosewinkel, T. Šantl-Temkiv, N. W. Nielsen, K. Finster, J. H. Sørensen, F. Stratmann, and U. S. Korsholm. 2016. On the usage of classical nucleation theory in quantification of the impact of bacterial INP on weather and climate. Atmos. Environ. 139:230–240.
- Santarpia, J. L., Y.-L. Pan, S. C. Hill, N. Baker, B. Cottrell, L. McKee, S. Ratnesar-Shumate, and R. G. Pinnick. 2012. Changes in fluorescence spectra of bioaerosols exposed to ozone in a laboratory reaction chamber to simulate atmospheric aging. Opt. Express 20 (28):29867. doi: 10.1364/OE.20.029867.
- Šantl-Temkiv, T., P. Amato, U. Gosewinkel, R. Thyrhaug, A. Charton, B. Chicot, K. W. Finster, G. Bratbak, and J. Löndahl. 2017. High-flow-rate impinger for the study of concentration, viability, metabolic activity, and ice-nucleation activity of airborne bacteria. Environ. Sci. Technol. 51 (19):11224–11234. doi: 10.1021/acs.est.7b01480.
- Šantl-Temkiv, T., K. Finster, T. Dittmar, B. M. Hansen, R. Thyrhaug, N. W. Nielsen, and U. G. Karlson. 2013. Hailstones: A window into the microbial and chemical inventory of a storm cloud. PLoS One 8 (1):e53550. doi: 10.1371/journal.pone.0053550.
- Šantl-Temkiv, T., K. Finster, B. M. Hansen, L. Pašić, and U. G. Karlson. 2013. Viable methanotrophic bacteria enriched from air and rain can oxidize methane at cloud-like conditions. Aerobiologia (Bologna) 29 (3):373–384. doi: 10.1007/s10453-013-9287-1.
- Šantl-Temkiv, T., U. Gosewinkel, P. Starnawski, M. Lever, and K. Finster. 2018. Aeolian dispersal of bacteria in southwest Greenland: Their sources, abundance, diversity and physiological states. Fems Micriobiol. Ecol. 94 (4):1–10.
- Šantl-Temkiv, T., R. Lange, D. Beddows, R. Rauter, S. Pilgaard, M. Dall’Osto, N. Gunde-Cimerman, A. Massling, and H. Wex. 2019. Biogenic sources of ice nucleation particles at the High Arctic Site Villum Research Station. Environ. Sci. Technol. 53 (18):10580–10590. doi: 10.1021/acs.est.9b00991.
- Šantl-Temkiv, T., M. Sahyoun, K. Finster, S. Hartmann, S. Augustin-Bauditz, F. Stratmann, H. Wex, T. Clauss, N. Woetmann, J. Havskov, et al. 2015. Characterization of airborne ice-nucleation-active bacteria and bacterial fragments. 109 :105–117. doi: 10.1016/j.atmosenv.2015.02.060.
- Sattler, B., H. Puxbaum, and R. Psenner. 2001. Bacterial growth in supercooled cloud droplets. Geophys. Res. Lett. 28 (2):239–242. doi: 10.1029/2000GL011684.
- Šaulienė, I., L. Šukienė, G. Daunys, G. Valiulis, L. Vaitkevičius, P. Matavulj, S. Brdar, M. Panic, B. Sikoparija, B. Clot, et al. 2019. Automatic pollen recognition with the rapid-E particle counter: The first-level procedure, experience and next steps. Atmos. Meas. Technol. 12 (6):3435–3452. doi: 10.5194/amt-12-3435-2019.
- Savage, N. J., and J. A. Huffman. 2018. Evaluation of a hierarchical agglomerative clustering method applied to WIBS laboratory data for improved discrimination of biological particles by comparing data preparation techniques. Atmos. Meas. Tech. 11 (8):4929–4942. doi: 10.5194/amt-11-4929-2018.
- Savage, N. J., C. E. Krentz, T. Könemann, T. T. Han, G. Mainelis, C. Pöhlker, and J. Alex Huffman. 2017. Systematic characterization and fluorescence threshold strategies for the wideband integrated bioaerosol sensor (WIBS) using size-resolved biological and interfering particles. Atmos. Meas. Tech. 10 (11):4279–4302. doi: 10.5194/amt-10-4279-2017.
- Saxena, V. K. 1983. Evidence of the biogenic nuclei involvement in Antarctic coastal cloudst. J. Phys. Chem. 87 (21):4130–4134. doi: 10.1021/j100244a029.
- Saxena, V. K., and D. C. Weintraub. 1988. Ice forming nuclei concentrations at Palmer Station, Antarctica. In Atmospheric Aerosols and Nucleation. Lecture Notes in Physics, ed. P. E. Wagner and G. Vali, vol. 309. Berlin, Heidelberg: Springer. doi: 10.1007/3-540-50108-8_1158.
- Schmale Iii, D. G., B. R. Dingus, and C. Reinholtz. 2008. Development and application of an autonomous unmanned aerial vehicle for precise aerobiological sampling above agricultural fields. J. Field Rob. 25 (3):133–147. doi: 10.1002/rob.20232.
- Schmale, III, D. G., S. D. Ross, T. L. Fetters, P. Tallapragada, A. K. Wood-Jones, and B. Dingus. 2012. Isolates of Fusarium graminearum collected 40–320 meters above ground level cause Fusarium head blight in wheat and produce trichothecene mycotoxins. Aerobiologia (Bologna) 28 (1):1–11. doi: 10.1007/s10453-011-9206-2.
- Schnell, R. C. 1977. Ice nuclei in seawater, fog water and marine air off the coast of Nova Scotia: Summer 1975. J. Atmos. Sci. 34 (8):1299–1305. doi: 10.1175/1520-0469(1977)034<1299:INISFW>2.0.CO;2.
- Schumacher, C. J., C. Pöhlker, P. Aalto, V. Hiltunen, T. Petäjä, M. Kulmala, U. Pöschl, and J. A. Huffman. 2013. Seasonal cycles of fluorescent biological aerosol particles in boreal and semi-arid forests of Finland and Colorado. Atmos. Chem. Phys. 13 (23):11987–12001. doi: 10.5194/acp-13-11987-2013.
- Sesartic, A., U. Lohmann, and T. Storelvmo. 2012. Bacteria in the ECHAM5-HAM Global Climate Model. Atmos. Chem. Phys. 12 (18):8645–8661. doi: 10.5194/acp-12-8645-2012.
- Šikoparija, B., O. Marko, M. Panić, D. Jakovetić, and P. Radišić. 2018a. How to prepare a pollen calendar for forecasting daily pollen concentrations of ambrosia, betula and poaceae? Aerobiologia (Bologna) 34 (2):203–217. doi: 10.1007/s10453-018-9507-9.
- Šikoparija, B., G. Mimić, M. Panić, O. Marko, P. Radišić, T. Pejak-Šikoparija, and A. Pauling. 2018b. High temporal resolution of airborne ambrosia pollen measurements above the source reveals emission characteristics. Atmos. Environ. 192:13–23. doi: 10.1016/j.atmosenv.2018.08.040.
- Šikoparija, B., C. A. Skjøth, K. Alm Kübler, A. Dahl, J. Sommer, L. Grewling, P. Radišić, and M. Smith. 2013. A mechanism for long distance transport of Ambrosia pollen from the Pannonian plain. Agric. For. Meteorol. 180:112–117. doi: 10.1016/j.agrformet.2013.05.014.
- Sikoparija, B., Galán, C. Smith, M. Abramidze, T. Adams-Groom, B. Albertini, R. Anelli, P. Bastl, K. Bigagli, V. Bonini, et al. 2017. Pollen-monitoring: Between analyst proficiency testing. Aerobiologia (Bologna) 33 (2):191–199.
- Smith, D. J., D. W. Griffin, R. D. McPeters, P. D. Ward, and A. C. Schuerger. 2011. Microbial survival in the stratosphere and implications for global dispersal. Aerobiologia (Bologna) 27 (4):319–332. doi: 10.1007/s10453-011-9203-5.
- Smith, D. J., J. D. Ravichandar, S. Jain, D. W. Griffin, H. Yu, Q. Tan, J. Thissen, T. Lusby, P. Nicoll, S. Shedler et al. 2018. Airborne bacteria in earth’s lower stratosphere resemble taxa detected in the troposphere: Results from a new NASA aircraft bioaerosol collector (ABC). Front. Microbiol. 9:1752. doi: 10.3389/fmicb.2018.01752.
- Smith, D. J., H. J. Timonen, D. A. Jaffe, D. W. Griffin, M. N. Birmele, K. D. Perry, P. D. Ward, and M. S. Roberts. 2013. Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl. Environ. Microbiol. 79 (4):1134–1139. doi: 10.1128/AEM.03029-12.
- Smith, B., M. Beman, D. Gravano, and Y. Chen. 2015. Development and validation of a microbe detecting UAV payload. Paper presented at 2015 Workshop on Research, Education and Development of Unmanned Aerial Systems (RED-UAS), IEEE, Cancun, Mexico, pp. 258–264.
- Sofiev, M., and K.-C. Bergmann, eds. 2013. Allergenic pollen: A review of the production, release, distribution and health impacts, 252 pp. Dordrecht Heidelberg: Springer. ISBN-13: 978-9400748804.
- Sofiev, M., P. Siljamo, H. Ranta, and A. Rantio-Lehtimäki. 2006. Towards numerical forecasting of long-range air transport of birch pollen: Theoretical considerations and a feasibility study. Int. J. Biometeorol. 50 (6):392–402. doi: 10.1007/s00484-006-0027-x.
- Sterflinger, K., and G. Piñar. 2013. Microbial deterioration of cultural heritage and works of art - tilting at windmills? Appl. Microbiol. Biotechnol. 97 (22):9637–9646. doi: 10.1007/s00253-013-5283-1.
- Sullivan, S. C., C. Hoose, A. Kiselev, T. Leisner, and A. Nenes. 2018. Initiation of secondary ice production in clouds. Atmos. Chem. Phys. 18 (3):1593–1610. doi: 10.5194/acp-18-1593-2018.
- Suski, K. J., T. C. J. Hill, E. J. T. Levin, A. Miller, P. J. DeMott, and S. M. Kreidenweis. 2018. Agricultural harvesting emissions of ice-nucleating particles. Atmos. Chem. Phys. 18 (18):13755–13771. doi: 10.5194/acp-18-13755-2018.
- Swanson, B. E., and J. A. Huffman. 2018. Development and characterization of an inexpensive single-particle fluorescence spectrometer for bioaerosol monitoring. Opt. Express 26 (3):3646–3660. doi: 10.1364/OE.26.003646.
- Tang, M., W. Gu, Q. Ma, Y. Jie Li, C. Zhong, S. Li, X. Yin, R. J. Huang, H. He, and X. Wang. 2019. Water adsorption and hygroscopic growth of six anemophilous pollen species: The effect of temperature. Atmos. Chem. Phys. 19 (4):2247–2258. doi: 10.5194/acp-19-2247-2019.
- Techy, L., D. G. Schmale, III, and C. A. Woolsey. 2010. Coordinated aerobiological sampling of a plant pathogen in the lower atmosphere using two autonomous unmanned aerial vehicles. J. F. Robot 27 (3):335–343.
- Temkiv, T. Š., K. Finster, B. M. Hansen, N. W. Nielsen, and U. G. Karlson. 2012. The microbial diversity of a storm cloud as assessed by hailstones. FEMS Microbiol. Ecol. 81 (3):684–695. doi: 10.1111/j.1574-6941.2012.01402.x.
- Tesson, S. V. M., and T. Šantl-Temkiv. 2018. Ice nucleation activity and aeolian dispersal success in airborne and aquatic microalgae. Front. Microbiol. 9:1–14.
- Thomson, E. S., D. Weber, H. G. Bingemer, J. Tuomi, M. Ebert, and J. B. C. Pettersson. 2018. Intensification of ice nucleation observed in ocean ship emissions. Sci. Rep. 8 (1):1111. doi: 10.1038/s41598-018-19297-y.
- Tobo, Y., K. Adachi, P. J. DeMott, T. C. J. Hill, D. S. Hamilton, N. M. Mahowald, N. Nagatsuka, S. Ohata, J. Uetake, Y. Kondo, et al. 2019. Glacially sourced dust as a potentially significant source of ice nucleating particles. Nat. Geosci. 12 (4):253–258. doi: 10.1038/s41561-019-0314-x.
- Tobo, Y., P. J. Demott, T. C. J. Hill, A. J. Prenni, N. G. Swoboda-Colberg, G. D. Franc, and S. M. Kreidenweis. 2014. Organic matter matters for ice nuclei of agricultural soil origin. Atmos. Chem. Phys. 14 (16):8521–8531. doi: 10.5194/acp-14-8521-2014.
- Tong, Y., and B. Lighthart. 2000. The annual bacterial particle concentration and size distribution in the ambient atmosphere in a rural area of the Willamette Valley, Oregon. Aerosol Sci. Technol. 32 (5):393–403. doi: 10.1080/027868200303533.
- Turnbull, P. C., P. M. Lindeque, J. Le Roux, A. M. Bennett, and S. R. Parks. 1998. Airborne movement of anthrax spores from carcass sites in the Etosha National Park, Namibia. J. Appl. Microbiol. 84 (4):667–676. doi: 10.1046/j.1365-2672.1998.00394.x.
- Uetake, J., Y. Tobo, Y. Uji, T. C. J. Hill, P. J. Demott, S. M. Kreidenweis, and R. Misumi. 2019. Seasonal changes of airborne communities over Tokyo and influence of local meteorology. Front. Microbiol. 10:1–12. doi: 10.3389/fmicb.2019.01572.
- United Nations. 2015. U.N. Sustainable Development Goals. Accessed September 10, 2019. https://sustainabledevelopment.un.org/
- Vaitilingom, M., L. Deguillaume, V. Vinatier, M. Sancelme, P. Amato, N. Chaumerliac, and A.-M. Delort. 2013. Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proc. Natl. Acad. Sci. 110 (2):559–564. doi: 10.1073/pnas.1205743110.
- Vaïtilingom, M., L. Deguillaume, V. Vinatier, M. Sancelme, P. Amato, N. Chaumerliac, and A.-M. Delort. 2013. Potential impact of microbial activity on the oxidant capacity and organic carbon budget in clouds. Proc. Natl. Acad. Sci. U. S. A. 110 (2):559–564. doi: 10.1073/pnas.1205743110.
- Vesala, T., N. Kljun, Ü. Rannik, J. Rinne, A. Sogachev, T. Markkanen, K. Sabelfeld, T. Foken, and M. Y. Leclerc. 2008. Flux and concentration footprint modelling: State of the art. Environ. Pollut. 152 (3):653–666. doi: 10.1016/j.envpol.2007.06.070.
- Villa, T. F., F. Salimi, K. Morton, L. Morawska, and F. Gonzalez. 2016. Development and validation of a UAV based system for air pollution measurements. Sensors (Switzerland) 16 (12):2202. doi: 10.3390/s16122202.
- Vinatier, V., N. Wirgot, M. Joly, M. Sancelme, M. Abrantes, L. Deguillaume, and A. M. Delort. 2016. Siderophores in cloud waters and potential impact on atmospheric chemistry: Production by microorganisms isolated at the Puy De Dôme Station. Environ. Sci. Technol. 50 (17):9315–9323. doi: 10.1021/acs.est.6b02335.
- von Blohn, N., S. K. Mitra, K. Diehl, and S. Borrmann. 2005. The ice nucleating ability of pollen: Part III: New laboratory studies in immersion and contact freezing modes including more pollen types. Atmos. Res. 78 (3–4):182–189. doi: 10.1016/j.atmosres.2005.03.008.
- Von Der Weiden, S. L., F. Drewnick, and S. Borrmann. 2009. Particle Loss Calculator - A new software tool for the assessment of the performance of aerosol inlet systems. Atmos. Meas. Tech. 2 (2):479–494. doi: 10.5194/amt-2-479-2009.
- Waggoner, P. E. 1973. The removal of Helminthosporium maydis spores by wind. Phytopathology 63 (10):1252–1255. doi: 10.1094/Phyto-63-1252.
- Wang, X., G. B. Deane, K. A. Moore, O. S. Ryder, M. D. Stokes, C. M. Beall, D. B. Collins, M. V. Santander, S. M. Burrows, C. M. Sultana, et al. 2017. The role of jet and film drops in controlling the mixing state of submicron sea spray aerosol particles. Proc. Natl. Acad. Sci. 114 (27):6978–6983. doi: 10.1073/pnas.1702420114.
- Wang, B., T. H. Harder, S. T. Kelly, D. S. Piens, S. China, L. Kovarik, M. Keiluweit, B. W. Arey, M. K. Gilles, and A. Laskin. 2016. Airborne soil organic particles generated by precipitation. Nature Geosci. 9 (6):433–437. doi: 10.1038/ngeo2705.
- Wei, K., Zou, Z. Zheng, J. Li, F. Shen, C.-Y. Wu, Y. Wu, M. Hu, and M. Yao. 2016. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 550:751–759. doi: 10.1016/j.scitotenv.2016.01.137.
- Weil, T., C. De Filippo, D. Albanese, C. Donati, M. Pindo, L. Pavarini, F. Carotenuto, M. Pasqui, L. Poto, J. Gabrieli, et al. 2017. Legal immigrants: Invasion of alien microbial communities during winter occurring desert dust storms. Microbiome 5 (1):32. doi: 10.1186/s40168-017-0249-7.
- Werchan, M., T. Sehlinger, F. Goergen, and K.-C. Bergmann. 2018. The pollator: A personal pollen sampling device. Allergo J. Int. 27 (1):1–3. doi: 10.1007/s40629-017-0034-y.
- Wex, H., L. Huang, W. Zhang, H. Hung, R. Traversi, S. Becagli, R. J. Sheesley, C. E. Moffett, T. E. Barrett, R. Bossi, et al. 2019. Annual variability of ice nucleating particle concentrations at different Arctic locations. Atmos. Chem. Phys. 19:1–31. doi: 10.5194/acp-19-5293-2019.
- Whitehead, J. D., E. Darbyshire, J. Brito, H. M. J. Barbosa, I. Crawford, R. Stern, M. W. Gallagher, P. H. Kaye, J. D. Allan, H. Coe, et al. 2016. Biogenic cloud nuclei in the central Amazon during the transition from wet to dry season. Atmos. Chem. Phys. 16 (15):9727–9743. doi: 10.5194/acp-16-9727-2016.
- Whitehead, J. D., M. W. Gallagher, J. R. Dorsey, N. Robinson, A. M. Gabey, H. Coe, G. McFiggans, M. J. Flynn, J. Ryder, E. Nemitz, et al. 2010. Aerosol fluxes and dynamics within and above a tropical rainforest in South-East Asia. Atmos. Chem. Phys. 10 (19):9369–9382. doi: 10.5194/acp-10-9369-2010.
- Wiedensohler, A., W. Birmili, J. P. Putaud, and J. Ogren. 2014. Recommendations for aerosol sampling. In Aerosol science: Technology and applications, ed. I. Colbeck and M. Lazaridis, 45–59. Hoboken, New Jersey: John Wiley & Sons, Ltd.
- Wilkinson, D. M., S. Koumoutsaris, E. A. D. Mitchell, and I. Bey. 2012. Modelling the effect of size on the aerial dispersal of microorganisms. J. Biogeogr. 39 (1):89–97. doi: 10.1111/j.1365-2699.2011.02569.x.
- Wilson, T. W., L. A. Ladino, P. A. Alpert, M. N. Breckels, I. M. Brooks, J. Browse, S. M. Burrows, K. S. Carslaw, J. A. Huffman, C. Judd, et al. 2015. A marine biogenic source of atmospheric ice-nucleating particles. Nature 525 (7568):234–238. doi: 10.1038/nature14986.
- Wirgot, N., V. Vinatier, L. Deguillaume, M. Sancelme, and A. M. Delort. 2017. H2O2 modulates the energetic metabolism of the cloud microbiome. Atmos. Chem. Phys. 17 (24):14841–14851. doi: 10.5194/acp-17-14841-2017.
- Wolf, R., I. El-Haddad, J. G. Slowik, K. Dällenbach, E. Bruns, J. Vasilescu, U. Baltensperger, and A. S. H. Prévôt. 2017. Contribution of bacteria-like particles to PM2.5 aerosol in urban and rural environments. Atmos. Environ. 160:97–106. doi: 10.1016/j.atmosenv.2017.04.001.
- Womack, A. M., P. E. Artaxo, F. Y. Ishida, R. C. Mueller, S. R. Saleska, K. T. Wiedemann, B. J. M. Bohannan, and J. L. Green. 2015. Characterization of active and total fungal communities in the atmosphere over the Amazon Rainforest. Biogeosciences. 12(21):6337–6349. doi: 10.5194/bg-12-6337-2015.
- Womack, A. M., B. J. M. Bohannan, and J. L. Green. 2010. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. B Biol. Sci. 365 (1558):3645–3653.
- Wright, T. P., J. D. Hader, G. R. McMeeking, and M. D. Petters. 2014. High relative humidity as a trigger for widespread release of ice nuclei. Aerosol Sci. Technol. 48 (11):i–v. doi: 10.1080/02786826.2014.968244.
- Wu, Y., A. Calis, Y. Luo, C. Chen, M. Lutton, Y. Rivenson, X. Lin, H. C. Koydemir, Y. Zhang, H. Wang, et al. 2018. Label-free bioaerosol sensing using mobile microscopy and deep learning. ACS Photonics 5 (11):4617–4627. doi: 10.1021/acsphotonics.8b01109.
- Wu, Y.-H., C.-C. Chan, C. Y. Rao, C.-T. Lee, H.-H. Hsu, Y.-H. Chiu, and H. J. Chao. 2007. Characteristics, determinants, and spatial variations of ambient fungal levels in the subtropical Taipei metropolis. Atmos. Environ. 41 (12):2500–2509. doi: 10.1016/j.atmosenv.2006.11.035.
- Xu, Z., Y. Wu, F. Shen, Q. Chen, M. Tan, and M. Yao. 2011. Bioaerosol science, technology, and engineering: Past, present, and future. Aerosol Sci. Technol. 45 (11):1337–1349. doi: 10.1080/02786826.2011.593591.
- Yamaguchi, N., T. Ichijo, A. Sakotani, T. Baba, and M. Nasu. 2012. Global dispersion of bacterial cells on Asian dust. Sci. Rep. 2 :525doi: 10.1038/srep00525.
- Yamamoto, N., Y. Matsuki, H. Yokoyama, and H. Matsuki. 2015. Relationships among indoor, outdoor, and personal airborne Japanese cedar pollen counts. PLoS One 10 (6):e0131710–14. doi: 10.1371/journal.pone.0131710.
- Yang, Y., S. Yokobori, J. Kawaguchi, T. Yamagami, I. Iijima, N. Izutsu, H. Fuke, Y. Saitoh, Y. Matsuzaka, M. Namiki, et al. 2008. Investigation of cultivable microorganisms in the stratosphere collected by using a balloon in 2005. JAXA Res. Dev. Rep. 35–42. https://repository.exst.jaxa.jp/dspace/handle/a-is/18956
- Yao, M. 2018. Reprint of bioaerosol: A bridge and opportunity for many scientific research fields. J. Aerosol Sci. 119:91–96. doi: 10.1016/j.jaerosci.2018.01.009.
- Zavala, J., K. Lichtveld, S. Ebersviller, J. L. Carson, G. W. Walters, I. Jaspers, H. E. Jeffries, K. G. Sexton, and W. Vizuete. 2014. The gillings sampler - An electrostatic air sampler as an alternative method for aerosol in vitro exposure studies. Chem. Biol. Interact. 220:158–168.
- Ziemba, L. D., A. J. Beyersdorf, G. Chen, C. A. Corr, S. N. Crumeyrolle, G. Diskin, C. Hudgins, R. Martin, T. Mikoviny, R. Moore, et al. 2016. Airborne observations of bioaerosol over the Southeast United States using a wideband integrated bioaerosol sensor. J. Geophys. Res. 121 (14):8506–8524. doi: 10.1002/2015JD024669.
- Ziska, L. H., L. Makra, S. K. Harry, N. Bruffaerts, M. Hendrickx, F. Coates, A. Saarto, M. Thibaudon, G. Oliver, A. Damialis, et al. 2019. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: A retrospective data analysis. Lancet Planet. Health 3 (3):124–131.
