?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Lessons learned: A unique airborne-virus transmission mode
In response to the COrona VIrus Disease 2019 (COVID-19) pandemic, extensive research has been conducted to explore, understand, and model the dynamics of expelled respiratory droplets during transmission events. The impetus for these efforts was the realization that respiratory droplets are the carriers of the infectious pathogen Severe Acute Respiratory Syndrome-Corona Virus 2 (SARS-CoV-2). The importance of respiratory droplets, and in particular those that are airborne, in transmitting pathogens responsible for respiratory infectious diseases was known to scientists prior to the COVID-19 pandemic (Morawska Citation2006, Weber and Stilianakis Citation2008, Drossinos and Stilianakis Citation2020). Nevertheless, the concerted effort of numerous aerosol scientists during the COVID-19 pandemic promulgated this knowledge to a broader audience with significant scientific and public policy repercussions (Morawska and Milton Citation2020).
The concerted effort was influential in convincing national and international health organizations (WHO 2021, US CDC 2021, Lewis Citation2022) to promote that SARS-CoV-2 infections arise predominantly via the airborne transmission route. These efforts led to a clarification of the terminology commonly used by the medical community to characterize respiratory-droplet transmission as either due to aerosol or large droplets. This terminology is at odds with the standard definition of an aerosol as defined by the physical sciences community as the suspension of solid or liquid particles in air (Hinds Citation1999). An artificial dichotomy used by the medical community to define airborne transmission as occurring for particles at or below 5 µm aerodynamic diameter versus large droplets was questioned by the physical sciences community and attributed to misinterpretation of early experiments results (Randall et al. Citation2021). As a result, a new consensus has emerged on the revision of the definition of airborne transmission either by setting the demarcation diameter at approximately 100 m or eliminating it (Drossinos, Weber, and Stilianakis Citation2021). The overestimation of the importance of large-droplet pathogen transmission may have arisen from the incorrect deduction that the observed prevalence of close-contact transmission implies large-droplet transmission, an epidemiological observation that does not necessarily exclude aerosol transmission.
There has been considerable scientific debate between aerosol scientists, microbiologists, and the medical community on the level of evidence required for proof of airborne transmission. This debate stemmed from observations that SARS-CoV-2 airborne transmission was initially inferred from epidemiological data to be due to close proximity in an indoor environment (Lu et al. Citation2020) and the experimental difficulties encountered in identifying infectious viruses in air samples. Virologists argued that the detection of viral RNA copies in air samples by molecular means, i.e., by RT-PCR (Reverse-Transcription Polymerase Chain Reaction), did not necessarily imply the presence of infectious viruses and that infectivity of air-sampled viruses must be demonstrated by the cytopathic effect in cell culture, irrespective of the difficulties encountered in air sampling and low airborne-virus concentrations. Indeed, since the pandemic started, there has been growing experimental evidence to suggest that infectious viruses may be found in air samples, see, for example, Lednicky et al. (Citation2020 and 2021), Santarpia et al. (Citation2021), and the recent review by Tellier (Citation2022). This issue remains an active area of research.
Research efforts in the aerosol physics community have concentrated on droplet transport and dispersion in the buoyant turbulent expelled multiphase flow, and on their evaporation and possibly growth under various environmental conditions. Droplet-diameter changes, induced by the change in relative humidity when air is exhaled from the humid respiratory tract into the ambient environment, are fundamental in predicting and understanding respiratory-droplet behavior (Drossinos, Weber, and Stilianakis Citation2021). These droplet-diameter changes impact on the transmission between infected and susceptible individuals by modifying droplet transport in the environment (distance from emitter, airborne lifetime, filtering efficiency) and droplet behavior within the host (deposition location in the respiratory tract). The importance of airborne transmission and successful mitigation strategies, e.g., the filtering efficiency of masks as protection and source control measures, indoor air ventilation and filtration, have been thoroughly investigated via sophisticated Computational Fluid Dynamics (CFD) simulations, see for example the review by Zhou and Zou (Citation2021), and via ingenious experimental studies, cf. Ratnesar-Shumate et al. (Citation2021), and experimental studies on mask filtration (Drewnick et al. Citation2021) and efficacy (Lindsley et al. Citation2021).
Droplet dynamics in itself is not sufficient to understand the spread and potential control of an epidemic. Studies on droplet dynamics address primarily pathogen transmissibility, i.e., the pathogen’s ability to pass from person-to-person, and how transmissibility changes with time and environmental conditions. An equally important issue is pathogen infectivity, namely the ability of the pathogen to enter and replicate in host cells and cause infection. Respiratory-droplet dynamics as determined by their time-dependent diameter and phase is only part of the puzzle; pathogen infectivity is an important contributor, even though the factors that determine it are still debated and currently being researched.
We believe that important remaining open research issues include the quantification and mechanistic understanding of airborne virus infectivity and decay; the specification of the infectious dose; the unequivocal identification of infectious viruses in air samples; and epidemiological population modeling at mesoscopic scales that captures these effects. The first topics would provide insights in the most efficient non-pharmaceutical intervention strategies, whereas the latter would help us to determine characteristics of evolving epidemics, and to provide health-risk assessment at intermediate (mesoscale) temporal and spatial scales.
Beyond the dynamics of respiratory droplets
Airborne virus infectivity and inactivation are affected by numerous biological and environmental factors. Viral activity varies with ambient conditions, temperature and absolute humidity (relative humidity includes both factors) and solar radiation. Humidity influences viral infectivity by modifying the within-host defense system and by inducing within-droplet physico-chemical processes that may lead to viral inactivation in airborne particles. The within-host response involves a change of the ability of the upper airways to eliminate deposited pathogens. Clearing the airways requires proper hydration and adequate fluid production (Moriyama, Hugentobler, and Iwasaki Citation2020). As indoor air becomes drier, nasal mucociliary clearance speed decreases, from a maximum 20 mm/sec speed of a well hydrated mucous layer to zero at a dried mucus layer (Williams et al. Citation1996), and infection risk increases. The response of the upper-airway mucosa, the first line of defense to infection, may provide a link between indoor dryness and seasonality of respiratory infections due to influenza-, human respiratory syncytial-, and corona- viruses (Moriyama, Hugentobler, and Iwasaki Citation2020). The probability of infection arising from a deposited pathogen in the respiratory tract is modulated by the effect of indoor humidity on the host. Animal studies have shown that breathing dry air not only impairs mucociliary clearance speed, but also the innate immune response and repair processes (Kudo et al. Citation2019, Kelly, Martinsen, and Tatkov Citation2021). The relevance of indoor relative humidity to respiratory infections has obvious implications for building and construction regulations in that current building regulations emphasize thermal control and comfort, perceived indoor air quality, and energy use with no consideration of infection control (as done, for example, in hospitals and specialized manufacturing establishments).
Environmental conditions influence the infectivity of viruses while airborne. Viral infectivity is modulated by ultraviolet (UV) radiation, ambient temperature, relative humidity (Dabisch et al. Citation2021) and within-droplet properties like salt and protein concentrations, and pH (Lin, Schulte, and Marr Citation2020). Reported dependencies of viral infectivity on ambient relative humidity (RH) have often been conflicting with some showing significant (Schaffer, Soergel, and Straube Citation1976; Yang, Elankumaran, and Marr Citation2012) and others limited (Kormuth et al. Citation2018, Dabisch et al. Citation2021) dependence. Recent studies seem to suggest a U-shaped dependence of viral infectivity on relative humidity both in suspended aerosols (Lin and Marr Citation2020) and on surfaces (Morris et al. Citation2021) such that infectivity of respiratory viruses is sustained at low and high relative humidity levels with maximum inactivation at approximately 50% RH. Indeed, a mechanistic understanding of the effect of relative humidity on pathogen infectivity is slowly emerging. As the ambient relative humidity decreases, the droplet radius decreases due to fast equilibration at the local microenvironmental conditions via water evaporation. This decrease leads to increased concentrations of salts, organics, proteins, and acids present in the droplet that have a detrimental effect on viral infectivity damaging the virus.
Niazi et al. (Citation2021), who investigated the inactivation of airborne influenza A viruses, proposed that at lower humidity as the droplet evaporates and shrinks, efflorescence may occur whereby the salt crystallizes out of solution. This process might provide a within-droplet means to conserve viral infectivity. Oswin et al. (Citation2022), however, observed that crystallization can lead to an immediate loss of ∼50% of infectious virus of SARS-CoV-2 in less than 10 s after emission; however, infectivity is then sustained for the fraction that survives the phase change at the longest time reported of 20 min. Indeed, droplet drying may lead to a protective organic layer due to constituents in the suspending droplet around the virus that may keep it active (Nenes Citation2021). This initial rapid loss of infectivity is relatively small when compared to the potential variability in exhaled viral load and amount of aerosol, both of which may span many orders of magnitude between individuals. The remaining infectious virus expelled by an individual who is a high emitter may still be capable of transmitting the virus via the airborne route for subsequent infection in a susceptible host.
At intermediate relative humidity (%), the equilibrated salt concentration in virus-containing droplets is supersaturated presenting conditions that can inactivate the virus. At even higher relative humidity (
%), the virus within the exhaled droplet is exposed to a dilute aqueous solution of solutes. At such RHs the infectious droplet is exposed to conditions that are similar to the physiological conditions at the generation location of the droplet (Oswin et al. Citation2022); studies suggest a slow viral inactivation rate (see, for example, Lin and Marr Citation2020).
Dabisch et al. (Citation2021), who investigated the combined effect of temperature, humidity, and simulated sunlight on SARS-CoV-2 infectivity in aerosols, found that simulated sunlight and temperature have a greater influence on viral infectivity than relative (or absolute) humidity. Their experiments were performed with particles of a mass median aerodynamic diameter of approximately 2 µm, a droplet size characteristic of normal oro-nasal activity (breathing, speaking). They argued that the persistence of the virus in larger droplets may change as the greater mass of noninfectious material could shield the virus from the changing local microenvironmental conditions.
It is apparent that recent experiments have started to provide valuable piecewise information on viral inactivation (survival) under different microenvironmental conditions and across relevant particle sizes. Results may depend on the experimental designs as different temporal regimes or within-droplet species concentrations may be experimentally achievable. Experiments that suggest a U-shaped dependence of viral infectivity on RH have been performed with a rotating Golberg drum, which might miss short-time virus inactivation, whereas the experiments of Oswin et al. (Citation2022), performed with an electrodynamic trap (CELEBS, controlled electrodynamic levitation and extraction of bioaerosols onto a substrate, Oswin et al. Citation2021) provide short-time information.
Oswin et al. (Citation2022) also argued that a significant decrease in infectivity of SARS-CoV-2 could occur over a timescale of seconds to minutes following exhalation as a consequence of the considerable disparity in gas phase carbon dioxide concentration between the lungs and the environment (40,000 vs 400 ppmv). Their measurements suggested bicarbonate anions rapidly and irreversibly partition into the gas phase as CO2 leading to an increase in pH to alkaline conditions in the droplet. These basic conditions lead to a loss of infectivity on a timescale shorter than typical for loading a rotating Goldberg drum, and hence are probably not observable using such experimental designs.
The importance of understanding the evolving pH of respiratory droplets in an environment that contains condensable acids has also been highlighted by Luo et al. (Citation2022). They argued that in the presence of trace gases, acids, e.g., nitric acid, may enter in the droplet leading to its acidification. As a direct consequence, they suggested that the exposure of expelled droplets to non-harmful acidic environmental conditions could be used as a disease control strategy. Therefore, recent studies of different within-droplet pH conditions have investigated both acidic (Luo et al. Citation2022) and alkaline conditions (Oswin et al. Citation2022). At the extremes of both conditions, the two groups found increased virus inactivation.
The experimental studies mentioned provide complementary and orthogonal information. They all share the underlying belief that understanding within-droplet physico-chemical processes, induced by changes in the local environment around a droplet, are required for a successful resolution of the viral infectivity riddle. The overarching aim is to go beyond a simplifying correlation of RH and airborne viral infectivity and to identify the underlying within-droplet causative mechanisms. We believe that within droplet-phase change along with pH or other chemical changes may be pivotal factors in viral inactivation.
Measurements of viral inactivation are hampered by numerous experimental challenges in collecting and characterizing expelled aerosol, as extensively discussed in Gregson et al. (Citation2022). Along the same lines, the detection of viable SARS-CoV-2 in air samples, an issue of disaccord as mentioned earlier, has received significant attention, see, among others, Tellier (Citation2022). With more experimental and epidemiological evidence appearing, it is increasingly becoming acceptable that airborne droplets expelled by an infected person contain infectious viruses. However, in the absence of quantitative data on airborne viral shedding and inhalation at the individual and population level, studies have frequently resorted to reporting exhaled aerosol concentrations and fluxes to provide some indication, however imperfect, of the relative risk of transmission during speaking, coughing, singing and even playing music instruments.
Discussions of viral infectivity and within-droplet viral load should consider that the presence of a viral RNA copy is inherently stochastic (Drossinos, Weber, and Stilianakis Citation2021). For a reasonable estimate (Stadnytskyi et al. Citation2020) of an average oral fluid concentration of SARS-CoV-2 viral RNA copies per cm
the probability that a 10 μm droplet at generation (which almost immediately upon expulsion shrinks to approximately half its size), contain one viral RNA copy is
% (assuming, as is usually done in biological estimates, that the number of viruses in a droplet is Poisson distributed). This probability increases rapidly with droplet size to reach 97.4% for a 100 μm droplet (size at generation). These estimates assume, for simplicity, that viral load is proportional to droplet volume, an assumption that has been questioned recently. Smaller droplets (less than 4 μm) possibly have a higher load than the volume-number of virus proportionality would predict (Santarpia et al. Citation2020). Lastly, note that the relevant quantity for health risk estimates is the total volume of respiratory droplets inhaled as determined by an individual’s breathing rate (a reasonable estimate of an adult male being 8 lt/min), not directly the number of viruses per droplet.
Particle dynamics as input to mesoscopic population modeling
An unresolved issue is how to use the extensive knowledge on droplet dynamics to predict the temporal and spatial development of an epidemic within a population of a given size, and to calculate its risk of infection. Currently, research efforts concentrate on the transmission of the virus in small, enclosed spaces (e.g., a room, restaurant, airplane cabin, grocery store, supermarket) where properties of respiratory droplets and their dynamics can be explicitly calculated. Airborne virus infectivity, as determined by biological parameters like viral load and inactivation, and behavioral parameters, like interpersonal contact rates, also influence the time-evolution of the transmission and how infection risk is calculated. Both aspects should be included in population modeling in a systematic, detailed, and explicit way that goes beyond the traditional split of the viral transmission rate to the product of a contact rate times a probability of infection.
Infection-risk analyses that include viral transmissibility and infectivity are usually performed via microenvironmental modeling (Buonanno, Stabile, et al. Citation2020; Buonanno, Morawska, et al., Citation2020; Peng et al. Citation2022). Infection risk is predominantly calculated by the Wells-Riley exponential, dose-response risk infection model (Riley, Murphy, and Riley Citation1978), or variants of it. This deterministic exposure model is based on Wells’ quantum of airborne infection stochastic model, see, for example, Rudnick and Milton (Citation2003) or Noakes et al. (Citation2006).
A natural and important extension of microenvironmental modeling is to consider intermediate mesoscopic temporal and spatial scales (mesoscales). Mesoscopic epidemiological modeling can incorporate explicitly particle dynamics and droplet properties. Appropriate scales are encountered at larger enclosed environments like boarding schools, cruise and military ships, hospitals, prisons, military camps, and nursing homes.
At much larger scales, for example at the spatial scale of a country, intimate microscopic knowledge of the behavior of a cloud of respiratory droplets might not provide the most efficient modeling approach. Instead, effective interaction constants, e.g., pathogen transmission rates, that implicitly incorporate the relevant particle physics would be more appropriate, as done, for example, in coarse-grained descriptions of physical systems that lead to mesoscopic descriptions. Population modeling at the macroscopic level (country- or province-wise scales) would involve the coupling of very disparate time and length scales to make it computationally tractable and the results interpretable. At large temporal and spatial scales Susceptible-Infected-Recovered (SIR) or Susceptible-Exposed-Infected-Recovered (SEIR) or similar models, the choice depending on the characteristics of the epidemic and the pathogen, without explicit droplet dynamics would be more appropriate (Cuevas-Maraver et al. Citation2021; Kevrekidis et al. Citation2021). We note that data quality is very important in all epidemiological models (Kevrekidis et al. Citation2022). In the early stages of the COVID-19 pandemic, the number of reported cases was neither reliable nor complete due to the limited number of tests available and the large number of asymptomatic individuals. In fact, model predictions are usually compared to hospitalization or fatality time series as these are deemed more accurate.
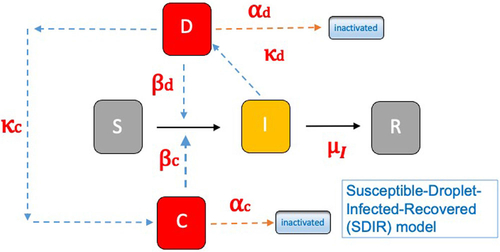
A mesoscopic epidemiological model that goes beyond microenvironmental modeling is the Susceptible-Droplet-Infected-Recovered (SDIR) model (Stilianakis and Drossinos Citation2010). The model introduces the population of infectious respiratory droplets, be they airborne or deposited (settled), as additional population compartments of the SIR model. The infectious-droplet compartments mediate between the infected and the susceptible compartments. Furthermore, by a judicious choice of parameters (e.g., transmission and droplet removal rates and viral inactivation) the model includes physical, behavioral and biological aspects of the transmissibility and infectivity of a respiratory virus. A schematic diagram of the model is shown in the left panel of : the airborne-droplet compartment is denoted by D and that of settled droplets by C.
Figure 1. Left panel: Schematic diagram of the Susceptible-Droplet-Infected-Recovered (SDIR) model. The infectious airborne-droplet compartment is denoted by D and that of the settled droplets by C. Subscripts denote airborne (d), settled (c), and infection (I). Right panel: Calculated contours of the basic reproduction number as a function of the evaporation factor
and ventilation rate
(air exchanges per hour). Figures based on Kioutsioukis, Stilianakis, and Drossinos (Citation2022).
The SDIR model allows the calculation (and prediction) of many characteristics of an epidemic spreading within an appropriately chosen population size. For example, the basic reproduction number the number of secondary infections when one infectious individual is placed within a totally susceptible population (hence, a quantity of relevance at the beginning of the epidemic) is predicted to be
where the subscript
denotes a droplet population characterized by its (post) evaporation diameter
the droplet diameter usually experimentally accessible, and the superscripts denote infectious airborne (
) and settled droplets (
). Explicit expressions for the various terms may be found in the relevant literature (Stilianakis and Drossinos Citation2010, Robinson, Stilianakis, and Drossinos Citation2012). Suffice it to say that infection transmission rates are denoted by
droplet production rates by
removal rates by
and the infection recovery rate is denoted by
The generation rate of settled droplets
is related to the airborne-droplet surface deposition rate that includes gravitational settling, inertial impaction, and possibly other particle deposition processes. Droplet evaporation (and possibly condensation) is modeled via the evaporation factor
such that
with
the pre-evaporation diameter, the droplet diameter at generation in the respiratory tract. The evaporation factor, reasonable values of which are [0–25 − 0.60], depends sensitively on relative humidity (and temperature), and it has numerous effects on the dynamics of the epidemic. It modifies the pathogen transmission rate, since the number of pathogens in a droplet depends on the diameter
it changes infectious-droplet transport properties and removal rates, via the change in the droplet diameter, and it modifies within-droplet viral infectivity, since as the droplet shrinks species concentration within the droplet increase, as discussed earlier.
Aerosol physics and dynamics are intimately coupled with viral transmissibility and infectivity in The infectious-droplet removal rates include ventilation, gravitational settling or, in general, indoor surface deposition (for airborne droplets) and virus inactivation. The transmission rate includes droplet evaporation (a physical property), probability of infection due to the viral load deposited on the airways (biological factor), and it depends on the number of daily contacts between susceptible and infectious individuals (behavioral factor). As an example of the predictive capabilities of such a mesoscale model we show how the ventilation rate modifies
right panel in . Indoor-air ventilation has been recognized as an efficient mitigation strategy, see, for example, the White House Brief (Citation2022). We considered two post-evaporation droplet diameters, 2.05 and 82.13 µm, with viral load 3.71e7 and 3.17e6 pathogens/cm3, respectively, and the remaining parameters were based on Stilianakis and Drossinos (Citation2010). We present contours of
as a function of the ventilation rate and the evaporation factor. The effect of indoor air ventilation is predicted to be dramatic. We chose an evaporation factor, vertical white line,
When the ventilation rate
(air exchanges per hour) changes from almost zero to slightly more than 5 exchanges rates per hour the basic reproduction number decreases from more than
to less than unity (and hence corresponding to containing the epidemic). The lower bound of the recommended air exchanges per hour rate to limit the spread of the disease is 4.
The time-dependent SDIR model was generalized to incorporate one spatial dimension in Robinson, Stilianakis, and Drossinos (Citation2012). The effect of ventilation was studied by imposing a uni-directional, horizontal external airflow. Numerical simulations showed that a critical ventilation velocity exists such that infection risk increases (downflow risk) for lower velocities, whereas at higher velocities it decreases. This threshold velocity reflects changes in the exposure time: as the airflow velocity increases exposure time decreases and vice versa. The effect of a uni-directional external air flow on the development of the epidemic depends strongly on the spatial, heterogeneous distribution of susceptible individuals.
The way forward
The recent extensive research effort by aerosol scientists, virologists, physicists, mathematicians, biologists and chemists, epidemiologists amongst many others helped elucidate some of the mechanisms of airborne transmission. Old and imprecise definitions used in the biomedical literature, for example the artificial dichotomy between aerosol and large-droplet transmission, have been redefined and associated previous misconceptions clarified. The proper identification of respiratory disease transmission was critically needed, as it led to confusing and conflicting public health decisions and perceptions. Research gaps still remain to understand at the single-particle level the identification of the physico-chemical within-droplet mechanisms of virus inactivation, and the within-host determination of the infectious dose. Experimental evidence suggests that relative humidity influences the particle physico-chemistry that, along with within-droplet highly acidic, basic, and salty concentrations, influence virus infectivity. The existence and extent of this relative humidity region depends on complex and within-droplet physico-chemical processes, triggered by changes in ambient relative humidity that are still under investigation. Phase transitions within the droplet (e.g., crystallization), and salt or protein or pH changes, induced by droplet shrinkage due to evaporation or volatilization of buffering ions such as bicarbonate, are part of the within-droplet viral infectivity puzzle.
Moreover, as we assume that the SARS-CoV-2 virus port of entry is the upper respiratory tract (nose and oropharynx), we need a better understanding of factors that reduce the mucosal barrier function, particularly hydration, age, comorbidities, immune deficiencies, and environmental stressors as air pollution.
An additional challenge is to go beyond microenvironmental modeling and to incorporate the available detailed information on virus transmissibility and infectivity into epidemiological models at mesoscale, and to calculate the health risk. This will demonstrate the epidemiological significance of the airborne transmission mode of respiratory diseases mediated by respiratory droplets containing infectious pathogens.
Thermal Hydraulics & Multiphase Flow Laboratory, Institute of Nuclear & Radiological Sciences and Technology, Energy & Safety, N.C.S.R. “Demokritos”, Agia Paraskevi, Greece
[email protected]
Jonathan P. Reid
School of Chemistry, Cantock' s Close, University of Bristol, Bristol, United Kingdom
[email protected]
Walter Hugentobler
Laboratory of Atmospheric Processes and their Impacts, School of Architecture, Civil and Environmental Engineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
[email protected]
Nikolaos I. Stilianakis
European Commission, Joint Research Centre, Ispra (VA), Italy Department of Biometry and Epidemiology, University of Erlangen-Nuremberg, Erlangen, Germany
[email protected]
Disclaimer
The views expressed are purely those of the authors and may not in any circumstances be regarded as stating an official position of the European Commission.
Acknowledgments
YD would like to thank the PEACoG (Physical Epidemiology Amherst Covid Group) members for their many insightful and helpful overarching discussions over the last two years. We thank AS&T Editor Shanna Ratnesar-Shumate for numerous valuable comments, both on the form and the content of this work.
References
- Buonanno, G., L. Stabile, and L. Morawska. 2020. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 141:105794. doi:10.1016/j.envint.2020.105794.
- Buonanno, G., L. Morawska, and L. Stabile. 2020. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 145:106112. doi:10.1016/j.envint.2020.106112.
- Cuevas-Maraver, J., P. G. Kevrekidis, Q. Y. Chen, G. A. Kevrekidis, V. Villalobos-Daniel, Z. Rapti, and Y. Drossinos. 2021. Lockdown measures and their impact on single- and two-age-structured epidemic model for the COVID-19 outbreak in Mexico. Math. Biosci. 336:108590. doi:10.1016/j.mbs.2021.108590.
- Dabisch, P., M. Schuit, A. Herzog, K. Beck, S. Wood, M. Krause, D. Miller, W. Weaver, D. Freeburger, I. Hooper, et al. 2021. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci. Technol. 55 (2):142–53. doi:10.1080/02786826.2020.1829536.
- Drewnick, F., J. Pikmann, F. Fachinger, L. Moormann, F. Sprang, and S. Borrmann. 2021. Aerosol filtration efficiency of household materials for homemade face masks: Influence of material properties, particle size, particle electrical charge, face velocity, and leaks. Aerosol Sci. Technol. 55 (1):63–79. doi:10.1080/02786826.2020.1817846.
- Drossinos, Y, and N. I. Stilianakis. 2020. What aerosol physics tells us about airborne pathogen transmission. Aerosol Sci. Technol. 54 (6):639–43. doi:10.1080/02786826.2020.175105.
- Drossinos, Y., T. P. Weber, and N. I. Stilianakis. 2021. Droplets and aerosols: An artificial dichotomy in respiratory virus transmission. Health Sci. Rep. 4 (2):e275. doi:10.1002/hsr2.275.
- Gregson, F. K. A., S. Sheikh, J. Archer, H. E. Symons, J. S. Walker, A. E. Haddrell, C. M. Orton, F. W. Hamilton, J. M. Brown, B. R. Bzdek, et al. 2022. Analytical challenges when sampling and characterising exhaled aerosol. Aerosol Sci. Technol. 56 (2)1: 60–175. doi:10.1080/02786826.2021.1990207.
- Hinds, W. C. 1999. Aerosol technology: Properties, behavior, and measurement of airborne particles. 2nd ed. New York: John Wiley & Sons.
- Kelly, S. J., P. Martinsen, and S. Tatkov. 2021. Rapid changes in mucociliary transport in the tracheal epithelium caused by unconditioned room air or nebulized hypertonic saline and mannitol are not determined by frequency of beating cilia. Intensive Care Med. Exp. 9 (1):8. doi:10.1186/s40635-021-00374-y.
- Kevrekidis, P. G., J. Cuevas-Maraver, Y. Drossinos, Z. Rapti, and G. A. Kevrekidis. 2021. Reaction-diffusion spatial modeling of COVID-19: Greece and Andalusia as case examples. Phys. Rev. E 104 (2-1):024412. doi:10.1103/PhysRevE.104.024412.
- Kevrekidis, G. A., Z. Rapti, Y. Drossinos, P. G. Kevrekidis, M. A. Barmann, Q. Y. Chen, and J. Cuevas-Maraver. 2022. Backcasting COVID-19: A physics-informed estimate for early case incidence. arXiv preprint 2202.00507v1. doi:10.48550/ARXIV.2202.00507.
- Kioutsioukis, I., N. I. Stilianakis, and Y. Drossinos. 2022. A modelling quantification of COVID-19 control strategies. Abstract, 11th International Aerosol Conference, September 4-9, Athens.
- Kormuth, K. A., K. Lin, A. J. Prussin, II, E. P. Vejerano, A. J. Tiwari, S. S. Cox, M. M. Myerburg, S. S. Lakdawala, and L. C. Marr. 2018. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J. Infect. Dis. 218 (5):739–47. doi:10.1093/infdis/jiy221.
- Kudo, E., E. Song, L. J. Yockey, T. Rakib, P. W. Wong, R. J. Homer, and A. Iwasaki. 2019. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. U S A 116 (22):10905–10. doi:10.1073/pnas.1902840116.
- Lednicky, J. A., M. Lauzard, Z. H. Fan, A. Jutla, T. B. Tilly, M. Gangwar, M. Usmani, S. N. Shankar, K. Mohamed, A. Eiguren-Fernandez, et al. 2020. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 100:476–82. doi:10.1016/j.ijid.2020.09.025.
- Lednicky, J. A., M. Lauzardo, M. M. Alam, M. A. Elbadry, C. J. Stephenson, J. C. Gibson, and J. G. Morris. Jr. 2021. Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. Int. J. Infect. Dis. 108:212–6. doi:10.1016/j.ijid.2021.04.063.
- Lewis, D. 2022. Why the WHO took two years to say COVID is airborne. Nature 604 (7904):26–31. doi:10.1038/d41586-022-00925-7.
- Lin, K., C. R. Schulte, and L. C. Marr. 2020. Survival of MS2 and Φ6 viruses in droplets as a function of relative humidity, pH, and salt, protein, and surfactant concentrations. PLoS One. 1512:e0243505. doi:10.1371/journal.pone.0243505.
- Lin, M, and L. C. Marr. 2020. Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environ. Sci. Technol. 54 (2):1024–32. doi:10.1021/acs.est.9b04959.
- Lindsley, W. G., F. M. Blachere, B. F. Law, D. H. Beezhold, and J. D. Noti. 2021. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Sci. Technol. 55 (4):449–57. doi:10.1080/02786826.2020.1862409.
- Lu, J., J. Gu, K. Li, C. Xu, W. Su, Z. Lai, D. Zhou, C. Yu, B. Xu, and Z. Yang. 2020. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 26 (7):1628–31. doi:10.3201/eid2607.200764.
- Luo, B., A. Schaub, I. Glas, L. K. Klein, S. C. David, N. Bluvshtein, K. Violaki, G. Motos, M. Pohl, W. Hugentobler, et al. 2022. Acidity of expiratory aerosols controls the infectivity of airborne influenza virus and SARS-CoV-2. medRxiv preprint doi:10.1101/2022.03.14.22272134.
- Morawska, L. 2006. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 16 (5):335–47. doi:10.1111/j.1600-0668.2006.00432.x.
- Morawska, L, and D. K. Milton. 2020. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 71 (9):2311–3. doi:10.1093/cid/ciaa939.
- Moriyama, M., W. J. Hugentobler, and A. Iwasaki. 2020. Seasonality of respiratory viral infections. Annu. Rev. Virol. 7 (1):83–101. doi:10.1146/annurev-virology-012420-022445.
- Morris, D. H., K. C. Yinda, A. Gamble, F. W. Rossine, Q. Huang, T. Bushmaker, R. J. Fischer, M. J. Matson, N. Van Doremalen, P. J. Vikesland, et al. 2021. Mechanistic theory predicts the effects of temperature and humidity on inactivation of SARS-CoV-2 and other enveloped viruses. eLife 10:e65902. doi:10.7554/eLife.65902.
- Nenes, A. 2021. Personal communication.
- Niazi, S., K. R. Short, R. Groth, L. Cravigan, K. Spann, Z. Ristovski, and G. R. Johnson. 2021. Humidity-dependent survival of an airborne influenza A virus: Practical implications for controlling airborne viruses. Environ. Sci. Technol. Lett. 8 (5):412–8. doi:10.1021/acs.estlett.1c00253.
- Noakes, C. J., C. B. Beggs, P. A. Sleigh, and K. G. Kerr. 2006. Modelling the transmission of airborne infections in enclosed spaces. Epidemiol. Infect. 134 (5):1082–91. doi:10.1017/S0950268806005875.
- Oswin, H. P., A. E. Haddrell, M. Otero-Fernandez, T. A. Cogan, J. F. S. Mann, C. H. Morley, D. J. Hill, A. D. Davidson, A. Finn, R. J. Thomas, et al. 2021. Measuring the stability of virus in aerosols under varying environmental conditions. Aerosol Sci. Technol 55 (12):1315–20. doi:10.1080/02786826.2021.1976718.
- Oswin, H. P., A. E. Haddrell, M. Otero-Fernandez, J. F. Mann, T. A. Cogan, T. Hilditch, J. Tian, D. Hardy, D. J. Hill, A. Finn, et al. 2022. The dynamics of SARS-CoV-2 infectivity with changes in aerosol microenvironment. Proc. Natl. Acad. Sci. (USA) 119 (27):e2200109119. doi:10.1073/pnas.2200109119.
- Peng, Z., A. L. Pineda Rojas, E. Kropff, W. Bahnfleth, G. Buonanno, S. J. Dancer, J. Kurnitski, Y. Li, M. L. C. Loomans, L. C. Marr, et al. 2022. Practical indicators for risk of airborne transmission in shared indoor environments and their application to COVID-19 outbreaks. Environ. Sci. Technol. 56 (2):1125–37. doi:10.1021/acs.est.1c06531. erratum 2022 ibid. 56.
- Randall, K., E. T. Ewing, L. C. Marr, J. L. Jimenez, and L. Bourouiba. 2021. How did we get here: what are droplets and aerosols and how far do they go? A historical perspective on the transmission of respiratory infectious diseases. Interface Focus. 11 (6):20210049. doi:10.1098/rsfs.2021.0049.
- Ratnesar-Shumate, R., K. Bohannon, G. Williams, B. Holland, M. Krause, B. Green, D. Freeburger, and P. Dabisch. 2021. Comparison of the performance of aerosol sampling devices for measuring infectious SARS-CoV-2 aerosols. Aerosol Sci. Technol. 55 (8):975–86. doi:10.1080/02786826.2021.1910137.
- Robinson, M., N. I. Stilianakis, and Y. Drossinos. 2012. Spatial dynamics of airborne infectious diseases. J. Theor. Biol. 297:116–26. doi:10.1016/j.jtbi.2011.12.015.
- Riley, E. C., G. Murphy, and R. L. Riley. 1978. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 107 (5):421–32. doi:10.1093/oxfordjournals.aje.a112560.
- Rudnick, S. N, and D. K. Milton. 2003. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 13 (3):237–45. doi:10.1034/j.1600-0668.2003.00189.x.
- Santarpia, J. L., V. H. Herrera, D. N. Rivera, S. Ratnesar-Shumate, S. P. Reid, P. W. Denton, J. W. S. Martens, Y. Fang, N. Conoan, M. V. Callahan, et al. 2020. The infectious nature of patient-generated SARS-CoV-2 aerosol. medRxiv preprint. doi:10.1101/2020.07.13.20041632.
- Santarpia, J. L., V. L. Herrera, D. N. Rivera, S. Ratnesar-Shumate, P. St, D. N. Reid, P. W. Ackerman, J. W. S. Denton, Y. Martens, N. Fang, et al. 2021. The size and culturability of patient-generated SARS-CoV-2 aerosol. J. Expo. Sci. Environ. Epidemiol 18:1–6. doi:10.1038/s41370-021-00376-8.
- Schaffer, F. L., M. E. Soergel, and D. C. Straube. 1976. Survival of airborne influenza virus: Effects of propagating host, relative humidity, and composition of spray fluids. Arch. Virol. 51 (4):263–73. doi:10.1007/BF01317930.
- Stadnytskyi, V., C. E. Bax, A. Bax, and P. Anfinrud. 2020. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A 117 (22):11875–7. doi:10.1073/pnas.2006874117.
- Stilianakis, N. I, and Y. Drossinos. 2010. Dynamics of infectious disease transmission by inhalable espiratory droplets. J. R Soc. Interface 7 (50):1355–66. doi:10.1098/rsif.2010.0026.
- Tellier, R. 2022. COVID-19: the case for aerosol transmission. Interface Focus. 12 (2):20210072. doi:10.1098/rsfs.2021.0072.
- US CDC update on airborne transmission of SARS-CoV-2 May 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html#anchor_1619805150492.
- Weber, T. P, and N. I. Stilianakis. 2008. Inactivation of influenza A viruses in the environment and modes of transmission: A critical review. J. Infect. 57 (5):361–73. doi:10.1016/j.jinf.2008.08.013.
- Williams, R., N. Rankin, T. Smith, D. Galler, and P. Seakins. 1996. Relationship between the humidity and temperature of inspired gas and the function of the airway mucosa. Crit. Care Med. 24 (11):1920–9. doi:10.1097/00003246-199611000-00025.
- White House Brief. 2022. “Let’s clear the air on COVID”. https://www.whitehouse.gov/ostp/news-updates/2022/03/23/lets-clear-the-air-on-covid/.
- WHO admits SARS-CoV-2 airborne transmission in May 2021. The latest update https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-how-is-it-transmitted.
- Yang, W., S. Elankumaran, and L. C. Marr. 2012. Relationship between humidity and Influenza A viability in droplets and implications for influenza’s seasonality. PLoS One. 7 (10):e46789. doi:10.1371/journal.pone.0046789.
- Zhou, M, and J. Zou. 2021. A dynamical overview of droplets in the transmission of respiratory infectious diseases. Phys Fluids (1994) 33 (3):031301. doi:10.1063/5.0039487.