Abstract
Carbon aerosol measurements from the Rupprecht & Patashnick Series 5400 carbon analyzer, the Magee Scientific AE-21 Aethalometer and a filter-based Andersen RAAS2.5-410 Chemical Speciation sampler with quartz filters analyzed by Thermal Optical Transmittance (TOT) were compared. The Series 5400 total carbon (TC) and organic carbon (OC) were moderately correlated (r = 0.64 and 0.67) with the RAAS TOT method and the elemental carbon (EC) was poorly correlated (r = 0.37). The 5400 underestimated the RAAS TC and OC by 64% and 78%, respectively. The underestimate is attributed in part to the positive OC artifact of the filter-based sampling method. Another difference between the 5400 measurements and the RAAS TOT is a correction for char. The lack of correction for any char that occurs in the 5400 could cause and underestimate of OC and an overestimate of EC. The 5400 overestimated RAAS EC by 89%. The Aethalometer black carbon (BC) was compared to the 5400 EC and the RAAS TOT EC measured. The Aethalometer BC correlated well (r = 0.86) with the RAAS EC, but the Aethalometer overestimated the RAAS EC by 30%. The 5400 EC was compared to Aethalometer BC both with and without a PM2.5 size selective inlet. The correlations were 0.92 (inlet) and 0.55 (no inlet). The 5400 overestimated the mean Aethalometer BC by 17% with the inlet and 39% without. The improvement in results may not be due to the addition of the PM2.5 inlet, but instead may be due to a difference in the amount of char formed by the 5400. Factors contributing to the differences in these results are discussed.
INTRODUCTION
A national monitoring program was implemented by the United States Environmental Protection Agency (USEPA) to support the PM2.5 (fine particulate matter) National Ambient Air Quality Standard (NAAQS) for gravimetric mass in 1997 (62 FR 38763). PM2.5 is the mass of particles that have an aerodynamic size of ≤ 2.5 μ m. The NAAQS was put in place to protect public health and the environment from excessive levels of PM2.5 mass. To support PM2.5 NAAQS monitoring efforts, a chemical speciation trends network (STN) was implemented to characterize the primary chemical components of mass in urban areas and provide data for trends across the U.S. Results from the STN and other PM speciation studies have shown carbon to be a major constituent of PM2.5 mass in many parts of the U.S. and may account for as much as 50% of the total fine particle mass in the atmosphere (CitationGray and Cass 1998; CitationTolocka et al. 2001; CitationUSEPA 2002). The carbon fraction consists of elemental carbon (EC), organic carbon (OC), and carbonate carbon. The inorganic carbon fraction or carbonate carbon found in minerals is assumed to be a very small component of the PM2.5 carbon mass and is not routinely measured (CitationChow and Watson 2002; CitationLim et al. 2003). EC is a primary emission from incomplete combustion of fossil fuels and biomass burning and serves as a tracer for combustion-derived particles. Particulate OC has both primary and secondary emission sources. Primary emissions originate from combustion, chemical, fossil fuel, and biogenic sources, whereas OC is secondarily formed from gas-to-particle transformation of anthropogenic and biogenic precursor gases to particulate matter (CitationSeinfeld and Pandis 1998). Both EC and OC are significant in relation to atmospheric visibility (EC as a light-absorbing material and OC as a light-scattering material), source attribution, influence of climate by radiative forcing, and the development of control strategies for attaining the NAAQS. Since carbon represents a significant portion of the total PM2.5 mass, control strategies for carbon will be considered for reductions in PM2.5.
Current routine methods of collection and analysis of carbon aerosol involve filter-based sample collection with thermal optical analysis (TOA). These methods provide low time resolution (24 h) data and are subject to both sampling artifacts and analysis method uncertainties (CitationTurpin et al. 2000; CitationChow et al. 1993). The nature and complexity of carbon aerosol requires time-resolved data for improved temporal resolution to help develop control strategies, determine and understand the sources, develop air quality models, and understand the relevance of carbon to the effect of PM2.5 on health. Continuous and semicontinuous monitoring techniques are commercially available for the collection of carbon aerosol at very high time resolution (as short as 1 min) and can play a key role in providing these data. However, before these technologies can be considered for use in routine monitoring networks, method demonstration and comparison studies are needed to determine how they compare with standard, filter-based sampling methods.
There are several published methods for OC and EC measurements in ambient air. Most of these techniques involve the use of time-integrated, filter-based collection on a quartz filter substrate and analysis by thermal optical analysis (CitationHuntzicker et al. 1982; CitationCachier et al. 1989; CitationNIOSH 1999; CitationBirch and Cary 1996; CitationChow et al. 1993; CitationPeterson and Richards 2002). Methods of this type are the predominant technique in use today for OC and EC determinations. Newly developed continuous and semicontinuous, automated measurement techniques are commercially available. There are two automated systems currently available in the U.S. for OC and EC determinations: the Series 5400 ambient particulate monitor from the Rupprecht & Patashnick Company, Inc. (Albany, NY, USA; CitationRupprecht et al. 1995) and the carbon aerosol analysis field instrument from Sunset Laboratories (Tigard, OR, USA).
In addition to methods for OC and EC, automated methods for the determination of the light-absorbing carbon aerosol or black carbon (BC) are also available. These include the Aethalometer from Magee Scientific (Berkeley, CA, USA) the particle soot absorption photometer (PSAP) from Radiance Research Inc. (Seattle, WA, USA), and the multiangle absorption photometer (MAAP) from Thermo Electron Corporation (Woburn, MA, USA). There are “research-grade” instruments available for BC measurements, including the resonant photoacoustic spectrometer (CitationMoosmuller et al. 1998; CitationArnott et al. 1999, Citation2003), the integrating plate (CitationBond et al. 1999; CitationLin et al. 1973), and the integrating sphere (CitationHitzenberger et al. 1996). Of the commercially available instruments, the Aethalometer, which measures BC collected on a quartz filter tape by optical absorption, is the most commonly studied BC measurement technique (CitationHansen and McMurry 1990; Kulbusch et al. 1998; CitationMoosmuller et al. 1998; CitationAllen et al. 1999; CitationHitzenberger et al. 1999; CitationLavanchy et al. 1999; CitationBabich et al. 2000; CitationWatson and Chow 2002; CitationLim et al. 2003). The MAAP is newly available and measures BC using a radiative transfer scheme on particle-loaded glass fiber filters (CitationPetzold et al. 2002). The PSAP operates in a manner similar to the Aethalometer and measures light absorption of particles collected on a glass fiber filter (CitationBond et al. 1999; CitationMertes et al. 2004).
For this study, the Thermo Andersen RAAS2.5-410, PM2.5 integrated, filter-based sampling system (Smyrna, GA, USA) was used. Quartz filters from the RAAS were analyzed by a thermal optical transmittance (TOT) analysis technique for carbon aerosol measurements. The RAAS has been evaluated in previous studies (CitationTolocka et al. 2001; CitationUSEPA 2001; CitationWatson and Chow 2002) and will be used with TOT as the reference for the automated methods. The Series 5400 Ambient Particulate Monitor (5400) from Rupprecht & Patashnick Company was selected for study. At the time of purchase, it was the only commercially available, continuous OC and EC monitor available. The Series AE-21 Aethalometer was selected to measure BC due to its wide use in previous studies.
provides a summary of previously published results for comparisons made in the last 15 years between the instruments selected for this study. Earlier comparisons used a thermal optical technique that did not use a laser transmittance or reflectance measurement for correction of char. More recent comparisons used filter-based TOA with TOT and thermal optical reflectance (TOR) as a reference for the automated methods. Comparisons of the Aethalometer BC with EC collected by TOR methods showed very good correlation that ranged from 0.87–0.98 (CitationAllen et al. 1999; CitationBabich et al. 2000; CitationWatson and Chow 2002). The Aethalometer BC/EC TOR in all cases showed some underestimation of EC TOR by the Aethalometer. Mean Aethalometer/EC TOR ratios ranged from 0.62 in Dallas, Texas (CitationBabich et al. 2000) to 0.92 in Fresno, CA (CitationWatson and Chow 2002). One study compared the Aethalometer to an in situ TOT method (CitationLim et al. 2003). Comparison of the Aethalometer to the in situ TOT method showed very good correlation (r = 0.95), but contrary to the results found in comparison to the EC TOR, the Aethalometer was found to overestimate the in situ TOT by 12% (mean Aethalometer BC/EC TOT = 1.12). There were three studies (CitationBerghmans et al. 1996; CitationLim et al. 2003; CitationWatson and Chow 2002) that reported results from comparison of the Aethalometer BC to the 5400 EC. The correlations were > 0.71 and the mean ratio of 5400 EC/BC showed the 5400 EC to overestimate the Aethalometer BC by as much as 63%. The 5400 incorporates a PM2.5 size-cut cyclone inlet as a standard device. Previous studies did not use a size-selective inlet on the Aethalometer, most likely because BC is thought to reside primarily in the submicron size fraction (CitationHeitzenberg 1982; CitationWolff et al. 1982; CitationMcMurry and Zhang 1989). Because all of the other measurement methods used in this study incorporate a PM2.5 inlet, it is of interest to evaluate the Aethalometer both with and without a size-selective inlet to determine any differences.
One study compared the 5400 to the TOR method (CitationWatson and Chow 2002), and one study compared the 5400 to an in situ TOT method (CitationLim et al. 2003). Both studies found good correlation (r = 0.74 and r = 0.86) of the 5400 EC with the TOA methods, but there was an overestimation of EC by the 5400 (mean 5400 EC/TOA ratios of 1.53 and 1.20). Watson and CitationChow (2002) found very poor correlation (r = 0.42 and r = 0.31) of the 5400 with TOR TC and OC. The regression slopes were much less than 1.00 and the intercepts were large (> 5 μg C m−3). The mean ratio of 5400/TOR TC and OC showed the 5400 be 48% higher than the TOR method. CitationLim et al. (2003) found very good agreement of the 5400 TC and OC with the in situ TOT method. The correlation regression slopes and the mean ratios of 5400/TOT TC and OC were all near 1.00.
TABLE 1 Summary of comparisons between automated and filter-based measurements
There have not been many comparisons between filter-based TOT methods and the automated instruments selected for this study. Additional comparisons are needed to demonstrate the use of automated systems for routine monitoring networks. The purpose of this article is to compare filter-based TOT and automated carbon measurements methods. The RAAS filter-based method (TOT analysis), the Series 5400 ambient particulate monitor, and the Series AE-21 Aethalometer were intercompared. A summary of the relationships between collocated carbon measurements collected during the summer and winter of 2002 in Research Triangle Park (RTP), NC are provided, along with a discussion of potential causes for the differences.
EXPERIMENTAL METHODS
The following section provides the monitoring site description, details on the filter-based and automated monitoring techniques, the definition of TC, OC, EC, and BC used in this study, and details regarding the data treatment prior to analysis.
Site Description
The site was located on a grass-covered field in a wooded area of the Research Triangle Park (RTP) of Durham, NC (35° 53′ N, 78° 52′ W). The population of Durham is approximately 203,000 and an estimated 50,000 people are employed in the RTP (CitationRTP 2002). The RTP is a suburban business park with one major interstate passing through it and several major highways leading into and out of the area. It is centrally located between the mountains in the west and the coast on the east and south. The area terrain is rolling and the site elevation is about 150 m. The filter-based sampling system was located on the wooden platform with the PM2.5 inlet located about 5 m above ground. The automated carbon sampling systems were housed about 10 m away in the temperature-controlled shelter, with the PM2.5 inlets located about 2 m above the trailer roof and about 6 m above ground. The sampling inlets were about 2 m apart. The immediate surrounding area consists of mixed deciduous and pine forest, highways and corporate and government research facilities. The site is located about 1200 m south of Interstate 40, a six-lane highway that carries significant traffic loads during the peak morning and evening rush-hour periods. It is also located about 100 m south of a four-lane road that parallels the Interstate and about 100 m east of a heavily traveled secondary road. Due to the close proximity of the site to roadways and forested areas, the expected primary and secondary sources of carbon aerosol include mobile sources, biogenic aerosol, and secondarily formed organic aerosol in the summer, and primarily mobile sources in the winter.
Carbon Measurement Methods
All monitors were operated at the recommended default settings specified by each manufacturer. A Thermo Andersen RAAS2.5-410, PM2.5 filter-based sampling system was used to collect 24 h integrated samples. The RAAS2.5-410 is a six-channel Speciation Sampler (Symrna, GA, USA). Only three channels were used to collect PM2.5 on 47 mm Teflon® -, nylon- and quartz-filter media at nominal flow rates of 16.7, 7.3, and 7.3 ± 10% liters per minute (lpm), respectively Quartz filters (Whatman, QM-A, Clifton, NJ, USA) were used for collection of carbon aerosol and were the only filters evaluated in this study. Gas-phase carbon denuders or backup filter and sorbent systems were not used for sample collection. Filters were prefired at 900°C for at least 3 h and placed in a freezer at ≤ −15°C until shipped to the field for use. Filters were returned to the analytical laboratory from the field in coolers at ≤4°C. Upon receipt in the lab, they were stored in the freezer until ready for analysis. The quartz filters were analyzed by Research Triangle Institute (RTI) International in Research Triangle Park, NC using the TOT method and a Sunset Laboratory Carbon analyzer (Tigard, OR, USA). The TOT method (CitationPeterson and Richards 2002) is a modification of the NIOSH 5040 method used for occupational exposure to particulate diesel emissions (CitationNIOSH 1999; CitationBirch and Cary 1996). The TOT method involves the evolution of OC in pure helium up to a temperature of 900°C and the evolution of EC in a 98%:2% helium:oxygen (He:O2) atmosphere up to 920°C. The temperature profile used for analysis was He for 60 s at 310°C, followed by He for 60 s at 480°C, He for 60 s at 615°C, He for 90 s at 900°C; then He:O2 for 35 s at 600°C, He:O2 for 45 s at 675°C, He:O2 for 45 s at 750°C, He:O2 for 45 s at 825°C, and He:O2 for 120 s at 920°C. The estimated method detection limit (MDL) provided by RTI for OC, EC, and TC was 0.13 μg C m−3. The sampling system was leak checked before every sample collection, and the sampling flow rate was calibrated monthly using a triCal volumetric flow calibrator (BGI, Inc., Waltham, MA, USA).
Three-hour samples were collected with the Series 5400 ambient particulate monitor. The 5400 Monitor (CitationRupprecht et al. 1995) collects continuous samples with dual, alternating collection streams. The sample inlet incorporates a PM2.5 sharp-cut cyclone operated at a flow rate of 16.7 ± 10% lpm. The air flows through the inlet to a multiple-holed, stainless-steel impaction plate. The PM2.5 cyclone and cutpoint of the impactor provide a particle collection range between 2.5 and 0.14 μ m (CitationRupprecht & Patashnick Co., Inc. 2002). No filter substrate material was used, and the baseline impactor temperature is maintained at 50°C. During the analysis phase, the particle-laden impactor is incorporated into a closed gas circulation loop that includes an afterburner, circulating pump, and nondispersive infrared (NDIR) CO2 detector. The NDIR CO2 detector measures the amount of CO2 released when the sample is oxidized at elevated temperatures. The CO2 value is used together with the sample volume in order to determine the concentration of carbon in the air during the sampling period. Air was present at all times during collection and analysis. The instrument measures the OC (the carbon evolved at 340°C) and TC (the carbon evolved at both 340°C and 750°C) concentrations in μg C m−3. Measurements of EC were obtained by subtracting the OC from the TC measured. The instrument was operated using the following temperature profile: an initial temperature increase from 50°C to 340°C (dwell time of 480 s) and then to a final temperature of 750°C (dwell time of 360 s). No temperature ramp control was used. The manufacturer's estimate of the MDL for the 5400 on a 3 h time base is 0.10 μg C m−3. The sample collection path was leak checked weekly. The CO2 detector was calibrated initially and audited weekly with 414 and 1417 ppm CO2 gas standards (Scott-Marrin Inc., Riverside, CA, USA). The collection flow rate was calibrated monthly with a triCal volumetric airflow calibrator.
Ten-minute measurements were made using the Series AE-21 Aethalometer. The Aethalometer (CitationHansen et al. 1982) is a semicontinuous method that measures light absorbing BC using continuous filtration and optical transmission to determine the attenuation of light at a fixed wavelength through a quartz filter tape. The Series AE-21 is a dual-channel unit that measures BC by optical absorption at 880 nm in the near-infrared and optical absorption at 370 nm in the near ultraviolet (UV). The BC measurement was of interest in this study; therefore, the readings from the UV channel were not evaluated. The Aethalometer converts light attenuation to BC mass using a fixed specific attenuation cross section (σ) of 16.6 m2 g−1 of BC. This was the default value set by the manufacturer for a wavelength of 880 nm (CitationHansen 2002). In previous studies, the Aethalometer was operated with a σ value of 19 m2 g−1 (CitationBabich et al. 2000; CitationLavanchy et al. 1999; CitationAllen et al. 1999; CitationLiousse et al. 1993; CitationPetzold et al. 1997; Kuhlbush et al. 1998; CitationMoosmuller et al. 1998; CitationSharma et al. 2002). The manufacturer's current default σ value of 16.6 m2 g−1 was used in this study and was also used by CitationLim et al. (2003) in Atlanta. The default is being used since the goal is to operate the instrument at the conditions recommended by the manufacturer for normal, routine operation. The flow rate was maintained at 5.0 ± 8% lpm for the study. The estimated MDL for this instrument collecting 10 min data at 5.0 lpm is 0.10 μg C m−3. This value was determined from the manufacturer's estimated instrument sensitivity (3 times the noise equivalent of 1 ng BC) and the sample volume for a 10 min measurement. The 10 min values are mathematically averaged to 3 h increments; therefore, the 3 h MDL is assumed to be the same since no additional sample volume is collected. The Aethalometer was operated both with and without a PM2.5 cyclone inlet manufactured by BGI, Inc. The BGI sharp-cut cyclone model 1.829 is designed for a 2.5 μ m aerodynamic aerosol size-cut at 5.0 lpm. The sharp-cut cyclone was cleaned monthly. The instrument flow rate was checked weekly and calibrated monthly with a triCal volumetric flow calibrator.
OC, EC, TC, and BC Definitions for this Study
The differentiation of OC from EC and BC is highly dependent on the specific measurement or analysis technique and operational procedures used. Different instrument operating procedures may lead to different results, as demonstrated by intercomparison studies for the TOA methods (CitationBirch 1998; CitationChow et al. 2001; CitationCurrie et al. 2002; CitationSchmid et al. 2001). In addition, there are currently no reference standards for assessing the measurement of true OC, EC, and BC or a standardized method for distinguishing between these species. Method differences also depend on the nature and source of aerosol collected.
For clarification and the purposes of the work reported here, 5400 OC is defined as the carbon thermally evolved by the Series 5400 monitor during the 50–340°C temperature step and measured by the CO2 detector. The carbon evolved during the second temperature step at 750°C plus the 5400 OC is defined as 5400 TC, and 5400 EC is the difference between the 5400 TC and 5400 OC measurements.
For the TOT method performed on quartz filters from the Andersen RAAS, the analysis is done in two phases. In the first phase, carbon is thermally evolved during the 310–900°C temperature steps in a pure helium atmosphere. The evolved carbon is oxidized to CO2 and then reduced to methane and measured by a flame ionization detector (FID). During the second phase, both pyrolysis correction and EC measurement are performed. The oven temperature is reduced to 600°C, an O2/He mix is added, and the temperature of the oven is raised to 920°C. The filter transmittance is monitored throughout the analysis. During the second phase, the point at which the transmittance reaches the initial value is considered the split between OC and EC. The carbon evolved in the first phase, and prior to the split, is OC and will be referred to as RAAS TOT OC. The carbon evolved after the split is EC and is referred to as RAAS TOT EC. For a detailed description and discussion of the TOT method, see CitationBirch and Cary (1996). RAAS TOT TC is the sum of the RAAS TOT OC and RAAS TOT EC measurements.
BC is defined as the light-absorbing carbon aerosol measured by the Aethalometer with the default optical absorption cross section of 16.6 m2 g−1 of BC. The Aethalometer BC, 5400 EC and RAAS TOT EC are treated as indicators of light-absorbing primary carbon aerosol and are compared directly in this study. The 5400 TC and OC measurements are compared to the RAAS TOT TC and OC measurements.
About the Data Sets
Twenty-four-hour, time-integrated RAAS TOT, 3 h 5400 carbon measurements, and 10 min Aethalometer BC measurements were collected during the summer of 2002. The most intensive sampling period was from 1 July through 2 October 2002, when the filter-based sampling system and both automated methods were operated. The intensive sampling was done in the summer because the highest TC aerosol concentrations were expected. Only the 5400 and Aethalometer with a PM2.5 inlet were operated during the winter (23 November through 31 December 2002). This comparison was done in the winter due to availability and delivery of the cyclone inlet for the Aethalometer.
Twenty-four-hour integrated filter samples were collected at a frequency of 1 every 3 days. For each 24 h RAAS TOT filter-sampling event, eight 3 h 5400 measurements were averaged to obtain 24 h values for comparison. The 24 h 5400 averages were considered valid where 75% completeness was obtained. Completeness is the percentage of valid data obtained from the amount of data expected to be collected. Data were excluded when measured below the MDL. Twenty-six valid matched data pairs were obtained for comparison of the 5400 and RAAS TOT OC and TC and 24 valid matched data pairs for EC.
Ten-minute Aethalometer BC measurements were averaged to obtain 24 h BC values to compare to the filter-based RAAS TOT EC measurements. Aethalometer BC averages were considered valid where 75% completeness was obtained and measurements were above the MDL. Twenty-one valid data pairs were obtained for the period.
The 5400 3 h EC measurements were compared to 10 min Aethalometer BC averaged over 3 h. The 75% completeness and MDL requirements were also applied. The Aethalometer BC data were collected both with and without a PM2.5 size-selective inlet. Of the 752 possible 3 h measurements that could be made over the period (1 July through 2 October) for comparison of the 5400 to the Aethalometer without a size-cut inlet, 558 valid matched data pairs were obtained for comparison. Beyond completeness and data below the MDL, additional missed data were due to downtime required to perform weekly instrument calibrations and maintenance. A smaller data set was obtained for those Aethalometer data collected with the PM2.5 inlet from 23 November through 31 December 2002. Of the 316 possible 3 h measurements 189 valid matched data pairs were obtained for comparison. The loss of data was due to the instruments being off-line for calibrations and maintenance, and 16 days (4–20 December) of lost data were due to an extended power outage resulting from a major ice storm. The missing data were primarily interspersed throughout the sampling period, except for the winter time comparison of 5400 EC to BC with PM2.5 inlet. The 16 days of consecutively lost data for this comparison occurred on all days of the week and not just on a particular day of the week or only on the weekend. Since no diurnal comparisons are being made, no affects on the data set are anticipated.
RESULTS AND DISCUSSION
Comparison of 5400 to RAAS TOT TC, OC, and EC
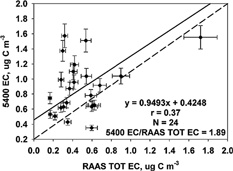
Three-hour 5400 measurements were averaged to obtain 24 h values for comparison to the RAAS filter measurements. A Deming, orthogonal regression and Pearson correlation (r) were used to compare the results. The Deming regression accounts for variability in both x and y variables. The regression slope, intercept, standard error, and correlation results are given in . The scatter plots are provided in . The 5400 TC to RAAS TOT TC correlation was 0.64, the regression equation was 5400 TC = 0.38 (± 0.09) RAAS TOT TC–0.26 (± 0.64) μg C m−3. The OC results were very similar to the TC results: 5400 OC = 0.22 (± 0.05) RAAS TOT OC −0.03 (± 0.33) μg C m−3, and the correlation was 0.67. The OC measured comprises a large part of the TC, so the similarity of results is not surprising. The mean RAAS TOT OC was 6.14 and the mean RAAS TOT TC was 6.56 μg C m−3 (). Comparison of the 5400 TC and OC with TOR in Fresno, CA (CitationWatson and Chow 2002) resulted in similar slopes (0.31 ± 0.02 for TC and 0.20 ± 0.02 for OC), but the correlation was not as good (r = 0.42 and r = 0.31) and the intercepts were much greater (6.22 and 5.42 μg C m−3) in Fresno. Comparison of the 5400 to an in situ TOT method in Atlanta, GA (CitationLim et al. 2003) resulted in much better slopes (0.96 for TC and 0.99 for OC), slightly larger intercepts (1.19 and 1.15 μg C m−3), and better correlations (r = 0.91 and 0.92).
TABLE 2 DemingFootnote a regression and Pearson correlation (r) results
TABLE 3 Minimum (Min.), maximum (Max.), mean (μ), and median (Med.) concentrations by comparison and method
FIG. 1 Comparison of the R&P 5400 TC to the RAAS TOT TC concentrations (μg C m−3) measured 1 July to 2 October 2002. The solid line represents the Deming linear regression, and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
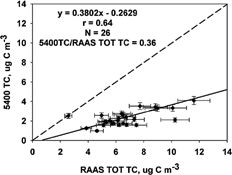
FIG. 2 Comparison of the R&P 5400 OC and RAAS TOT OC concentrations (μg C m−3) measured 1 July to 2 October 2002. The solid line represents the Deming linear regression and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
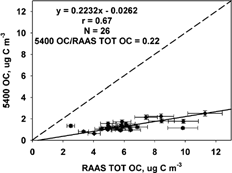
FIG. 3 Comparison of the R&P 5400 EC and RAAS TOT EC OC concentrations (μg C m−3) measured 1 July to 2 October 2002. The solid line represents the Deming linear regression and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
The 5400 TC and OC measurements significantly underestimated the RAAS TC and OC results in this study. The mean 5400/RAAS ratios of TC and OC (0.36 and 0.22) were much smaller than those found CitationWatson and Chow (2002) using the TOR method (1.48 and 1.48) and those found by CitationLim et al. (2003) using the in situ TOT method (1.02 and 0.92). The underestimation of TC and OC is attributed in part to the positive OC artifact expected from the filter-based sampling method. The filter sampling method did not incorporate gas-phase carbon denuders or backup filters/sorbents, and blank subtraction was not applied. The TOR method routinely incorporates a blank subtraction for OC measurements (CitationChow et al. 1993). The in situ TOT method used by CitationLim et al. (2003) incorporated a dynamic blank for the measurements collected. Another difference between the 5400 measurements and the RAAS TOT is a correction for char that occurs during pyrolysis. The RAAS TOT method use transmittance for correction of char; however, the 5400 does not incorporate a char correction. Lack of correction for char causes an underestimate of OC and overestimate of EC. These issues are discussed in more detail below.
The 5400 EC-to-RAAS TOT EC regression was 5400 EC = 0.95 (± 0.51) RAAS TOT EC + 0.43 (± 0.30) μg C m−3, and the correlation was 0.37. The ratio of mean 5400 EC is much higher than the mean RAAS TOT EC (ratio of 1.89). Both CitationWatson and Chow (2002) and CitationLim et al. (2003) reported an overestimation of EC when comparing of the 5400 to the TOR and the in situ TOT method (ratio of 1.53 and 1.20, respectively). This overestimation of EC is indicative of the bias associated with the 5400 carbon measurements due to the lack of correction for char that may occur.
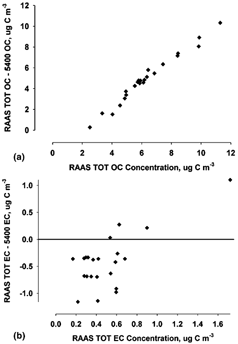
CitationBerghmans et al. (1996) found an underestimate of carbon measured by the 5400 and performed lab experiments to identify a “saturation effect” for the 5400. A similar effect is seen in this study when assessing the difference in RAAS TOT OC and 5400 OC measurements as a function of RAAS TOT OC concentration (). There is a strong relationship between the differences in measurements as a function of concentration. As the RAAS TOT OC value increases, the 5400 OC concentration does not increase accordingly. An inverse trend was apparent for the EC data (increasing difference as concentration decreased); however, the EC concentrations for both measurements were very low (less than 1.8 μg C m−3).
FIG. 4 Difference in RAAS TOT and R&P 5400 measurements as a function of RAAS TOT concentration (μg C m−3) for (a) OC and (b) EC. The sampling period was 1 July through 2 October 2002. The number of samples for was (a) 26 and (b) 24.
The RAAS is a filter-based sampling system that collects aerosol on quartz filters that are subsequently analyzed by the TOT method. The 5400 collects aerosol on a heated metal impaction place with a 0.14–2.5 μ m cutpoint. Neither method uses a gas-phase carbon denuder or backup filter sorbents or filters. Factors to consider regarding the difference between the two types of measurements include the differences in thermal evolution dwell times, temperatures, and atmospheres; correction for char.; adsorption of gas-phase organics on the quartz-filter substrate; variation in the amount of volatilization of organics from the particles due to the differences in sampling flow rates (7.3 lpm for the RAAS and 16.7 for the 5400) and resulting pressure drops across the filter and impaction plate; the low-end cutpoint of the 5400 (0.14 μ m); and possible volatilization of organics from the particles due to the 5400 baseline collection temperature of 50°C. Losses due to particle bounce and resulting “blow-off” associated with classic impaction are reported not to be of consequence due to containment of the impaction plate (CitationRupprecht et al. 1995). The quantitative impact of each of these effects is unknown; however, additional discussion of these issues is provided below.
Different Operational Definitions
The differentiation of OC from EC is highly dependent on the specific measurement or analysis technique and operational procedures used the method. Different operational procedures can lead to highly variable results, as demonstrated by intercomparison studies for the TOA methods such as TOT (CitationBirch 1998; CitationChow et al. 2001; CitationCurrie et al. 2002; CitationSchmid et al. 2001). The RAAS TOT method determines OC in a pure helium atmosphere up to a temperature of 900°C, while the 5400 determines OC in air up to a temperature of 340°C. In addition, the TOT method determines EC in an O2/He atmosphere up to 920°C, while the 5400 determines EC in air from 340 to 750°C. Different evolution temperatures, atmospheres, and dwell times for the 5400 would contribute to the differences in OC, EC, and TC when compared to the TOT method.
Correction for Char
An important difference between the 5400 measurements and the RAAS TOT is the correction for OC char. Some organic species in aerosol samples produce pyrolytically generated EC or char during thermal analyses (CitationHuntzicker et al. 1982). The RAAS TOT method uses transmittance for correction of char; however, the 5400 does not incorporate a char correction. Although the formation of char during heating in air is minimized, it is not completely eliminated (CitationCadle et al. 1980) and could lead to an underestimation of OC and an overestimation of EC. With very low measured EC concentrations, the uncertainty due to char is magnified. An underestimation of OC and an overestimation of EC and poor correlation between the 5400 EC and RAAS TOT EC were found in this study. This is indicative of a bias associated with the 5400 carbon measurements due to the lack of a correction for char. The amount of char formed is not a constant and depends on a variety of factors, including the amount of OC, the temperature used in analysis, the residence time at each temperature, the presence of certain inorganic constituents, and the carrier gas used (CitationCadle et al. 1980; CitationYu et al. 2002). All of these factors, except the amount of OC and inorganic constituents, present in the sampled atmosphere, are different between the 5400 and RAAS TOT thermal methods.
Positive and Negative Artifacts
Sampling for OC is subject to positive and negative artifacts that are difficult to separate and quantify. Estimates of the positive (adsorption) and negative (volatilization) biases range from +50 to −80%, respectively (CitationEatough et al. 1990; CitationTurpin et al. 1994). Adsorption of condensable organics on the quartz sampling filter is dependent on ambient temperature, surface area of the filter, partitioning coefficient (vapor pressure), and the presence of other competing adsorbates (CitationMader et al. 2001). However, metal impaction plates do not absorb gas-phase organics (CitationZhang and McMurry 1987). The volatilization of particle-phase semivolatile organic compound (SVOC) can be a function of the pressure drop (filter-face–velocity) across the filter or the impaction plate (CitationMcDow and Huntzicker 1990). Increasing pressure drop due to increasing flow rate can drive volatilization (CitationTurpin et al. 2000). Positive artifacts can be reduced for filter sampling through the use of gas-phase denuders and backup filters and sorbents, but these may disrupt the gas–particle equilibrium and cause negative biases in the measurements. No denuders or backup filters and sorbents were used for filter sampling. The RAAS TOT method used in this study also does not incorporate an OC blank subtraction. The typical average annual OC blank value found for a RAAS sampler in the EPA STN is 1.3 μg C m−3 (CitationFlanagan et al. 2002). This amounts to a positive artifact that is 23% of the mean OC and 21% of the mean TC measured in this study and does not fully account for the difference in results.
The 5400 uses an impaction plate for sample collection. Impactor OC measurements have been found to be 45–55% lower than quartz filter sampling methods (CitationHering et al. 1990). CitationZhang and McMurry (1991) predicted lower impactor carbon measurements due to losses of SVOCs as a result of the pressure drop across the impactor and the equilibrium ratio of gas-to-particle concentrations of the evaporative SVOC species. CitationMcMurry et al. (1996) shows very poor agreement between an impactor and filter carbon measurements. Impactor OC was higher than filter OC at two locations (Meadview, AZ and Hopi Point, AZ) and lower than filter OC for a site in Los Angeles, CA. CitationCabada et al. (2004), compared micro-orifice uniform deposit impactors (MOUDIs) to filter-based samples and found that about 50% of the organic mass was lost by the MOUDI in summer and good agreement (no loss) was found in winter. This was attributed to the greater loss of SOA (secondary organic aerosol, assumed to be SVOCs) present at higher levels in the summer. The 5400 TC and OC measurements in RTP were much lower than the quartz filter samples and are partly attributed to the lower OC expected to be measured by the impactor.
The 50°C baseline collection temperature of the 5400 monitor, used to prevent condensation of moisture, could cause additional loss of SVOCs. Zhang and McMurry (1989) tested the effect of 20°C versus 40°C temperature on the volatilization loss from impactors. They saw no loss for ambient aerosols but did see losses of SOA produced in smog chambers at the higher temperature. A loss of SVOC mass was demonstrated in the literature for the 50°C taper element oscillating microbalance (TEOM), which uses a filter and not an impaction plate. As much as 37% of the PM2.5 mass was lost during data collection in summer (CitationAyers et al. 1999; CitationAllen et al. 1997; CitationCyrys et al. 2001). Losses of SVOC mass at 50°C in the 5400 could account for a portion of the lower response in comparison to filter OC measurements, which are collected near ambient temperature. The loss of SVOC is expected to vary considerably as a function of ambient conditions and aerosol type, and therefore it is not expected to be constant.
Low-End Impaction Cutpoint
The 5400 collects aerosol on a heated metal impaction place with a 0.14–2.5 μ m cut point. The low-end impaction cutpoint is an important issue in the comparison of the 5400 to filter-based methods. In theory, the filter collects all particles ≤ 2.5 μ m (with a PM2.5 size-selective inlet) and has no loss of the ultrafine fraction (< 0.1 μ m) of particle mass. The 5400 loses all particle mass < 0.14 μ m. How much mass is unknown; however, very small particles do not contribute significantly to the mass and rarely account for more than a few percent of the total mass of airborne particles (CitationSeinfeld and Pandis 1998; CitationKeywood et al. 1999; CitationSturm et al. 2003). Mass distribution is a function of aerosol type and age. In the summertime (when these samples were collected), it is expected that SOA is present in the atmosphere due to photochemical activity. SOA is expected to be higher in both the ultrafine mode (< 0.18 μ m) and the accumulation mode (> 0.18 μ m and < 2.5 μ m) in the summer (CitationFine et al. 2004), so it is conceivable that losses of ultrafine particle mass by the 5400 due to the low-end cutpoint of 0.14 μ m would be greater in the summer.
Comparison of Aethalometer BC to RAAS TOT EC
Ten-minute Aethalometer BC measurements were averaged to obtain 24 h BC values to compare to the RAAS EC measurements. Refer to for the correlation and regression results and for the scatter plot. The correlation of Aethalometer 24 h BC to RAAS filter EC was 0.89. The regression equation was BC = 1.17 (± 0.16) RAAS TOT EC + 0.06 (± 0.10) μg C m−3. Other studies have shown that filter-based EC measurements compare well with Aethalometer BC (CitationHansen and McMurry 1990; CitationAllen et al. 1999). CitationHansen and McMurry (1990) reported filter EC = 1.05 (± 0.14) BC – 0.09 (± 0.45) μg m−3 and correlation of 0.89; CitationAllen et al. (1999) reported BC = 0.95 (±0.04) TOR EC – 0.2 (± 0.4) μg m−3 and correlation of 0.96. The filter EC values are not subject to the same positive and negative sampling artifacts expected with OC (and subsequently TC). The RAAS TOT EC method used to analyze the EC is fundamentally different from the optical absorption method used by the Aethalometer. The Aethalometer overestimated the RAAS TOT EC on average by 30% (BC/RAAS TOT EC ratio of 1.30). CitationAllen et al. (1999), CitationBabich et al. (2000), and CitationChow and Watson (2002) reported mean BC/TOR EC ratios that were all < 1.00 (), which shows an underestimation of TOR EC by the Aethalometer. Measurements of EC by TOR have been shown to be greater (as much as a factor of 2) than EC measured by TOT (CitationChow et al. 2001). Lower EC by the TOT method would cause the ratio to be higher in this study and could explain the difference in results seen here versus those found previously for EC by TOR.
FIG. 5 Comparison of Aethalometer BC and Andersen RAAS filter EC concentrations (μg C m−3) measured 1 July to 2 October 2002. The solid line represents the Deming linear regression and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
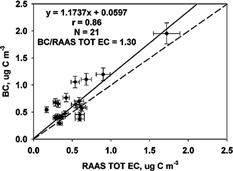
CitationLiousse et al. (1993) CitationPetzold et al. (1997), and CitationHitzenberger et al. (1999) suggest that the specific attenuation cross section (σ) used by the Aethalometer to determine BC mass is highly variable, changes with location, carbon source, mass fraction, and particle size, and that a site-specific value should be used. The σ of the Aethalometer was calibrated against the EC as determined by the TOT method. Multiplying the slope of the BC to TOT EC regression times the σ used to determine BC provides a site-specific estimate (CitationBabich et al. 2000). Multiplying the slope (1.17) by the σ value of 16.6 m2 g−1 results in σ of 19.4 m2 g−1 for RTP, NC during the summer. When comparing the Aethalometer BC to mass of BC using a thermal method, CitationLiousse et al. (1993) reported a range of σ from 5 m2 g−1 for a remote area to 20 m2 g−1 for an area dominated by biomass burning. CitationPetzold et al. (1997) reported a range of values from 8 m2 g−1 for rural sites to 19 m2 g−1 for a street crossing in Germany, and CitationSharma et al. (2002) reported a range of values from 6.4 to 20 m2 g−1 for 6 sites in Canada. The σ of 19.4 m2 g−1 for RTP, NC is consistent with the σ of 19 m2 g−1of BC typically used for the Aethalometer (CitationBabich et al. 2000; CitationLavanchy et al. 1999; CitationAllen et al. 1999; CitationLiousse et al. 1993; CitationPetzold et al. 1997; Kuhlbush et al. 1998; CitationMoosmuller et al. 1998; CitationSharma et al. 2002). It is slightly higher than the σ of 18 m2 g−1 found by CitationLiousse et al. (1993) for an area dominated by anthropogenic sources with an aerosol high in sulfur and carbon and the value of 18 m2 g−1 identified by CitationAllen et al. (1999) for a site in southwestern PA dominated by vehicular emissions and open burning. The aerosol at the RTP site in the summer is to be influenced by motor vehicle emissions due to its very close proximity to three major roadways and SOA due to photochemical activity.
Comparison of 5400 EC to Aethalometer BC
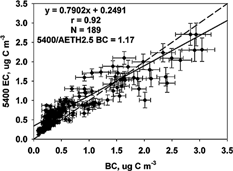
The 5400 3 h EC measurements were compared to 10 min Aethalometer BC measurements averaged over 3 h. The Aethalometer is typically operated without any size-selective inlet; however, the 5400 incorporates a PM2.5 cyclone in its standard configuration. Since the 5400 incorporates a PM2.5 cyclone inlet, it was of interest to determine the effects of a PM2.5 inlet on the 5400 EC to Aethalometer BC comparison. The Aethalometer BC data were collected both with and without a PM2.5 size-selective inlet. See for the correlation and regression results. Data were collected without the PM2.5 inlet (AETH) from 1 July through 2 October 2002 and with the PM2.5 inlet (AETH2.5) from 23 November through 31 December 2002. The correlation of 5400 to AETH BC during summer was 0.55, and the regression equation was 5400 EC = 0.94 (± 0.06) AETH BC + 0.29 (± 0.05) μg C m−3. The correlation of 5400 EC to AETH2.5 BC during winter was 0.92, with a regression of 5400 EC = 0.79 (± 0.02) AETH2.5 BC + 0.25 (± 0.02) μg C m−3. The mean 5400 EC/AETH BC concentration ratio was 1.39, and the mean 5400 EC/AETH2.5 BC was 1.17. Without a PM2.5 inlet on the Aethalometer, the mean 5400 EC concentration was 39% higher than the AETH BC. With the PM2.5 inlet added to the Aethalometer, the mean 5400 EC concentration was only 17% higher. show the scatter plots both with and without the inlet. The addition of the PM2.5 sharp-cut cyclone to the Aethalometer seemed to improve the correlation with the 5400 EC.
FIG. 6 Comparison of R&P 5400 EC and Aethalometer BC, without PM2.5 inlet (AETH BC) concentrations (μg C m−3) measured 1 July to 2 October 2002. The solid line represents the Deming linear regression and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
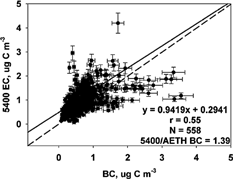
FIG. 7 Comparison of R&P 5400 EC and Aethalometer BC with PM2.5 inlet (AETH2.5) concentrations (μg C m−3) measured 23 November to 31 December 2002. The solid line represents the Deming linear regression and the dashed line represents the 1:1 line. The correlation (r), number of samples (N), and mean ratio are also shown.
As mentioned in the discussion earlier for the comparison of the 5400 to RAAS TOT, the 5400 does not incorporate any type of correction for char. This would lead to an underestimation of OC and overestimation of EC. The improvement in results for the comparison in winter may not be due to the addition of the PM2.5 inlet but may be due to the lower amounts of OC in the winter and potentially less amount of char formed by the 5400. Less char would result in less overestimation of EC and less variability (scatter) in the data. When visually comparing , there is less scatter in and more data points with high 5400 EC relative to BC concentration in . The affect of char is also supported by the difference in mean and maximum EC measurements by the 5400 and Aethalometer. The 5400 EC mean and maximum concentrations in the summer were 0.92 and 4.20 μg C m−3, versus 0.77 and 2.71 μg C m−3 in the winter. The Aethalometer BC concentrations were essentially unchanged (). The 5400 measured more EC in summer than in winter. The concentration of EC as a primary emission is not expected to change unless there are more primary EC emissions in the winter (e.g., woodsmoke). The site in RTP is not in a residential area and is mostly influenced by motor vehicle traffic due to its proximity to highways; therefore, primary EC emissions are not expected to change significantly.
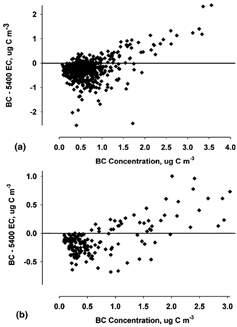
CitationBerghmans et al. (1996) found that for high concentrations measured by the Aethalometer, relatively low concentrations were measured by the 5400. Concentrations of BC in the RTP study did not reach very high levels (none > 5 μg C m−3); however, the slope of the 5400 to the AETH BC () dropped below the 1:1 line at values higher than 2 μg C m−3. This indicates a lower response by the 5400 at these concentrations, although there was a very large amount of scatter in the data set. The same trend was seen in the 5400 to AETH2.5 comparison (), where much less scatter was observed and the trend below the 1:1 line is easier to discern. This trend can be seen through assessing the difference between AETH2.5 BC and 5400 EC measurements as a function of AETH2.5 BC concentration. The downward trend in AETH2.5 BC–5400 EC can be seen in as the BC concentration exceeds 2 μg m−3. This trend is also seen when no inlet was used (). The difference between measurements increases with increasing BC concentration, suggesting confirmation of a “saturation” effect as also seen in the comparison of the 5400 TC and OC to the RAAS TOT in this study.
FIG. 8 Difference in Aethalometer BC and 5400 EC as a function of Aethalometer BC concentration for (a) Aethalometer with the PM2.5 inlet and (b) Aethalometer without the PM2.5 inlet. The sampling period was 1 July through 2 October 2002, and the number of samples was (a) 558 and (b) 189. The sampling period was 23 November through 31 December 2002.
Test of Population Means
In order to evaluate the differences in the mean concentrations provided by the various methods statistically, a t test was used. The t test was selected to compare the mean concentration values obtained from each pair of measurement techniques compared. The null hypotheses (H0) that the difference between the mean values μ 1 and μ 2 is not different from zero (H0: μ 1-μ 2 = 0), was tested at the 5% significance level (α = 0.05). Equal variances were assumed. Based on these results, the null hypothesis is rejected at the 5% level of significance for all but two cases: the comparison of means between the 5400 EC and the AETH2.5 BC (p = 0.0720) and the AETH BC and the filter-based RAAS EC (p = 0.1149). The population means for the first case were marginally significantly different at the 0.05 level. In all but these two comparisons, the measurement methods provide significantly different mean concentrations for the aerosol tested. It is important to note that although the mean EC values measured in all cases are well above the MDL, the mean concentrations are all < 1 μg m−3. Where statistically significant differences in the mean EC concentrations were found, there were not necessarily large differences in the actual mean concentration.
CONCLUSIONS
All of the methods used in this study provide measurements of carbon either by thermal evolution or optical absorption. The dissimilarity in sampling and analysis methods contributes to the difference in results obtained. The 5400 did not compare well with the filter-based method. The 5400 TC and OC measurements significantly underestimated the RAAS TC and OC results by 64 and 78%, respectively. The underestimation of TC and OC in this study could be attributed in part to the positive OC artifact of the filter-based sampling method. No filter blank was used to correct for positive filter artifacts for the RAAS TOT method. Sampling to address positive filter artifacts and OC blank subtraction should be considered when using filter-based methods. Another important difference between the 5400 measurements and the RAAS TOT is a correction for char. Although oxidation in air is expected to minimize char, any charring that occurs would cause an underestimation of OC and overestimation of EC by the 5400. Other factors that contribute to the underestimation of TC and OC by the 5400 include the difference in operational procedures (evolution temperatures and atmosphere, and dwell times) used to define OC and EC by the two methods, the potential for loss of SVOCs from the impactor, particle mass lost as a result of the low-end cutpoint of the 5400 (0.14 μ m), and possible volatilization of organics from the particles due to the 5400 baseline collection temperature of 50°C. The volatilization of organics from the particles due to the differences in sampling flow rates and resulting pressure drop across the filter or impactor also play a role. The 5400 overestimated RAAS EC by 89%. The overestimation of EC by the 5400 is attributed to the lack of correction for char and the dissimilarity in sampling and analysis methods.
The Aethalometer overestimated mean RAAS TOT EC concentration by 30% using the default σ of 16.6 m2 g−1 set by the manufacturer. A t test showed that the mean concentration values from Aethalometer BC and the filter-based RAAS TOT EC are not significantly different. Since α is expected to change with location, carbon source, and mass fraction a site-specific value was determined. The Aethalometer was calibrated against RAAS TOT EC in this study. A value for σ of 19.4 m2 g−1 was determined for the aerosol measured at the RTP site.
For comparisons between the 5400 and the Aethalometer BC, data were collected with and without a PM2.5 size selective inlet. This was done to be consistent with the 5400, which incorporates a PM2.5 cyclone, and to explore any difference in measurements made with the Aethalometer configured with a PM2.5 inlet. The correlation and mean ratio improved when the PM2.5 size-selective inlet was installed on the Aethalometer during the winter. The improvement in results for the comparison may not be due to the addition of the PM2.5 inlet, but may be due to lower amounts of OC in the winter and potentially lesser amounts of char formed by the 5400. It is unclear whether the PM2.5 inlet on the Aethalometer had any affect on the comparison. Additional experiments are needed to evaluate the effect of a PM2.5 cyclone inlet on the Aethalometer over different seasons.
Acknowledgments
This article has been subject to agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Notes
a Standard error.
b Not reported.
a Orthogonal regression for method comparison when imprecision is present in both variables.
b Standard error.
REFERENCES
- Allen , G. A. , Lawrence , J. and Koutrakis , P. 1999 . Field Validation of a Semi-Continuous Method for Aerosol Black Carbon (Aethalometer) and Temporal Patterns of Summertime Hourly Black Carbon Measurements in Southwestern PA . Atmos. Environ. , 33 : 817 – 823 .
- Allen , G. A. , Sioutas , C. , Koutrakis , P. , Reiss , R. , Lurmann , F. W. and Roberts , P. T. 1997 . Evaluation of the TEOM Method For Measurement of Ambient Particulate Mass in Urban Areas . J. Air Waste Manage. Assoc. , 47 : 682 – 689 .
- Arnott , W. P. , Moosmuller , H. , Rogers , C. F. , Jin , T. and Bruch , R. 1999 . Photoacoustic Spectrometer for Measuring Light Absorption by Aerosol: Instrument Description . Atmos. Environ. , 33 : 2845 – 2852 .
- Arnott , W. P. , Moosmuller , H. , Sheridan , P. J. , Ogren , J. A. , Raspet , R. , Slaton , W. V. , Hand , J. L. , Kreidenweis , S. M. and Collett , J. L. Jr. 2003 . Photoacoustic and Filter-Based Ambient Aerosol Light Absorption Measurements: Instrument Comparisons and the Role of Relative Humidity, . J. Geophys. Res. , 108 ( D1 ) : 1 – 11 .
- Ayers , G. P. , Keywood , M. D. and Gras , J. L. 1999 . TEOM vs. Manual Gravimetric Methods for Determination of PM2.5 Aerosol Mass Concentrations . Atmos. Environ. , 33 : 3717 – 3721 .
- Babich , P. , Davey , M. , Allen , G. and Koutrakis , P. 2000 . Method Comparisons for Particulate Nitrate, Elemental Carbon, and PM2.5 Mass in Seven U.S. Cities . J. Air Waste Manage. Assoc. , 50 : 1095 – 1105 .
- Berghmans , P. , Pauwels , J. , Roekens , E. and Bogaert , R. 1996 . Comparison of Methods for the Concentrations Measurement of Black (Carbonaceaous) Aerosol in Ambient Air, . J. Aerosol Sci. , 27 ( Suppl. 1 ) : S689 – S690 .
- Birch , M. E. 1998 . Analysis of Carbonaceous Aerosols: Interlaboratory Comparison . Analyst , 123 : 851 – 857 .
- Birch , M. E. and Cary , R. A. 1996 . Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust . J. Aerosol. Sci. Technol. , 25 : 221 – 241 .
- Bond , T. C. , Anderson , T. L. and Campbell , D. 1999 . Calibration and Intercomparison of Filter-Based Measurements of Visible Light Absorption by Aerosols . Aerosol Sci. Technol. , 30 : 582 – 600 .
- Cabada , J. C. , Rees , S. , Takahama , S. , Khlystov , A. , Pandis , S. N. , Davidson , C. I. and Robinson , A. L. 2004 . Mass Distributions and Size Resolved Chemical Composition of Fine Particulate Matter at the Pittsburgh Supersite . Atmos. Environ. , 38 : 3127 – 3141 .
- Cachier , H. , Bremond , M. P. and Buat-Menard , P. 1989 . Determination of Atmospheric Soot Carbon with a Simple Thermal Method, . Tellus , 41B : 379 – 390 .
- Cadle , S. H. , Groblicki , P. J. and Stroup , D. P. 1980 . Automated Carbon Analyzer for Particulate Samples . Anal. Chem. , 52 : 2201 – 2206 .
- Chow , J. C. and Watson , J. G. 2002 . PM2.5 Carbonate Concentrations at Regionally Representative Interagency Monitoring of Protected Visual Environment Sites, . J. Geophys. Res. , 107 ( D21 ) : 6/1 – 9 .
- Chow , J. C. , Watson , J. G. , Crow , D. , Lowenthal , D. H. and Merrifield , T. 2001 . Comparison of IMPROVE and NIOSH Carbon Measurements . Aerosol Sci. Technol. , 34 : 23 – 34 .
- Chow , J. C. , Watson , J. G. , Pritchett , L. C. , Pierson , W. R. , Frazier , C. A. and Purcell , R. G. 1993 . The DRI Thermal Optical Reflectance Carbon Analysis System: Description, Evaluation and Applications in U.S. Air Quality Studies, . Atmos. Environ. , 27A ( 8 ) : 1185 – 1201 .
- Currie , L. A. , Benner , B. A. Jr. , Cachier , H. , Cary , R. , Chow , J. C. , Druffel , E. R. M. , Eglinton , T. I. , Gustafsson , O. , Hartmann , P. C. , Hedges , J. I. , Kessler , J. D. , Kirchstetter , T. W. , Klinedinst , D. B. , Klouda , G. A. , Marolf , J. V. , Masiello , C. A. , Novakov , T. , Pearson , A. , Prentice , K. M. , Puxbaum , H. , Quinn , J. G. , Reddy , C. M. , Schmid , H. , Slater , J. F. , Watson , J. G. and Wise , S. A. 2002 . A Critical Evaluation of Interlaboratory Data on Total, Elemental, and Isotopic Carbon in the Carbonaceous Particle Reference Material, NIST SRM 1649a . J. Res. Nat. Inst. Standards Technol. , 107 ( 3 ) : 279 – 298 .
- Cyrys , J. , Dietrich , G. , Kreyling , W. , Tuch , T. and Heinrich , J. 2001 . PM2.5 Measurements in Ambient Aerosol: Comparison between Harvard Impactor (HI) and the Tapered Element Oscillating Microbalance (TEOM) System . Sci. Tot. Environ. , 278 : 191 – 197 .
- Eatough , D. J. , Aghdaie , N. , Cottam , M. , Gammon , T. , Hansen , L. D. , Lewis , E. A. and Farber , R. J. 1990 . “ Loss of Semi-Volatile Organic Compounds from Particles during Sampling on Filters ” . In Transaction of Visibility and Fine Particles , Edited by: Mathai , C. V. pp. 146 – 156 . Pittsburgh, PA : Air and Waste Management Association .
- Flanagan , J. B. and Peterson , M. R. 2002 . Analysis of Speciation Network Carbon Blank Data , Research Triangle Park, NC : RTI International . prepared under USEPA contract 68-D-98–032, 30 August 2002
- Fine , P. M. , Chakrabarti , B. , Krudysz , M. , Schauer , J. J. and Sioutas , C. 2004 . Diurnal Variations of Individual Organic Compound Constituents of Ultrafine and Accumulation Mode Particulate Matter in the Los Angeles Basin . Environ. Sci. Technol. , 38 : 1296 – 1304 .
- Gray , H. A. and Cass , G. R. 1998 . Source Contributions to Atmospheric Fine Carbon Particle Concentrations . Atmos. Environ. , 32 ( 22 ) : 3805 – 3825 .
- Hansen , A. D. A. 2002 . The Aethalometer , Berkeley, CA : Magee Scientific Company . available at
- Hansen , A. D. A. and McMurry , P. H. 1990 . An Intercomparison of Measurements of Aerosol Elemental Carbon During the 1986 Carbonaceous Species Method Comparison Study . J. Air Waste Manage. Assoc. , 40 ( 6 ) : 894 – 895 .
- Hansen , A. D. A. , Rosen , H. and Novakov , T. 1982 . Real-Time Measurement of the Absorption Coefficient of Aerosol Particles . Appl. Opt. , 21 ( 17 ) : 3060 – 3062 .
- Heitzenberg , J. 1982 . Size Segregated Measurements of Particulate Elemental Carbon and Aerosol Light Absorption at Remote Arctic Locations . Atmos. Envion. , 16 : 2461 – 2469 .
- Hering , S. V. , Appel , B. R. , Cheng , W. , Salaymeh , F. , Cadle , S. H. , Mulawa , P. A. , Cahill , T. A. , Eldred , R. A. , Surovik , M. , Fitz , D. , Howes , J. E. , Knapp , K. T. , Stockburger , L. , Turpin , B. J. , Huntzicker , J. J. , Zhang , X.-Q. and McMurry , P. H. 1990 . Comparison of Sampling Methods for Carbonaceous Aerosols in Ambient Air . Aerosol Sci. Technol. , 12 : 200 – 213 .
- Hitzenberger , R. , Dusek , U. and Berner , A. 1996 . Black Carbon Measurements Using an Integrating Sphere, . J. Geophys. Res. , 101 ( D14 ) : 606 – 19 .
- Hitzenberger , R. , Jennings , S. G. , Larson , S. M. , Dillner , A. , Cachier , H. , Galambos , Z. , Rouc , A. and Spain , T. G. 1999 . Intercomparison of Measurement Methods for Black Carbon Aerosols . Atmos. Environ. , 33 : 2823 – 2833 .
- Huntzicker , J. J. , Johnson , R. L. , Shah , J. J. and Cary , R. A. 1982 . “ Analysis of Organic and Elemental Carbon in Ambient Aerosol by a Thermal-Optical Method ” . In Particulate Carbon: Atmospheric Life Cycle , Edited by: Wolff , G. T. and Klimisch , R. L. pp. 79 – 88 . New York : Plenum Press .
- Keywood , M. D. , Ayers , G. P. , Gras , J. L. , Gillett , R. W. and Cohen , D. D. 1999 . Relationships between Size Segregated Mass Concentration Data and Ultrafine Particle Number Concentration in Urban Areas . Atmos. Environ. , 33 : 2907 – 2913 .
- Kuhlbusch , T. A. J. , Hertlein , A.-M. and Schutz , L. W. 1998 . Sources, Determination, Monitoring, and Transport of Carbonaceous Aerosols in Mainz, Germany . Atmos. Environ. , 32 ( 6 ) : 1097 – 1110 .
- Lavanchy , V. M. H. , Gaggeler , H. W. , Nyeki , S. and Baltensperger , U. 1999 . Elemental Carbon (EC) and Black Carbon (BC) Measurements with a Thermal Method and an Aethalometer at the High-Alpine Research Station Jungfraujoch . Atmos. Environ. , 33 : 2759 – 2769 .
- Lim , H.-J. , Turpin , B. J. , Edgerton , E. , Hering , S. V. , Allen , G. , Maring , H. and Solomon , P. 2003 . Semicontinuous Aerosol Carbon Measurements: Comparison of Atlanta Supersite Measurements, . J. Geophys. Res. , 108 ( D7 ) : 7/1 – 11 .
- Lin , C.-I. , Baker , M. and Charlson , R. J. 1973 . Absorption Coefficient of Atmospheric Aerosol: A Method for Measurement . Appl. Opt. , 12 ( 6 ) : 1356 – 1363 .
- Liousse , C. , Cachier , H. and Jennings , S. G. 1993 . Optical and Thermal Measurement Methods of Black Carbon Aerosol Content in Different Environments: Variation of The Specific Attenuation Cross-Section, Sigma (σ) . Atmos. Environ. , 27 ( 8 ) : 1203 – 1211 .
- Mader , B. T. and Pankow , J. F. 2001 . Gas/solid Partitioning of Semivolatile Organic Compounds (SOCs) to Air Filters. 3. An Analysis of Gas Adsorption Artifacts in Measurements of Atmospheric SOCs When Using Teflon Membrane Filters and Quartz Fiber Filters . Environ. Sci. Technol. , 35 : 3422 – 3432 .
- McDow , S. R. and Huntzicker , J. J. 1990 . Vapor Adsorption Artifact in the Sampling of Organic Aerosol: Face Velocity Effects, . Atmos. Environ. , 24A ( 10 ) : 2563 – 2571 .
- McMurry , P. H. and Zhang , X. Q. 1989 . Size Distributions of Ambient Organic and Elemental Carbon . Aerosol Sci. Technol. , 10 : 430 – 437 .
- McMurry , P. H. , Zhang , X. Q. and Lee , C.-T. 1996 . Issues in Aerosol Measurements for Optics Assessments, . J. Geophys. Res. , 101 ( D14 ) : 197 – 19 .
- Mertes , S. , Dippel , B. and Schwarzenbock , A. 2004 . Quantification of Graphitic Carbon in Atmospheric Aerosol Particles by Raman Spectroscopy and First Application for the Determination of Mass Absorption Efficiencies . Aerosol Sci. , 35 : 347 – 361 .
- Moosmuller , H. , Arnott , W. P. , Rogers , C. F. , Chow , J. C. , Frazier , C. A. , Sherman , L. E. and Dietrich , D. L. 1998 . Photoacoustic And Filter Measurements Related to Aerosol Light Absorption During the Northern Front Range Air Quality Study (Colorado 1996/1997), . J. Geophys. Res. , 103 ( D21 ) : 157 – 28 .
- NIOSH . 1999 . “ National Institute for Occupational Safety and Health (NIOSH) Method 5040, Elemental Carbon (Diesel Particulate) ” . In NIOSH Manual of Analytical Methods (NMAM) , 4th Ed.
- Peterson , M. R. and Richards , M. H. 2002 . “ Thermal-Optical-Transmittance Analysis for Organic, Elemental, Carbonate, Total Carbon, and OCX2 in PM2.5 by the EPA/NIOSH Method ” . In Air and Waste Management Association Symposium on Air Quality Measurement Methods and Technology—2002 San Francisco, CA 13–15 November
- Petzold , A. , Kopp , C. and Niessner , R. 1997 . The Dependence of the Specific Attenuation Cross-Section on Black Carbon Mass Fraction and Particle Size . Atmos. Environ. , 31 ( 5 ) : 661 – 672 .
- Petzold , A. , Kramer , H. and Schonlinner , M. 2002 . Continuous Measurement of Atmospheric Black Carbon Using a Mult-angle Absorption Photometer . Environ Sci. & Pollut. Res. , 4 : 78 – 82 .
- Research Triangle Park (RTP) . 2002 . Research Triangle Park Facts, 28 February 2002 Available at
- Rupprecht , G. , Patashnick , H. , Beeson , D. E. , Green , R. N. and Meyer , M. B. 1995 . “ A New Automated Monitor for the Measurement of Particulate Carbon in the Atmosphere ” . In Particulate Matter: Health and Regulatory Issue Pittsburgh, PA 4–6 April 1995
- Ruprecht and Patashnick Co., Inc. 2002 . Operating Manual, Series 5400 Ambient Particulate Monitor, January 2002, Revision B
- Schmid , H. P. , Laskus , L. , Abraham , H. J. , Baltensperger , U. , Lavanchy , V. M. H. , Bizjak , M. , Burba , P. , Cachier , H. , Crow , D. J. , Chow , J. C. , Gnauk , T. , Even , A. , ten Brink , H. M. , Giesen , K. P. , Hitzenberger , R. , Hueglin , C. , Maenhaut , W. , Pio , C. A. , Puttock , J. , Putaud , J. P. , Toom-Sauntry , D. and Puxbaum , H. 2001 . Results of the Carbon Conference International Aerosol Carbon Round Robin Test: Stage 1, . Atmos. Envrion. , 35 ( 12 ) : 2111 – 2121 .
- Seinfeld , J. H. and Pandis , S. N. 1998 . Atmospheric Chemistry and Physics: From Air Pollution to Climate Change , New York, NY : John Wiley & Sons .
- Sharma , S. , Brook , J. R. , Cachier , H. , Chow , J. , Gaudenzi , A. and Lu , G. 2002 . Light Absorption and Thermal Measurements of Black Carbon in Different Regions of Canada, . J. Geophys. Res. , 107 ( D24 ) : 11/1 – 11 .
- Sturm , P. J. , Baltensperger , U. , Bacher , M. , Lechner , B. , Hausberger , S. , Heiden , B. , Imhof , D. , Weingartner , E. , Prevot , A. S. H. , Kurtenbach , R. and Weisen , P. 2003 . Roadside Measurements of Particulate Matter Size Distribution . Atmos. Environ. , 37 : 5273 – 5281 .
- Tolocka , M. P. , Solomon , P. A. , Mitchell , W. , Norris , G. A. , Gemmill , D. B. , Weiner , R. W. , Vanderpool , R. W. , Homolya , J. B. and Rice , J. 2001 . East versus West in the US: Chemical Characteristics of PM2.5 During Winter of 1999 . Aerosol Sci. Technol. , 34 ( 1 ) : 88 – 96 .
- Turpin , B. J. , Huntzicker , J. J. and Hering , S. V. 1994 . Investigation of Organic Aerosol Sampling Artifacts in the Los Angeles Basin . Atmos. Environ. , 28 ( 19 ) : 3061 – 3071 .
- Turpin , B. J. , Saxena , P. and Andrews , E. 2000 . Measuring and Simulating Particulate Organics in the Atmosphere: Problems and Prospects, . Atmos. Environ. , 34 : 2983 – 3013 .
- U.S. Environmental Protection Agency (USEPA) . 1997 . 62 FR 38763, Revised Requirements for Designation of Reference and Equivalent Methods for PM2.5 and Ambient Air Quality Surveillance for Particulate Matter, Final Rule U.S. Environmental Protection Agency, 40 Code of Federal Regulations Parts 53 and 58, Federal Register, 18 July 1997
- U.S. Environmental Protection Agency (USEPA) . May 2001 . Evaluation of PM2.5 Chemical Speciation Samplers for use in the EPA National Chemical Speciation Network , Research Triangle Park, NC : USEPA, Office of Air Quality Planning and Standards . EPA-454/R-01–008
- U.S. Environmental Protection Agency (USEPA) . September 2002 . Latest Findings on National Air Quality: 2001 Status and Trends , Research Triangle Park, NC : USEPA, Office of Air Quality Planning and Standards . Available at
- Watson , J. G. and Chow , J. C. 2002 . Comparison and Evaluation of In Situ and Filter Carbon Measurements at the Fresno Supersite, . J. Geophys. Res. , 107 ( D21 ) : 3/1 – 15 .
- Watson , J. G. , Cooper , J. A. and Huntzicker , J. J. 1984 . The Effective Variance Weighting for Least Squares Calculations Applied to the Mass Balance Receptor Model . Atmos. Environ. , 18 : 1347 – 1355 .
- Wolff , G. T. , Groblicki , P. J. , Cadle , S. H. and Countess , R. J. 1982 . “ Particulate Carbon at Various Locations in the United States ” . In Particulate Carbon Atmospheric Life Cycle , Edited by: Wolff , G. T. and Klimisch , R. L. New York : Plenum Press .
- Yu , J. Z. , Xu , J. and Yang , H. 2002 . Charring Characteristics of Atmospheric Organic Particulate Matter in Thermal Analysis . Environ. Sci. Technol. , 36 : 754 – 761 .
- Zhang , X. Q. and McMurry , P. H. 1987 . Theoretical Analysis of Evaporative Losses from Impactor and Filter Deposits . Atmos. Environ. , 21 : 1779 – 1789 .
- Zhang , X. Q. and McMurry , P. H. 1991 . Theoretical Analysis of Evaporative Losses of Adsorbed and Absorbed Species During Atmospheric Aerosol Sampling . Environ. Sci. Technol. , 25 : 456 – 459 .