 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The condensational growth of water droplets in technical and natural systems varies with environmental conditions such as droplet size, droplet composition, air humidity, temperature, and turbulence. This contribution addresses the influence of external electrical fields on the condensation process. Although electrical fields exist in the atmosphere, for example in thunderstorm clouds, and although it is generally accepted that electrical fields exert an influence on the condensation process, no quantitative description of this influence at ambient temperatures exists. We present laboratory experiments and a theoretical model to further develop understanding of the influence of electric fields on condensation at various temperatures. The levitated droplet cluster technology is applied to study the kinetics of droplet growth in an external electric field at temperatures between 50 °C and 70 °C. The theoretical model is designed to mirror the experimental conditions as precisely as possible. Experimental and kinetic model results are in qualitative agreement in that the relative contribution of the electrically induced contribution to total condensation reduces with increasing temperature. Vice versa, the electrically induced condensation significance is expected to further increase with decreasing temperatures below 50 °C. We expect that electro-condensation will be the dominating process at room temperatures and even more at temperatures near 0 °C, at electric field strengths typical for clouds. Further studies are needed to extend the experimental and theoretical temperature range to conditions typical for clouds in the atmosphere.
Copyright © 2020 American Association for Aerosol Research
EDITOR:
1. Introduction
The interrelationships between atmospheric electricity and precipitation have been studied for centuries (Franklin Citation1751; Kelvin Citation1872; Foote Citation1878; Zeleny Citation1917). Electric fields typical for thunderstorm clouds affect the condensation processes in clouds (Isard Citation1977; Murino Citation1979; Warshavsky and Shchekin Citation1999; Vorob’ev and Malyshenko Citation2001; Amiri, Pourabadeh, and Khatibi Citation2002; Singh and Kumar Citation2003; Sharma Citation2010; Butt et al. Citation2011). Field strengths of about 105 V m−1 occur which accelerate the condensational growth of droplets (Gabyshev et al. Citation2019) specifically in the lower zone of the clouds, leading to a more rapid formation of precipitation (Shishkin Citation1954).
This contribution addresses the topic of condensational growth of water droplets in electric fields. The issue is not only of fundamental interest in cloud physics, but also receives increasing attention during the development of devices for collecting atmospheric moisture (Reznikov Citation2015; Cruzat and Jerez-Hanckes Citation2018; Damak and Varanasi Citation2018) and condensational heat exchangers (Saha et al. Citation2020; Shahriari et al. Citation2017) using electric fields. We apply the levitated droplet cluster technology to study microphysical processes in and around droplets, which allows to control single droplets in laboratory conditions (Fedorets Citation2004). A detailed description of this technology is provided in paragraph 2.1. The droplets levitate freely and in self-organized, hexagonal clusters above a flat surface of water. The ascending steam-air flow (Fedorets et al. Citation2017a) establishes a vertical velocity of about 0.1 m s−1 and thus keeps the droplets levitated for several minutes, mimicking a gravitational sedimentation distance of tens of meters in a natural cloud. Likewise, under natural conditions, the laboratory droplets fall at a terminal velocity relative to the surrounding air. Besides the sedimentation flow, the droplets are almost motionless, yet creating microscopic turbulences leading to small, virtually random vibrational motions (Fedorets et al. Citation2019a). The experimental setup has proven useful to study the impact of external electrical fields on the condensational growth of such droplets (Gabyshev et al. Citation2019; Fedorets et al. Citation2019b; Fedorets et al. Citation2020).
In earlier studies, both the mathematical model and the experimental conditions were limited to a fixed temperature of (Gabyshev et al. Citation2019). First attempts to study microbiological processes in droplets (Fedorets et al. Citation2019c) extended the temperature range down to
(Fedorets, Dombrovsky, and Ryumin Citation2017b). It is obvious that the description of condensational growth of droplets in natural clouds needs to cover temperatures down to 0 °C and below. There are still a number of technical issues preventing such experiments. Particularly, at a lower temperature, only smaller droplets levitate, which rapidly become mechanically unbalanced in electric fields. It is therefore the goal of this contribution to derive a theoretical concept to describe the temperature dependence of the condensational growth of water droplets in an electric field. We will verify the results with experimental data between 50 °C and 70 °C using levitated droplet clusters in laboratory experiments. Finally, we will extrapolate the conclusions to room temperatures. In a sense, this study tries to build a bridge between the droplet cluster technology and real-world cloud droplets, between high-temperature experiments on the cluster on the one hand and room temperature natural precipitation conditions on the other.
2. Details of experiment
2.1. Experimental setup
An experimental setup to generate droplet clusters was used to study the impact of an external electric field on the condensational growth of water droplets (Fedorets, Aktaev, and Dombrovsky Citation2018; Fedorets and Dombrovsky Citation2017). Briefly, a self-organizing, hexagonal array of droplets levitates above a water surface (). The liquid layer is distributed over a sitall substrate of 400 μm thickness, which is lower side is heated by a laser beam focused on a spot of 1 mm diameter. The confocal chromatic sensor, which provides feedback for the peristaltic pump, helps to maintain a water layer thickness of 400 ± 2 μm. The thermostat maintains the layer temperature far from the heating point at
Figure 1. Schematics of a side view of a laboratory set-up with top-view photograph of a droplet cluster (dashed line shows the central group in the cluster used to measure the average growth rate).
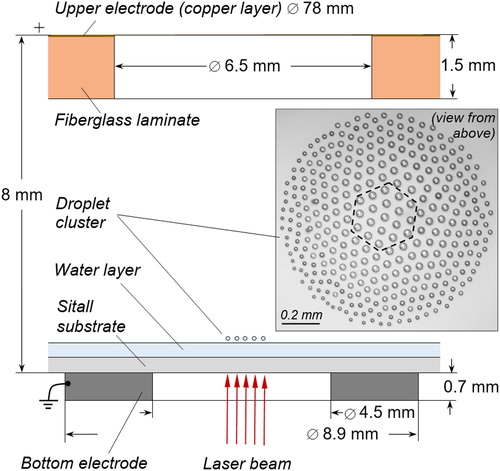
The water layer evaporates in the area of local laser heating and forms a convective, rising plume of a heated mixture of steam and air (Fedorets Citation2012; Fedorets, Marchuk, and Kabov Citation2014). A horizontal airflow from the periphery to the center of the heated area feeds the plume (Fedorets et al. Citation2017c). There is a vertical temperature gradient within the plume (Arinshtein and Fedorets Citation2010), which leads to a cooling of the moist air to temperatures below the dew point. Since there is no shortage of condensation nuclei in the laboratory air, water droplets spontaneously form through heterogeneous condensation. While these droplets grow, the smallest droplets are blown away and too heavy droplets fall out of the steam back to water surface. Some droplets of intermediate size find a balance with the vertical flow and self-assemble into an organized, flat, hexagonal cluster (). The distance between droplets and the height of their levitation are of the order of the droplets’ diameters 2 R.
An external electric field is established with a pair of electrodes. The lower electrode is a thin metal washer (0.7 mm thick, 8.9 mm outer diameter, 4.5 mm inner diameter), glued directly to the lower surface of the sitall substrate. The upper electrode is a disk of metalized fiberglass (copper layer, 18 μm thick) with an outer diameter of 78 mm and a diameter of the observational hole in its center of 6.5 mm. The HVLAB3000 high-voltage source (ET Enterprises, UK) creates an electric field with potential difference in the range from 0.2 to 3.0 kV. Throughout the experiment, the upper electrode has a positive potential and the lower electrode is grounded. The condensational growth rate was measured experimentally at five temperatures from 50 °C up to 70 °C in increments of 5 °C.
2.2. Electric fields
The electric field strength near the droplet cluster E0 is related to the potential at the upper electrode U by the ratio (Gabyshev et al. Citation2019; Fedorets et al. Citation2019b):
(1)
(1)
where U0 = 1 kV. According to the numerical calculations performed to establish (Equation1
(1)
(1) ), the field inhomogeneity is small and it varies by less than 10−2 V m−1 near the droplets.
The external field polarizes the droplet, which levitates above the water layer surface. In addition, according to the concept of mirror charges (Jackson Citation1962), the droplet has a reflection in water with image charges of signs opposite to those on the droplet. Assuming that all induced charges within the droplet are concentrated at its upper and lower poles (Fedorets et al. Citation2020), the contribution of the image charges qim to the total electric field is, based on the solution presented in (Sivukhin Citation1977),
(2)
(2)
where ε0 is the electric constant, and R is the droplet radius. The indices 1, 2, and 3 of the relative permittivity ε stand for moist air, the water layer, and the droplet, respectively. The image charges create an electric field intensity near the height of the center of the original cluster droplet, which can be formulated as:
(3)
(3)
Here, h is the distance from the water surface to the droplet’s base. Since the gap h typically is of the order of the droplet radius R and therefore
the proximity of the water surface does not significantly affect the electric field in the region of the levitated cluster.
In an external electric field, the cluster lifetime reduces due to electrokinetic forces that tend to disrupt the cluster’s mechanical equilibrium (Gabyshev Citation2018; Andreev and Gabyshev Citation2018). Therefore, in earlier experiments, the voltage at the upper electrode did not exceed 700 V (Gabyshev et al. Citation2019; Fedorets et al. Citation2019b; Fedorets et al. Citation2020). In the present study, the voltage was limited to U = 400 V. The measured condensational growth rate was averaged over a group of droplets in the cluster center (). Within the group, the difference in the diameters of maximum and minimum droplets did not exceed 5%.
3. Calculation model
3.1. General considerations
The mass flow of water vapor deposited on a droplet with surface S consists of two terms:
(4)
(4)
The first term I0 is diffusion. The second term IE occurs in the presence of an electric field due to the drift motion of water vapor molecules
(5)
(5)
where n0 is the concentration of water vapor molecules of mass m0 moving onto the elementary area dS of the droplet surface S at normal velocity
In our model, the droplet temperature is considered to be in equilibrium with the temperature of the surrounding air (the thermal stabilization characteristic time scale of a cluster droplet is of the order of 10−2 s (Fedorets et al. Citation2020)).
Expression (Equation4(4)
(4) ) was used in Gabyshev et al. (Citation2019) at a fixed temperature. However, a considerable temperature dependence exists depending on the relative permittivity ε, the density ρ of water in the droplet, the mean free path
the diffusion coefficient D of water vapor molecules in air, as well as the absolute humidity c (see Appendix). At the same time, the polarization of water vapor molecules condensing on a droplet depends on both the temperature and the external electric field. In addition to the external field, the droplet has an intrinsic electric field, which we will take into account in the description of its growth rate. For simplicity, the influences of the neighboring droplets’ fields are neglected as before (Gabyshev et al. Citation2019).
3.2. Diffusional term
The mass flux to the droplet surface (Fuchs Citation1959) is determined by taking into account the Stefan flow responsible for hydrodynamic entrainment of air by the steam flow
(6)
(6)
where D is the water vapor diffusion coefficient (Gebhart et al. Citation1988), r is the distance from the droplet center,
is the total molar concentration of the medium equal to the sum of the molar concentration c/M of water vapor (with an absolute humidity c and a molar mass M) and the molar concentration of the remaining components of the air c'/M' (as dry air with the mass concentration c' and a molar mass M'), respectively. The value of cs differs very slightly near and far from the droplet.
We have no knowledge of the exact law for dc/dr, which is the change in the mass concentration of steam c with increasing distance r from the droplet center. Thence, after transformation, integration, and substitution the limits of integration from (Fuchs Citation1959), we define the effective mass concentration difference for water vapor
(7)
(7)
and obtain the expression for the steam flux condensed on the droplet: where c0 and
are the absolute humidities in the vicinity of the droplet surface and at the water layer surface directly under the droplet, respectively. It is convenient to use the form:
(8)
(8)
where
is the water vapor mixing ratio (ratio of the water vapor mass concentration c to that of dry air c'). The ratio χ characterizes the intensity of the Stefan flow. It is small at room temperature or below, but cannot be neglected at high temperatures (appendix).
From other side, the increase of the droplet mass per unit time is
(9)
(9)
After transformation, separation of variables, and integration we obtain the equation for the droplet radius R
(10)
(10)
The droplet’s surface area is S = 4πR2. From (Equation10(10)
(10) ), the surface area growth rate per unit time is
(11)
(11)
Limit χ ≪ M/M' yields with (Equation11
(11)
(11) ) in classical Maxwellian form (Maxwell Citation1890).
The Kelvin equation describes the absolute humidity near the curved surface of a droplet (Jakubczyk et al. Citation2012)
(12)
(12)
where
is the Boltzmann constant,
is the absolute humidity above a flat surface at absolute temperature T, σ is the surface tension coefficient of water, and M is the molar mass of bulk water. The Kelvin formula does not allow analytical solutions of the respective equations. However, it is significant only at radii less than 10−7 m and our droplets are in the range of 4 µm < R < 14 μm. We therefore neglect droplets’ curvature effects.
The calculation of the absolute humidities and
deserve special attention. An experimentally observed droplet cluster levitates in the center of the low-pressure region bounded by a local heating spot. The pressure is higher on the rim of this region rather than in its center so that the cluster is locked in a potential well (Fedorets et al. Citation2019a; Aktaev et al. Citation2018). Inside this well, the air humidity changes with height above the water surface. The steam near a flat water surface is close to saturation. When ascending, it cools so that it becomes more saturated and even supersaturation occurs. Since a droplet cluster has formed spontaneously, the dew point was reached in the region of its levitation. A more detailed estimate of the height of initial condensation is not possible from the experimental results alone. At the same time, some authors (Fedorets Citation2004; Shavlov, Dzhumandzhi, and Romanyuk Citation2011) describe a heuristic picture of a cluster’s droplet existing at essentially non-equilibrium conditions, at which only half of the droplet (γ1 = 1/2) contributes to condensation.
As the condensation appears to happen mainly in the bottom half of the droplet due to the direction of the oncoming steam-air flow, we have to estimate the temperature difference between the droplet and the water layer surface. When, for example, the condensational rate is m2 s−1 (Gabyshev et al. Citation2019), the temperature difference is 0.06 °C from the relation (Equation11
(11)
(11) ). This is some effective value characterizing the difference in temperature of the lower edge of the droplet and that of water layer surface. This calibration allows to ignore a number of poorly controlled yet important parameters such as the variable levitation height, the spatial anisotropy due to the proximity of the droplet to the surface of thermostated water, the presence of random impurities in laboratory air, the variability of the steam-air flow speed with height, and the absolute humidity gradient. The value 0.06 °C is small because the condensation mainly occurs on the droplet’s bottom half, which is next to the water layer surface. Apparently, this value is adequate, because even weak bursts of the surface temperature of the layer were not detected by the thermal imaging camera during the collapse of the cluster.
We will calculate the single droplet growth rate in the absence of an electric field by using formula (Equation11(11)
(11) ), taking into account the area factor γ1 as discussed above. However, the individual droplets of a levitated cluster exert a mutual aerodynamic influence on each other and the growth rate of these droplets is only half (γ2 = 1/2) as compared to free, single droplets (Fedorets et al. Citation2017c). The reduction of the growth rate is caused by the competition of droplets for the steam and by an increased aerodynamic resistance of the cluster array. So far, neither an analytical nor a numerical description of this empirical finding has been presented. Therefore, experimental data of Ṡ0 will be compared to computational data of γṠ0, where γ = γ1 γ2. This approach is certainly not applicable to droplets at the cluster’s rim, because the conditions for condensation are not homogeneous in this region ().
Finally, the droplet levitates due to the steam-air flow from below. Therefore, the Frössling approximation (Frössling Citation1938; Mordy Citation1959) may be used to account for the ventilation in changes of the diffusion flux in (Equation4(4)
(4) ) by
where Pr is the Prandtl number and Re is the Reynolds number. The Reynolds number depends on the speed of the steam-air flow supporting the droplet levitation. Since there is no information available about the exact temperature dependence of this steam-air speed, we have to neglect it. The Prandtl number of air in the temperature range of liquid water does not exceed Pr = 0.7. The Reynolds number usually did not exceed Re = 0.5 in experiments with small droplets. Therefore, the error due to the ventilation estimates is way below 15%.
3.3. Drift term
In this section, the contribution of the external electric field to the condensational growth is estimated. We follow the kinetic scheme proposed in (Guerrini and Murino Citation1990), because it led to reasonable results in earlier studies (Gabyshev et al. Citation2019).
Droplets are microscopic spherical capacitors. Various electrification mechanisms occur (Lenard Citation1892; Shavlov Citation2008; Shavlov, Dzhumandzhi, and Yakovenko Citation2018; Saranin Citation1998; Shishkin Citation1951; Frenkel Citation1963) and the resulting charge affects the kinetics of condensation growth (Leontovich Citation1983). The charge q of a droplet with radius R is determined by
(13)
(13)
where k is the electrification coefficient depending on atmospheric conditions such as the cloud shape and stage of its development (Pruppacher and Klett Citation2010). In the levitated cluster, the droplets’ charge is negative, and k is 0.1 e.s.u. cm−2 or 3.4 · 10−7 C m−2 (Fedorets et al. Citation2020). It leads to q ≈ –500e for a droplet with R = 15 μm. This charge creates a field with the strength of about 103 V m−1. Therefore, the intrinsic charges are of weak significance both in natural thunderclouds and the laboratory experiment.
The external electric field, in which the droplet cluster is localized, is almost uniform (Gabyshev et al. Citation2019). The total field near the droplet surface E is equal to the sum of the external field the field of intrinsic charges of the droplet
and the polarization field of the droplet in the external field
(14)
(14)
In the projection onto the normal to the droplet surface, the strength is
(15)
(15)
The water vapor molecules are dipoles with a constant dipole moment p0 = 6.138 · 10−30 C · m. The total polarization of the water molecule is equal to the sum of the electronic polarization and orientational one (Singh and Singh Citation2004):
(16)
(16)
We use a value of for the electronic polarizability of a water molecule (Nir, Adams, and Rein Citation1973). Thus, its dipole moment, as induced in an external electric field, is
(17)
(17)
Its potential energy is
(18)
(18)
The process of vapor condensation onto a droplet occurs within a thin near-surface Knudsen layer around the droplet with a thickness of the order of the mean free path The presence of a local electric field (Equation14
(14)
(14) ) in this layer leads to acceleration of water vapor molecules. Those molecules, which fall onto the droplet in the absence of any external field, determine the diffusion flux in (Equation4
(4)
(4) ). They have non-zero initial normal velocity component directed to the droplet surface. Other steam molecules do not have that normal velocity, as they move tangentially to the droplet surface in the absence of the electric field. According to the kinetic theory of gases, such molecules make up exactly 2/3 of the total number under the assumption that all directions of their motion are equally probable. They dominate the drift flux (Equation5
(5)
(5) ), when the electric field is on. A molecule moving along a tangent can make its path both at a distance
and in close proximity to the surface of the droplet. Therefore, when the electric field is switched on, such molecules have different yet equally probable values of the distance from the surface of the droplet (within the range from zero to
). In order to take the likelihood of the initial distances into account, the decrease in potential energy should be calculated with half the length
(19)
(19)
where m0 is the mass of one water molecule,
is the component of the molecular velocity directed normal to the surface of the droplet. Thus, the normal velocity of the deposited molecules depends on the exact location of impact on the droplet’s surface. This location is determined by the colatitude angle η and the azimuthal angle φ:
(20)
(20)
The drift flux is thus equal to
(21)
(21)
where the 2/3 coefficient in front of the integral takes into account the equiprobable motion of vapor molecules according to the kinetic theory of gases.
From (Equation4(4)
(4) ), the total vapor mass flow dm accumulated on the droplet during the time dt is
(22)
(22)
where
(23)
(23)
The area growth rate is
(24)
(24)
And the radius growth rate is
(25)
(25)
The last expression is implicit, because the radius is in both the left and right hand sides. After the permutation, time is finally expressed as:
(26)
(26)
where γ = 1/4 is the parameter introduced and discussed in paragraph 3.2, resulting from the aerodynamic drag of the droplet cluster as a whole. The dependence (Equation26
(26)
(26) ) describes the relation between t and R. The dependence on the droplet area S introduces by substituting
in the upper limit of the outward integral.
From (Equation21(21)
(21) ), the ratio of the drift flux IE to the diffusion flux I0, introduced in (Equation4
(4)
(4) ), depends on the temperature and the electric field:
(27)
(27)
4. Results and discussion
The experimental data were obtained in the absence of an electric field as well as at U = 300 V and U = 400 V. The last two values, according to (Equation1(1)
(1) ), correspond to the electric field strengths of 0.65 · 105 and 0.87 · 105 V m−1 in the region of the droplet cluster, respectively. The average radius of droplets in the clusters was 8 μm.
As the basic quantities characterizing the condensational growth, we chose the average absolute droplet’s growth rate Ṡ [μm2 s−1] and the relative rate Ṡr = Ṡ/Ṡ0 (divided by Ṡ0 in the absence of an electric field). The secondary quantities are ΔṠr/ΔE [V−1 μm] (change in the relative rate Ṡr over the interval of strength ΔE between 0.65 · 105 and 0.87 · 105 V m−1, i.e., between U = 300 V and 400 V, according to (Equation1(1)
(1) )), ΔṠr/ΔT [K−1] (change in the relative growth rate Ṡr for equal temperature intervals ΔT = 5 K). The use of relative quantities is convenient in that the subtraction of unity from Ṡr yields the fraction (Equation27
(27)
(27) ) of the contribution of the drift flux caused by the electric field: Ṡr − 1 = (Ṡ – Ṡ0)/Ṡ0 = IE/I0. On average, the relative error in measuring the surface area growth rate Ṡ was about 7%. In the graphs of , the corresponding confidence intervals, in fact, do not go beyond the markers and therefore are not shown.
Figure 2. Experimental points (with solid lines) and calculated functions for a droplet with radius 8 μm (dotted lines): (a) the average absolute growth rate Ṡ, (b) the relative growth rate Ṡr, (c) the change in Ṡr in the interval E between 0.65 · 105 and 0.87 · 105 V m−1, and (d) the change in Ṡr in the temperature intervals ΔT = 5 K.
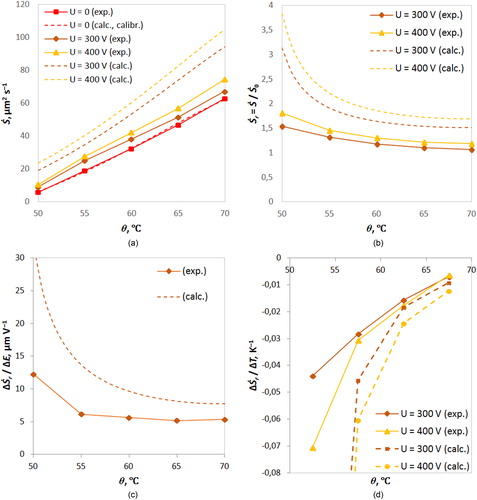
We can conclude that the electric field accelerates the growth of droplets. The stronger the field, the faster the growth. The absolute value of Ṡ increases with increasing temperature θ (), which is caused by an increase in the maximum absolute humidity around the droplet. At the same time, the rate Ṡ is higher at a higher magnitude of the applied electric field due to the acceleration of steam molecules in the near-surface layer and an intensification of the drift flow (section 3.3). According to (Equation27(27)
(27) ), the contribution of electro-condensation linearly depends on the field strength modulus (Gabyshev et al. Citation2019).
The relative rate Ṡr and the fraction of the drift flux Ṡr − 1 (contribution of electro-condensation) decrease with increasing temperature (). This agrees with the disordering of the steam molecular dipoles as predicted above at a higher temperature, which suppresses the orientation polarization of the molecules. In a stronger field, the curve runs higher because the field partially compensates for disordering of steam molecules that occurs with increasing temperature (Equation16(16)
(16) ).
The transition of the field strength from E = 0.65 · 105 V m−1 (U = 300 V) to E = 0.87 · 105 V m−1 (U = 400 V) is accompanied by a decrease of the relative rate Ṡr with increasing temperature (). In other words, it is more difficult to achieve an increase in Ṡr by increasing the field strength at higher temperatures. From a different point of view, a change of the field strength by the same amount causes a smaller change in Ṡr at higher temperatures than at lower ones (the slopes decrease with increasing temperature in ).
The relative rate increment per unit temperature increment ΔṠr/ΔT decreases in modulus with increasing temperature (). Since the experiment provides discrete values with a step width of ΔT = 5 K, the model calculation also had to be discretized with this step width for better comparability (). This consequently introduces coarsening of the result. Negative values in mean a decrease of Ṡr along the positive direction of the horizontal axis in .
Finally, the graphs () can be interpreted in the opposite direction, which is more interesting from the point of view of cloud physics. Namely, with decreasing temperature θ, the droplet growth rate becomes more and more temperature-sensitive: the lower the temperature, the faster the increase of the relative rate Ṡr. This means that with decreasing temperature, the significance of electro-condensation increases because the contribution of the drift flow (equal to IE/I0 = Ṡr − 1) increases (). At low temperatures, condensation is more sensitive to changes of the field strength than at high temperatures (). Altogether, this indicates that in clouds, where temperatures are much lower than in the experiment, the influence of the electric field must be substantional. The experimental results give reasons to suggest that at a temperature below 50 °C the contribution of electro-condensation exceeds the diffusion flux in the absence of an electrical field ().
The model results are in qualitative agreement with the experimental results (the behavior and mutual arrangement of the curves). The discrepancy between theory and experiment seems to occur due to the highly non-equilibrium and anisotropic thermophysical conditions, in which the droplet cluster is located, and which could not be described in detail by the calculation now. We presume that under the conditions of an atmospheric cloud, the reaction of droplets to electrical fields is even more pronounced than in the experiment.
5. Conclusions
We studied the temperature dependence of the condensational growth rate of water droplets in a uniform electric field with an intensity typical for natural atmospheric clouds. The droplet cluster technology was employed. The experimental data were in qualitative agreement with a theoretical model. In addition to previous studies (Gabyshev et al. Citation2019), the present study takes into account the orientational polarization of steam molecules, uses a more accurate description of their electronic polarizability (Equation16(16)
(16) ), and accepts the equiprobable motion of steam molecules according to the kinetic theory of gases. The droplet growth rate in the absence of an electric field is now calculated analytically with calibration by experimental points. The Stefan flow is taken into account, which allows to apply the model over the liquid water temperature range, including high temperatures and even metastable states.
The model shows that the electric field enhances condensational growth. The growth rate depends on the temperature. The most significant driver of the temperature dependence is the humidity. In general, the higher the temperature, the higher the absolute humidity can be, and the faster a droplet grows in moist air. The stream flux, which leads to deposition onto a droplet and thus to condensation, consists of two parts. The diffusion part I0 is present in the absence of an electric field as well. In the presence of the electric field, the drift part IE adds to it. The contribution of the drift part or, in other words, the electro-condensation, is dependent on temperature in two ways. On the one hand, the absolute humidity c is proportional to the temperature and it accelerates electro-condensation. On the other hand, the orientational polarization of water vapor molecules (Equation16(16)
(16) ) decreases with temperature. The first of these two opposing mechanisms is dominant, so that the contribution of electro-condensation grows with increasing temperature and the droplet grows faster. However, the relative contribution of electro-condensation IE/I0 decreases with temperature because I0 grows faster with increasing temperature than IE does. This means that the higher the temperature, the less effective is the electro-condensation. Therefore, its contribution to total condensation is expected to be large at low temperatures.
We conclude that the previously discovered phenomenon of acceleration of condensational growth (Gabyshev et al. Citation2019; Fedorets et al. Citation2019b) has the most significant effect at low temperatures, ceteris paribus, when ordinary diffusion processes are rather slow. Presumably, the electro-condensation will be the dominating process at low temperatures near 0 °C as well as at room temperatures and at electric field strengths typical for clouds. The presence of an electric field of about 105 V m−1 accelerates condensation approximately by a factor of two. Further experimental studies should be carried out to develop a levitated droplet cluster technology for lower temperatures than possible so far (Fedorets, Dombrovsky, and Ryumin Citation2017b).
Authors’ contributions
D.G. developed the calculation model and drafted the manuscript. A.F. developed and carried out the experiment. O.K. reviewed and edited the manuscript. All authors participated in the discussion of results and contributed to the manuscript.
Additional information
Funding
References
- Aktaev, N. E., A. A. Fedorets, E. Y. Bormashenko, and M. Nosonovsky. 2018. Langevin approach to modeling of small levitating ordered droplet clusters. J. Phys. Chem. Lett. 9 (14):3834–8. doi:10.1021/acs.jpclett.8b01693.
- Amiri, M. C., J. Pourabadeh, and M. A. A. Khatibi. 2002. Effects of electric field on condensation. J. Appl. Sci. 2 (2):136–40. doi:10.3923/jas.2002.136.140.
- Andreev, S. N., and D. N. Gabyshev. 2018. Oscillatory motion of microdroplets of a Droplet cluster in a linearly nonuniform electric field. Bull. Lebedev Phys. Inst. 45 (9):257–62. doi:10.3103/S1068335618090014.
- Arinshtein, E. A., and A. A. Fedorets. 2010. Mechanism of energy dissipation in a droplet cluster. Jetp Lett. 92 (10):658–61. doi:10.1134/S0021364010220042.
- Borman, V. D., B. I. Nikolaev, N. I. Nikolaev, and V. A. Chuzhinov. 1971. The influence of an electric field on the thermal diffusion coefficient for gases. JETP 33 (5):881. http://www.jetp.ac.ru/cgi-bin/r/index/e/33/5/p881?a=list.
- Butt, H.-J., M. B. Untch, A. Golriz, S. A. Pihan, and R. Berger. 2011. Electric-field-induced condensation: an extension of the Kelvin equation. Phys Rev E: Stat Nonlin Soft Matter Phys. 83 (6 Pt 1):061604. doi:10.1103/PhysRevE.83.061604.
- Cruzat, D., and C. Jerez-Hanckes. 2018. Electrostatic fog water collection. J. Electrostat. 96:128–33. doi:10.1016/j.elstat.2018.10.009.
- Damak, M., and K. K. Varanasi. 2018. Electrostatically driven fog collection using space charge injection. Sci. Adv. 4 (6):eaao53232018. doi:10.1126/sciadv.aao5323.
- Fedorets, A. A. 2004. Droplet cluster. JETP Lett. 79 (8):373. doi:10.1134/1.1772434.
- Fedorets, A. A. 2012. Mechanism of Stabilization of a Droplet Cluster above the Liquid–Gas Interface. Tech. Phys. Lett. 38 (11):988–90. doi:10.1134/S1063785012110077.
- Fedorets, A. A., N. E. Aktaev, and L. A. Dombrovsky. 2018. Suppression of the condensational growth of droplets of a levitating cluster using the modulation of the laser heating power. Int. J. Heat Mass Transf. 127:660–4. doi:10.1016/j.ijheatmasstransfer.2018.07.055.
- Fedorets, A. A., N. E. Aktaev, D. N. Gabyshev, E. Bormashenko, L. A. Dombrovsky, and M. Nosonovsky. 2019a. Oscillatory motion of a Droplet cluster. J. Phys. Chem. C 123 (38):23572–6. doi:10.1021/acs.jpcc.9b08194.
- Fedorets, A. A., E. Bormashenko, L. A. Dombrovsky, and M. Nosonovsky. 2019c. Droplet clusters: nature-inspired biological reactors and aerosols. Philos. Trans. A Math. Phys. Eng. Sci. 377 (2150):20190121doi:10.1098/rsta.2019.0121.
- Fedorets, A. A., and L. A. Dombrovsky. 2017. Generation of levitating droplet clusters above the locally heated water surface: a thermal analysis of modified installation. Int. J. Heat Mass Transf. 104:1268–74. doi:10.1016/j.ijheatmasstransfer.2016.09.087.
- Fedorets, A. A., L. A. Dombrovsky, E. Bormashenko, and M. Nosonovsky. 2019b. On relative contribution of electrostatic and aerodynamic effects to dynamics of a levitating droplet cluster. Int. J. Heat Mass Transf. 133:712–7. doi:10.1016/j.ijheatmasstransfer.2018.12.160.
- Fedorets, A. A., L. A. Dombrovsky, D. N. Gabyshev, E. Bormashenko, and M. Nosonovsky. 2020. Effect of external electric field on dynamics of levitating water droplets. Int. J. Thermal Sci. 153:106375–83. doi:10.1016/j.ijthermalsci.2020.106375.
- Fedorets, A. A., L. A. Dombrovsky, and P. I. Ryumin. 2017b. Expanding the temperature range for generation of droplet clusters over the locally heated water surface. Int. J. Heat Mass Transf. 113:1054–8. doi:10.1016/j.ijheatmasstransfer.2017.06.015.
- Fedorets, A. A., M. Frenkel, E. Bormashenko, and M. Nosonovsky. 2017c. Small levitating ordered droplet clusters: stability, symmetry, and Voronoi entropy. J. Phys. Chem. Lett. 8 (22):5599–602. doi:10.1021/acs.jpclett.7b02657.
- Fedorets, A. A., M. Frenkel, E. Shulzinger, L. A. Dombrovsky, E. Bormashenko, and M. Nosonovsky. 2017a. Self-assembled levitating clusters of water droplets: pattern-formation and stability. Sci. Rep. 7 (1):1888doi:10.1038/s41598-017-02166-5.
- Fedorets, A. A., I. V. Marchuk, and O. A. Kabov. 2014. Application of a Droplet cluster to visualize microscale gas and liquid flows. Jetp Lett. 99 (5):266–9. doi:10.1134/S0015462808060124.
- Foote, E. 1878. Electricity in thunder-storms. Popular Sci. Mon. 13 (689):689–93.
- Franklin, B. 1751. Experiments and observations on electricity, 43. St. John's Gate, London: E. Cave.
- Frenkel, I. I. 1963. Theory and phenomena of atmospheric electricity. Wright-Patterson Air Force Base, Translation Division, Foreign Technology Division.
- Frössling, N. 1938. Über die Verdunstung fallender Tropfen. Gerlands Beiträge zur Geophysik 52:170–216.
- Fuchs, N. A. 1959. Evaporation and droplet growth in gaseous medium, ed. R. S. Bradley. Pergamon.
- Gabyshev, D. N. 2018. Damping oscillations of microdroplets of a Droplet cluster in an external electric field. Phys. Wave Phen. 26 (3):221–33. doi:10.3103/S1541308X1803007X.
- Gabyshev, D. N., A. A. Fedorets, N. E. Aktaev, O. Klemm, and S. N. Andreev. 2019. Acceleration of the condensational growth of water droplets in an external electric field. J. Aerosol Sci. 135:103–12. doi:10.1016/j.jaerosci.2019.06.002.
- Gebhart, B., Y. Jaluria, R. L. Mahajan, and B. Sammakia. 1988. Buoyancy-induced flows and transport. Hemisphere, Washington: Springer.
- Guerrini, A., and G. Murino. 1990. Electric Forces and Physics of Clouds. Il Nuovo Cimento C 13 (3):663–8. doi:10.1007/BF02507630.
- IAPWS. 1997. Release on the static dielectric constant of ordinary water substance for temperatures from 238K to 873K and pressures up to 1000 MPa. IAPWS R8-97. http://www.iapws.org/relguide/Dielec.html
- Isard, J. O. 1977. Calculation of the influence of an electric field on the free energy of formation of a nucleus. Philos. Mag. 35 (3):817–9. doi:10.1080/14786437708236010.
- Jackson, J. D. 1962. Classical electrodynamics. New York: Wiley. doi:10.1063/1.3057859.
- Jakubczyk, D., M. Kolwas, G. Derkachov, K. Kolwas, and M. Zientara. 2012. Evaporation of Micro-Droplets: the “Radius-Square-Law” Revised. Acta Phys. Pol. A 122 (4):709–16. doi:10.12693/APhysPolA.122.709.
- Jennings, S. G. 1988. The mean free path in air. J. Aerosol Sci. 19 (2):159–66. doi:10.1016/0021-8502(88)90219-4.
- Kelvin, W. T. 1872. Royal Institution Friday evening lecture May. 18, 1860. In Reprint of Papers on electrostatics and magnetism. New York: Macmillan.
- Kulmala, M., and T. Vesala. 1991. Condensation in the continuum regime. J. Aerosol Sci. 22 (3):337–46. doi:10.1016/S0021-8502(05)80011-4.
- Lenard, P. 1892. Ueber die Electricität der Wasserfälle. Ann. Phys. Chem. 282 (8):584–636. doi:10.1002/andp.18922820805.
- Leontovich, M. A. 1983. Introduction to thermodynamics. Statistical physics. Moscow: Nauka Press, 136–40. [in Russian].
- Maxwell, J. C. 1890. Theory of the wet bulb thermometer. In Scientific papers of James Clerk Maxwell, ed. W. D. Niven, vol. 2, 636–640. Cambridge: C. J. Clay, M. A. and Sons, University Press.
- Mordy, W. 1959. Computation of the growth by condensation of a population PF cloud Droplets. Tellus 11 (1):16–44. doi:10.3402/tellusa.v11i1.9283.
- Murino, G. 1979. Influence of electric field on condensation of water vapour. South African Journal of Physics 2; 113.
- Nir, S., S. Adams, and R. Rein. 1973. Polarizability calculations on water, hydrogen, oxygen, and carbon dioxide. J. Chem. Phys. 59 (6):3341–55. doi:10.1063/1.1680478.
- Pruppacher, H. R., and J. D. Klett. 2010. Microphysics of clouds and precipitation. Dordrecht: Springer.
- Reid, R. C., J. M. Prausnitz, and B. E. Poling. 1987. The properties of gases and liquids. New York: McGraw-Hill.
- Reznikov, M. 2015. Electrically enhanced condensation I: effects of corona discharge. IEEE Trans. Ind. Appl. 51 (2):1137–45. doi:10.1109/TIA.2014.2354734.
- Saha, S. K., H. Ranjan, M. S. Emani, and A. K. Bharti. 2020. Electric fields, additives and simultaneous heat and mass transfer in heat transfer enhancement. Cham, Switzerland:Springer.
- Saranin, V. A. 1998. Possibility of the levitation of Droplets in the atmosphere when they are charged by induction in an electric field under nonuniform evaporation conditions. Tech. Phys. 43 (2):145–50. doi:10.1134/1.1258958.
- Shahriari, A., P. Birbarah, J. Oh, N. Miljkovic, and V. Bahadur. 2017. Electric field–based control and enhancement of boiling and condensation. Nanoscale Microscale Thermophys. Eng. 21 (2):102–21. doi:10.1080/15567265.2016.1253630.
- Sharma, M. 2010. Role of electric field in the nucleation phenomenon. PhD thesis, Chaudhary Charan Singh University. http://shodhganga.inflibnet.ac.in/handle/10603/45166.
- Shavlov, A. V. 2008. The mechanism of interphase electrization at evaporation and vapor growth of ice and water. Earth's Cryosphere 12 (2):52.
- Shavlov, A. V., V. A. Dzhumandzhi, and S. N. Romanyuk. 2011. Electrical properties of water drops inside the dropwise cluster. Phys. Lett. A 376 (1):39–45. doi:10.1016/j.physleta.2011.10.032.
- Shavlov, A. V., V. A. Dzhumandzhi, and A. A. Yakovenko. 2018. Charge separation at the evaporation (condensation) front of water and ice. Charging of spherical Droplets. Tech. Phys. 63 (4):482–90. doi:10.1134/S1063784218040205.
- Shishkin, N. S. 1951. Studies of the formation of summer rainfall and lightning electricity. Phys. Usp. 45:313. doi:10.3367/UFNr.0045.195111a.0313.[in Russian]
- Shishkin, N. S. 1954. Clouds, precipitation, and thunderstorm electricity. Moscow: GITTL. [in Russian].
- Singh, N., and A. Kumar. 2003. Equivalence between external electric field with temperature and supersaturation in nucleation process. Indian J. Radio Space Phys. 32 (6):379–81.
- Singh, N., and D. Singh. 2004. Polarizability affecting nucleation of water vapour condensation and ice glaciation in presence of external electric field. Indian J. Radio Space Phys. 33 (1):43–9.
- Sivukhin, D. V. 1977. General physics course. V. 3. Electricity. Moscow: Nauka, 95–6. [in Russian].
- Someshwar, A. V., and B. W. Wilkinson. 1985. Study of electric field-induced effects on water vapor adsorption in porous adsorbents. Ind. Eng. Chem. Fund. 24 (2):215–20. doi:10.1021/i100018a014.
- Vargaftik, N. B. 1972. Thermophysical properties of gases and liquids: handbook. Moscow: Nauka. [in Russian]
- Vorob’ev, V. S., and S. P. Malyshenko. 2001. New phase nucleation in electric fields. J. Exp. Theor. Phys. 93 (4):753–9. doi:10.1134/1.1420443.
- Warshavsky, V. B., and A. K. Shchekin. 1999. The effects of external electric field in thermodynamics of formation of dielectric droplet. Colloids Surf. A. 148 (3):283–90. doi:10.1016/S0927-7757(98)00769-9.
- Wei, S., C. Zhong, and H. Su-Yi. 2005. Molecular dynamics simulation of liquid water under the influence of an external electric field. Mol. Simul. 31 (8):555–9. doi:10.1080/0892702500138483.
- Zeleny, J. 1917. Instability of electrified liquid surfaces. Phys. Rev. 10 (1):1–6. doi:10.1103/PhysRev.10.1.
Appendix.
Material expressions
To calculate the temperature dependence of the dielectric permittivity of water, we make a linear approximation over the entire range of liquid water at normal atmospheric pressure instead of expression in (IAPWS, Citation1997):
where T is the absolute temperature (correlation coefficient 0.998). We do the same with the 100% (highest possible) absolute humidity (correlation coefficient 0.999)
and with water density (correlation coefficient 0.999)
where θ is the temperature in units It is more convenient to use these more precise expressions rather than a power dependence (Vargaftik Citation1972). The ratio of the densities of water vapor and air is set by approximation (correlation coefficient 0.997 in the range from
to
):
For the mean free path of molecules in 100% moist air, we will extrapolate from a set of values from (Jennings Citation1988)
We approximate the diffusion coefficient of water vapor in air using data from (Gebhart et al. Citation1988):
The diffusion coefficient, like other transport coefficients, weakly depends on the electric field within the range under study (Borman et al. Citation1971; Someshwar and Wilkinson Citation1985; Wei, Zhong, and Su-Yi Citation2005). Alternative representations of the temperature dependence of the diffusion coefficient may also be used (Vargaftik Citation1972; Kulmala and Vesala Citation1991; Reid, Prausnitz, and Poling Citation1987).
