?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Particles composition has an important influence on heterogeneous nucleation of water vapor on the particles surface and subsequent particles abatement by heterogeneous condensation. In this work, three kinds of single-component particles of typical chemical composition of coal-fired fine particles are selected. First, Environmental Scanning Electron Microscopy (ESEM) system is adopted to directly visualize the heterogeneous condensation process of water vapor on the particles surface. It can be found that when water vapor condenses on the particles, spherical cap-shaped embryo droplets occur, which is significantly consistent with the embryo model theory proposed by Fletcher (1958). Then, according to Fletcher’s nucleation theory, a numerical experimental platform is established by MATLAB programming to predict nucleation parameters. The theoretical critical supersaturation and the experimental results are compared at the same time. It is noted that the classical theory of heterogeneous nucleation underestimates particles nucleation ability and predicts a higher critical supersaturation than that experimentally measured. Finally, the growth rate of droplets on three kinds of particles is obtained experimentally. The results show that with the same initial particle size, the final growth size of CaSO4 is larger than that of SiO2 and Fe2O3 particle, and the growth rate of droplets on CaSO4 is also the largest, showing the best growth performance.
Copyright © 2022 American Association for Aerosol Research
EDITOR:
1. Introduction
Coal-fired “PM2.5” is one of the main sources of atmospheric particulate pollution based on the source apportionment of “PM2.5,” especially in China (Song et al. Citation2006; Wu et al. Citation2014). The emissions of a large amount of coal-fired fine particles from power plants not only cause losses to the country’s economic development, but also pose a threat to human health (Tecer et al. Citation2008). Therefore, the effective capture and removal of coal-fired “PM2.5” is an urgent problem to be solved. However the conventional dust removal technology has a low efficiency in removing fine particles (Fan et al. Citation2009; Bao et al. Citation2012; De Joannon et al. Citation2013). Pretreatment technology is usually used to enlarge fine particles, which is currently one of the effective and economic measures to improve the efficiency of particles abatement (Jun-Ho et al. Citation2004; Rajniak et al. Citation2007; Yan, Chen, and Yang Citation2016). Heterogeneous condensation of water vapor on the particles is proven to be a promising preconditioning technique to enlarge and remove particles efficiently (Chen et al. Citation1993; Chen et al. Citation1998; Chen and Tao Citation2000; Yang et al. Citation2010; Xu et al. Citation2017). The principle is that supersaturated water vapor condenses on the surface of particles which are considered as the condensation nuclei to form embryo droplets and then particles size will be enlarged for subsequent removal. Therefore, a supersaturated water vapor environment is the prerequisite basis, which requires a suitable humidity and temperature of particles-containing flue gas. At present, the widely used wet flue gas desulfurization (WFGD) system provides favorable physical conditions for creating a water vapor supersaturation field and promoting the condensational growth of fine particles. Yang’s group (Yang et al. Citation2010; Bao et al. Citation2013) from Southeast University initially applied the technology of water vapor condensation to promote fine particles abatement in a wet ammonia desulfurization industrial plant with the scale of 50 × 104 Nm3/h. The results showed that a supersaturated environment could be created by adding 0.08 kg of steam per N m3 of flue gas and a reduction of 30%–50% in particles emission concentration could be attained successfully.
Heterogeneous condensation of water vapor on particles is generally divided into two steps (Smorodin and Hopke Citation2006; Tammaro et al. Citation2012). The first step is the nucleation process of water vapor to form embryo droplets with the critical size. The second step is the droplets growth process in which water vapor continues to condense on the droplets. Therefore nucleation is the basis and prerequisite of the condensation process. In addition to lots of theoretical researches of heterogeneous nucleation (Krastanov Citation1957; Fletcher Citation1958), a large number of nucleation experiments have also been carried out. Sharoichenko (Citation2000) used an electric field to suspend a single particle in a thermal diffusion cloud chamber and investigated vapor nucleation on the surface of smooth glass beads. The result was that the critical saturation required for nucleation was between 1.0066–1.0420 for particles with diameter of 4.05 ± 0.4 µm, which was consistent with previous experimental results on glass surfaces (Mccormick and Westwater Citation1965; Mahata and Alofs Citation1975). Hogrefe and Keesee (Citation2002) combined upward thermal diffusion with electrostatic levitation to study vapor-to-liquid nucleation on individual glass particles by using a Charge Coupled Device (CCD) camera. The critical supersaturation could be determined by measuring the period and amplitude of particles vibration with the CCD camera. It was found that the variability in the critical supersaturation was sensitive to the effect of surface electric charge density from particle to particle of nominally the same size and material. However, the heterogeneous condensation process could not be visualized by this CCD camera. Porstendorfer et al. (Citation1985) investigated heterogeneous nucleation of water vapor on the surface of Ag and NaCl particles with different nanoscales in a cloud chamber, and found that the critical supersaturations on the surface of soluble and insoluble particles were completely different. For hydrophobic insoluble Ag particles, the critical supersaturation was greater than that predicted by Kelvin equation; but for hydrophilic soluble NaCl particles, the critical supersaturation was much smaller than that predicted by Kelvin equation. Chen et al. (Citation1993) used a flow cloud chamber to investigate the heterogeneous nucleation process on SiO2, Al2O3, TiO2, and carbon black particles, and determined the critical supersaturation according to the particles’ removal efficiency experimentally. The results showed that particles’ removal efficiency would increase when increasing supersaturation and particles’ chemical composition had an important influence on the nucleation process. It could be found from these nucleation experiments that particles’ composition plays an important role on the nucleation process, and the required critical supersaturation for nucleation on particles with different chemical compositions would be quite different. Although researchers have conducted a large number of experimental studies on the surface of different particles, there are few reports on heterogeneous nucleation on the surface of coal-fired fine particles. However, the particle size, chemical composition and surface properties of coal-fired particles are rather different from the particles listed above. Moreover, these nucleation experiments aim at determining the critical supersaturation, lacking a direct observation of the nucleation process. Thus the inconsistency between the critical supersaturation based on theoretical prediction and the experimental results cannot be reasonably explained.
In this work, three kinds of single particles with a single component of the chemical composition of typical coal-fired fine particles are utilized. The nucleation process on these three particles is studied experimentally and numerically by using Environmental Scanning Electron Microscopy (ESEM) system and the classical nucleation theory separately. The results are presented here.
2. Visualization investigation on heterogeneous nucleation
2.1. Experimental
Visualized observation of heterogeneous nucleation of water vapor on particles surface can be realized by ESEM (model, Quanta 200, made in USA). Different from traditional scanning electron microscopy, a gas environment is allowed in the sample chamber of ESEM, which creates favorable conditions for the research of phase change process of water vapor. Details about the experimental setup and operating methods were given in our previous work (Lv et al. Citation2020b). Here only important accessories and experimental procedures are illustrated.
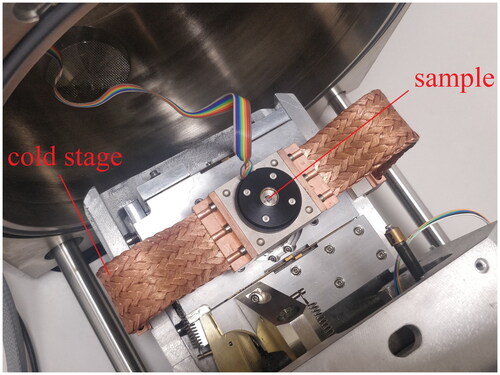
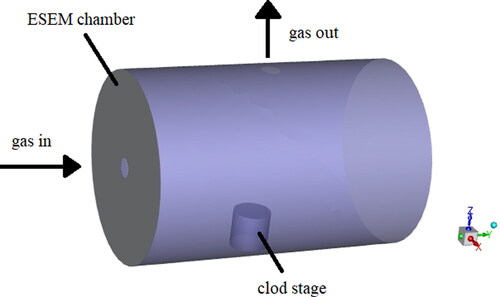
Particles composition has an important influence on the nucleation process (Chen et al. Citation1993). According to the main chemical composition of coal-fired fine particles, three kinds of micron-sized particles with different components and wetting properties are selected, which are SiO2, Fe2O3 and CaSO4. The particles are required to be as spherical as possible. Then particles are dispersed separately on the surface of a superhydrophobic substrate to prepare the mixed superhydrophobic/fine particles surface. After that, the mixied surface is placed on the base of the cold stage in ESEM. shows distributions of the cold stage and sample in the ESEM chamber. By controlling the temperature of the cold stage and adjusting the water vapor pressure, a sufficient supersaturated water vapor atmosphere is created for heterogeneous nucleation. Apart from the cold stage, a Gaseous Secondary Electron Detector (GSED) is also an important accessory of ESEM. shows the appearance of GSED. In imaging process, the high-energy electron beam interacts with samples surface to produce initial secondary electrons with weaker signals. However the initial secondary electrons will accelerate upward movement under the action of electric field because an acceleration voltage of 300–500 V is applied to GSED. Then these secondary electrons will collide with the gas molecules in the sample chamber to ionize the gas molecules. After that, positive ions and additional environmental secondary electrons will be generated. This acceleration and ionization process is repeated continuously, so that initial secondary electronic signal is continuously amplified and finally received by the GESD probe and successfully converted into video and image signals.
2.2. Results and discussion
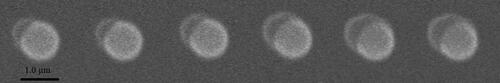
The ESEM images of heterogeneous condensation on SiO2, Fe2O3 and CaSO4 particles are illustrated in , respectively. In , the particle size of SiO2 particle is 1.0 µm. The water vapor pressure is maintained at 600 Pa and the temperature of the cold stage is −0.7 °C. It can be found that when water vapor condenses on a single insoluble particle surface, a spherical cap-shaped embryo droplet would occur first. This phenomenon is highly consistent with Fletcher’s embryo model of heterogeneous nucleation on the surface of insoluble particles. When water vapor continues to condense, this embryo drop grows subsequently. It should be noted that the temperature of the clod stage here is actually a critical temperature. That is to say, if the temperature of the cold stage is higher than this critical value, no obvious nucleation could be observed because vapor supersaturation at this time is very small to activate the nucleation process. If the temperature is lower than this critical value, nucleation process would occur significantly because vapor supersaturation at this time is very large to complete the nucleation process. The following temperatures of the cold stage on Fe2O3 and CaSO4 particles are all critical temperature.
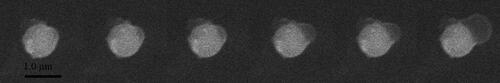
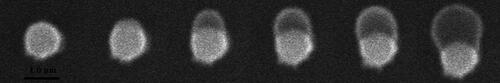
The time-resolved images of heterogeneous condensation of water vapor on a single Fe2O3 particle are illustrated in . The particle size is 1.0 µm. The water vapor pressure is maintained at 600 Pa with the temperature of the cold stage is at −1.9 °C. Once an embryo drop formed on the particle surface, it keeps developing and grows into a large droplet eventually. shows the ESEM images of heterogeneous condensation on a single CaSO4 particle. When condensing on CaSO4, the particle diameter is 1.0 µm and the vapor pressure is maintained at 600 Pa. But the temperature of the cold stage is at −0.3 °C at this time. A spherical cap-shaped embryo randomly occurs on the particle surface and continues to enlarge, which is basically the same as the condensation processes on the surface of SiO2 and Fe2O3 particles.
3. Numerical investigation on heterogeneous nucleation
As described above, heterogeneous condensation process is divided into two processes: the nucleation process and the droplets growth process. Based on the ESEM images in Sec. 2, it can be found that the initial size of the embryo droplet occurring on the surface of a single particle is about tens of nanometers. And according to the classical nucleation theory (Fletcher Citation1958), the critical size of embryo droplet on a 1.0 µm single particle is 2.0–3.0 nm. In other words, at the moment when the appearance of embryo droplets formation was observed, water vapor condensation has already entered into the second step where droplets continue to enlarge after nucleation. Although the theoretical resolution of ESEM could reach 3.0 nm when an accelerating voltage of 30.0 kV is applied, the actual resolution cannot capture the appearance of the critical embryo in the nucleation process due to the influence of water vapor in the gas environment on the electron bombardment. Therefore, MATLAB programming is used in this article to establish a numerical experimental platform for the numerical experiments of heterogeneous nucleation of water vapor on the surface of SiO2, Fe2O3 and CaSO4 particles.
3.1. Characteristics of supersaturated environment in ESEM
Heterogeneous condensation of water vapor is actually an energy-unfavorable process since the formation of droplets requires an increase in the free energy of water molecules. In order to overcome the free energy barrier and activate particles to become condensation nuclei, water vapor must be supersaturated. The degree of supersaturation S is defined as the ratio of vapor partial pressure to the saturation vapor pressure
at the gas temperature T.
(1)
(1)
Based on the theoretical model of heat and mass transfer, the supersaturation distribution can be solved in the sample chamber. Since the vapor pressure in the chamber is always maintained at a known constant value, only the gas temperature distribution needs to be solved according to the theoretical heat transfer model, and then the supersaturation level can be obtained using EquationEquation (1)(1)
(1) . Establish a three-dimensional geometric model of the sample chamber, as shown in . The analysis of heat transfer balance is based on the following assumptions. (1) The gas temperature and vapor partial pressure in the sample chamber are considered in thermal equilibrium; (2) The gas flowing into the chamber is not considered to contain fine particles, and the influence of fine particles on the degree of supersaturation is ignored. (3) The influence of heat transfer coupling term in the chamber on the supersaturation is ignored. (4) The temperature and vapor pressure of the inlet gas flow are evenly distributed. The boundary conditions in the sample chamber are shown in .
Table 1. The boundary conditions in ESEM chamber.
The energy equation for heat transfer balance is described:
(2)
(2)
where
is the water vapor density,
is the specific heat capacity of water vapor at constant pressure,
is the gas flow rate, T is the gas temperature, k is the water vapor thermal conductivity and
is the gradient.
Expand EquationEquation (2)(2)
(2) into a partial differential form to obtain:
(3)
(3)
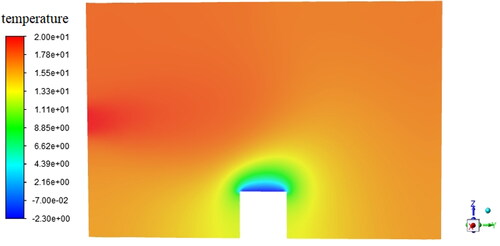
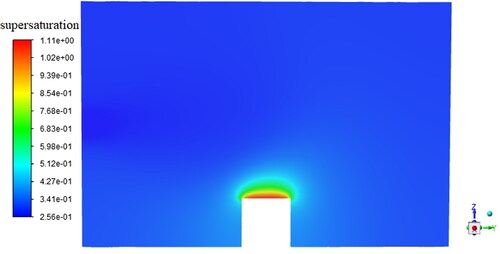
Based on the finite volume method in FLUENT, the energy equation in the geometric model is solved to obtain the gas temperature distribution in the sample chamber, as shown in . It can be seen that when the temperature of the cold stage is set to be −2.3 °C, there is an obvious temperature gradient between the water vapor near the cold stage and the ambient water vapor since the inlet vapor temperature is 20.0 °C. According to the temperature distribution in , the vapor saturation pressure at this temperature can be obtained. Since the partial vapor pressure in the chamber is a certain value, the supersaturation distribution can be then determined, as shown in . It can be found that when the temperature of the cold stage is −2.3 °C and the vapor pressure inside the chamber is 600 pa, a maximum supersaturation of 1.11 can be obtained near the wall of the cold stage.
3.2. Theoretical nucleation model
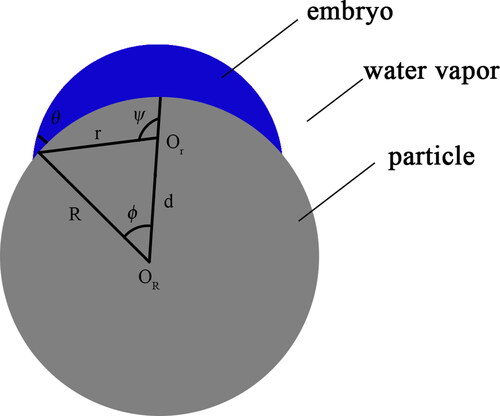
shows the embryo model of heterogeneous nucleation of water vapor on the surface of a smooth spherical and insoluble particle proposed by Fletcher (Fletcher Citation1958). This is also the most widely accepted classical nucleation model. As illustrated in , when water vapor condenses on the surface of a smooth spherical particle, a spherical cap-shaped embryo droplet is formed. The vapor phase, the embryo droplet and the particle are denoted as v, l and s respectively.
When droplets stay on the particle’s surface, interface balance of the vapor phase, the embryo droplet and the particle satisfies the Young equation:
(4)
(4)
where θ is the contact angle and is usually used to characterize the wettability of particle surface, m is the cosine of the contact angle, and σij is the interface free energy between phase i and j.
To form an embryo droplet with radius r, the free energy barrier ΔG in the system is required to be overcome:
(5)
(5)
where ΔGv is the free energy change per unit volume when vapor changes from gas phase to liquid phase, Vl is the volume of the embryo droplet, and Aij is the contact area between phase i and j. The volume of the embryo, the contact area between embryo and vapor phase and the contact area between embryo and particle could be solved:
(6)
(6)
(7)
(7)
(8)
(8)
According to geometry, the angle ϕ, ψ and the distance d between the centers of the particle and the embryo could be found:
(9)
(9)
(10)
(10)
(11)
(11)
In order to obtain the critical radius of the embryo, the derivative of free energy barrier with respect to the droplet radius is required to be 0.
(12)
(12)
Then the critical radius of the embryo r* is
(13)
(13)
Substitute EquationEquation (13)(13)
(13) into Equation(5)
(5)
(5) and obtain the free energy of formation of a critical embryo.
(14)
(14)
Here f (m, x) is the Fletcher geometrical factor,
(15)
(15)
(16)
(16)
(17)
(17)
(18)
(18)
where Vwm is the volume of a single water molecule, kB is the Boltzmann constant, T is the vapor temperature, R is the particle radius, and S is the saturation of water vapor.
According to the Boltzmann distribution of statistical physics, the nucleation rate J can be obtained.
(19)
(19)
where Kc is a kinetic constant and is uncertain in range of 1028–1031m−2·s−1. It is set to be 1029 m−2·s−1 in this work.
According to the above, in order to overcome free energy limitation and activate particles to become condensation nuclei, water vapor must be supersaturated. The probability to cause heterogeneous nucleation can be identified by evaluating vapor supersaturation. As long as the supersaturation in the gas environment exceeds the critical supersaturation, vapor condensation will occur. The definition of critical supersaturation Scr is the saturation ratio when the nucleation rate is J = 1 s−1, which is
(20)
(20)
3.3. Contact angle measurement of SiO2, Fe2O3 and CaSO4 particles
There are many ways to characterize the wettability of particle surface and contact angle measurement is a common method to directly characterize the wettability. The contact angles are measured via a contact angle goniometer (model, JC2000D, made in China) with high resolution camera (model, Guppy pro, made in Germany) by employing the sessile drop method. shows the results of contact angle measurement of SiO2, Fe2O3 and CaSO4 particles. It can be seen that the contact angle of SiO2 particles is about 40°, showing moderate hydrophilicity. The contact angle of CaSO4 particles is only 17°, showing good wettability. While the contact angle of Fe2O3 particles is 55° with poor wettability.
Table 2. Contact angle of component particles.
3.4. Numerical prediction results on SiO2, Fe2O3 and CaSO4 particles
3.4.1. Nucleation free energy
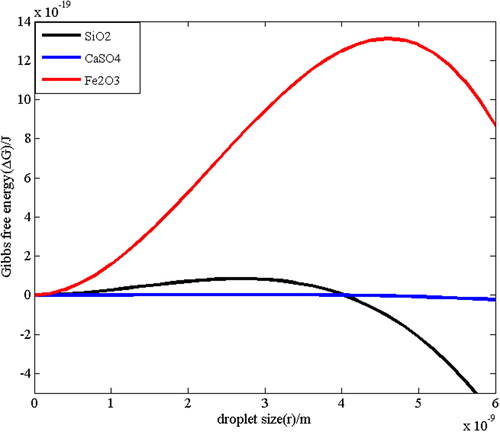
When ambient water vapor reaches a certain degree of saturation, the phase transition process from water vapor to the embryo droplets begins to occur. The dependence of Gibbs free energy change ΔG of an embryo droplet formation on droplet radius r is shown in . According to the characteristics of supersaturated environment in ESEM in Sec. 3.1, it is found that the maximum water vapor supersaturation in the sample chamber is very close to 1.11. Therefore the supersaturation value is set to be 1.11 in the numerical calculation of the free energy change. The parameters for numerical calculation are listed in . shows the nucleation free energy on SiO2, Fe2O3 and CaSO4 particles. It can be seen that there is a maximum value of ΔG with the change of r, which is the critical nucleation free energy ΔG*. The critical free energy of Fe2O3 particles is much greater than that of SiO2 particles, and the critical free energy of SiO2 particles is greater than that of CaSO4 particles at the same time. This is because under a certain vapor supersaturation, the critical free energy reflects the nucleation ability of water vapor. The smaller the critical free energy, the stronger the nucleation ability of water vapor on the particles. According to the above, Fe2O3 has the largest contact angle and the worst surface wettability, so the nucleation ability is also the worst. On the contrary, the contact angle of CaSO4 is the smallest with only 17°, so the critical free energy of CaSO4 is the smallest and the nucleation ability is the strongest.
Table 3. The parameters for numerical calculation of ΔG.
3.4.2. Nucleation rate
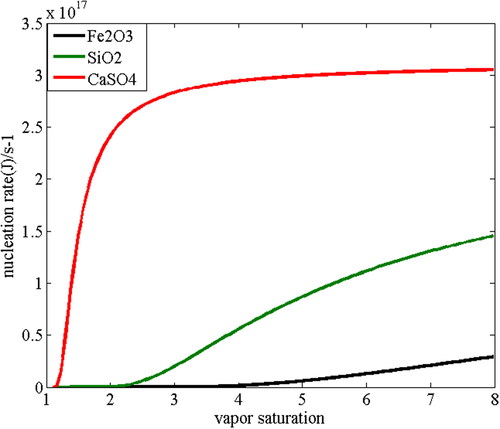
Nucleation rate of heterogeneous condensation of water vapor on particles surface is affected by the gas-liquid interfacial tension and is related to water vapor saturation. shows the dependence of nucleation rate on vapor saturation when water vapor condenses on the surface of SiO2, Fe2O3 and CaSO4 particles with the diameter of 1.0 µm. It can be seen that nucleation rate on the surface of CaSO4 particles is greater than that on the surface of SiO2 particles and Fe2O3 particles. For SiO2 particles, when the vapor saturation is less than 1.34, the nucleation rate is less than 1.0 per second. When the vapor saturation reaches 1.34, the nucleation rate can be 2.92 per second. Importantly when the supersaturation is slightly increased to 1.38, the nucleation rate at this time is 2.82 × 103 per second with an increase of three orders of magnitude. Therefore the nucleation rate J is extremely sensitive to the saturation S. Similarly, for Fe2O3 particles, when the saturation is less than 1.66, the nucleation rate is less than 1.0 per second. But with a slight increase of the saturation from 1.66 to 1.74, a sharp increase of nucleation rate can be detected from 1.23 per second to 853.62 per second with an increase of three orders of magnitude. This is because the contact angle between SiO2 and Fe2O3 particles is relatively close, so the cosine value of the contact angle is also similar. However the contact angle of CaSO4 particles is relatively small. When the vapor saturation reaches 1.1, the nucleation rate of CaSO4 particles is as high as 1.71 × 1011, showing good nucleation ability.
3.4.3. Critical saturation
Particles with different chemical compositions have different surface wettability, and the critical supersaturation required for heterogeneous nucleation of water vapor on the particles surface is also different. The critical supersaturation can be determined according to EquationEquation (20)(20)
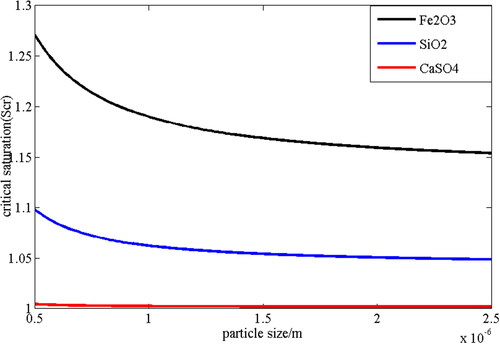
(20) and the iterative method. The parameters for numerically calculating the critical saturation are listed in below, where Scr0 is the initial critical saturation given at the beginning of the iteration. Due to the difference in particle’s chemical composition, the surface wettability is also different, as shown in the value of m in . Then in this work, when particle size is set to vary from 0.5 µm to 2.5 µm, the relationship between the critical saturation and the particle size on the surface of different particles can be obtained, as shown in . shows the relationship of the required critical supersaturation on the surface of SiO2, Fe2O3 and CaSO4 particles as a function of particle size. It can be found that no matter what kind of particles, the critical supersaturation required for nucleation will decrease as the particle size increases. At the same time, for particles with the same diameter, the critical supersaturation required by Fe2O3 is greater than that required by SiO2, and the critical supersaturation required by SiO2 is greater than that required by CaSO4 particles. For example, for particles with size of 1.0 µm, the critical saturation on the surface of Fe2O3, SiO2 and CaSO4 particles is 1.1897, 1.0623 and 1.0025, respectively. This is because the contact angle of Fe2O3 particles is the largest while the contact angle of CaSO4 particles is the smallest of these three kinds of particles. By reducing the contact angle, the gas-liquid interfacial tension can be reduced, thereby improving the nucleation performance of the particles. The critical saturation will also decrease accordingly.
Table 4. The parameters for numerical calculation of Scr.
4. Comparison of the theoretical and experimental critical supersaturation
According to the experimental process in Sec. 2.1, when the chamber vapor pressure is constant, a supersaturated vapor environment can be created by slowly lowering the temperature of the cold stage. However, no significant droplets are observed in the beginning process of lowering the temperature, which also means that the supersaturation at this time is not high enough to activate enough particles to become condensation nuclei. Until the temperature of the cold stage decreases to a certain critical value, obvious nucleation will occur at this time when 50% of the particles have droplets on the surface. As the temperature decreased further, it can be found that all particles are activated to condensational growth. Therefore, the onset of heterogeneous nucleation is investigated by recording this “critical” temperature Tcr as a function of the experimental “critical” supersaturation Scr in this work. The results of the “critical” temperature and supersaturation on Fe2O3, SiO2 and CaSO4 particles are shown in and . The uncertainty due to non-absolute spherical and microscopic impurities of particles surface is indicated by the error bar.
Table 5. The experimental “critical” temperature Tcr and supersaturation Scr.
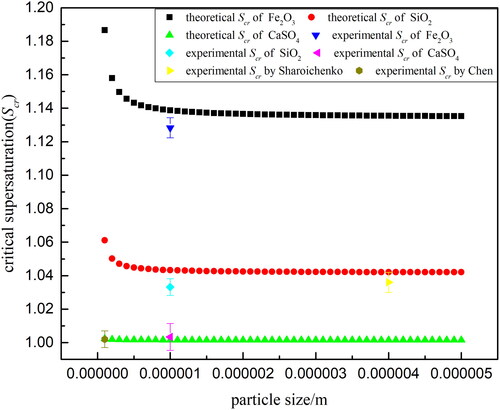
In addition to the experimental critical supersaturation of the three kinds of particles, the variation in the Scr as a function of particle size predicted by Fletcher’s nucleation theory in Sec. 3.4.3 is also shown in . The other two discrete points in are the experimental critical supersaturation of glass particles by Sharoichenko (Citation2000) as described in Introduction and the experimental critical supersaturation of sub-micrometer SiC particles by Chen et al. (Citation1998), respectively. These two sets of experimental data are basically consistent with the theoretical predictions qualitatively. When particle size increases, the required critical supersaturation will increase.
Importantly, in this work of heterogeneous nucleation on Fe2O3, SiO2 and CaSO4 particles, it can be found that the theoretical prediction results basically match with the experimental results. Apart from the experimental Scr of CaSO4 particles is very close to theoretical results, the experimental Scr on the surface of SiO2 and Fe2O3 particles are lower than the theoretical value. For example, for SiO2 and Fe2O3 particles with a diameter of 1.0 µm, the theoretical Scr predicted based on the classical nucleation theory are 1.0433 and 1.1388, respectively, while the experimental Scr are 1.0332 and 1.1283, respectively. This phenomenon is not only consistent with our previous comparison between experimental critical supersaturation and theoretical results on SiO2 particles with size range of 1.0–10.0 µm (Lv, Zhang, and Xu Citation2020a), but also consistent with Chen's results (1998). Chen investigated heterogeneous nucleation of water vapor on sub-micrometer particles in a flow cloud chamber and determined the size dependence of experimental critical supersaturation on SiC, SiO2 and naphthalene particles. The results showed that the experimental critical supersaturation is smaller than that predicted by the Fletcher’s theory of heterogeneous nucleation even considering the effects of the line tension and surface diffusion. So it was concluded that the macroscopic theory of heterogeneous nucleation underestimated the nucleation rate and predicted a higher critical supersaturation than that experimentally measured. In this work, water depletion is not considered in the ESEM chamber since a continuous supply of supersaturated water vapor required for nucleation is created sufficiently by water evaporation from a built-in water reservoir. Thus the nucleation ability of Fe2O3, SiO2 and CaSO4 particles in the experiment is much stronger than theoretical predictions.
5. Droplet growth rate
The time-resolved images of the condensation process on three kinds of particle surfaces are processed by image processing method to obtain the number of pixels and equivalent droplet diameter. The growth rates of droplets on the surface of particles with three different components are obtained. show the image processing results of condensation process on the surface of SiO2, CaSO4 and Fe2O3 respectively. shows the number of pixels and the calculated droplet equivalent diameter after the image processing method.
Table 6. Number of pixels and droplet equivalent diameter after image processing.
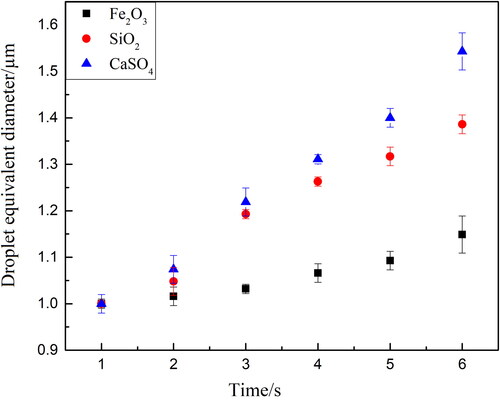
According to the calculated droplet equivalent diameter, the evolution of droplet size with time can be obtained, as shown in . It can be found that the condensational growth rate of droplets on CaSO4 particle is slightly higher than that on SiO2 particle, while the growth rate on SiO2 is significantly higher than that on Fe2O3. For the three kinds of particles with initial particle size of 1.0 µm, when the growth time is in 6th second, Fe2O3, SiO2 and CaSO4 particles can enlarge into 1.149 µm, 1.386 µm and 1.543 µm respectively, which shows obvious differences. This is because the wettability relationship of three particles is: Fe2O3 < SiO2 < CaSO4. According to the droplet condensational growth kinetic model deduced by Kulmala (Citation1993), the better wettability of particle surface, the greater probability of water vapor condensation on the particle. Among these three particles, CaSO4 has the best wettability, and the probability of heterogeneous condensation on its surface is the greatest. Therefore, the overall growth performance of droplets is the best on CaSO4.
6. Conclusions
The heterogeneous condensation process on SiO2, Fe2O3 and CaSO4 particles has been visualized by ESEM system. The time-resolved images reveal that a spherical cap-shaped embryo could be observed at a certain edge of the particle surface regardless of the types of particles employed in this study. This phenomenon is in great accordance with Fletcher’s embryo model on spherical and insoluble particles. Based on Fletcher’s nucleation theory, numerical prediction of heterogeneous nucleation has been investigated. The results show that both the critical free energy and the critical saturation required for nucleation on the surface of CaSO4 particles are the smallest among these three kinds of particles. In addition, nucleation rate on the surface of CaSO4 particles is greater than that on the surface of SiO2 particles and Fe2O3 particles. By comparing the theoretical predicted critical supersaturation with the experimental results, it can be found that the experimental critical supersaturation is lower than the theoretical value. The nucleation ability of Fe2O3, SiO2 and CaSO4 particles in the experiment is much stronger than theoretical predictions. The time-resolved images of heterogeneous condensation process on the surface of three kinds of particles are then processed to obtain the evolution law of droplet growth rate with time. The results show that the droplet growth rate and final growth diameter on the surface of CaSO4 particles are the largest and Fe2O3 is the smallest with the same growth time. The investigation on heterogeneous condensation of water vapor on three kinds of single-component particles of typical chemical composition of coal-fired fine particles can bring important reference value for the effective removal of coal-fired “PM2.5.”
Additional information
Funding
References
- Bao, J., L. Yang, S. Song, and G. Xiong. 2012. Separation of fine particles from gases in wet flue gas desulfurization system using a cascade of double towers. Energy Fuels 26 (4):2090–7. doi:10.1021/ef201868q.
- Bao, J., L. Yang, J. Yan, G. Xiong, B. Lu, and C. Xin. 2013. Experimental study of fine particles removal in the desulfurated scrubbed flue gas. Fuel 108:73–9.
- Chen, C., L. Hung, and H. Hsu. 1993. Heterogeneous nucleation of water vapor on particles of SiO2, Al2O3, TiO2, and carbon black. J. Colloid Interface Sci. 157 (2):465–77. doi:10.1006/jcis.1993.1209.
- Chen, C., G. Ming-Sheng, T. Yi-Jer, and H. Chong-Cheng. 1998. Heterogeneous nucleation of water vapor on submicrometer particles of SiC, SiO2, and naphthalene. J. Colloid Interface Sci. 198 (2):354–67. doi:10.1006/jcis.1997.5298.
- Chen, C., H. K. Shu, and Y. K. Yang. 1993. Nucleation-assisted process for the removal of fine aerosol particles. Ind. Eng. Chem. Res. 32 (7):1509–19. doi:10.1021/ie00019a027.
- Chen, C., and C. Tao. 2000. Condensation of supersaturated water vapor on submicrometer particles of SiO2 and TiO2. J. Chem. Phys. 112 (22):9967–77. doi:10.1063/1.481633.
- De Joannon, M., G. Cozzolino, A. Cavaliere, and R. Ragucci. 2013. Heterogeneous nucleation activation in a condensational scrubber for particulate abatement. Fuel Process. Technol. 107:113–8. doi:10.1016/j.fuproc.2012.10.004.
- Fan, F., L. Yang, J. Yan, J. Bao, and X. Shen. 2009. Experimental investigation on removal of coal-fired fine particles by a condensation scrubber. Chem. Eng. Process 48 (8):1353–60. doi:10.1016/j.cep.2009.06.011.
- Fletcher, N. H. 1958. Size effect in heterogeneous nucleation. J. Chem. Phys. 29 (3):572–6. doi:10.1063/1.1744540.
- Hogrefe, O. V., and R. G. Keesee. 2002. Heterogeneous vapor-to-liquid nucleation of water on individual glass particles. Aerosol Sci. Technol. 36 (2):239–47. doi:10.1080/027868202753504092.
- Jun-Ho, J., H. Jungho, B. Gwi-Nam, and K. Yong-Gin. 2004. Particle charging and agglomeration in DC and AC electric fields. J. Electrostat. 61:57–68.
- Krastanov, L. 1957. Effectiveness of semi-adsorbent condensation nuclei. Russian, Idöjárás 61:390–4.
- Kulmala, M. 1993. Condensational growth and evaporation in the transition regime. Aerosol Sci. Technol. 19 (3):381–8. doi:10.1080/02786829308959645.
- Lv, L., J. Zhang, and J. Xu. 2020a. Microscopic visualization of heterogeneous nucleation process on smooth spherical particle: Method and results. Chem. Eng. Sci. 213:115411. doi:10.1016/j.ces.2019.115411.
- Lv, L., J. Zhang, J. Xu, and J. Yin. 2020b. Heterogeneous condensation process observed by environmental scanning electron microscopy (ESEM): On smooth single aerosol particle. Aerosol Sci. Technol. 54 (12):1515–26. doi:10.1080/02786826.2020.1796460.
- Mahata, P. C., and D. J. Alofs. 1975. Insoluble condensation nuclei: The effect of contact angle, surface roughness and adsorption. J. Atmos. Sci. 32 (1):116–22. doi:10.1175/1520-0469(1975)032<0116:ICNTEO>2.0.CO;2.
- Mccormick, J. L., and J. W. Westwater. 1965. Nucleation sites for dropwise condensation. Chem. Eng. Sci. 20 (12):1021–36. doi:10.1016/0009-2509(65)80104-X.
- Porstendorfer, J., H. G. Scheibel, F. G. Pohl, O. Preining, G. Reischl, and P. E. Wagner. 1985. Heterogeneous nucleation of water vapor on monodispersed Ag and NaCl particles with diameters between 6 and 18 nm. Aerosol Sci. Technol. 4 (1):65–79. doi:10.1080/02786828508959039.
- Rajniak, P., C. Mancinelli, R. T. Chern, F. Stepanek, L. Farber, and B. T. Hill. 2007. Experimental study of wet granulation in fluidized bed: Impact of the binder properties on the granule morphology. Int. J. Pharm. 334 (1–2):92–102. doi:10.1016/j.ijpharm.2006.10.040.
- Sharoichenko, O. V. 2000. A new apparatus for study of heterogeneous nucleation on single micron-sized insoluble particles. New York: State University of New York.
- Smorodin, V. Y., and P. K. Hopke. 2006. Relationship of heterogeneous nucleation and condensational growth on aerosol nanoparticles. Atmos. Res. 82 (3–4):591–604. doi:10.1016/j.atmosres.2006.02.015.
- Song, Y., Y. Zhang, S. Xie, L. Zeng, M. Zheng, L. Salmon, M. Shao, and S. Slanina. 2006. Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmos. Environ. 40 (8):1526–37. doi:10.1016/j.atmosenv.2005.10.039.
- Tammaro, M., F. Di Natale, A. Salluzzo, and A. Lancia. 2012. Heterogeneous condensation of submicron particles in a growth tube. Chem. Eng. Sci. 74:124–34. doi:10.1016/j.ces.2012.02.023.
- Tecer, L., O. Alagha, F. Karaca, G. Tuncel, and N. Eldes. 2008. Particulate matter (PM(2.5), PM(10-2.5), and PM(10)) and children's hospital admissions for asthma and respiratory diseases: A bidirectional case-crossover study. J. Toxicol. Environ. Health A 71 (8):512–20. doi:10.1080/15287390801907459.
- Wu, S., F. Deng, H. Wei, J. Huang, X. Wang, Y. Hao, C. Zheng, Y. Qin, H. Lv, M. Shima, et al. 2014. Association of cardiopulmonary health effects with source-Appointed ambient fine particulate in Beijing, China: A combined analysis from the healthy volunteer natural relocation (HVNR) study. Environ. Sci. Technol. 48 (6):3438–48. doi:10.1021/es404778w.
- Xu, J., Y. Yu, Y. Yin, J. Zhang, and H. Zhong. 2017. Heterogeneous condensation coupled with partial gas circulation for fine particles abatement. Chem. Eng. J. 330:979–86. doi:10.1016/j.cej.2017.08.047.
- Yan, J., L. Chen, and L. Yang. 2016. Combined effect of acoustic agglomeration and vapor condensation on fine particles removal. Chem. Eng. J. 290:319–27. doi:10.1016/j.cej.2016.01.075.
- Yang, L., J. Bao, J. Yan, J. Liu, S. Song, and F. Fan. 2010. Removal of fine particles in wet flue gas desulfurization system by heterogeneous condensation. Chem. Eng. J. 156 (1):25–32. doi:10.1016/j.cej.2009.09.026.