?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Particles and droplets produced from pharmaceutical inhaler devices are naturally charged. Previous in silico and in vitro studies have shown that the levels of these electrostatic charges may potentially affect deposition in the airways but this has not been confirmed in vivo. Human lung scintigraphic studies using radiolabelled particles with controlled charges would provide crucial data on locating the deposition sites with respect to the particle charge level. An aerosol charging rig has been developed for this purpose. 99mTc-radiolabelled droplets from an Aerogen® Solo vibrating mesh nebulizer were charged by induction and then dried to yield positively charged particles. Particles with near-neutral charges were produced at an induction voltage of −0.4 kV, while those with 10-4,000 elementary charges per particle were generated at −4.5 kV, depending on the particle size. The number of elementary charges per particle generally decreased with radioactivity, especially for solutions at 400 and 800 MBq/mL. This was attributed to the indirect ionizing effect of the gamma radiation in the air, which produced bipolar ions that neutralized the initially charged particles. Radioactivity at 100 MBq/mL was found to be optimal in generating the highest particle charges that could potentially affect in vivo deposition in the lungs. The aerosol charging rig is suitable for use in human scintigraphy studies which we will conduct in the near future.
Copyright © 2021 American Association for Aerosol Research
EDITOR:
Introduction
Pharmaceutical aerosols generated from dry powder inhalers, metered dose inhalers, and nebulizers naturally carry electrostatic charges in the absence of applied electric fields. Particles become charged from physical contacts between each other and between particles and solid surfaces, a phenomenon known as triboelectrification (Bailey Citation1984; Citation1993; Matsusaka and Masuda Citation2003; Peart Citation2001). On the other hand, for aqueous formulations, breakup of the electrical double layer or nonequilibrium surface potential in the liquid surface during flow and atomization produces spontaneously charged droplets (Bailey Citation1988; Iribarne and Klemes Citation1974; Iribarne and Mason Citation1967; Jonas and Mason Citation1968; Jonassen Citation1998; Lopatenko, Kontush, and Kolpakov Citation1987; Matteson Citation1971; Yatsuzuka, Higashiyama, and Asano Citation1996; Yatsuzuka, Mizuno, and Asano Citation1994). These mechanical processes occur inside inhalers during aerosol generation and can lead to charged particles or droplets. Evidence from a number of in silico, in vitro, and in vivo aerosol studies suggests that these charges may affect particle deposition in the airways, but the in vivo effect has not been confirmed.
The five mechanisms of particle deposition in the airways include inertial impaction, gravitational sedimentation, diffusion, interception, and electrostatic attraction (Hinds Citation1999). Charges enhance deposition by increasing attractive forces to airway surfaces. Although the airways are normally neutral in charge, an image charge of the opposite polarity to that of the charged particle may be induced on its surface, especially in the small airways in the peripheral lung (Bailey, Hashish, and Williams Citation1998; Balachandran et al. Citation1997; Finlay Citation2019). This results in deposition through attraction between the particle and its image charge. It has been estimated that a 3 µm particle would need a few tens of elementary charges for charge effects to be more significant than inertial impaction in affecting deposition in a 12th generation airway 5 mm in diameter under tidal breathing (Finlay Citation2019). A 1 µm particle would need similar elementary charges to be more significant than sedimentation in affecting deposition in an alveolus 400 µm in diameter (Finlay Citation2019). In silico models have explored the deposition of unipolarly charged particles in dichotomous and realistic airway models (Bailey Citation1997; Bailey, Hashish, and Williams Citation1998; Balachandran et al. Citation1997; Koullapis et al. Citation2016; Majid et al. Citation2012; Yu Citation1985). Balachandran et al. (Citation1997) showed that the deposition of 2.2 µm particles increased 10- to 16-fold in all airway generations when the number of elementary charges per particle was increased from 1 to 200 (Balachandran et al. Citation1997). Similar effects of electrostatic forces on deposition were obtained for 0.5 and 5 µm particles (Bailey, Hashish, and Williams Citation1998). Majid et al. (Citation2012) predicted that 1 µm particles have approximately 20% higher deposition in the alveolar region once charge levels exceed approximately 100 elementary charges per particle (Majid et al. Citation2012). In vitro studies have shown that particles produced from various commercial pharmaceutical inhalation products possess numbers of elementary charges per particle that are within or above the ranges examined in silico (Byron, Peart, and Staniforth Citation1997; Kwok and Chan Citation2008; Kwok, Collins, and Chan Citation2006; Kwok, Glover, and Chan Citation2005; Kwok et al. Citation2010). Therefore, those charges may potentially influence deposition in vivo.
There have been few in vivo human experiments on the effects of particle charge, all of which were conducted in the 1970s and 1980s (Melandri et al. Citation1975; Melandri et al. Citation1983; Prodi and Mularoni Citation1985). The total deposition of monodisperse 0.3-1 µm carnauba wax particles in the subjects increased with the amount of charges carried, up to about 200 positive or negative elementary charges per particle (Melandri et al. Citation1975; Melandri et al. Citation1983; Prodi and Mularoni Citation1985). It was reported that electrostatic effects became nonnegligible compared to gravitational sedimentation for 1 µm particles with approximately ≥30 elementary charges (Finlay Citation2019; Melandri et al. Citation1983). However, since only the net deposition was measured in these studies, the specific location of deposition within the respiratory tract was unclear. It was assumed from the small particle size that the enhanced deposition occurred in the alveoli (Melandri et al. Citation1983). Better-designed in vivo studies, preferably with imaging data to locate particle deposition, are needed to obtain more informative data.
Since the presence of water vapor in the air is known to reduce electrostatic charges, it has been hypothesized that electrostatic charges may be neutralized in the fully humidified air in the lungs (Finlay Citation2019). However, the time needed for humid air to neutralize electrostatic charges is too long for such an effect to occur during transit in a single breath (Finlay Citation2019). Consequently, deposition in the lungs may be potentially affected by electrostatic effects. Nevertheless, well-controlled in vivo studies are required to test this hypothesis.
There are hitherto no human in vivo studies employing the administration of aerosols carrying known charges with mapping of their deposition in the lungs. Such studies may be achievable by inhaling radiolabelled aerosols with characterized charges, followed by lung scintigraphy. The data obtained, whether positive or negative, will constitute a major milestone in the advancement of this field.
This article presents an aerosol charging rig for producing inhalable, radiolabelled particles with controllable charges suitable for in vivo human lung scintigraphy studies.
Materials and methods
Aerosol charging rig
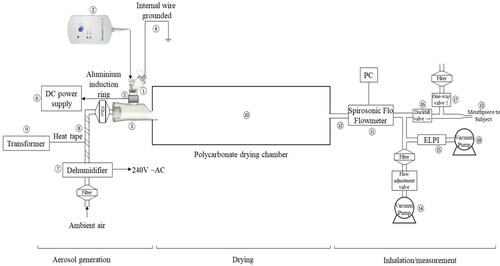
The schematic of the experimental rig for aerosol generation and charging is shown in . It comprised three sections according to their functions, namely, aerosol generation, drying, and inhalation/measurement. All filters indicated in the figure were SureGard® respiratory low resistance filters (Bird Healthcare, Bayswater, Victoria, Australia). They were used to prevent the entry of ambient particles into the rig, and to avoid contamination of the environment by particles generated during the experiments.
An Aerogen® Solo nebulizer (①) powered by an Aerogen® Pro-X Controller (②) was connected to an Aerogen® Ultra (③) with the vibrating mesh facing downwards (all from Aerogen, Galway, Ireland). An earthed stainless steel wire (④) was inserted into the nebulizing solution in each run for grounding. Aluminum foil tape (3 M, St. Paul, MN, USA) was wrapped around the outside of the aerosol inlet on the Aerogen® Ultra on which the nebulizer sat (⑤). This foil tape served as the conductive ring around the nebulizer vibrating mesh for induction charging of the droplets. An electric cable connected it to the negative electrode of a direct current power supply (⑥), which was a Model 9185B (BK Precision, Yorba Linda, CA, USA) or Model EAN 581 direct current control unit (Topas, Dresden, Germany) for charging the ring at −0.4 or −4.5 kV, respectively. Polyvinyl chloride insulating tape was wrapped around the foil ring to prevent electrical shocks. The silicone flap that serves as a one-way valve in the Aerogen® Ultra was removed to maximize the airflow through the rig and reduce turbulence. The resultant opening was connected to a Büchi B-296 dehumidifier (⑦) by Polypress tubing (both from Büchi, Flawil, Switzerland). A KM-HT-BS 30 glass fibre-insulated heating tape (⑧) (SAF Wärmetechnik, Mörlenbach, Germany) was connected to a Variac® SRV-5 variable laboratory autotransformer (⑨) (ISE, Cleveland, OH, USA) and wrapped around the Polypress tubing to warm the cold air from the dehumidifier to prevent condensation in the Aerogen® Ultra during nebulization. The autotransformer output voltage was 80 V.
The outlet of the Aerogen® Ultra was inserted without the mouthpiece into a custom-made, medical grade polycarbonate drying chamber (⑩) (Plastix Australia, Arncliffe, New South Wales, Australia). The chamber was a 1 m long hexagonal cylinder, with each side of the hexagon being 14 cm and a volume of 50.9 L. The circular openings at both ends had an internal diameter of 1.32 cm. One of the hexagonal end panels of the drying chamber was detachable to facilitate cleaning and sterilization between use by human subjects. A hole 33.4 mm in diameter was in the middle of both ends of the chamber. One of these was attached to the outlet of the Aerogen® Ultra for droplets to enter the chamber. The other hole served as the exit for the particles and was connected to a SpiroSonic Flo flow meter (⑪) (Uscom, Budapest, Hungary) via a static-control tubing (⑫) (1/4”, McMaster-Carr, Douglasville, GA, USA), followed by a three-way piece that led to a mouthpiece for a human subject ⑬, a vacuum pump for removing unsampled aerosols (⑭), and a modified Electrical Low Pressure Impactor (⑮) (ELPI; Dekati, Kangasala, Finland). Duckbill and one-way valves (⑯ and ⑰) were placed before the mouthpiece to direct exhaled air from the subject to exit the line during tidal breathing. The aerosol rig was designed for future in vivo studies so ⑬, ⑭, ⑯, and ⑰ were included in the schematic but were not used in the in vitro aerosol charging experiments in this article. Only the Model RB 0021 C vacuum pump for the ELPI (⑱) (Busch, Maulburg, Germany) was operated to generate the airflow for aerosol sampling. The design and operation principle of the modified ELPI has been described elsewhere (Keskinen, Pietarinen, and Lehtimäki Citation1992; Kwok, Collins, and Chan Citation2006; Kwok, Glover, and Chan Citation2005; Kwok, Noakes, and Chan Citation2008). It is a 13-stage cascade impactor with femto-ammeters connected to the electrically insulated stages to measure the net current on those stages. The corona charger was removed and replaced with a straight stainless steel tube (Kwok, Noakes, and Chan Citation2008). The ELPI was placed sideways to allow the aerosols to enter horizontally so that a right-angled throat was not required. This was to avoid particle loss and alteration of particle charge due to impaction in the throat. The impactor stages were thinly coated with silicone before use by spraying with Slipicone® Release Spray (DC Products, Mount Waverley, Victoria, Australia) to minimize particle bounce and re-entrainment.
Preparation of 99mTc-DTPA complexes in saline
Radiopharmaceuticals were prepared by the Department of Nuclear Medicine at St George Hospital and delivered to The University of Sydney on the same day as the aerosol charging experiments. Technetium-99m (99mTc) was eluted in 0.9% w/v sodium chloride for injections from a Gentech® Molybdenum [99Mo]/Technetium [99mTc] Sterile Generator (ANSTO Health, Kirrawee, New South Wales, Australia). Six milliliters of this saline with a known amount of radioactivity was injected into a Pentastan Diethylenetriaminepentaacetic Acid (DTPA) Reagent Multi Dose Vial (ANSTO Health, Kirrawee, New South Wales, Australia). Each vial contained 13.25 mg of sodium DTPA monohydrate, 0.8 mg stannous chloride dihydrate, and 7.1 mg sodium chloride (ANSTO Health Citation2017). After 30 s of mixing, the solution was left to stand for 10 min at ambient temperature to allow formation of 99mTc-DTPA complexes. Quality control was performed by thin layer chromatography to ensure that the amount of free pertechnetate in the solutions accounted for <5% of the total measured radioactivity. The procedure followed the 99mTc-DTPA quality control protocol for clinical studies in the Department of Nuclear Medicine at St George Hospital. The solutions were made by accounting for the radioactivity decay between preparation and use so that they were 25, 50, 100, 400, and 800 MBq/mL at the time of the aerosol charging experiments.
Aerosol charging experiments
The whole rig depicted in was shielded with at least 2.4 mm of lead to protect operators from radiation exposure. The radioactivity of the 6-mL solution in the Pentastan vial was measured by a Model CRC®-15R dose calibrator (Capintec, Florham Park, NJ, USA) at the start of the experiment. Two and a half milliliters of the radioactive solution was added to an Aerogen® Solo nebulizer. The grounding wire was inserted into the solution in the nebulizer, which was then attached to the Aerogen® Ultra in the rig. The vacuum pump for the ELPI was switched on to establish an airflow of 30 L/min and the ELPIvi™ 4.0 software (Dekati, Kangasala, Finland) opened to save the data at a current range of 400,000 fA. After zeroing the current signals, the nebulizer was switched on and the charging voltage increased gradually from 0 V to −0.4 or −4.5 kV within half a minute. Solutions with 25, 50, 100, 400, 800 MBq/mL radioactivity were tested at −4.5 kV with two Aerogen® Solo nebulizers (Mesh# 1158 and 1326), whereas only the 100 and 800 MBq/mL solutions were tested at −0.4 kV with one nebulizer (Mesh# 1326). Six minutes of nebulization was performed in each run. ELPI sampling continued after switching off the nebulizer until the current signals returned to baseline. The runs were conducted in duplicate for each nebulizer unit. The radioactivity of the particles deposited on the ELPI stages after sampling were measured by placing each stage into a sealable plastic bag and then into the scintillation counter. The time of measurement was noted for each stage.
Data analysis
The radioactivity measured for all the ELPI stages was converted to the radioactivity at the time when the 6-mL solution was measured at the start of the experiment (Time 0) by the following equation:
(1)
(1)
where A0 is the radioactivity at Time 0 for a given ELPI stage, At the radioactivity for that stage measured at t hours after Time 0, and λ the decay constant, which is:
(2)
(2)
where t1/2 is the half-life of 99mTc (6.0067 h) (Bé et al. Citation2004). The charge on a given stage (q) was derived from the electric current data by calculating the area under the curve for the entire sampling period in the current-versus-time plot for that stage. The number of elementary charges per particle (n) for a given ELPI stage was estimated by the following equation:
(3)
(3)
where ρ is the theoretical true density of the dried particles (see below), V the volume of a particle calculated using the physical particle diameter, which was in turn obtained by dividing the geometric mean aerodynamic cutoff diameter of that ELPI stage (the square root of the product of the aerodynamic cutoff diameters of the stage and its previous stage) by the square root of ρ, m the mass of particles collected on that ELPI stage, and e the elementary charge (1.602 × 10−19 C). The use of geometric mean aerodynamic cutoff diameters is to account for the imperfect collection efficiency of the stages (Lopez-Cuesta and Longuet Citation2014; Marjamäki et al. Citation2000). The particles were assumed to be spherical, non-agglomerated, and non-porous. The theoretical true density was estimated by the following equation:
(4)
(4)
where ρ1, ρ2, and ρ3 are the true densities of sodium DTPA monohydrate, stannous chloride dihydrate, and sodium chloride, respectively, and m1, m2, and m3 the masses of these three ingredients, respectively, in the final solution after 6 mL of 0.9% w/v sodium chloride was added. The true densities, ρ1, ρ2, and ρ3 were taken to be 1.30, 2.71, and 2.16 g/cm3, respectively. The theoretical true density of the dried particles derived from EquationEquation (4)
(4)
(4) was 2.01 g/cm3. The mass of particles (in mg) collected on an ELPI stage was calculated as:
(5)
(5)
where A0 is the radioactivity at Time 0 for a given ELPI stage and Av the activity of the 6-mL solution was measured at Time 0. The constant in the numerator is the total mass of solutes in the 6-mL solution (i.e., m1 + m2 + m3), which was 75.15 mg.
Electrical mobility (µe) was calculated as follows (Finlay Citation2019).
(6)
(6)
(7)
(7)
where n is the number of elementary charges e that the particle carries, C the Cunningham slip correction factor, η the viscosity of air, d the physical diameter of the particle and λ the mean free path of gas molecules (0.066 µm).
Results
On a typical day when the aerosol charging rig was in operation, the relative humidity and temperature of the air in the ambient environment and in the line just before the ELPI were measured (HM34 handheld humidity and temperature meter, Vaisala, Helsinki, Finland) to be 64% and 16% and 19.0 °C and 19.1 °C, respectively. Thus, the dehumidifier sufficiently dried the air in the rig and heating of the tubing from the dehumidifier did not affect the temperature downstream, with no observable condensation in the Aerogen® Ultra resulting from the nebulization.
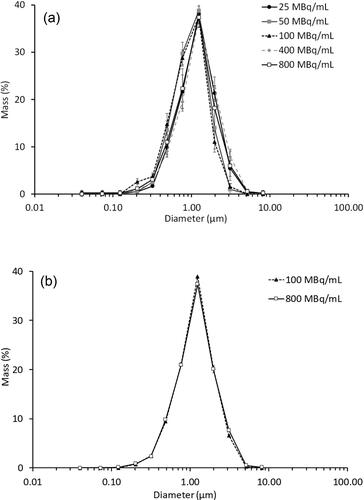
The nebulized droplets were observed as powder deposits, rather than wet blots when collected on the ELPI stages indicating the drying chamber operated as intended. The aerodynamic particle size distributions are shown in . Since radioactivity was proportional to the mass of solutes in the nebulizing solutions, the distribution of radioactivity amongst the ELPI stages was taken as the distribution by mass because they were equivalent. The overall particle size distribution was not affected by the radioactivity or induction voltage, with most particles collected on Stage 8 (corresponding to 0.96 µm aerodynamic cutoff diameter).
Figure 2. Aerodynamic particle size distribution profiles by mass in the ELPI at (a) -4.5 kV (n = 4) and (b) -0.4 kV (n = 2). Data presented as mean ± standard deviation for (a) and mean ± range/2 for (b).
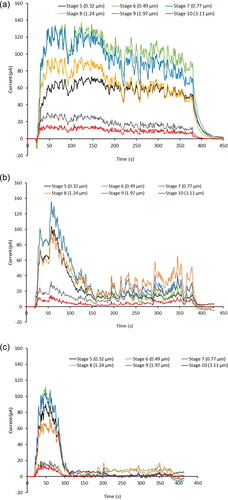
Typical current profiles for 100, 400, and 800 MBq/mL solutions nebulized with Aerogen® Solo Mesh# 1326 at −4.5 kV are shown in . All the electric currents detected were net positive in polarity for both charging voltages, with −4.5 kV producing higher currents from the particles. Most of the currents were detected on Stages 5-10 (geometric mean aerodynamic diameter between 0.32-3.11 µm), especially on Stages 6 and 7 (0.49-0.77 µm). Stage 8 (1.24 µm) had the highest amount of deposits (). The lower and upper stages showed minimal deposits under visual inspection, which was reflected in their low measured radioactivity. The charges carried by the particles were relatively stable for 100 MBq/mL over the whole nebulization period (). However, although the charges for 400 and 800 MBq/mL were similar in magnitude to those for 100 MBq/mL, they started to decrease after the first minute of nebulization and settled to an “equilibrium” level from the second minute onwards. The charge reduction was faster and the equilibrium currents were lower with increasing radioactivity (). These trends were observed for both nebulizer units tested. In a preliminary study with 1 GBq/mL radioactivity, the first 2 min of the nebulized aerosol was removed to waste by the spare vacuum pump (⑭ in ) before diverting the airflow to the ELPI. Thus the ELPI sampled the radioactive particles from the second to the sixth minute of nebulization. The currents measured over that period were similar to those in the equilibrium phase for 800 MBq/mL () but without the initial peak. Therefore, the peaking of currents at the start of nebulization was not an artifact from the ELPI.
Figure 3. Typical current profile of particles from Aerogen® Solo Mesh# 1326 with (a) 100, (b) 400, and (c) 800 MBq/mL solutions at -4.5 kV.
shows the effect of radioactivity on the number of elementary charges per particle. The actual radioactivity at the time of the aerosol charging experiments was slightly different to those stated in the Materials and Methods section (25, 50, 100, 400, 800 MBq/mL) due to logistical constraints in timing the delivery and use of the radioactive solutions on the days of experiments. The charges produced from the two nebulizer units (Mesh# 1158 and 1326) were similar so their data at −4.5 kV were considered together, hence n = 4. The number of elementary charges per particle at −4.5 kV increased with aerodynamic particle size and ranged from tens to thousands (). On the other hand, particles produced at −0.4 kV with 100 or 800 MBq/mL solutions carried very low charges that were nearly neutral (). The negative charges on Stage 10 were due to an anomalous baseline shift on the electrometer for that stage in one of the runs. Despite the relatively high variability in the data at −4.5 kV, the mean number of elementary charges per particle in all size fractions increased with increasing radioactivity from 25 MBq/mL, and peaked at 100 MBq/mL, then decreased with further increases in radioactivity (). All particles produced from 400 MBq/mL solutions carried <10 elementary charges and were nearly uncharged for 800 MBq/mL solutions. Charge reduction was also observed between 100 and 800 MBq/mL at −0.4 kV, even though the number of elementary charges per particle for 100 MBq/mL was already very low at this voltage (). The emitted radioactivity fractions at 100 and 800 MBq/mL charged at −4.5 kV were calculated by dividing the total radioactivity collected in the ELPI by the total radioactivity loaded in the nebulizer corrected to Time 0 (). This was done to check whether the decrease in the lower electric currents measured by the ELPI after 100 s of nebulized 800 MBq/mL solutions was due to lower aerosol output. The emitted radioactivity fractions for 100 MBq/mL and 800 MBq/mL solutions were 4.7 ± 0.4% and 29.7 ± 3.6%, respectively. The 6.3-fold increase in the emitted fraction at higher concentration indicated that the mass of aerosols collected in ELPI also increased to the same extent.
Figure 4. The effect of radioactivity on the number of elementary charges per particle for (a) Stages 5-10 at -4.5 kV (n = 4), (b) Stages 5-10 at -0.4 kV (n = 2). Data presented as mean ± standard deviation for (a) and as mean ± range/2 for (b).
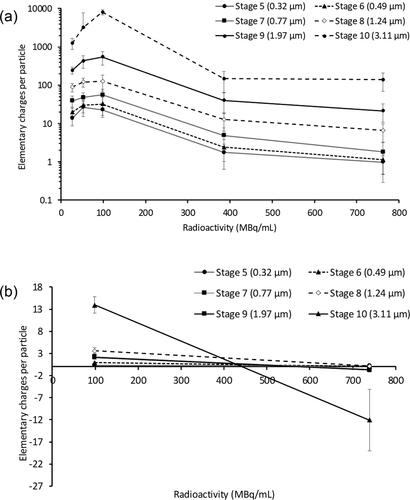
Figure 5. The number of elementary charges per particle with respect to the aerodynamic diameter for (a) Stages 5-10 at -4.5 kV, (b) Stages 5-10 at -0.4 kV. Data presented as mean ± standard deviation for (a) and as mean ± range/2 for (b).
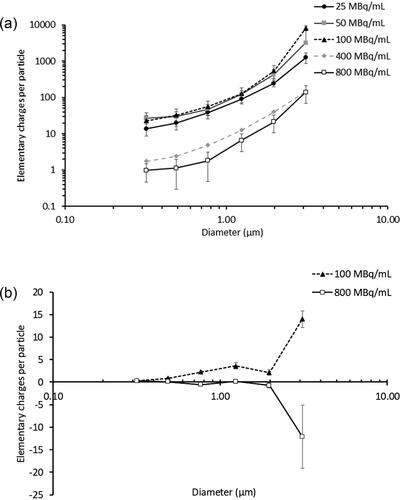
Table 1. Emitted fraction of the total radioactivity collected in the ELPI with respect to the total activity loaded in the nebulizer corrected to Time 0 for 100 and 800 MBq/mL solutions charged at -4.5 kV.
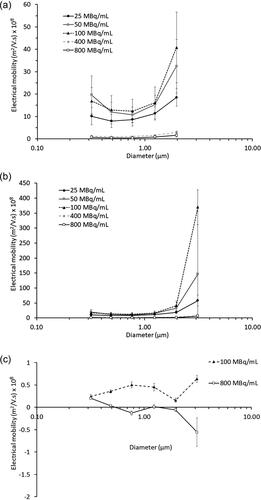
Electrical mobility () when −4.5 kV was induced showed similar values for Stages 5-9 (0.32-1.97 µm) regardless of the radioactivity concentrations used. However, the aerosols collected on stage 10 (3.11 µm) showed the largest mobility value at 100 MBq/mL and decreased at other radioactive concentrations. At lower voltage of −0.4 kV (), there was little variation of electrical mobility across Stages 5-10 for 100 and 800 MBq/mL.
Discussion
The present aerosol charging rig was designed with the goal of using it to study the effect of electrostatic charges on lung deposition in human subjects. This fundamental study will be the first step toward further studies that would investigate the effect of charge as well as other factors linked with charge (e.g., particle size, aerosol concentration, charge polarity, relative humidity) on particle deposition. The more that is known about the fundamental effects of these factors, the better that future studies can be designed to mimic clinical aerosols. It is important to produce particles of inhalable sizes and controllable charges, which is difficult to achieve simultaneously with current pharmaceutical inhalers. Dry particles are preferred because droplets may evaporate and change in size as they traverse the air, complicating data interpretation. Humidifying the air fully can minimize droplet evaporation but it may also affect aerosol charging. A simple way to standardize the particle size distribution is by drying the droplets completely before inhalation. This was achieved by introducing dry air into the rig via a dehumidifier and the inclusion of a drying chamber to allow for evaporation. Powder deposits on the ELPI stages indicated that drying was complete by the time the aerosol was to be inhaled. The dehumidifier could cool to 1 °C which caused unwanted liquid condensation in the Aerogen® Ultra when the incoming air was left cold in some preliminary studies, particularly on the vibrating mesh and the inlet around which the aluminum foil tape for induction charging was wrapped. This hindered aerosol charging through short circuiting, thus a heating tape (⑧ in ) was used to warm the incoming air and prevent condensation. The temperature at the point of inhalation remained ambient despite heating the air from the dehumidifier because the air cooled passively in the drying chamber (⑩ in ).
Aerogen® Solo is a vibrating mesh nebulizer that can be used in breathing circuits with a ventilator/nasal cannula or used directly with a mouthpiece/face mask (Aerogen Citation2016). The Aerogen Ultra® acts as an aerosol holding chamber when the Aerogen® Solo is used alone. It has a port for external air entrainment and optional supplemental oxygen supply. This port was connected to the dehumidifier in the aerosol charging rig after having the silicone flap removed to minimize turbulence, as described in the Materials and Methods section. The nebulizer mesh plate, 5 mm in diameter and 60 µm thick, is made from a nickel-palladium alloy with 1000 precision-formed orifices (Sweeney et al. Citation2019). It vibrates at 128 kHz to extrude micron-sized droplets for inhalation. Depending on the nebulizer unit and liquid formulation, the volume median droplet diameter and output rate may vary from 3.2 to 4.9 µm and 0.3 to 0.5 mL/min, respectively (Aerogen Citation2016; Bennett et al. Citation2018; Sidler-Moix et al. Citation2015; Sweeney et al. Citation2019). Since the nebulized droplets are aqueous, the aerodynamic size range is similar to that based on volume. The residual volume in the liquid reservoir after nebulization is <0.1 mL for a 3 mL load so the aerosol output is efficient (Aerogen Citation2016). These characteristics are useful for our application. Since over half of the nebulized droplets are already are <5 µm by volume (Sidler-Moix et al. Citation2015), the dried particles obtained in the present study were even smaller. In fact, virtually all of them were <5 µm in aerodynamic diameter, with a size distribution that was not affected by the radioactivity or induction voltage (). This offers good pulmonary targeting in scintigraphy studies as deposition by inertial impaction in the oropharynx and upper respiratory tract should be minimal. Consequently, changes in the deposition pattern due to electrostatic charge should be more distinguishable.
The droplets in the rig were charged by induction using a two-electrode arrangement, with the grounding wire and the aluminum foil tape serving as the electrodes (④ and ⑤ in ). Induction charging is a common electrification method for conductive and semi-conductive liquids (Zhao, Castle, and Adamiak Citation2005). The liquid and nozzle (or vibrating mesh in the case of Aerogen® Solo) are grounded in this mode of charging, while the direct current power supply is connected to the induction electrode near the site of droplet breakup (Hopkinson Citation1980; Kim et al. Citation2017; Marchewicz et al. Citation2019; Zhao, Castle, and Adamiak Citation2005). The voltage required is usually in the kV range. The electric field induces charging of the liquid surface in this area to the opposite polarity to that of the induction electrode. The droplets then carry these charges when they are detached mechanically from the bulk liquid (Hopkinson Citation1980; Kim et al. Citation2017; Marchewicz et al. Citation2019). The saline solution for lung scintigraphy is conductive so positively charged droplets were formed upon nebulization by charging the aluminum foil tape ring (i.e., the induction electrode) negatively in the rig in the present study. Since charges remain on the droplet during evaporation, the resultant particles were also positively charged (). A similar electrode arrangement was employed in a charger device with an Aeroneb® Lab vibrating mesh nebulizer in an earlier study (Golshahi et al. Citation2015). However, instead of an induction ring around the mesh, the counter electrode was a small disk positioned directly opposite to the mesh. This counter electrode was charged positively from 0-5 kV to produce negatively charged particles (Golshahi et al. Citation2015). The number of elementary charges per particle in that study ranged from 10 to 10,000, increasing with particle size and induction voltage. These trends were also seen in the present study, with the number of elementary charges per particle between 10 and 4,000. The difference in the upper charge levels may be due to differences in the induction voltage and geometry of the aerosol charging rigs, which would change the electric field and hence the charging capability. It may also be caused by the different solutions nebulized as charging can be affected by their composition (Kwok et al. Citation2010). Golshahi et al used solutions containing albuterol sulfate only (0.05 and 0.1% w/v) or 0.05% w/v albuterol sulfate with sodium chloride (0.0005% and 0.01% w/v) (Golshahi et al. Citation2015). On the other hand, the 99mTc-DTPA solutions used in the present study had different ionic solutes at comparatively higher concentrations (see Materials and Methods). The charging of sprayed liquids is known to be inversely related to the conductivity, and hence the concentration of ionic species in the liquid, so the lower charges may be attributed to this (Vaaraslahti, Laitinen, and Keskinen Citation2002). Overall, the electrical mobility of the particles >0.5 µm at the induction voltage of −4.5 kV increased with particle size (). Thus the movement, and hence deposition, of the larger particles are more susceptible to the influence from electric fields than the smaller ones. On the other hand, the electrical mobility of the particles at the induction voltage of −0.4 kV was near-neutral so the electric field effects should be minimal. The implication of the electrical mobility of the particles on their deposition in vivo requires further investigation as the detailed electrical environment in the airways is complicated by the intricate air-tissue geometry of the lungs so that its influence on charged particles is unclear.
Preliminary runs in the present study at 0 V produced low negatively charged particles. This was the natural charging by nebulization because droplet formation disrupts the electrical double layer or nonequilibrium surface potential in liquid surfaces, resulting in charge separation (Kwok et al. Citation2010). Interestingly, various commercial nebulizing solutions containing drugs and other electrolytes also naturally charged negatively by jet nebulization (Kwok et al. Citation2010). Since charge levels of the same polarity should be compared in the in vivo study, neutral-to-low positive charges were obtained by charging the induction ring at −0.4 kV (). Variable charges were measured, even though all the experimental conditions were kept constant. The low number of replicates (n = 2) may also contribute to the observed variability. However, it is well-known that electrostatic charging is a highly variable phenomenon that is susceptible to many factors, some of which are known (e.g., relative humidity, temperature, surface contamination) but many others are not obvious (Blacker and Birley Citation1991; Peart Citation2001). The variability in the aerosol charges could be accounted for by measuring the particle charges simultaneously for each subject because the airflow could be split and diverted between the subject and the ELPI (). Therefore, scintigraphic data of a subject could be matched with the charge profile of the inhaled aerosol.
The gamma emitter, 99mTc (as 99mTc-DTPA complexes) is the most common radionuclide used in in vivo lung deposition studies (Devadason et al. Citation2012). Gamma scintigraphy, also known as planar imaging, and single-photon emission computed tomography (SPECT) have been employed to measure the two-dimensional and three-dimensional distributions of inhaled aerosol in the lungs, respectively (Scheuch et al. Citation2010). Planar imaging is simpler than SPECT and requires a lower inhaled radioactivity for data acquisition. Its recommended lung dose for 99mTc is 2-10 MBq (Newman et al. Citation2012), compared to 25-200 MBq for SPECT (Fleming et al. Citation2012). The time required to inhale enough radiopharmaceutical particles to reach the target lung dose should be as short as possible, preferably within a few minutes. For longer times, the radioactivity inhaled earlier in the dosing period will start to be cleared from the lungs by systemic absorption and/or mucociliary clearance, depending on the site of particle deposition. This will reduce the spatial and temporal fidelity of the imaging data.
Since particles are continuously produced by the nebulizer in the aerosol charging rig, human subjects can breathe tidally during dosing. The higher the radioactivity carried by the particles, the shorter will be the dosing period. However, it was found that aerosol charging was decreased with increasing radioactivity and the optimal charging was achieved at 100 MBq/mL (). Solutions with radioactivity >100 MBq/mL will lengthen the dosing period so they are less practical. Furthermore, particles carried lower charges when the radioactivity was low so 100 MBq/mL is preferable. If the volumetric output rate of the Aerogen® Solo is 0.3 mL/min (see above), then the radioactivity output rate would be 30 MBq/min. Assuming that the subject breathes tidally with a 1:1 inhalation-exhalation cycle, with no particles retained in the aerosol charging rig (although this is unlikely) and all of them deposit in the lungs, then the dosing rate would be 15 MBq/min. Therefore, the lungs should theoretically receive the minimum radioactivity required for SPECT within 2 min. It would take less than a minute to achieve sufficient radioactivity for planar imaging. A few in silico models with micron-sized particles indicate that deposition in various airway generations increased measurably when the number of elementary charges per particle increased above approximately 100 (Bailey, Hashish and Williams Citation1998; Balachandran et al. Citation1997; Hashish, Bailey and Williams Citation1994). The charges carried by particles produced from 100 MBq/mL solutions in the present study are thus sufficiently high to potentially affect lung deposition (). Therefore, it is feasible to use the aerosol charging rig with solutions at this radioactivity for in vivo scintigraphic studies.
The reduction in aerosol charging at high radioactivity could be due to the indirect ionizing effect of gamma radiation. Although gamma radiation has a weaker ionizing ability than alpha and beta particles, it can eject fast-moving electrons upon energy transfer by photoelectric absorption, Compton scattering, or pair production (Bigelow Citation1995; Gensdarmes, Boulaud and Renoux Citation1998). These fast-moving electrons are essentially beta particles that produce a strong electric field in their immediate surroundings and cause the separation of orbital electrons from gas molecules in its trajectory. Consequently, bipolar ions are formed in the atmosphere that can then diffuse and adsorb onto particles in the vicinity and neutralize the charges originally carried by those particles (Clement and Harrison Citation1991). Alpha particles have the same ionizing effect as beta particles, but they work over a shorter distance due to their low penetrating power. This is the mechanism of action of commercial aerosol neutralizers that commonly use alpha (e.g., polonium-210) or beta emitters (e.g., krypton-85) (TSI Incoporated Citation2020). Neutralizers with gamma emitters are rare because gamma radiation is highly penetrating and dense shielding is needed for safety. Nevertheless, an electrostatic discharger using gamma radiation from cesium-137 or cobalt-60 has been reported for neutralizing charges generated in non-conductive liquids (e.g., petroleum) flowing through pipes and vessels (Rhodes Citation1977). Alpha and beta emitters actually become charged by themselves due to charge separation through emission of positive or negative charges, respectively (Clement and Harrison Citation1991, Citation1992; Gensdarmes, Boulaud and Renoux Citation2001). The charges left behind on the parent material would then be neutralized by the external bipolar ions generated by the radiation. Although gamma radiation is uncharged (hence no charge separation occurs upon emission), the aerosol particles in the present study may self-charge through interactions with the emitted photons, ionic diffusion, or ionic space charges, in addition to induction charging (Gensdarmes, Boulaud and Renoux Citation1998).
The air in the present aerosol charging rig, particularly that in the drying chamber, could be ionized by the mechanism discussed above and neutralize incoming particles that were initially charged. The neutralizing effect became prominent when the radioactivity was >100 MBq/mL. The level of charging in the first minute of nebulization with 400 and 800 MBq/mL at −4.5 kV was similar to that with 100 MBq/mL (). Initially the air in the rig was radiation-free so there was no charge neutralization and the sampled particles carried high charges. However, as more radioactive particles traverse the rig, the level of bipolar ions in the air increased and neutralization became noticeable. This was seen in the decreasing charges over the second minute, with the rate of decline faster for the higher radioactivity solutions. Then the amount of bipolar ions, i.e., the extent of neutralization, reached equilibrium. This was reflected in the lower and relatively more stable charge levels for the rest of the runs. The neutralizing effect was not as significant at 100 MBq/mL so the charges remained high (). However, particle charges seemingly decreased with decreasing radioactivity for 25 and 50 MBq/mL (). The neutralizing effect was expected to be even lower at these radioactivity levels so the particles should initially carry higher charges. These highly charged particles were more likely to be retained in the rig due to electrostatic deposition so that only particles with sufficiently low charges apparently exited the rig. This is similar to the preferential retention of higher charged particles from metered dose inhalers in spacers and was the reason for the apparently lower particle charges measured by the ELPI (Kwok, Collins and Chan Citation2006). shows that the activity collected in the ELPI from 800 MBq/mL solutions was approximately 6.3 times higher than that from 100 MBq/mL solutions. This implies that the decreased electric currents measured by the ELPI after 100 s of nebulization () was due to the neutralizing effect of the radioactivity and not to a lower amount of aerosols collected in the ELPI.
No in vivo lung scintigraphic study has been reported in the literature on the electrostatic properties of radiolabelled aerosols tested, as particle charge was not of primary interest in those studies. The data show that particle charges could be decreased to different extents, depending on the amount of radioactivity employed. Since the charge levels generated naturally from pharmaceutical inhalers are high enough to potentially affect in vivo deposition (see Introduction), those charges might have been unintentionally reduced or neutralized by the ionizing radiation in previous in vivo scintigraphic studies. If electrostatic charges could indeed influence in vivo deposition, then the scintigraphy data may not show the true deposition pattern of the corresponding non-radiolabelled, but naturally charged, aerosols normally inhaled by patients. This has important implications for scintigraphic studies because the discrepancy in the charge levels may affect the clinical relevance of the data if radioactive aerosols deposit differently to their non-radioactive counterparts. The in vivo effects of electrostatic charges should be investigated to clarify these points. The authors intend to conduct human scintigraphic studies in the near future using the aerosol charging rig presented in this article.
Conclusion
An aerosol charging rig has been developed to study lung deposition of charged particles in humans by gamma scintigraphy. The charges were neutralized through indirect ionization by gamma radiation for solutions of 99mTc-DTPA in saline with radioactivity >100 MBq/mL. Solutions with 100 MBq/mL radioactivity generated positively charged particles with aerodynamic diameters <5 µm carrying 10-4,000 elementary charges per particle. This charge range is sufficiently high to potentially affect deposition in the lungs.
Acknowledgments
Dr Jim Fink of Aerogen Pharma Corporation is thanked for his helpful advice on the use of the Aerogen® Solo vibrating mesh nebulisers.
Additional information
Funding
References
- Aerogen. 2016. Aerogen® Solo System Instruction Manual. Galway, Ireland: Aerogen.
- ANSTO Health. 2017. Pentastan DTPA reagent multi dose vials product information. Kirrawee, NSW: ANSTO Health.
- Bailey, A. G. 1984. Electrostatic phenomena during powder handling. Powder Technol. 37 (1):71–85. doi: https://doi.org/10.1016/0032-5910(84)80007-8.
- Bailey, A. G. 1988. Electrostatic spraying of liquids. Somerset, England: Research Studies Press.
- Bailey, A. G. 1993. Charging of solids and powders. J. Electrostat. 30:167–80. doi: https://doi.org/10.1016/0304-3886(93)90072-F.
- Bailey, A. G. 1997. The inhalation and deposition of charged particles within the human lung. J. Electrostat. 42 (1-2):25–32. doi: https://doi.org/10.1016/S0304-3886(97)00134-4.
- Bailey, A. G., A. H. Hashish, and T. J. Williams. 1998. Drug delivery by inhalation of charged particles. J. Electrostat. 44 (1-2):3–10. doi: https://doi.org/10.1016/S0304-3886(98)00017-5.
- Balachandran, W., W. Machowski, E. Gaura, and C. Hudson. 1997. Control of drug aerosol in human airways using electrostatic forces. J. Electrostat. 40-41:579–84. doi: https://doi.org/10.1016/S0304-3886(97)00106-X.
- Bé, M.-M., V. Chisté, C. Dulieu, E. Browne, V. Chechev, N. Kuzmenko, R. Helmer, A. Nichols, E. Schönfeld, and R. Dersch. 2004. Table of radionuclides, Vol 1 - A = 1 to 150. Sèvres, France: Bureau international des Poids et Mesures.
- Bennett, G., M. Joyce, L. Sweeney, and R. MacLoughlin. 2018. In vitro determination of the main effects in the design of high-flow nasal therapy systems with respect to aerosol performance. Pulm. Ther. 4 (1):73–86. doi: https://doi.org/10.1007/s41030-018-0054-x.
- Bigelow, R. A. 1995. Radiation interactions in matter. In Nuclear and particle physics simulations: The consortium of upper-level physics software, ed. R. A. Bigelow, M. J. Moloney, J. Philpott, and J. Rothberg. New York: John Wiley and Sons.
- Blacker, R. S., and A. W. Birley. 1991. Electrostatic charge occurrence, significance and measurement. Polym. Test. 10 (4):241–62. doi: https://doi.org/10.1016/0142-9418(91)90020-X.
- Byron, P. R., J. Peart, and J. N. Staniforth. 1997. Aerosol electrostatics. I: Properties of fine powders before and after aerosolization by dry powder inhalers. Pharmaceutical Research 14 (6):698–705. doi: https://doi.org/10.1023/A:1012181818244.
- Clement, C. F., and R. G. Harrison. 1991. Self-charging of radioactive aerosols. J. Aerosol Sci. 22:S175–S178. doi: https://doi.org/10.1016/S0021-8502(05)80063-1.
- Clement, C. F., and R. G. Harrison. 1992. The charging of radioactive aerosols. J. Aerosol Sci. 23 (5):481–504. doi: https://doi.org/10.1016/0021-8502(92)90019-R.
- Devadason, S. G., H.-K. Chan, S. Haeussermann, C. Kietzig, P. J. Kuehl, S. Newman, K. Sommerer, and G. Taylor. 2012. Validation of radiolabeling of drug formulations for aerosol deposition assessment of orally inhaled products. J. Aerosol Med. Pulmon. Drug Deliv. 25 (S1):S-6–S9. doi: https://doi.org/10.1089/jamp.2012.1Su3.
- Finlay, W. H. 2019. The mechanics of inhaled pharmaceutical aerosols: An introduction. 2nd ed. London: Academic Press.
- Fleming, J., D. L. Bailey, H.-K. Chan, J. Conway, P. J. Kuehl, B. L. Laube, and S. Newman. 2012. Standardization of techniques for using single-photon emission computed tomography (SPECT) for aerosol deposition assessment of orally inhaled products. Journal of Aerosol Medicine and Pulmonary Drug Delivery 25 (S1):S29–S51. doi: https://doi.org/10.1089/jamp.2012.1Su5.
- Gensdarmes, F., D. Boulaud, and A. Renoux. 1998. Aerosol charging under gamma radiation. J. Aerosol Sci. 29:S851–S852. doi: https://doi.org/10.1016/S0021-8502(98)90608-5.
- Gensdarmes, F., D. Boulaud, and A. Renoux. 2001. Electrical charging of radioactive aerosols – Comparison of the Clement-Harrison models with new experiments. J. Aerosol Sci. 32 (12):1437–58. doi: https://doi.org/10.1016/S0021-8502(01)00065-9.
- Golshahi, L., P. W. Longest, L. Holbrook, J. Snead, and M. Hindle. 2015. Production of highly charged pharmaceutical aerosols using a new aerosol induction charger. Pharm. Res. 32 (9):3007–17. doi: https://doi.org/10.1007/s11095-015-1682-6.
- Hashish, A. H., A. G. Bailey, and T. J. Williams. 1994. Modelling the effect of charge on selective deposition of particles in a diseased lung using aerosol boli. Phys. Med. Biol. 39 (12):2247–62. doi: https://doi.org/10.1088/0031-9155/39/12/008.
- Hinds, W. C. 1999. Aerosol Technology: Properties, behavior, and measurement of airborne particles. New York: John Wiley and Sons.
- Hopkinson, P. R. 1980. Induction charging electrostatic spraying device and method. USA Patent 4215818.
- Iribarne, J. V., and M. Klemes. 1974. Electrification associated with droplet production from liquid jets. J. Chem. Soc, Faraday Trans. 1. 70 (0):1219–27. doi: https://doi.org/10.1039/f19747001219.
- Iribarne, J. V., and B. J. Mason. 1967. Electrification accompanying the burst of bubbles in water and dilute aqueous solution. Trans. Faraday Soc. 63:2234–45. doi: https://doi.org/10.1039/tf9676302234.
- Jonas, P. R., and B. J. Mason. 1968. Systematic charging of water droplets produced by break-up of liquid jets and filaments. Trans. Faraday Soc. 64:1971–82. doi: https://doi.org/10.1039/tf9686401971.
- Jonassen, N. 1998. Electrostatics. New York: Chapman and Hall.
- Keskinen, J., K. Pietarinen, and M. Lehtimäki. 1992. Electrical low pressure impactor. J. Aerosol Sci. 23 (4):353–60. doi: https://doi.org/10.1016/0021-8502(92)90004-F.
- Kim, H.-U., A. Kulkarni, S. Ha, D. Shin, and T. Kim. 2017. Note: Electric field assisted megasonic atomization for size-controlled nanoparticles. Rev. Sci. Instrum. 88 (7):076106. doi: https://doi.org/10.1063/1.4984825.
- Koullapis, P. G., S. C. Kassinos, M. P. Bivolarova, and A. K. Melikov. 2016. Particle deposition in a realistic geometry of the human conducting airways: Effects of inlet velocity profile, inhalation flowrate and electrostatic charge. J. Biomech. 49 (11):2201–12. doi: https://doi.org/10.1016/j.jbiomech.2015.11.029.
- Kwok, P. C. L., and H.-K. Chan. 2008. Effect of relative humidity on the electrostatic charge properties of dry powder inhaler aerosols. Pharm. Res. 25 (2):277–88. doi: https://doi.org/10.1007/s11095-007-9377-2.
- Kwok, P. C. L., R. Collins, and H.-K. Chan. 2006. Effect of spacers on the electrostatic charge properties of metered dose inhaler aerosols. J. Aerosol Sci. 37 (12):1671–82. doi: https://doi.org/10.1016/j.jaerosci.2006.08.008.
- Kwok, P. C. L., W. Glover, and H.-K. Chan. 2005. Electrostatic charge characteristics of aerosols produced from metered dose inhalers. J. Pharm. Sci. 94 (12):2789–99. doi: https://doi.org/10.1002/jps.20395.
- Kwok, P. C. L., T. Noakes, and H.-K. Chan. 2008. Effect of moisture on the electrostatic charge properties of metered dose inhaler aerosols. J. Aerosol Sci. 39 (3):211–26. doi: https://doi.org/10.1016/j.jaerosci.2007.11.004.
- Kwok, P. C. L., S. J. Trietsch, M. Kumon, and H.-K. Chan. 2010. Electrostatic charge characteristics of jet nebulized aerosols. J. Aerosol Med. Pulm. Drug Deliv. 23 (3):149–59. doi: https://doi.org/10.1089/jamp.2009.0795.
- Lopatenko, S. V., S. M. Kontush, and A. V. Kolpakov. 1987. Optimization of the process of the charging of a liquid during dispersion. Colloid J. USSR 49:691–6.
- Lopez-Cuesta, J.-M., and C. Longuet. 2014. Thermal degradation, flammability, and potential toxicity of polymer nanocomposites. In Health and environmental safety of nanomaterials: Polymer nancomposites and other materials containing nanoparticles, ed. J. Njuguna, K. Pielichowski, and H. Zhu. Sawston, UK: Woodhead Publishing.
- Majid, H., P. Madl, W. Hofmann, and K. Alam. 2012. Implementation of charged particles deposition in stochastic lung model and calculation of enhanced deposition. Aerosol Sci. Technol. 46 (5):547–54. doi: https://doi.org/10.1080/02786826.2011.645957.
- Marchewicz, A., A. T. Sobczyk, A. Krupa, and A. Jaworek. 2019. Electrostatic charging of water spray by induction. J. Phys: Conf. Ser. 1322:012032. doi: https://doi.org/10.1088/1742-6596/1322/1/012032.
- Marjamäki, M., J. Keskinen, D.-R. Chen, and D. Y. H. Pui. 2000. Performance evaluation of the Electrical Low Pressure Impactor (ELPI). J. Aerosol Sci. 31 (2):249–61. doi: https://doi.org/10.1016/S0021-8502(99)00052-X.
- Matsusaka, S., and H. Masuda. 2003. Electrostatics of particles. Adv. Powder Technol. 14 (2):143–66. doi: https://doi.org/10.1163/156855203763593958.
- Matteson, M. J. 1971. The separation of charge at the gas-liquid interface by dispersion of various electrolyte solutions. J. Colloid Interface Sci. 37 (4):879–90. doi: https://doi.org/10.1016/0021-9797(71)90369-9.
- Melandri, C., V. Prodi, G. Tarroni, M. Formignani, T. De Zaiacomo, G. F. Bompane, and G. Maestri. 1975. On the deposition of unipolarly charged particles in the human respiratory tract. Inhaled Particles 4:193–201.
- Melandri, C., G. Tarroni, V. Prodi, T. De Zaiacomo, M. Formignani, and C. C. Lombardi. 1983. Deposition of charged particles in the human airways. J. Aerosol Sci. 14 (5):657–9. doi: https://doi.org/10.1016/0021-8502(83)90070-8.
- Newman, S., W. D. Bennett, M. Biddiscombe, S. G. Devadason, M. B. Dolovich, J. Fleming, S. Haeussermann, C. Kietzig, P. J. Kuehl, B. L. Laube, et al. 2012. Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J. Aerosol Med. Pulmon. Drug Deliv. 25 (S1):S10–S28. doi: https://doi.org/10.1089/jamp.2012.1Su4.
- Peart, J. 2001. Powder electrostatics: Theory, techniques, and applications. KONA 19 (0):34–45. doi: https://doi.org/10.14356/kona.2001009.
- Prodi, V., and A. Mularoni. 1985. Electrostatic lung deposition experiments with humans and animals. Ann. Occup. Hyg. 29:229–40.
- Rhodes, D. F. 1977. Electrostatic charge reducer. USA Patent 4057071.
- Scheuch, G., G. Bennett, L. Borgström, A. Clark, R. Dalby, M. Dolovich, J. Fleming, P. Gehr, I. Gonda, C. O'Callaghan, et al. 2010. Deposition, imaging, and clearance: What remains to be done? J. Aerosol Med. Pulmon. Drug Deliv. 23 (S2):S39–S57. doi: https://doi.org/10.1089/jamp.2010.0839.
- Sidler-Moix, A.-L., E. R. Di Paolo, U. Dolci, M. Berger-Gryllaki, J. Cotting, and A. Pannatier. 2015. Physicochemical aspects and efficiency of albuterol nebulization: Comparison of three aerosol types in an in vitro pediatric model. Respir. Care. 60 (1):38–46. doi: https://doi.org/10.4187/respcare.02490.
- Sweeney, L., A. P. McCloskey, G. Higgins, J. M. Ramsey, S.-A. Cryan, and R. MacLoughlin. 2019. Effective nebulization of interferon-γ using a novel vibrating mesh. Respir. Res. 20 (1):66. doi: https://doi.org/10.1186/s12931-019-1030-1.
- TSI Incoporated. 2020. Choosing the right aerosol neutralizer. Shoreview, MN: TSI Incorporated.
- Vaaraslahti, K., A. Laitinen, and J. Keskinen. 2002. Spray charging of droplets in a wet scrubber. J. Air Waste Manag. Assoc. 52 (2):175–80. doi: https://doi.org/10.1080/10473289.2002.10470776.
- Yatsuzuka, K., Y. Higashiyama, and K. Asano. 1996. Electrification of polymer surface caused by sliding ultrapure water. IEEE Trans. Ind. Applicat. 32 (4):825–31. doi: https://doi.org/10.1109/28.511638.
- Yatsuzuka, K., Y. Mizuno, and K. Asano. 1994. Electrification phenomena of pure water droplets dripping and sliding on a polymer surface. J. Electrostat. 32 (2):157–71. doi: https://doi.org/10.1016/0304-3886(94)90005-1.
- Yu, C. P. 1985. Theories of electrostatic lung deposition of inhaled aerosols. Ann. Work Exposures Health 29:219–27.
- Zhao, S., G. S. P. Castle, and K. Adamiak. 2005. Comparison of conduction and induction charging in liquid spraying. J. Electrostat. 63 (6-10):871–6. doi: https://doi.org/10.1016/j.elstat.2005.03.048.