Abstract
Condensational growth of ultrafine particles under saturation conditions in conjunction with virtual impaction technology has been used recently as a method to concentrate ultrafine particles for conducting inhalation toxicological studies. The Harvard Ultrafine Concentrated Ambient Particle System (HUCAPS) was challenged with a variety of artificially generated aerosols of different chemistry, solubility, and hygroscopicity as well as by ambient-origin ultrafine particles. The system's ability to concentrate these different particles was evaluated for a wide range of saturation ratios. It was found that hygroscopic particles grow and are concentrated more efficiently than hydrophobic ones. The effect of aerosol-hygroscopicity and water solubility on the overall aerosol concentration enrichment factor diminishes with increasing saturation ratios. Results indicated that a saturation ratio higher than that predicted by theory, with a value of about 3, is needed to assure that all particles grow and are efficiently concentrated by the same enrichment factor, regardless of particle hygroscopicity and solubility. Under such an optimum saturation ratio, the performance of the HUCAPS was evaluated using ambient-origin ultrafine particles. The results show that the HUCAPS concentrates ambient ultrafine particles by a factor of 40–50 with a little size distortion. Its overall low-pressure drop (2.2 kPa) and its high concentration enrichment factor make the HUCAPS a versatile device suitable for in vitro and in vivo inhalation toxicological studies.
INTRODUCTION
Many epidemiological studies have linked particulate matter (PM) and especially its PM2.5 fraction (dp[aerodynamic diameter] < 2.5 μm) to respiratory and cardiovascular adverse health effects, including premature mortality, respiratory and cardiovascular disease, exacerbation of asthma, decreased lung function, and increased risk of myocardial infraction (CitationSchwartz and Dockery 1992; CitationDockery et al. 1993; CitationDockery and Pope 1994; CitationPeters et al. 2000; CitationGamble et al. 1998; CitationPope et al. 1999).
Ultrafine particles (dp < 0.1 μm) are formed through gas-to-particle conversion mechanisms. Ultrafine particles are associated both with natural and anthropogenic sources (CitationWhitby and Svendrup 1980). Due to phase transformation processes that become more enhanced as particle size decreases, ultrafine particle sizes change continuously (CitationSeinfeld 1991). Several studies exploring the chemical composition of ambient ultrafine particles in both urban and rural settings have been reported. Earlier studies have indicated that typically 50–70% of ultrafine mass consists of carbonaceous (mostly organic) materials (CitationPuxbaum and Wopenka 1984; CitationBerner et al. 1996). CitationCass et al. (2000) also reported results from measurements of ultrafine particle mass concentration made in seven Southern California cities. The chemical composition of these ultrafine particle samples averaged 50% organic compounds, 14% trace metal oxides, 8.7% elemental carbon, 8.2% sulphate, 6.8% nitrate, and 3.7% ammonium ion.
Recent toxicological studies have suggested that ultrafine particles may play an important role in explaining the underlying biological mechanisms for the adverse health effects of PM2.5. Inhaled ultrafine particles deposit in the respiratory tract almost exclusively by diffusional mechanisms (CitationICRP 1994). Inhalation of fumes, consisting mainly of ultrafine particles, lead to the well-known effects of metal or polymer fume fever (CitationDrinker et al. 1927; CitationGordon et al. 1992). Scientists have attributed the greater pulmonary effects of ultrafine particles, compared to larger submicrometer particles, to their larger specific surface area, the greater interstitial access, and their altered biopersistence (CitationOberdorster et al. 1994; CitationJohnston et al. 2000). In addition, acids or catalytic metals on the surfaces of irritant particles can be more efficiently transferred deeper in the lungs by ultrafines than by an equal mass of larger particles. Finally, investigators have suggested that trace metals distributed widely throughout the lung by ultrafine particles could catalyze the formation of oxidants within the lung and cause tissue damage (CitationDreher et al. 1997).
Laboratory animal inhalation (in vivo) toxicological studies using artificial aerosols of various sizes have demonstrated moderate or no effects, even at concentrations much higher than those typically found in ambient air (CitationAvol et al. 1988; CitationHackney et al. 1989; CitationAnderson et al. 1992). This is inconsistent with results from epidemiological studies that have shown adverse health effects even at PM concentration levels below the national ambient air quality standards. This discordance between laboratory and epidemiological studies may be explained by the reliance of the laboratory studies on controlled-chamber exposures to single chemical component particles or simple mixtures of a few components. Such artificial particles do not adequately represent the heterogeneous mixture of ambient particles. The various components of ambient particles may also synergistically interact to produce toxic health effects, not seen with artificially generated atmospheres. It was also shown that the oxidative stress of real particles differs significantly from that of the artificially generated particles (CitationLippmann 1989). Despite the tremendous progress, which has been made over the last few years in terms of revealing biological mechanisms of PM, there is a great need to do inhalation exposure studies using ambient ultrafine particles.
Ambient particle concentrators based on virtual impaction technology have been used successfully during recent years to conduct in vivo animal and human inhalation exposures to concentrated accumulation-mode (PM0.1−2.5) and coarse-mode particles (PM2.5−10) (CitationSioutas et al. 1995; CitationGodleski et al. 1996; CitationClarke et al. 2000; CitationDemokritou et al. 2003). However, virtual (inertial) impaction technology cannot be applied to separate and concentrate ultrafine particles because adequate inertia cannot be induced without supersonic flows and excessive pressure drops. Subjecting the ultrafine particles to such a substantial vacuum will increase volatilization of labile species and change the chemical and toxicological characteristics of the particles. In addition, such a substantial vacuum at the chamber side is prohibited for inhalation studies.
Recently, condensational growth of ultrafine particles using water vapor as a condensing medium in conjunction with virtual impaction technology has been used as a method to concentrate ultrafine particles (CitationSioutas et al. 1999; CitationKim et al. 2000; CitationDemokritou et al. 2002; CitationGupta et al. 2004). Subsequently, a thermal restoration method can be used to remove the excess water from the grown droplets and return the particles to their original ultrafine particle size mode (CitationDemokritou et al. 2002; CitationGupta et al. 2004).
Condensational growth of particles is a well-known atmospheric phenomenon responsible for cloud formation. Various theoretical and experimental studies have investigated the nucleating ability of inorganic salt particles and carbonaceous combustion residues like diesel soot particles for typical atmospheric conditions. Their results have revealed that inorganic hygroscopic particles like NaCl and (NH4)2SO4 show significant growth even at subsaturation (RH < 100%) conditions, whereas hydrophobic particles (such as soot particles) show growth only at much higher saturation ratio (CitationNiessner et al. 1990; CitationFinlayson-Pitts and Pitts 1986). However, aging of the hydrophobic particles in the atmosphere can lead to an increase in the particle hygroscopicity. This remarkably improved nucleation property of the aged aerosol was shown in many atmospheric studies (CitationLammel and Novakov 1995; CitationKoutrakis et al. 1989; CitationKotzick et al. 1997; CitationWeingartner et al. 1997; CitationCruz and Pandis 1997).
In this article, we investigate the effect of physicochemical properties of the ultrafine particles on the condensational growth and concentration for the unique simulated atmospheric conditions of an ultrafine particle concentrator. In such a system, an ultrafine particle is subjected to water vapor saturation, growth, concentration, and thermal removal of the excess water in a matter of a few seconds. These unique conditions are significantly different, in terms of aerosol residence time, from those occurring in the natural atmosphere. It is also important for us to determine the optimum saturation ratio for the Harvard ultrafine concentrator, at which all ultrafine particles regardless of their hygroscopicity are grown and concentrated by the same enrichment factor.
METHODS
Description of the System
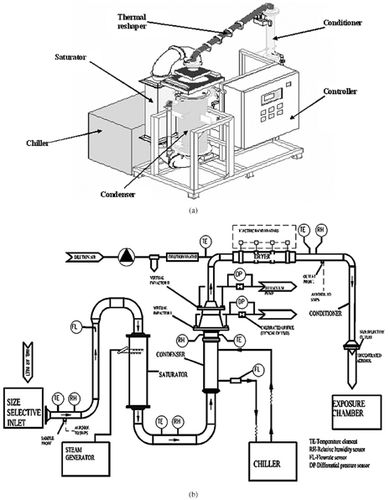
A schematic diagram of the Harvard ultrafine concentrated ambient particle system (HUCAPS) is shown in . It consists of the following basic components: (1) size-selective device, (2) condensational growth unit, (3) series of two virtual impactors, and (4) thermal dilution-dryer section. The system operates with a 5000 lpm input flow and delivers 58 lpm of concentrated aerosol (CitationGupta et al. 2004).
The size-selective device is a high-volume (5000 lpm), two-stage inertial impactor, which overall removes particles larger than 2.5 μm from the incoming aerosol stream (). The condensational growth unit is comprised of a saturator and a condenser. The steam injection tube is located inside the saturator, near its entrance. As the air passes by, steam is injected and mixed with the air. Steam is generated using a fully modulated, electric humidifier. The condenser is a shell-and-tube, coolant-to-air heat exchanger, with the air flowing through the tubes and the coolant (glycol–water solution) through the shell side. There is an external primary refrigeration unit (chiller), which is used to cool the secondary coolant (glycol–water solution).
Condensationally grown particles are subsequently drawn through a series of two virtual impactors to increase their concentration by 40–50 times (Stages I and II; ). Both virtual impactors have a cutpoint of 1.0 μm (CitationDemokritou et al. 2003; CitationGupta et al. 2004). A thermal restoration method is used to restore the original size distribution of the concentrated aerosol by removing the excess water from the particle droplets. This thermal restoration method has been shown to remove the excess water from the grown particles adequately (CitationDemokritou et al. 2002). According to this method, the concentrated aerosol (at a flow of 50 lpm) is first diluted with a small volume (8 lpm) of particle-free air and is then heated up to 90°F (). Thus the overall concentrated aerosol output flow from HUCAPS is 58 lpm. The concentrated stream of aerosol emerging from HUCAPS is then passed through a size-selective outlet before it can be supplied to the inhalation chambers. The size-selective outlet is a conventional inertial impactor designed to remove accumulation-mode particles (2.5 μm > dp > 0.15 μm). HUCAPS is fully automated with all operational parameters, including relative humidity, aerosol temperature, refrigerant temperature, and volumetric flow in every component of the system controlled within acceptable tolerances by feedback devices. A detailed description of the performance characterization of the HUCAPS can be found elsewhere (CitationGupta et al. 2004).
Experimental Setup
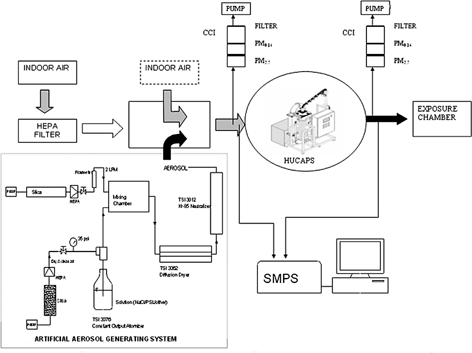
shows the basic experimental setup used to conduct a series of performance evaluation tests on the HUCAPS. Polydisperse aerosol (nominal size 10–400 nm) was generated from aqueous solution/suspension using a constant output atomizer (nanoparticle generator, model 3076 TSI Inc., St. Paul, MN, USA). After passing through a Kr85 charge neutralizer to bring charge distribution to the Boltzmann equilibrium, the aerosol was introduced into the duct where it was mixed with High Efficiency Particulate Air (HEPA)-filtered room air. The duct was used to achieve complete aerosol mixing prior to entry into the HUCAPS. Aerosols with different chemical composition and hygroscopicity were generated presents the details of all artificial aerosols used in these experiments.
TABLE 1 Salient features of different aerosols used to challenge the HUCAPS
Particle number concentration and size distribution were measured upstream and downstream of the HUCAPS (alternating sequentially) using a scanning mobility particle sizer (SMPS; TSI Inc., St. Paul, MN, USA) to evaluate the system's ability to increase the concentration and restore the size distribution of the ultrafine particles. In addition to the real-time measurements, ultrafine particles were sampled using two identical compact cascade impactors (CCI; CitationDemokritou et al. 2004) upstream and downstream of the ultrafine particle concentrator for gravimetric measurements. The 30 lpm CCIs consisted of two impaction stages, a PM2.5 and a PM0.16, for removing particles above 2.5 μm and between 2.5–0.16 μm, respectively. Polyurethane foam (PUF) substrates were used for impaction, whereas particles smaller than 0.16 μm were collected on a 47 mm backup Teflon membrane filter (TEFLO w/ring; Pall Lifesciences, Ann-Arbor, MI, USA). The pressure drops across the two impaction stages and the backup filter were 0.06, 5.73, and 3.3 kPa, respectively (CitationDemokritou et al. 2004).
Saturation Ratio and Growth as a Function of Physicochemical Properties of Aerosol
The Sr (defined as the ratio of vapor pressure to the saturation vapor pressure) is expected to play an important role on the activation and growth of ultrafine particles (CitationHinds 1999). In order for the ultrafine particles to be uniformly concentrated, they all have to grow regardless of their size, hygroscopicity, and chemical composition to sizes well above the cutpoint of the virtual impactors. A selective growth of certain particles based on their physicochemical properties will result in a distorted size distribution of the concentrated aerosol, which may potentially affect its toxicological characteristics. In order to address this issue, the saturation conditions inside HUCAPS were varied and the system's ability to grow and concentrate ultrafine particles was evaluated for each type of aerosol. A saturation ratio up to 3.5 was obtained by adjusting the aerosol temperatures in the saturator and the supersaturator (condenser).
The minimum (optimum) Sr under which all particles, regardless of hygroscopicity and chemistry, grew and concentrated the same was also experimentally determined for the system.
HUCAPS Performance Evaluation under Optimum Saturation Conditions
Using the optimum value of Sr as it was found from the above experiments, more tests were performed to evaluate performance of the HUCAPS with the same set of artificial particles and indoor air. The concentration enrichment factor (CEF), defined as the ratio of the outlet (downstream) to sample (upstream) aerosol number concentration, was determined using an SMPS for these experiments.
In addition, ultrafine particle mass collected on Teflon filters was measured using the compact cascade impactors. At least two sets of mass measurements were taken for each type of artificial aerosol. The sampling durations for the mass measurements with ammonium sulphate, sodium chloride, and potassium sulphate were 8 h, 4 h, and 12 h, respectively. For indoor air, four sets of mass measurements were taken. Since, the typical indoor ultrafine aerosol concentrations were only about 1 μg/m3, to collect enough mass during each set of measurements the system was operated for a total duration of 24 h. At least 10% of the Teflon filters were used as procedural blanks for both of the samplers. Teflon filters were pre- and postweighed after 48 h temperature (21± 2°C) and humidity (40 ± 5%) equilibration in a room having controlled environmental conditions. An electronic microbalance (Mettler-Toledo model MT-5) was used for weighing. The results from these measurements were used to calculate the overall aerosol concentration enrichment factors based on mass concentrations for each type of aerosol.
Filter Reflectance Analysis for Black Carbon
Reflectance analysis was performed on the Teflon filters for the indoor air sampling experiments to obtain concentration levels for the least hygroscopic constituent of indoor air, the black carbon (BC).
Light absorption by particles is almost exclusively caused by elemental carbon (also called black or graphitic carbon; CitationHorvath 1989). Prior work (CitationEdwards et al. 1983; CitationKinney et al. 2000) demonstrated that filter reflectance can be a good proxy for BC concentrations for outdoor air samples. For example, in CitationKinney et al. (2000) reflectance measurements on Teflon filters and BC analyses on colocated quartz fiber filters using the thermal desorption method (CitationBirch and Cary 1996), resulted in a high correlation coefficient (r = 0.95). Reflectance measurements were made inside a class-100 flow bench using an EEL smoke stain reflectometer (Model 43D by Diffusion Systems Ltd, London, UK). During the reflectance analysis process, the filter holder only touched the outer plastic support ring, holding the filter in a fixed, flat geometry. This enabled reflectance measurements to be made without a significant risk of contaminating the filter for later chemical analysis. Reflectance measurements are expressed as the absorption coefficient (Abs*) in reciprocal meters. Absorption coefficient was calculated based on the reflectance, volume of air sampled, and the active area of the filter (CitationKinney et al. 2000).
A useful parameter for combining the optical absorption and chemical composition of atmospheric aerosols is specific absorption, defined as the ratio of absorption coefficient to the mass concentration of a particular chemical species (analogous to molar absorptivity). The average specific absorption for BC obtained for different studies ranges between 8 and 12 m2/g (CitationEdwards et al. 1983; CitationKinney et al. 2000). Therefore, an average specific absorption of 10 m2/g was used to estimate BC mass concentration from measurements of the absorption coefficient for the current study.
RESULTS AND DISCUSSION
Saturation Ratio and Particle Growth as a Function of Physicochemical Properties of Aerosol
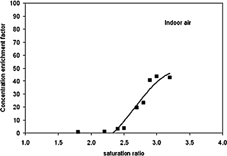
summarizes the results on the effect of the Sr on the concentration of ultrafine particles for aerosols with different hygroscopicity and chemical characteristics. It is evident that all of the measured aerosol concentration enrichment factors do not achieve their maximum value at Sr ∼ 2.0–2.5 predicted by theory based on the Kelvin's equation (CitationHinds 1999; CitationSioutas et al. 1999; CitationDemokritou et al. 2002). This is an indication that at such a theoretical sr, not all of the particles activated and grew above the 1.0 μm cutpoint size of the virtual impactors. One explanation for this discrepancy may be that inside the multitube supersaturator (condenser), water vapor condenses not only on the ultrafine particles but also on the walls of the heat exchanger. This competing mechanism may significantly lower the availability of water vapor for droplet growth, thus requiring a higher-than-theoretical saturation ratio for the particles to grow for this simulated atmospheric process. (e.g., for an upstream aerosol concentration of about 1300 particles/cm3, total particle surface area available for condensation is ∼1.2 × 10−6 m2 compared to the total surface area ∼ 9.4 m2 of the condenser tubes)
TABLE 2 Effect of saturation ratio on the concentration enrichment factor as a function of aerosol hygroscopicity
At lower Sr, a preferential particle growth due to hygroscopic differences is suggested (). However, the effect of particle-hygroscopicity on the overall CEF diminishes with increasing saturation ratio. A similar finding has been shown by atmospheric studies, suggesting that hydrophobic particles such as soot particles need a much higher Sr than that predicted by theory to initiate growth (CitationKotzick et al. 1997). It is obvious from the results presented in that a minimum Sr (∼ 3.0) is necessary to ensure that particles grow, regardless of their hygroscopic properties, to satisfactory sizes and are subsequently concentrated by similar enrichment factors by the ultrafine particle concentrator. It is important to note that in our study Sr is calculated based on temperature and relative humidity (RH) conditions at the entrance and exit of the condenser. However, the actual Sr at different points inside the condenser (radial and longitudinal gradient), may differ due to differences in temperature and water vapor concentration (CitationZhang and Liu 1990).
The above results also agree with those from previous studies exploring the hygroscopic properties of atmospheric aerosols at different urban settings. CitationSaxena et al. (1995) suggested that the approach to droplet equilibrium when the Sr is increased is expected to be rapid for pure salts or mixtures of hygroscopic compounds, whereas it may be much slower for aerosols containing hydrophobic components. For example, if an ultrafine particle contains an organic film or coating, that film may impede the transport of water across the particle surface, thus increasing the time required to achieve equilibrium between a droplet and its surroundings. For the unique atmospheric conditions simulated in the ultrafine particle concentrator, aerosol residence time in the supersaturator (condenser) where the growth takes place is approximately 0.6 s and is significantly smaller than the typical residence time, that occurs during cloud formation. This extremely short residence time, during which particle growth occurs in the concentrator, may be an additional factor that explains why higher-than-theoretical Sr are needed to achieve maximum CEFs.
shows the CEF as a function of saturation ratio for indoor air. The indoor air used for these experiments has ultrafine particles predominantly of outdoor origin and is a complex heterogeneous mixture of particles possessing a wide range of morphological, chemical, physical, and thermodynamic properties. The experimental points on the curve in represent the average data from a single day; however, the size distribution and total ultrafine number concentration varies from hour to hour and is different on different days. Nonetheless, data from other days than the one reported in this figure showed the same pattern of CEF versus Sr. This consistent behavior indicates that the growth pattern of the indoor particles within the concentrator is relatively independent of the day-to-day variation in particle properties of indoor air.
The maximum CEFs of 40–50 times is obtained at Sr of approximately 3.0. It is important to note that these CEFs were the values obtained after diluting the 50 lpm output of the virtual impactors with a small amount of particle-free air (8 lpm) as part of the thermal reshaping process. Therefore, the original CEFs from the HUCAPS before dilution were actually higher by a factor of about 15%. Increasing the Sr to any higher value did not result in an increase in the CEFs. Also, the actual CEFs are lower than the ideal CEF (100 times, based on the nominal flow ratios) due to the expected levels of particle losses within the two virtual impactor stages. However, as will be visible later in this text, these particle losses were found to be constant for the entire particle size range (< 300 nm) of interest. The details of the performance evaluation tests and results for the two virtual impactor stages can be found elsewhere (CitationDemokritou et al. 2003; CitationGupta et al. 2004).
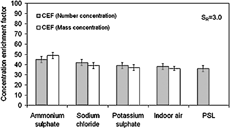
summarizes the comparison of CEFs for particle number and mass for each type of artificially generated test aerosol and indoor air for the optimum saturation ratio of 3.0. The excellent agreement between the gravimetric and SMPS measurement provides strong validation of these independent CEF measurement techniques. Due to very low aerosol mass concentrations, no mass measurements were performed for PSL particles. For this study, PSL particles were used to simulate the overall concentration enrichment for hydrophobic carbonaceous particles. However, use of surfactant does alter the true hygroscopicity of the pure PSL particles and makes them less hydrophobic. It is also true that in a natural environment it is rare to find a truly hydrophobic carbon particle. The hydrophobic carbonaceous particles (like soot) are always loaded with some polar (as well as some nonpolar) organics and trace metal oxides that significantly alter their true hygroscopicity.
Filter Reflectance Analysis for Elemental Carbon
shows the result from reflectance analysis on the aerosol mass collected on the Teflon filters. Despite the uncertainty involved with the assumption of high degree of correlation between the measured absorption coefficient and actual BC mass concentration, the CEFs based on elemental carbon mass concentration showed good agreement with the CEFs based on the total particulate mass collected (). This further indicates that, at the optimum Sr = 3.0, the least-hygroscopic component of indoor air aerosol, BC, was concentrated by the same high enrichment factor as the rest of the aerosol components. This is one more confirmation that the HUCAPS maintains the integrity of the ultrafine particles and is therefore appropriate for concentration of ambient ultrafine particles for inhalation toxicological studies.
TABLE 3 Results from filter reflectance analysis for BC on the aerosol mass collected
Restoration of Aerosol Size Distribution
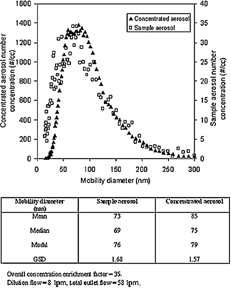
It is also important to maintain the same size distribution between sample and concentrated ultrafine aerosol for inhalation toxicological studies. shows the representative size distributions for both the sample and concentrated aerosols at Sr = 3.0 for indoor air. The mean, median, and modal diameters for sample and concentrated aerosols agreed reasonably well. This is an indication that the thermal restoration method satisfactorily restored the size distribution of the concentrated aerosol. The small distortion of the size distribution suggests that physicochemical properties of ultrafine particles do not influence the HUCAPS performance significantly. If there had been any substantial preferential concentration enrichment of particles with a specific chemical composition, there could have been a significant distortion of the aerosol size distribution. In the near future, experiments will be performed to investigate whether there are any serious chemical transformations (aerosol surface or bulk) occurring during the aerosol growth, enrichment, and restoration processes. Specifically, semivolatile components (such as lower molecular weight PAHs) of the aerosols will be of special interest with respect to any potential changes in particle toxicity.
CONCLUSIONS
Condensational growth of ultrafine particles under saturation conditions in conjunction with virtual impaction technology has been recently used as a method to concentrate ultrafine particles for inhalation toxicological studies. In the present study, the effect of particle hygroscopicity, on the overall concentration enrichment was experimentally evaluated. The results show that hygroscopicity of ultrafine particles significantly affects the final droplet size to which particles grow and hence the overall CEFs. Hygroscopic particles grow and are concentrated more efficiently than those that are hydrophobic. It is necessary to use a Sr of 3.0, which is higher than that predicted by theory, to achieve uniform condensational growth that is independent of particle hygroscopicity. This is particularly important because typically about 50–70% of atmospheric ultrafine mass consists of hydrophobic carbonaceous materials. Finally, the HUCAPS concentrates ambient ultrafine particles by a factor of 40–50 times with small distortion between sample and concentrated aerosol size distributions. The overall low-pressure drop (2.2 kPa) and high CEF make the HUCAPS a versatile device suitable for in vitro and in vivo inhalation toxicological studies.
Acknowledgments
This study was supported by NIEHS HARVARD-CENTER grant No. ES 0002. Also, we would like to thank Dr. Michael Wolfson and Stephen Ferguson for their helpful comments and technical support.
Notes
*Relative humidity value at which phase transfer into liquid form takes place.
*The concentration enrichment data correspond to averages of at least three measurements.
REFERENCES
- Anderson , K. R. , Avol , E. L. , Edwards , S. A. , Shamoo , D. A. , Peng , R. C. , Linn , W. S. and Hackney , J. D. 1992 . Controlled Exposures of Volunteers to Respirable Carbon and Sulfuric Acid Aerosols . J. Air Waste Manag. Assoc. , 42 : 437 – 442 .
- Avol , E. L. , Linn , W. S. , Whynot , J. D. , Anderson , K. R. , Shamoo , D. A. , Valencia , L. M. , Little , D. E. and Hackney , J. D. 1988 . Respiratory Dose-response Study of Normal and Asthmatic Volunteers Exposed to Sulfuric Acid Aerosol in the Submicrometer Range . Toxicol. Ind. Health , 4 : 173 – 184 .
- Berner , A. , Sidla , S. , Galambos , Z. , Kruisz , C. , Hitzenberger , R. , ten Brink , H. M. and Kos , G. P. A. 1996 . Modal Character of Atmospheric Black Carbon Size Distributions . J. Geophys. Res. , 101 : 19559 – 19565 .
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . Aerosol. Sci. Technol. , 25 : 221 – 241 .
- Cass , G. R. , Hughes , L. A. , Bhave , P. , Kleeman , M. J. , Allen , J. O. and Salmon , L. G. 1775 . The Chemical Composition of Atmospheric Ultrafine Particles, . Philos. Trans. Royal Soc. London Series A-Math. Phy. Eng. Sci. , 358 : 2581 – 2592 .
- Clarke , R. W. , Coull , B. , Reinisch , U. , Catalano , P. , Killingsworth , C. , Koutrakis , P. , Kavouras , I. , Murthy , G. G. K. , Lawrence , J. , Lovett , E. , Wolfson , M. , Verrier , R. and Godleski , J. 2000 . Inhaled Concentrated Ambient Particles Induce Pulmonary Inflammation and Hematological Changes in Canines . Environ. Health Persp. , 108 : 1179 – 1187 .
- Cruz , C. N. and Pandis , S. N. 1997 . A Study of the Ability of Pure Secondary Organic Aerosol to Act as Cloud Condensation Nuclei . Atmos. Environ. , 31 : 2205 – 2214 .
- Demokritou , P. , Gupta , T. , Ferguson , S. and Koutrakis , P. 2003 . Development of a High-volume Concentrated Ambient Particles System (CAPS) for Human and Animal Inhalation Toxicological Studies . Inhalat. Toxicol. , 15 : 111 – 129 .
- Demokritou , P. , Lee , S. J. , Ferguson , S. and Koutrakis , P. 2004 . A Compact Multistage (Cascade) Impactor for the Characterization of Atmospheric Aerosols . J. Aerosol Sci. , 35 : 281 – 299 .
- Demokritou , P. , Gupta , T. and Koutrakis , P. 2002 . A High Volume Apparatus for the Condensational Growth of Ultrafine Particles for Inhalation Toxicological Studies . Aerosol. Sci. Technol. , 36 : 1061 – 1072 .
- Dockery , D. W. and Pope , C. A. 1994 . Acute Effects of Particulate Air Pollution . Ann. Rev. Pub. Health , 15 : 107 – 132 .
- Dockery , D. W. , Pope , C. A. , Xu. , X. , Spengler , J. D. , Ware , J. H. , Fay , M. E. , Ferris , B. G. and Speizer , F. E. 1993 . An Association Between Air Pollution and Mortality in Six U.S. Cities . New Eng. J. Med. , 329 : 1753 – 1808 .
- Dreher , K. L. , Jaskot , R. H. , Lehmann , J. R. , Richards , J. H. , McGee , J. K. , Ghio , A. J. and Costa , D. L. 1997 . Soluble Transition Metals Mediate Residual Oil Fly Ash Induced Acute Lung Injury . J. Toxicol. Environ. Health , 50 : 285 – 305 .
- Drinker , P. , Thomson , R. M. and Finn , J. L. 1927 . Metal Fume Fever II: Resistance Acquired by Inhalation of Zinc Oxide on Two Successive Days . J. Ind. Hyg. Toxicol. , 9 : 98 – 105 .
- Edwards , J. D. , Ogren , J. A. , Weiss , R. E. and Charlson , R. J. 1983 . Particulate Air Pollutants: A Comparison of British “Smoke” with Optical Absorption Coefficient and Elemental Carbon Concentration . Atmos. Environ. , 17 : 2337 – 2341 .
- Finlayson-Pitts , B. J. and Pitts , J. N. 1986 . Atmospheric Chemistry: Fundamentals and Experimental Techniques , New York : Wiley .
- Gamble , J. 1998 . PM2.5 and Mortality in Long Term Prospective Cohort Studies: Cause Effects or Statistical Associations? . Environ. Health Perspect. , 106 ( 9 ) : 535 – 549 .
- Godleski , J. , Sioutas , C. , Katler , M. and Koutrakis , P. 1996 . Death from Inhalation of Concentrated Ambient Air Particles in Animal Models of Pulmonary Disease, . Resp. Crit. Care Med. , 155 ( 4 ) : A246
- Gordon , T. , Chen , L. C. , Fine , J. M. , Schleisinger , R. B. , Su , W. Y. , Kimmel , T. A. and Amdur , M. O. 1992 . Pulmonary Effects of Zinc Oxide in Human Subjects, Guinea Pigs, Rats, and Rabbits . Am. Ind. Hyg. Assoc. J. , 53 : 503 – 509 .
- Gupta , T. , Demokritou , P. and Koutrakis , P. 2004 . Development and Performance Evaluation of a High Volume Ultrafine Particle Concentrator for Inhalation Toxicological Studies . Inhal. Toxicol. , 16 : 1 – 12 .
- Hackney , J. D. , Linn , W. S. and Avol , E. L. 1989 . Acid Fog: Effects on Respiratory Function and Symptoms in Healthy and Asthmatic Volunteers . Environ. Health Perspect. , 79 : 159 – 162 .
- Hinds , W. 1999 . Aerosol Technology , New York : John Wiley & Sons Inc. .
- Horvath , H. and Habenreich , T. A. H. 1989 . The Absorption Coefficient of the Vienna Aerosol: Comparison of Two Methods . Aerosol Sci. Technol. , 10 : 506 – 514 .
- International Commission on Radiological Protection (ICRP) . 1994 . Human Respiratory Tract Model for Radiological Protection: A Report of Committee 2 of the ICRP , Oxford : Pergamon Press .
- Johnston , C. J. , Finkelstein , J. N. , Mercer , P. , Corson , N. , Gelein , R. and Oberdorster , G. 2000 . Pulmonary Effects Induced by Ultrafine PTFE Particles . Toxicol. Appl. Pharmacol. , 168 : 208 – 215 .
- Kim , S. , Chang , M. C. , Kim , D. and Sioutas , C. 2000 . A New Generation of Portable Coarse, Fine and Ultrafine Particle Concentrators for Use in Inhalation Toxicology, . Inhal. Toxicol. , 12 ( suppl. ) : 121 – 137 .
- Kinney , P. L. , Aggarwal , M. , Northridge , M. E. , Janssen , N. A. H. and Shepard , P. 2000 . Airborne Concentrations of PM2.5 and Diesel Exhaust Particles on Harlem Sidewalks: A Community-based Pilot Study . Environ. Health Persp. , 108 : 213 – 218 .
- Koutrakis , P. , Wolfson , J. M. , Spengler , J. D. , Stern , B. and Franklin , C. A. 1989 . Equilibrium Size of Atmospheric Aerosol Sulfates as a Function of the Relative Humidity . J. Geophys. Res. , 94 : 6442 – 6448 .
- Kotzick , R. , Panne , U. and Niessner , R. 1997 . Changes in Condensation Properties of Ultrafine Carbon Particles Subjected to Oxidation by Ozone, . J. Aerosol Sci. , 28 : 725 – 735 .
- Lammel , G. and Novakov , T. 1995 . Water Nucleation Properties of Carbon Black and Diesel Soot Particles . Atmos. Environ. , 29 : 813 – 823 .
- Lippmann , M. 1989 . Progress, Prospects, and Research Needs on the Health Effects of Acid Aerosols . Environ. Health Perspect. , 79 : 203 – 205 .
- Niessner , R. , Daeumer , B. and Klockow , D. 1990 . Investigation of Surface Properties of Ultrafine Particles by Application of a Multistep Condensation Nucleus Counter . Aerosol Sci. Technol. , 12 : 953 – 963 .
- Oberdorster , G. , Ferin , J. and Lehnert , B. E. 1994 . Correlation Between Particle Size, in vivo Particle Persistence and Lung Injury . Environ. Health Perspect. , 102 : 173 – 179 .
- Peters , A. , Verrier , R. L. , Schwartz , J. , Gold , D. R. , Mittleman , M. , Baliff , J. , Oh , J. A. , Allen , G. , Monahan , K. and Dockery , D. W. 2000 . Air Pollution and Incidence of Cardiac Arrythmia . Epidemiology , 11 : 11 – 17 .
- Pope , C. A. , Verrier , R. L. , Lovett , E. G. , Larson , A. C. , Raizenne , M. E. , Kanner , R. E. , Schwartz , J. , Villegas , M. , Gold , D. R. and Dockery , D. W. 1999 . Heart Rate Variability Associated with Particulate Air Pollution . Amer. Heart. J. , 138 : 890 – 899 .
- Puxbaum , H. and Wopenka , B. 1984 . Chemical Composition of Nucleation and Accumulation Mode Particles Collected in Vienna, Austria . Atmos. Environ. , 18 : 573 – 580 .
- Saxena , P. , Hildemann , L. M. , McMurry , P. H. and Seinfeld , J. H. 1995 . Organics Alter Hygroscopic Behavior of Atmospheric Particles . J. Geophys. Res. , 100 : 188755 – 18770 .
- Schwartz , J. and Dockery , D. W. 1992 . Increased Mortality in Philadelphia Associated with Daily air Pollution Concentrations . Am. J. Epidemiol. , 135 : 12 – 19 .
- Seinfeld , J. H. 1991 . Atmospheric Chemistry and Physics of Air Pollution , New York : Wiley .
- Sioutas , C. , Kim , S. and Chang , M. 1999 . Development and Evaluation of a Prototype Ultrafine Particle Concentrator . J. Aerosol Sci. , 30 : 1001 – 1017 .
- Sioutas , C. , Koutrakis , P. and Burton , R. M. 1995 . A Technique to Expose Animals to Concentrated Fine Ambient Aerosols, . Environ. Health Perspect. , 103 : 172 – 177 .
- Weingartner , E. , Burtscher , H. and Baltensperger , U. 1997 . Hygroscopic Properties of Carbon and Diesel Soot Particles . Atmos. Environ. , 31 : 2311 – 2327 .
- Whitby , K. T. and Svendrup , G. M. 1980 . California Aerosols: Their Physical and Chemical Characteristics . Environ. Sci. Technol. , 10 : 477
- Zhang , Z. Q. and Liu , B. Y. H. 1990 . Dependence of the Performance of TSI 3020 Condensation Nucleus Counter on Pressure, Flow Rate, and Temperature . Aerosol Sci. Technol. , 13 : 493 – 504 .