Abstract
Fume agglomeration frustrates electron microscopy of individual primary particles. Lauric acid was added to an ethanol suspension of metal oxide fume particles collected from arc welding in order to create a dense suspension that minimized interparticle contact. Evaluation of transmission electron micrographs demonstrated that the surfactant aided dispersion and therefore analysis of individual particles.
INTRODUCTION
Fumes are comprised of agglomerates of metal oxide nanoparticles formed during combustion or high-temperature metallurgical processing, such as welding. Primary particles in welding fumes range from a few nanometers to a few hundred nanometers. The agglomerates are 0.01–2 micrometers (CitationVoitkevich 1995).
Agglomeration impedes examination of individual primary particles. Since successful microscopy requires many particles per micrograph, dilution is not an efficient solution. This paper describes a method for the preparation of a dense, contact–free suspension of welding fumes by adding a surfactant.
Such a suspension would ease the investigation of the chemical composition of individual primary particles, which is important in determining the possible toxicity of fumes inhaled by workers.
This study also demonstrates that agglomerates on microscope grids are not necessarily the same shape or size as agglomerates in the air or in the lungs because the environment may affect agglomeration. This is relevant to the characterization of aerosol inhalation. CitationRudell et al. (1988) compared welding fume agglomerates before and after inhalation and found that agglomerate size changes, presumably from the humidity. CitationKendall et al. (2002) proposed that fluid in the lungs increases agglomeration of inhaled particles. Studying the effect of surfactants on aerosol particle agglomeration outside the human body may shed light on inhalation toxicology.
Experimentation with lauric acid (CitationMoore et al. 2001) as a surfactant in ethanol (CitationKimura and Bandow, 1983) yielded promising results for the dispersion of iron/manganese oxide welding fume particles.
EXPERIMENTAL METHODS
Fume was collected during gas metal arc welding (GMAW) of mild steel electrode wire (AWS ER70S-3, 98.0 wt% Fe, 1.2 wt% Mn, 0.6 wt% Si, 0.2 wt% Cu) in a welding chamber (CitationQuimby and Ulrich 1999) by drawing air at a rate of ∼2 l/min through polycarbonate filters (0.2 μm pores) stationed ∼30 cm above the arc. Samples were prepared for transmission electron microscopy (TEM) by shaking fume off filters. For each sample, 0.09 g of fume was added to 30 ml of ethanol. The fume was suspended through 30 s of ultrasonication. Drops of the suspension were pipetted onto TEM grids (copper film reinforced with carbon) where the ethanol evaporated. The fume–ethanol ratio controlled the particle number density.
Lauric acid–ethanol solutions of various concentrations were added to fume suspensions as surfactant. Surfactant solutions were prepared by dissolving 0.01 moles of lauric acid (C12H24O2) in ethanol in concentrations from 10−5 to 1 mol/l. The solutions were mixed with an ultrasonic probe for 2 min to ensure dissolution. 2.5 ml of fume suspension (containing 0.0075 g fume) was combined with 1 ml of surfactant solution followed by ultrasonication for 2 min. Droplets of this mixture was then dripped onto TEM grids and allowed to dry.
To quantify dispersion, micrographs of two suspensions were analyzed. The former came from 2 mg of fume in 1 ml of ethanol without surfactant. The latter was created from 2 mg of fume in 1 ml of ethanol with 5 × 10−4 mol/l lauric acid. Twenty-one micrographs of each sample at 10,000x magnification were captured. Each micrograph covered a randomly selected area (9.3 μm by 6.7 μm). They were scanned and analyzed on a Macintosh Powerbook G4 computer using the public domain NIH Image program (developed at the US National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
The perimeter of the image of each agglomerate was measured, except when intersected by the micrograph edge. This was accomplished by counting the number of pixels along lines of varying contrast. The areas of the two-dimensional images of the primary particles in each agglomerate were also measured.
Because NIH Image is limited in the number of pixels it can measure at once, the images were scanned at 300 pixels per inch of micrograph, which translated to 8.5 nm (of the magnified image) per pixel. Therefore, noise in the images was often interpreted as small nanoparticles. Also, particles smaller than ∼50 nm were difficult to distinguish because of a lack of contrast and focus at that scale. Therefore, all features that had both a perimeter and an area of less than that of a diameter circle (∼0.16 μm and ∼0.002 μm2, respectively) were ignored. Since the goal was to study agglomeration, ignoring the smallest particles did not alter the analysis, because particles smaller than 50 nm are almost exclusively single particles.
The summed area of the same number of similarly sized primary particles does not change with the degree of dispersion. However, the summed perimeter increases with dispersion, because there is less particle-to-particle contact. If the surfactant dispersed the fume, then the ratio of the total perimeter to the total area of the primary particles would be greater in the surfactant-aided suspension.
RESULTS AND DISCUSSION
The primary particles prepared without surfactant (top halves of , ) can be seen to be in close contact with each other, often overlapping above and below.
FIG. 1 (top) Transmission electron micrograph of 7.5 mg mild steel GMAW fume in 2.5 ml ethanol. (bottom) Transmission electron micrograph of 7.5 mg mild steel GMAW fume in 3.5 ml of lauric acid–ethanol solution (0.29 moles/l).
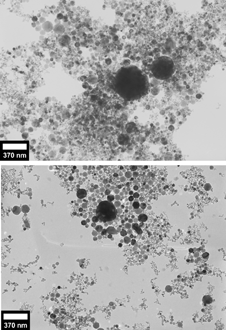
FIG. 2 (top) Transmission electron micrograph of 7.5 mg of mild steel GMAW fume in 2.5 ml ethanol. (bottom) Transmission electron micrograph of 6.0 mg mild steel GMAW fume in 3 ml of lauric acid–ethanol solution (3.33 × 10−5 moles/l).
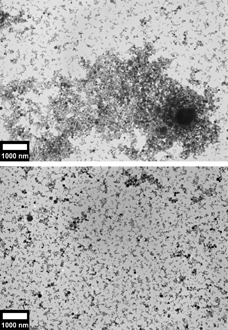
This contrasts with the images of the suspensions created by adding lauric acid (bottom halves of , ). Large agglomerates cannot be found in these suspensions. In regions where the particles are densely packed, the particles are easily distinguishable.
The data in confirms that the surfactant did indeed disperse the fume particles. The average area covered by agglomerates was essentially the same, but the ratio between the summed perimeter and summed particle area was noticeably different.
TABLE 1 Image analysis of two groups of 21 micrographs (10000×) of fume particles, one prepared without surfactant, one prepared with 5 × 10−4 moles/l lauric acid in ethanol
CONCLUSIONS
A dense suspension of metal oxide fume nanoparticles formed from arc welding was prepared in ethanol, with the addition of lauric acid as a surfactant. It was found that lauric acid limited agglomeration, which can aid the investigation of individual fume primary particles.
Acknowledgments
The U.S. Navy, Office of Naval Research, funded this project. Professors John Vander Sande and Dr. Lenore Rainey of Massachusetts Institute of Technology and Professor Kwadwo Osseo-Asare of the Pennsylvania State University provided advice, for which the authors are grateful.
REFERENCES
- Kendall , M. , Tetley , T. D. , Wigzell , E. , Hutton , B. , Nieuwenhuijsen , M. and Luckham , P. 2002 . Lung Lining Liquid Modifies PM2.5 In Favor Of Particle Aggregation: A Protective Mechanism . Am. J. Physiol. – Lung C , 282 : L109 – L114 .
- Kimura , K. ,. and Bandow , S. 1983 . The Study Of Metal Colloids Produced By Means Of Gas Evaporation Technique. I. Preparation Method And Optical Properties In Ethanol . B. Chem. Soc. Jpn. , 56 : 3578 – 3584 .
- Moore , R. , Evans , S. D. , Shen , T. and Hodson , C. 2001 . Room-Temperature Single-Electron Tunneling In Surfactant Stabilized Iron Oxide Nanoparticles . Physica E. , 9 : 253 – 261 .
- Quimby , J. B. ,. and Ulrich , G. D. 1999 . Fume Formation Rates In Gas-Shielded Metal Arc Welding . Weld. J. , 78 : 142 – 149 .
- Rudell , B. , Akselsson , K. R. and Berlin , M. H. 1988 . Growth of Welding Aerosol Particles in High Relative Humidity and Particle Deposition in the Human Respiratory Airways . J. Aerosol Sci. , 19 : 1153 – 1156 .
- Voitkevich , V. 1995 . Welding Fumes: Formation, Properties and Biological Effects , Cambridge , , England : Abington Publishing .