?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Air quality on board the International Space Station (ISS) is a significant concern for the long-term health of astronauts living on the station. Aerosolized particles are generated from a variety of sources, such as on-board equipment, electronics, and the astronauts themselves. Airborne particles can remain suspended for significant amounts of time due to the absence of gravitational settling. In this work, we examine the particulate matter on board the ISS through scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM/EDX) and single-particle multi-element inductively coupled plasma time-of-flight mass spectrometry (sp-ICP) analysis of two vacuum bag dust samples collected with a handheld vacuum cleaner used to clean surfaces and filter faces inside the ISS. SEM/EDX analysis shows that many large particles were collected. These particles are most commonly clothing fibers or of biological origin, along with smaller numbers of carbonaceous particles that may be from polymer and halopolymer materials. sp-ICP analysis quantitatively determined metal concentrations in particles approximately <10 µm in diameter and was able to identify several distinct particle compositions. Some of these, such as stainless steel (Fe-Cr-Ni-Mn), antiperspirant (Al-Zr), silver (Ag), and titanium oxide (Ti) particles, are consistent with particle types identified through past aerosol and vacuum bag sampling and analyses. Other types, including Fe-Al and other types of Al and Zr particles, do not have a clear origin consistent with past work. SEM/EDX and sp-ICP provide different but complementary information regarding the composition of particles obtained from vacuum bag dust samples. The material analyzed was obtained through the Divert Unwanted Space Trash (DUST) experiment on ISS, which returned vacuum bags to Earth for the purpose of understanding airborne particles in the unique ISS environment.
Copyright © 2021 American Association for Aerosol Research
EDITOR:
Introduction
Aerosol particles including dust from skin cells, hair, particles from many other sources are generated during normal work and habitation within the International Space Station (ISS). The aerosol particles that contribute to dust can originate from a number of sources, including the astronauts themselves, objects and materials used on a regular basis, and the underlying materials, electronics, and components of the station. Large-scale mixing of the ventilation system with HEPA filtration is designed to control the airborne particulate matter, although localized higher concentrations can exist near sources. The mechanisms by which particles are generated, persist in the air, and interact with surfaces on the ISS differ from mechanisms that are understood to work at the Earth’s surface owing to the zero-gravity environment (Meyer et al. Citation2018; Meyer Citation2015; Mulholland et al. Citation2015). Gravitational settling is the primary means by which large particles (with diameters on the order of tens to hundreds of µm) are removed from the atmosphere near the Earth’s surface, a process which occurs over timescales of seconds to minutes (Haines et al. Citation2019). Respirable particles are typically considered to be particles with aerodynamic diameters less than 20–100 µm (Brown et al. Citation2013; WHO Citation1999), though particles with aerodynamic diameters greater than 20–30 µm are often not considered significant exposure risks due to short lifetimes (Brown et al. Citation2013; WHO Citation1999). The potential negative health impacts of inhaled particles tend to increase as particle size decreases, as smaller particles are able to penetrate further into the respiratory system (Brown et al. Citation2013; Oberdörster Citation2000; WHO Citation1999). Larger particles are cleared through mucociliary transport and do not penetrate into the respiratory system, but these particles are often swallowed and transported to the gastrointestinal tract (Vignal et al. Citation2017). Exposure to air pollution may also be associated with a variety of gastrointestinal issues (Vignal et al. Citation2017 and references therein). However, the necessary caveat to these studies is that they were performed near the Earth’s surface with the associated particle microphysics and gravitational effects. Gravitational settling processes will not occur on-board the ISS, allowing large particles to persist in the ISS internal atmosphere and potentially creating unique exposure environments for the swallowing and ingestion of larger particles. Inhalation of particles is a known health risk and may be a concern on the space station as well due to the months-long time periods that astronauts spend in the enclosed environment of the ISS. These issues will also not be unique to the ISS and would be present in other zero-gravity environments related to future long-term habitation and travel in space.
An analysis of the air and dust on board the ISS is necessary to understand the prevalence and characteristics of any airborne particles. This analysis can then be used to assess any potential health risks to astronauts and to gain an understanding of particle sources, which may provide insight into the condition of materials on board the ISS. Identification of particle sources can also help direct mitigation equipment and activities to reduce particle emissions that impair air quality in space environments and habitats. Two significant parameters relevant to evaluating these particle properties are knowledge of the aerosol particle elemental composition and size. In recent years, airborne particles on the ISS have been studied by analyzing the contents of vacuum bag samples and by deploying a passive aerosol sampling system to collect aerosols as they are about to enter the air filtration system as part of the Aerosol Sampling Experiment (Haines et al. Citation2019; Meyer Citation2018, Citation2019; Perry et al. Citation2014; Macatangay and Perry Citation2007). Additional experiments and instruments dedicated to real-time particle concentration monitoring and collection are scheduled for deployment and validation in the near future (Meyer Citation2020; Tryner et al. Citation2019). The vacuum bag stores dust and debris that is collected using a handheld vacuum that is regularly used to clean the cabin air filters and surfaces within the ISS. This preliminary work was done with samples taken from vacuum bags that were collected during recent operation on the ISS.
Vacuum bags returned from ISS have proven to be a useful source of information for airborne particulate matter in the spacecraft environment. This is based on the crew members’ use of the on-orbit vacuum cleaners to clean debris from filter faces in weekly housekeeping activities. The assumption is that all the material on the filter face was once airborne, and followed the ventilation system flow patterns until reaching the filters, where this material built up a layer which was subsequently vacuumed. However, some other vacuuming practices may collect materials in the bag that were not previously airborne, so analysis results cannot be extrapolated to quantify astronaut aerosol exposure. Examples include haircuts with a clipper tool vacuum attachment, and the practice of vacuuming wall panels and acoustic insulation. The latter activity significantly increases the quantities of materials used as fire retardants and in fabric treatments. Occasionally, a vacuum cleaner is required as part of a crew procedure to clean up materials at the end of a payload experiment. In this situation, there could be some unique materials in the vacuum bag that have never been airborne at all, or never in the relative quantities which occur in the bag debris. For this reason, vacuum bag may vary widely from one mission to another and have nonhomogeneous composition within each bag. In general, the dust particles in vacuum bag debris have an uncertain history and cannot be directly correlated to crew exposure and health effects, nonetheless, materials analyses have provided valuable insight on various environmental contaminants and trends.
Previous work as part of the Aerosol Sampling Experiment and analysis of vacuum bag samples has shown that the ISS airborne particle population is diverse (Haines et al. Citation2019; Meyer Citation2018, Citation2019; Perry et al. Citation2014; Macatangay and Perry Citation2007). Passive samplers designed to capture airborne supermicron aerosols show that large particles, with diameters from several tens to several hundreds of µm, are common (Meyer Citation2018, Citation2019). Carbonaceous particles from clothing fiber, skin, food, and other sources were the most common types of particles sampled. A variety of heavy metal-containing particles were also observed and typically existed as a metallic inclusion within a carbonaceous particle matrix. The most common particle types were Al-Cl-Zr particles that likely originate from antiperspirant products and Fe-Cr-Ni particles that likely originate from stainless steel. Passive samplers were also deployed to collect airborne particles from several µm to 10 nm in diameter and collected fewer particles than the active samplers (Meyer Citation2018, Citation2019), limiting the number of particles in this smaller size range that could be analyzed. TiO2 particles and fluffy Fe-Cu and Fe-Cr particles characteristic of particles generated through evaporation-condensation processes were the most common particle types. Dendritic Ag-S particles were also observed. Previous analysis of vacuum bag samples showed the presence of many large particles, consisting of fiber, hair, food, skin, plastic, and undetermined debris (Perry et al. Citation2014). These samples were sieved in an attempt to determine the size-resolved particle composition, but very few particles passed through a 75-µm sieve and no particles passed through a 53-µm sieve. This potentially indicates that small particles adhered to larger particles and aggregates, thereby preventing passage through the sieves.
Two techniques were used to analyze the residue from these vacuum bag samples: scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM/EDX) (Sullivan, Gorkowski, and Jahn Citation2018; Ault and Axson Citation2017; Laskin et al. Citation2016) and multi-element single-particle inductively coupled plasma time-of-flight mass spectrometry (ME-sp-ICP-ToF-MS, hereafter referred to as sp-ICP) (Loosli et al. Citation2019; Hendriks et al. Citation2017). Electron microscopy can be used to determine particle size and elemental composition, but is time-intensive, entails non-ideal sample preparation, and semivolatile components are lost in the high vacuum environment. There is no easy way to size-segregated particles after they are collected by the vacuum, and size-segregated sampling of airborne particles on board the ISS is difficult. sp-ICP is only relevant for analyzing metallic elements, but the single-particle and time-of-flight (ToF) mass spectrometry aspects of this technique allow for high sample throughput and determination of all metallic elements within an individual particle. The sp-ICP-TOF-MS is a recently developed instrument (Hendriks et al. Citation2017) that is currently only available at a few institutions in the United States. It is capable of analyzing all elements in individual particles, as opposed to having to select just a few elements for analysis when compared to the more traditional quadrupole mass analyzer. Both SEM and sp-ICP were used to compile the data presented herein and provide an analysis of the particles present in the vacuum bag sample collected from the ISS.
Methods
Two vacuum bag dust samples were analyzed. The first sample bag was returned on SpaceX-12 (17 September 2017, hereafter referred to as ISS-12) and the second one was returned on SpaceX-16 (13 January 2019, hereafter referred to as ISS-16). The NASA Divert Unwanted Space Trash (DUST) experiment allowed the return of some vacuum bags to Earth to shed light on the types of particles that are airborne on ISS, and to provide larger quantities of material vs. previous sampling activities. These bags are typically disposed of with other garbage; however, some were prioritized for return as part of an ongoing effort to characterize spacecraft cabin aerosols for future long-duration missions. The samples are thick masses of dust, hair, and debris () taken from the full vacuum bags using sterilized tools. A small amount of vacuum bag dust from ISS-12 was analyzed using SEM/EDX on an FEI/Quanta 600 instrument operating at an accelerating voltage of 20 keV. The sample was prepared by using a spatula to take a small amount of dust that is free of visibly large particles, depositing the dust onto a piece of conductive carbon tape, and then sputter-coating with a 2 nm layer of Pt to allow for accurate imaging. Compositions are from single-spot analysis on individual particles and are reported as a relative weight percentage that is calculated from the measured atomic percentage of each detected element. Only supermicron particles were able to be analyzed in this manner.
Figure 1. Photograph of vacuum bag sample ISS-16 from the Divert Unwanted Space Trash (DUST) experiment. The sample consists of variety of materials, including hair, skin, fibers, dust, and unidentified debris indistinguishable to the naked eye. The vacuum bag sample ISS-12 was similar in appearance.

Multi-element single-particle ICP analysis was conducted with a commercially available instrument (icpTOF, TOFWERK AG, Thune, Switzerland; hereafter referred to as sp-ICP) whose design and operation has been described previously (Loosli et al. Citation2019; Hendriks et al. Citation2017). Briefly, particles are introduced to the instrument by aspirating a liquid particle suspension, ionized by the ICP plasma source, and the resulting ions are analyzed with the ToF mass analyzer. The ToF mass analyzer and ion optics enable multi-element detection of ion-generation events resulting from the ionization of individual particles. Each particle that is ionized by the instrument produces a spike in the measured intensity of its analyzable constituent ions, which is referred to as a particle event. The instrument has mass range of 7–275 amu and a mass resolving power of ∼2500. Detection limits in the tenths of a femtogram range (10−16 g) are feasible and are determined individually for each isotope following IUPAC guidelines by calculating the threshold ion signal above the mean background (Loosli et al. Citation2019), which is done through EquationEquation (1)(1)
(1) .
(1)
(1)
Data points with signal exceeding this threshold are counted as particle events while data points with signal that do not exceed this threshold are considered background signal. This calculation is performed in windows of 100 data points for the duration of sampling and analysis. The limit of detection for each isotope is measured as the average threshold value during the sampling duration. The average limits of detection for each analyte, along with the standard deviation of these averages, are discussed in more detail later in the work and are visible in and . Accurate mass calibrations with R2 > 0.995 are achieved for most elements and are performed using a series of multi-element solutions (Loosli et al. Citation2019; Hendriks et al. Citation2017). Mass calibrations for the lighter elements 24Mg, 27Al, and 28Si are less accurate with 0.88 < R2 < 0.90. In this work, an He/H2 reaction gas mixture was added to a post-ionization collision cell to remove signal from ArO+ and ArO2+ and reduce background interference at m/z + 56 and +28 to enable more accurate quantification of 56Fe and 28Si.
Particles from a small amount (0.03 g) of vacuum bag dust of ISS-12 and ISS-16 were analyzed through sp-ICP. Samples were prepared by suspending 0.03 g of dust in 5 mL of filtered HPLC-grade water in a polypropylene tube, vortexing for 2 min, resting for 30 min, and then taking a 1 mL aliquot from the middle of the tube. HPLC-grade water (Sigma-Aldrich HPLC Plus #34877) was filtered through a 0.02 µm pore size filter (Anotop 25 plus 0.02 µm pore size, Whatman #6809-4102) to remove any residual particles and impurities. The sample was allowed to rest to allow large particles to settle to the bottom, which is desirable as especially large particles (diameter >∼10 µm) may clog the instrument sampling line or generate an ion signal that is too large for the instrument detector to accurately measure. The 1 mL aliquot was diluted to 3 mL and then passed through a 200 nm pore size polytetrafluoroethylene filter in polypropylene housing, which was then rinsed with 3 mL of ethanol and another 3 mL of water. The filter housing was partially opened with a rotary tool, cleaned with compressed air for several minutes, and then opened by hand. The filter was then detached from the housing with Teflon-coated tweezers and placed in the sp-ICP sample tube, which was filled with 10 mL of filtered HPLC water and vortexed for 5 min. This procedure is intended to remove organic material and soluble salts from the sample (such as described in the SEM images in ) before entering the sp-ICP to avoid saturation of the detector. The elements typically comprising organic material and soluble salts (C, O, N, S, Na, K, Cl) are not easily detected or quantitatively analyzed through sp-ICP and the absence of such particles will not negatively impact the overall analysis. The first procedure attempted simply diluted the 1 mL aliquot instead of rinsing with water and ethanol. This sample had such high amounts of organic and salt material, either dissolved or as particles, that the ion signal generated during analysis was sufficiently high to be potentially damaging to the detector and caused the instrument software to freeze. As such, the second procedure attempted to dissolve organic particles and separate organic material and dissolved salts from the metallic particles of interest.
An sp-ICP method blank was also performed, incorporating the same procedure as described above but without the suspension of any solid mass in the initial solvent. The mass distribution frequency for the most common elements detected in the blank is shown in . Overall, the mass frequency distributions show that some elements detected in the ISS samples are also detected in the blank, but these elements are detected less frequently and at lower masses in the blank. The exceptions to this are Fe and Al, which are more common in the blank than the ISS samples but are present at relatively small masses, almost exclusively less than 10−14 g. The higher number of Fe and Al particles observed in the blank than the ISS samples results from higher limits of detection for Fe and Al in the ISS sample relative to the blank. A higher limit of detection is likely due to small amounts of soluble Fe and Al present in the ISS samples, which raises the baseline signal at the relevant m/z and requires a larger particle mass for detection above the baseline. Fe- and Al-containing particles detected in the ISS samples with masses below 10−14 g have been removed from the sample datasets because it could not be determined whether such particles originate from the ISS material or are a result of the method background.
Results and discussion
SEM/EDX analysis
Several SEM images and accompanying single-point EDX spectra are shown in for vacuum bag dust particles from ISS-12. Particles visible in this sample are large with the longest dimension in most particles >50 µm in diameter and >250 µm in diameter in many particles. Smaller particles are also visible but can’t be accurately analyzed because the surrounding large particles block transmission of the escaping electrons and X-rays. Particles with diameter >50 µm would be expected to have a short atmospheric lifetime near the Earth’s surface as they will be rapidly removed through gravitational settling, but their lifetime and behavior on board the ISS are less clear. Most particles are irregular ovoids with rough surface textures. C, O, and N are the most prevalent elements in these particles and many also contain small amounts (<10 weight percentage of Na, Cl, S, and Si). This composition suggests that these are biologically derived particles, possibly originating from the astronauts or their food. Other common particles are long and thin and appear fibrous, suggesting that they may originate from fabric or hair. EDX analysis shows these particles contain C and O, which is consistent with fabric fibers. A particle composed of C and O in approximately a 95:5 wt.% ratio was also observed near several fiber particles (, spectrum 7), suggesting a non-biological origin for some of the non-fiber carbonaceous material.
Figure 3. SEM image 1 from ISS-12. Five particles are composed of varying amounts of organic carbon, nitrogen, sulfur, silicon, and salts, suggesting they are biological particles originating from astronauts or food (spectra 2, 3, 4, 5, and 6). In the lower left is visible a particle with a much higher C:O ratio of 95:5 (spectrum 7), suggesting a different origin.
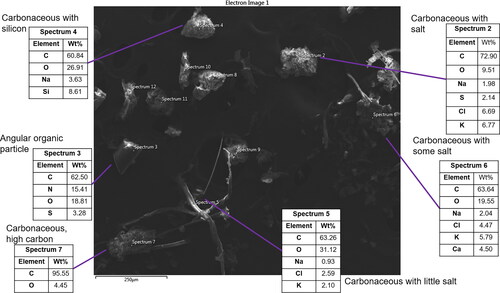
Figure 4. SEM image 1, view 2 from ISS-12. Three particles with organic and salt compositions indicative of biological origin (spectra 9, 10, and 12). A particle with a C:O ratio of nearly 1:1 is also visible (spectrum 11), potentially indicating it is from a polymeric material. Another particle with a high amount of F (26%, spectrum 8) is present and may potentially originate from a fluoropolymer material. The origin of Mg is not clear, but past work observed small amounts of Mg that likely originated from talc adhered to other particles (Meyer Citation2018).
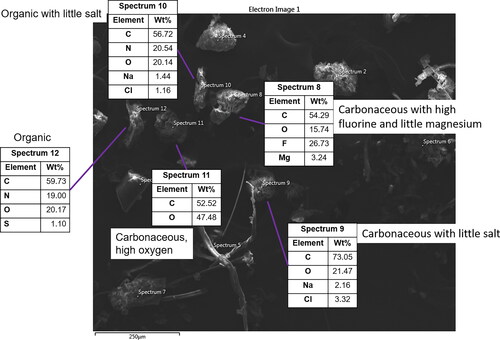
Figure 5. SEM image 2 from ISS-12. Three particles are displayed with organic carbon and salt compositions consistent with biological origins (spectra 17, 18, and 20).
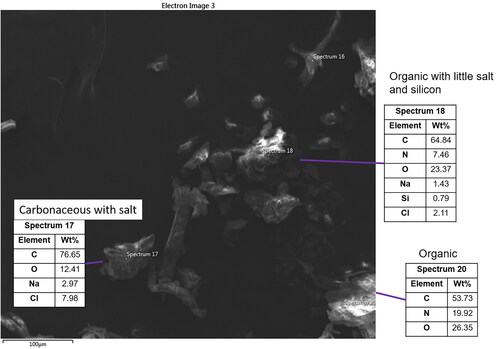
Figure 6. SEM image 3 from ISS-12. A single particle with organic carbon and salt composition consistent with biological origin (spectrum 24). EDX of two fiber particles (spectra 23 and 25) indicates that these contain only C and O, which along with their ribbon-like twisting morphology suggests they are likely cotton fabric fibers. A particle with large amounts of F and Cl (12%–26%, spectra 21 and 22) is also visible. The co-occurrence of these two elements and variable weight percentage at different points within the particle suggest this particle may be a blend of chlorofluoropolymers.
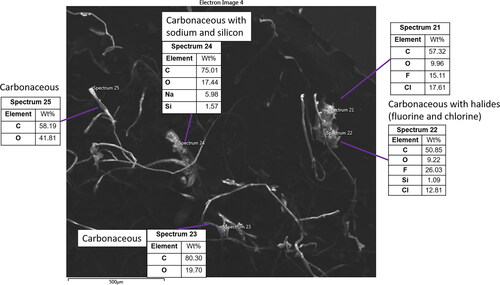
Several particles ( and , spectra 8 and 21 + 22, respectively) also contain high (>15 weight percentage) amounts of the halide elements F and/or Cl without a corresponding salt cation such as Na, suggesting that these elements are covalently bound. These particles could result from fluoropolymers or chlorofluoropolymers. Such halopolymers have a wide array of materials applications and are highly chemical- and flame-resistant, both desirable characteristics for materials used on board the ISS (Orndoff Citation2016). The F- and Cl-bearing particle in exhibits different F:Cl ratios at two points within the particle (spectra 21 and 22), potentially indicating that this particle is composed of a blend of more than one halopolymer. Materials containing a high number of halogen atoms tend to bioaccumulate within living organisms (Houde et al. Citation2011; Bohlin, Jones, and Strandberg Citation2007) due to the increased compound hydrophobicity and decreased water solubility and typically have some degree of toxicity (Butenhoff and Rodricks Citation2015; Bohlin, Jones, and Strandberg Citation2007). The inhalation of halopolymer particles could potentially lead to adverse health effects in astronauts, however, inhalation is not the typical route of human exposure for these compounds (Bohlin, Jones, and Strandberg Citation2007). The potential inhalation and ingestion of halopolymer particles of this size range is a situation that is likely unique to habitation on board the ISS, as these particles would remain airborne for a very short time near the Earth’s surface. It is thus not clear to what degree these particles will accumulate in and be cleared from the respiratory tract following inhalation (Haines et al. Citation2019) and how they may accumulate after swallowing and transport to the gastrointestinal tract (Vignal et al. Citation2017).
sp-ICP-TOF-MS analysis
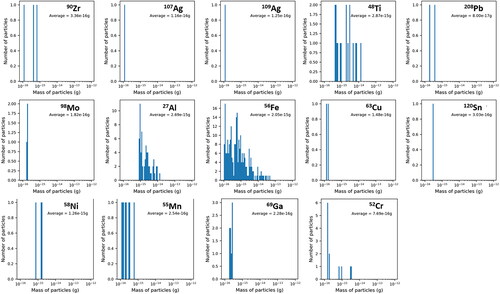
The most common elements identified through sp-ICP are shown in . A series of plots showing the mass frequency distributions of these elements are shown in and . Each of these element masses may come from a particle with other measured elements; these associations are discussed in subsequent paragraphs. The mass measured in each particle for any individual element is on the order of 1 × 10−16 to 1 × 10−12 g with most elements between 1 × 10−15 and 1 × 10−14 g. The average detection limit of each element during analysis is indicated on each plot ( and ) with a red shaded region that corresponds to one standard deviation around the average detection limit. Masses that fall within or below this region may result from noise in the signal background rather than from real detection events and should be regarded critically, and are therefore not used to inform discussion of particle compositions and sources in the following sections. The software used to analyze the sp-ICP data utilizes an established algorithm based on Poisson statistics to separate signal from noise (Hendriks et al. Citation2017), however, this procedure may be more prone to error in samples such as ours with significant amounts of dissolved background material. For many elements, there are a small number of particle events detected near this region and a higher number of particles at larger masses.
Figure 7. Absolute (top) and relative (bottom) abundances of the most common elements in each sample. Relative abundance is shown as the relative frequency of particle events with the specified isotope out of total particle events detected (e.g., 30% of particles analyzed in sample ISS-12 contained 90Zr). The relative frequency of each element is not normalized and particle events can contain more than one isotope.
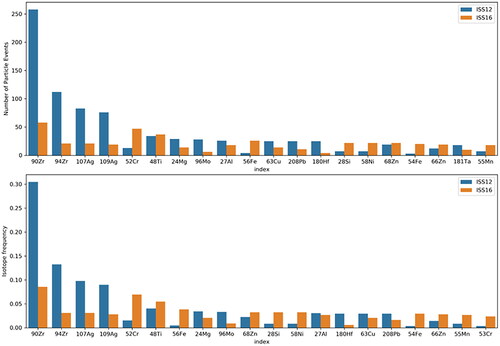
Figure 8. Mass frequency distributions for common elements in ISS-12. The red shaded region indicates one standard deviation around the average limit of detection; particles within this region may result from background signal noise. The x-axis mass-scale ranges from 10−16 to 10−12 g.
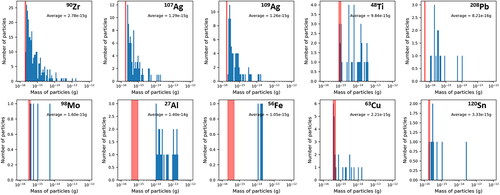
Figure 9. Mass frequency distributions for common elements in ISS-16. The red shaded region indicates one standard deviation around the average limit of detection; particles within this region may result from background signal noise. The x-axis mass-scale ranges from 10−16 to 10−12 g.
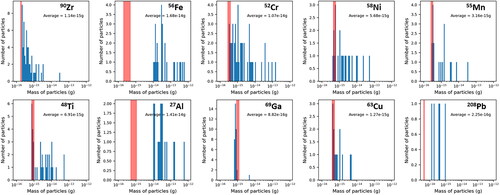
The corresponding particle size cannot be known with certainty as the chemical form in which the element occurs is unknown but a range can be estimated by assuming that the metal is present in its pure metallic form or as a simple oxide. This calculation is done by assuming a spherical particle shape and then taking into account the density and mass fraction of the metal in the phase. For example, the average mass of Al in a particle from ISS 12 is 1.62 × 10−14 g. If the particle or particle region contains pure Al this corresponds to a particle or inclusion 225 nm in diameter, while if the particle contains Al2O3 this corresponds to a particle or inclusion 245 nm in diameter. These and other diameters calculated from element masses that are ten times larger and ten times smaller (max and min diameters, respectively) than the average mass are listed in and for the elements displayed in . The masses used to calculate the 10× more and 10× less diameters span most of the range of masses observed in and and are intended to give a sense of how particle size may vary over the observed mass ranges. These estimates do not account for the presence of other elements within a particle and are therefore a lower bound for a number of the particles.
Table 1. Average masses measured in particle events reported for several of the most abundant elements in ISS-12.
Table 2. The same quantities are reported as in for ISS-16.
Similar sets of elements are present in both the ISS-12 and ISS-16 samples. Each of these elements is detected at levels higher than what is observed in the blank (). Particles containing Zr, Al, Cr, and Ti, are common in both samples. Fe particles are common in ISS-16 but not in ISS-12, while Ag-containing particles are observed in both samples but are more common in ISS-12. Al, Ti, Fe, and Cr are common elements that are used in a variety of materials and alloys, with Fe and Cr together being characteristic of stainless steel (Meyer Citation2018; Schauwinhold et al. Citation2008). Most of the other elements have comparatively more limited uses, but many have been detected in prior work (Meyer Citation2018, Citation2019). Zr may be used as an antiperspirant (Meyer Citation2018) or in heat-resistant applications (Nielsen Citation2000) and Ag may be used in electrical applications, as solder, or for antimicrobial applications (Brumby et al. Citation2008). The elements Pb, Cu, Mo, Sn, Ga, Mn, and Ni are also displayed in and but are present to a lesser extent in the samples. The exact origin for each of these elements is unclear but they could conceivably have roles in a variety of material, alloy, or electronics applications. Three Ga particles are present in both ISS samples above the detection limit and were not consistently associated with other elements. Ga could originate from Ga-As semiconductor materials, and As may not be detected in these particles due to their small mass and the relatively high detection limits for As (average ∼1.2 × 10−15 g).
Elements present in both ISS samples and not shown in and include Zn, Ba, Mg, and Cd (online supplementary information [SI]), which are consistent with prior observations (Meyer Citation2018). Mg was not associated with other elements but may originate from talc, which was observed in prior work (Meyer Citation2018). Zn was sometimes associated with Fe and the elements with which Fe was commonly associated as discussed above, which could be similar to Fe-Zn fume-like particles that were previously observed (Meyer Citation2019). Fume-like is a morphological characterization that is consistent with particles that form and grow through condensed vapor growth and agglomeration (Kulkarni, Baron, and Willeke Citation2011). A small number of Ba particles are also present in both ISS samples (three total above the detection limit) but are notable in that each is associated with Pb and two of the three are also associated with Cd and Zn. Other elements are also associated with the Ba particles, suggesting they may be aggregates of some sort, although their origin is unknown.
A series of bar charts showing how often the more common elements shown in and are associated with other elements are displayed in ; these associations are discussed in more detail in the following paragraphs. Associations may occur as particles aggregate during sample collection or workup and when this seems a likely origin for particle associations it is discussed in the text. However, the consistent associations observed also suggest a singular origin for many of the particle types that are discussed below.
Figure 10. Percentage of measured particles of the most common elements in ISS-12 (blue) and ISS-16 (orange) where another element is associated with that particle event. The element of interest is noted in the upper right of each plot and the 10 most commonly associated elements (with the element of interest) are arrayed along the x-axis while the percentage of particle events with that element measured along the y-axis. Particle events can contain more than one additional element. The percentage of particle events is not normalized.
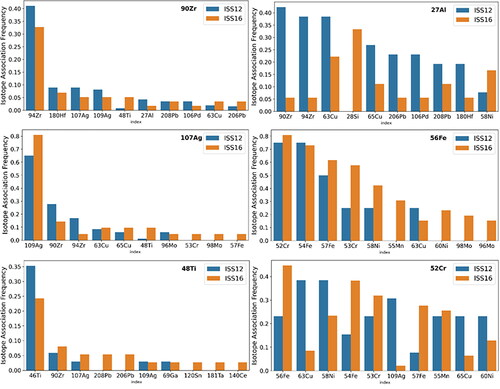
In ISS-12 and ISS-16, we see that Fe is often associated with Cr and is also sometimes associated with Ni, Mn, Cu, and Mo. There are only four 56Fe particles in ISS-12 above the 1 × 10−14 g mass cutoff, which is comparable to the particle numbers in the blank. Therefore, Fe-bearing particles do not appear to have a significant presence in ISS-12. Fe and Cr together are indicative of stainless steel, while the other elements detected in ISS-16 are all potential additives to steel alloys. Other potential material sources for these particles are wiring materials such as Kanthal, an Fe-Cr-Al material. Cr, Ni, and Mn are more commonly observed in larger Fe particles (SI), which makes sense as these elements are a relatively minor component of steel and most Fe alloys (Schauwinhold et al. Citation2008) and their ion signal may not register above the instrumental detection limit in smaller particles. For example, the common SAE 304 stainless steel alloy contains 15%–20% Cr and 2%–10.5% Ni. This is the same reason why isotopes of Fe (54Fe and 57Fe) are not observed in all 56Fe-containing particles. Some Fe particles are associated with Cu, which could originate from wiring and be similar to fume-like Fe-Cu particles that were observed in previous active sampling aerosol samples (Meyer Citation2018, Citation2019).
Many Zr particles appear to be pure Zr, while some are associated with Hf, Ag, Al, and Ti. Hf may simply be associated with Zr due to chemical similarity and natural co-occurrence (Nielsen Citation2000) or may be an intended component of materials such as filaments or electrodes. The association with Al likely occurs due to the presence of Al-Cl-Zr particles that are similar to those observed in samples collected by the passive aerosol sampler (Meyer Citation2018, Citation2019); however, the analytical constraints of the sp-ICP mean that these particles are likely smaller than most of those collected by the passive sampler. Associations with other elements may be due to the presence of other materials or may result from aggregation of Al-Zr-Cl particles with other particles during sampling or processing. In ISS-12, Zr particles are much more common (). However, similar to the Zr particles analyzed in ISS-16, they are often pure but sometimes associated with Al, Ag, and Hf. Pb and Fe are also present in a small number of Zr particles. The Zr particles in ISS-12 and ISS-16 also have similar mass frequency distributions ( and ), which suggests a consistent source for this particle type.
Ag particles are common in the ISS-12 sample, unlike ISS-16. Some of these particles appear sufficiently small that they are close to the limit of detection of the instrument, which gives rise to the <100% overlap between particles that contain both 107Ag and 109Ag (). Many Ag particles appear pure, some contain Zr or Cu, and some contain a variety of other elements ( and SI). Some of the clothing fibers collected by the passive aerosol sampler are composed of nylon with silver coatings (presumably for antimicrobial properties) and in some of these particles, the silver coating had begun to wear off, generating Ag aerosols (Meyer Citation2019). One possible explanation for the presence of Ag in these samples is that Ag flakes and aerosols of a wide range of sizes aggregated with carbonaceous or other metallic particles during sample collection or workup. Pure Ag dendritic particles of unclear origin were also observed during prior analysis (Meyer Citation2019), so the exact origin of Ag particles detected during sp-ICP analysis is undetermined.
Al particles in ISS-12 and ISS-16 have some similarities in composition but these particle types occur with different frequencies. Associations with Zr, Cu, Hf, and Pb are present in both, consistent with the previously described associations for Zr, but this particle type is relatively more common in ISS-12. Al-Si particles are present in ISS-16 but completely absent in ISS-12. Similar particles have been observed in prior work (Meyer Citation2018) and may originate from fiberglass or personal care products (Meyer Citation2018). Al-Cu particles may originate from wiring or Al-Li alloys, which have long been used in aerospace applications and often contain small amounts of Cu (Dorin, Vahid, and Lamb Citation2018); Li is too light to analyze through sp-ICP. An association between Al and Pd is also present in ISS-12. Pd is present in the ISS-12 particles that contain the greatest amount of Zr, suggesting it may result from aggregation of small amounts of Pd by the large Zr-Al particles. Pd is present in quantities near the detection limit, and therefore may be absent in ISS-16 because the Zr mass frequency distribution tends toward smaller particle masses ( and ).
Cr is consistently associated with Fe as well as with Mn, Ni, and Mo, consistent with Cr originating from stainless steel or other alloys that contain these elements. A small number of Cr-rich particles without other elements are also present, consistent with prior observations (Meyer Citation2018). Ti appears mostly as pure particles but is also associated with several elements to a small extent. This is consistent with the presence of submicrometer pure TiO2 particles previously observed with the active airborne sampler (Meyer Citation2018, Citation2019). Association with Zr and Ag may be due to aggregation of common Zr and Ag particles with other materials, as mentioned above. Associations with other elements do not follow a clear trend in terms of the relative masses and may also result from aggregation of TiO2 particles with other materials.
Conclusions and outlook
Together, SEM/EDX and sp-ICP are techniques that provide significant information on the composition and sources of collected particle samples in the ISS through analysis of vacuum bag dust samples. SEM/EDX analysis of ISS-12 vacuum bag dust identified particles >50 µm in diameter as highly prevalent in the vacuum bag samples. The most common types observed were organic particles of biological origin, organic carbon-based particles, and fibrous particles. Particles <50 µm were also observed but could not be imaged or analyzed in detail because of heavy particle loading and nearby larger particles. Particles that appear to result from fluoropolymers and chlorofluoropolymers were also identified. Visible light microscopy analysis would also likely be useful in the analysis of these larger particles, particularly incorporating Raman or FTIR vibrational spectroscopy or colorimetry to obtain greater information on particle chemical composition and potential origin. If halopolymer particles are determined to be a potential health risk, then future computer-controlled scanning electron microscopy (CCSEM) analyses of active and passive collection aerosol samples should include F or Cl-rich carbonaceous particles as a separate class to assess their prevalence and size characteristics. Better constraints of particle size will be necessary to assess particle fate after they enter the body. If some form of size separation is not performed on the vacuum bag dust, then electron microscopy can only easily analyze large particles. Post-sampling size separation will likely be difficult, as previous attempts at sieving vacuum bag dust samples were unable to separate small particles from large debris aggregates (Perry et al. Citation2014). ISS samples could be re-aerosolized as a first step toward size separation or to enable collection on substrates with decreased particle loading. There are a number of techniques that can be used to aerosolize dry material, but doing so in a controlled manner that does not introduce compositional artifacts or alter particle morphologies may be difficult.sp-ICP analysis of smaller particles from the ISS-12 and ISS-16 vacuum bag dusts identified several common types of particles, some of which were present in both samples. The most consistently visible types of particle compositions were Fe-Cr-Mn-Ni-Mo that likely originated from stainless steel or other Fe alloys, Fe-Cu, Al-Cu, Al-Si, Zr-Al-Hf/Zr-Al-Hf-Pd, pure Ag, pure Ti, and Pb-bearing. Several of these particle compositions are characteristic of known materials such as stainless steel, silver fabric coatings, semiconductor and electronics materials, and antiperspirants. Others are consistent with previously described particles (Meyer Citation2018, Citation2019) of unknown origin such as fume-like Fe-Cu/Zn particles, pure Ti particles, Pb-bearing particles, and Cr-rich particles. Some compositions, for example, Ba-bearing particles, do not have a clear origin or similarity to previously described particles. A number of elements (Sn, Zn, Ga, Ba, and Cd) are present in only a small number of particles and more detailed conclusions about their size ranges and origins are not feasible without further analysis. While an exact origin cannot be pinpointed for many particle compositions, the elements identified are not unexpected based on previous observations of passive and active collection aerosol samples (Meyer Citation2018, Citation2019) and the wide-ranging applications of all of these elements. These particles may be out-of-place in most environments but a wide variety of specialized materials and unique particle generation mechanisms are relevant to air quality on the ISS. There were some particle compositions identified in each sample that were not present in the other, suggesting that this technique can be used to detect potentially unique particle-generating events or diagnose changes in the composition of sampled particle populations that occur with time. The identification of many heterogeneous particles and many particle types also demonstrates the utility of a high-throughput, multi-element, single-particle technique to analyze ISS vacuum bag samples.
The sp-ICP results demonstrate that this technique can be used as a high-throughput method to detect and quantify the masses of metallic elements in submicron particles. Supermicron particles were also likely analyzed but exact upper and lower limits on the size of particles analyzed are unknown. Dozens to hundreds of particles were analyzed in the two ISS samples and resulted from 0.03 g of material, a very small proportion of the total vacuum bag material collected (∼10 s to 100 s of g). However, there likely exists room for improvement in the procedure used to prepare vacuum bag dust for sp-ICP analysis. Centrifugation or other methods can be used to separate excessively large particles from the suspension and longer vortexing or another method may be more effective at extracting metallic particles from the large dust and fiber aggregates present in the vacuum bag samples. A procedure to remove dissolved ions and organic material was employed during sample preparation. Ideally, future preparation would utilize a smaller pore size filter to retain more insoluble particles and this filter would not be in a sealed housing to simplify filter removal. Other filtration procedures may also be useful in isolating specific size fractions of particles. Combining other analytical techniques with sp-ICP, such as a dynamic light scattering measurement of the size and number distribution of particles within the liquid suspension or post-filtration analysis of particles through SEM/EDX, may also be useful. Such measurements would provide better size constraints on the particles analyzed and inform whether metals exist as pure metallic particles or are embedded in a particle matrix that cannot be detected through sp-ICP such as carbonaceous material.
Supplemental Material
Download MS Excel (58.4 KB)Acknowledgment
We thank Bernard Crimmins of Clarkson University for bulk ICP-MS analysis and advice on sample preparation. We also thank R. J. Lee Group for SEM/EDX analysis of other ISS particle samples that helped inform our analysis.
Additional information
Funding
References
- Ault, A. P., and J. L. Axson. 2017. Atmospheric aerosol chemistry: Spectroscopic and microscopic advances. Anal. Chem. 89 (1):430–52. doi: https://doi.org/10.1021/acs.analchem.6b04670.
- Bohlin, P., K. C. Jones, and B. Strandberg. 2007. Occupational and indoor air exposure to persistent organic pollutants: A review of passive sampling techniques and needs. J. Environ. Monit. 9 (6):501–9. doi: https://doi.org/10.1039/b700627f.
- Brown, J. S., T. Gordon, O. Price, and B. Asgharian. 2013. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 10 (1):1–12.
- Brumby, A., P. Braumann, K. Zimmermann, F. Van Den Broeck, T. Vandevelde, D. Goia, H. Renner, G. Schlamp, K. Zimmermann, W. Weise, et al. 2008. Silver, silver compounds, and silver alloys. In Ullmann’s encyclopedia of industrial chemistry, ed. H. Renner, 9–12. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA.
- Butenhoff, J. L., and J. V. Rodricks. 2015. Human health risk assessment of perfluoroalkyl acids. In Toxicological effects of perfluoroalkyl and polyfluoroalkyl substances, ed. J. C. DeWitt, Chapter 15, 363–418. Cham: Humana Press (Springer International Publishing).
- Dorin, T., A. Vahid, and J. Lamb. 2018. Aluminium lithium alloys. In Fundamentals of aluminium metallurgy, ed. R. N. Lumley, 387–438. Amsterdam, The Netherlands: Elsevier.
- Haines, S. R., A. Bope, J. M. Horack, M. E. Meyer, and K. C. Dannemiller. 2019. Quantitative evaluation of bioaerosols in different particle size fractions in dust collected on the International Space Station (ISS). Appl. Microbiol. Biotechnol. 103 (18):7767–82. doi: https://doi.org/10.1007/s00253-019-10053-4.
- Hendriks, L., A. Gundlach-Graham, B. Hattendorf, and D. Günther. 2017. Characterization of a new ICP-TOFMS instrument with continuous and discrete introduction of solutions. J. Anal. At. Spectrom. 32 (3):548–61. doi: https://doi.org/10.1039/C6JA00400H.
- Houde, M., A. O. De Silva, D. C. G. Muir, and R. J. Letcher. 2011. Monitoring of perfluorinated compounds in aquatic biota: An updated review. Environ. Sci. Technol. 45 (19):7962–73. doi: https://doi.org/10.1021/es104326w.
- Kulkarni, P., P. A. Baron, and K. Willeke. 2011. Introduction to aerosol measurement. In Aerosol measurement, ed. P. Kulkarni, P. A. Baron, and K. Willeke, 1–13. Hoboken, NJ: John Wiley & Sons.
- Laskin, A., M. K. Gilles, D. A. Knopf, B. Wang, and S. China. 2016. Progress in the analysis of complex atmospheric particles. Annu. Rev. Anal. Chem. 9 (1):117–43. doi: https://doi.org/10.1146/annurev-anchem-071015-041521.
- Loosli, F., J. Wang, S. Rothenberg, M. Bizimis, C. Winkler, O. Borovinskaya, L. Flamigni, and M. Baalousha. 2019. Sewage spills are a major source of titanium dioxide engineered (nano)-particles into the environment. Environ. Sci. Nano. 6 (3):763–77. doi: https://doi.org/10.1039/C8EN01376D.
- Macatangay, A. V., and J. L. Perry. 2007. Cabin air quality on board Mir and the International Space Station—A comparison. Paper presented at the 37th International Conference on Environmental Systems (ICES 2007), Chicago, IL, USA, July 9–12, 1–20. doi: https://doi.org/10.4271/2007-01-3219.
- Meyer, M. E. 2015. Particle morphology and elemental composition of smoke generated by overheating common spacecraft materials. NASA Technical Memorandum 2015-218891, NASA Glenn Research Center, Cleveland, OH.
- Meyer, M. E. 2018. Results of the aerosol sampling experiment on the International Space Station. Paper presented at the 48th International Conference on Environmental Systems (ICES 2018), No. ICES-2018-100, 20180004817, Albuquerque, NM, USA, July 8–12.
- Meyer, M. E. 2019. Characterization and measurement of spacecraft airborne particulate matter. Paper presented at the 70th International Astronautical Congress (IAC), No. 20190032657, Washington, DC, USA, October 21–25.
- Meyer, M. E. 2020. Airborne particulate monitor: A real-time reference quality aerosol instrument payload for ISS air pollution quantification. Paper presented at the 2020 International Conference on Environmental Systems, No. ICES-2020-65, Lisbon, Portugal, July 12–16.
- Meyer, M. E., D. L. Urban, G. W. Mulholland, V. Bryg, Z. G. Yuan, G. A. Ruff, T. Cleary, and J. Yang. 2018. Evaluation of spacecraft smoke detector performance in the low-gravity environment. Fire Saf J. 98 (March):74–81. doi: https://doi.org/10.1016/j.firesaf.2018.04.004.
- Mulholland, G. W., M. Meyer, D. L. Urban, G. A. Ruff, Z-g Yuan, V. Bryg, T. Cleary, and J. Yang. 2015. Pyrolysis smoke generated under low-gravity conditions. Aerosol Sci. Technol. 49 (5):310–21. doi: https://doi.org/10.1080/02786826.2015.1025125.
- Nielsen, R. 2000. Zirconium and zirconium compounds. In Ullmann’s encyclopedia of industrial chemistry, ed. H. Renner, 1–204. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA.
- Oberdörster, G. 2000. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 74 (1):1–8.
- Orndoff, E. 2016. Fabrics for space travel. In Textile design: From interior space to outer space, ed. D. Schneiderman and A. G. Winton, 171–8. New York: Bloomsbury Publishing.
- Perry, J. L., J. E. Coston, G. C. Marshall, and S. Flight. 2014. Analysis of particulate and fiber debris samples returned from the International Space Station. Paper presented at the 44th International Conference on Environmental Systems, No. 20140012506, Tucson, AZ, USA, July 13–17.
- Schauwinhold, D., M. Toncourt, R. Steffen, D. Janke, K. Schäfer, H. Jacobi, R. Hammer, R. Hentrich, L. Kucharcik, H. Wiegels, et al. 2008. Steel. In Ullmann’s encyclopedia of industrial chemistry, ed. H. Renner., 181–222. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA.
- Sullivan, R. C., K. Gorkowski, and L. Jahn. 2018. Characterization of individual aerosol particles. In Physical chemistry of gas-liquid interfaces, ed. J. A. Faust and J. E. House, 353–402. Amsterdam, The Netherlands: Elsevier.
- Tryner, J., C. Quinn, B. C. Windom, and J. Volckens. 2019. Design and evaluation of a portable PM2.5 monitor featuring a low-cost sensor in line with an active filter sampler. Environ. Sci. Process. Impacts 21 (8):1403–15. doi: https://doi.org/10.1039/c9em00234k.
- Vignal, C., M. Pichavant, L. Y. Alleman, M. Djouina, F. Dingreville, E. Perdrix, C. Waxin, A. Ouali Alami, C. Gower-Rousseau, P. Desreumaux, et al. 2017. Effects of urban coarse particles inhalation on oxidative and inflammatory parameters in the mouse lung and colon. Part. Fibre Toxicol. 14 (1):1–13.
- WHO. 1999. Hazard prevention and control in the work environment: Airborne dust. Geneva, Switzerland: WHO.