Abstract
Real-time continuous PM2.5 mass measurements were made by a continuous ambient mass monitor (CAMM), a real-time total ambient mass sampler (RAMS), and a tapered element oscillating microbalance (TEOM) in Houston during summer and in Seattle during winter. Hourly PM2.5 mass concentrations measured by the three samplers were compared with one another to evaluate the sampling performance for total ambient PM2.5 mass measurements. Reasonably good agreement was observed between pairs of the continuous mass samplers. The regression parameters were calculated between PM2.5 mass concentrations of the instruments.
The mass difference of PM2.5 between the continuous adjusted RAMS and 30°C TEOM in Houston with relative humidity and temperature suggests that the loss of semivolatile ammonium nitrate contributed to the mass difference. The results also suggest that setting the TEOM at a lower temperature than the standard configuration still could lose semivolatile materials and thus would not be enough to measure total ambient PM2.5 mass.
The continuous PM2.5 mass samplers showed consistently higher PM mass concentrations than the integrated PM2.5 mass sampler measurements in Houston. The majority of the mass difference between the continuous and integrated PM2.5 mass resulted from the loss of semivolatile ammonium nitrate and organic compounds from the integrated PM2.5 samples. The results in Seattle showed more comparable values between the continuous and integrated PM2.5 masses, which was probably due to decreased volatilization loss of semivolatile materials from the integrated sample filters at the colder winter temperature. Some of the 30°C TEOM samples showed lower mass concentrations than the integrated mass monitor because of loss of semivolatile materials at 30°C. There was a suggestion that some of the RAMS mass measurements underestimated the total ambient PM2.5 mass concentration in Seattle.
INTRODUCTION
Atmospheric aerosol is a mixture of inorganic and organic species that contributes to adverse public health effects, visibility impairment, regional haze, and acid rain. The United States Environmental Protection Agency (USEPA) promulgated new National Ambient Air Quality Standards (NAAQS) for PM2.5 (fine particulate matter with an aerodynamic diameter less than 2.5 μm) in 1997 to protect public health from air pollution. With this promulgation, a federal reference method (FRM) was required to measure PM2.5 mass concentrations, as a consistent method that resembled the dichotomous samplers. The 24 h integrated single-filter-based mass measurement is the most common method used to collect airborne particulate matter (PM).
Several drawbacks of single-filter measurement of PM mass have been proposed (CitationChung 2001). The main disadvantage is the fact that they do not provide information in real time. There are often days or weeks of time lag between when samples are collected and when PM2.5 data become available. The second is that they require long sampling times of hours to acquire sufficient PM mass for accurate analysis. Long sampling time averages periods of high and low PM2.5 concentrations, making it difficult to identify the concentration spikes of short duration that may be responsible for some of the health effects that are observed to be associated with increased concentrations of airborne PM (CitationChung 2001). The possibility of sample contamination during sampling and postsample handling and processing, its labor-intensive nature, and relatively expensive cost are considered to be other drawbacks related to filter-based aerosol-sampling techniques.
One important issue associated with long sampling intervals of single-filter samplers is that semivolatile materials such as ammonium nitrate and organic compounds may be lost from particles that deposit on a filter of an integrated sampler. It could possibly lead to inaccurate measurement of ambient aerosol mass, which in turn hinders the determination of the attainment area of PM2.5 in compliance with the NAAQS.
Several real-time particle monitors have been developed, and their performances have been evaluated on how capable they are of accurately measuring PM2.5 concentrations under the different conditions. The Rupprecht and Patashnick tapered element oscillating microbalance (TEOM) method was compared with the FRM method in measurements of PM2.5 and PM10 in urban US areas in different seasons (CitationAllen 1997; CitationChung 2001). Their results showed that the TEOM had a greater tendency to underestimate PM2.5 mass than did the FRM due to heating the filters at 50°C to dry particle-bound water, which also resulted in volatilization of ammonium nitrate from particles collected on the filter. The continuous ambient mass monitor (CAMM) has also been compared with integrated samplers. Good agreements were shown between the CAMM and integrated PM2.5 mass concentrations (CitationBabich 2000; CitationChung 2001). A different type of real-time continuous sampler, the real-time total ambient mass sampler (RAMS), has also been compared with the TEOM and the FRM in US cities (CitationPang 2002; CitationLong 2003). The TEOM PM2.5 measurements were generally lower than the RAMS measurements due to the temperature issue mentioned above. The FRM PM2.5 mass appeared to be comparable with the RAMS mass or even higher during cold and humid periods due to the decreased mass contribution of semivolatile materials and large contribution by water. However, at warmer and drier conditions, the RAMS measurements had higher PM2.5 mass than the FRM.
Few studies have been conducted for the cross comparison of the three continuous samplers (i.e., the RAMS, CAMM, and 30°C TEOM with the sample equilibration system (SES)) for their evaluation of performance. In addition, since most TEOM filters were heated to 50°C to remove particle-bound water, loss of semivolatile compounds is expected to be dominant in the measurement of PM2.5 in urban areas, making it difficult to evaluate the performance of the TEOM compared with other continuous samplers. The SES is a Nafion drier that selectively removes water vapor from the air stream and reduces the amount of particle-bound water (CitationMeyer 2000).
The main purpose of this study is to provide comprehensive evaluation for the performance of these three real-time continuous PM2.5 measurements, including the 30°C TEOM for which filters were heated at a lower temperature than the standard configuration. PM2.5 mass concentrations from the continuous samplers were compared to each other and were also compared to the integrated PM2.5 mass concentrations. In addition, aerosol composition data from colocated continuous and integrated measurements were used to examine the effects of semivolatile aerosol composition on the measured PM2.5 mass values.
MEASUREMENTS
Site Description
A series of intensive measurements of PM2.5 mass concentrations were conducted from12 August to 15 September 2000 at LaPorte Municipal Airport (29.6461N, 95.0564W, elevation 6 m) in Houston, TX as part of the Texas Air Quality Study 2000 (TexAQS 2000; CitationDaum 2002). The TexAQS 2000 study was designed to improve the understanding of the factors that control the formation and transportation of ozone and particulate matter along the Gulf Coast of southeast Texas. The Houston site was supported by the USEPA to improve the understanding of airborne particulate matter and the atmospheric processes occurring in this industrialized area. The Houston-Galveston urban area are the focus of this study because it has a significant ozone pollution problem and would presumably have particle compositions dominated by organic carbon species. The Houston site is located on the shore of Galveston Bay, near the Houston Ship Channel, about 16 km east of downtown Houston. This area is heavily industrialized and the eastern half of Texas, including the LaPorte site, includes major urban areas. The eastern half of the state experiences its highest concentrations of ozone and fine particles during summer.
Sampling in Seattle was performed from 28 January to 21 February 2001 at Beacon Hill (47.5693N, 122.3106W, elevation 91 m) in Seattle, WA. The site, in an urban area near the Beacon Hill Reservoir, is located near US Route 50 and interstate highway I-90, north of the site, and is about 8 km southeast of downtown Seattle. Seattle is considered to be a site typically affected by wood smoke in winter when wood stoves are commonly used. During the colder periods in winter, layered haze from wood smoke can be easily seen in low-lying areas (CitationSheppard 2001). Seattle was chosen in the hope that there would be some days dominated by wood smoke. However, during the time of this sampling campaign weather was relatively mild. This site was operated in conjunction with the EPA Northwest Center for Particulate Matter and Health. Aerosol number-size distribution was also monitored at the site in order to measure and identify the variability of aerosol number and concentration, but it was not used in this study.
These locations were selected in order to obtain easily intensive chemical data so that any differences in the observed mass values among the continuous mass monitors or between the integrated and continuous monitors could be related to the composition of the ambient aerosol. Other PM2.5 mass and chemical species data, either continuous or integrated, were obtained simultaneously by other groups who participated in the measurements. These locations permitted direct comparisons with a variety of other continuous measurements of chemical components in the ambient aerosol.
Mass Measurements
Three different particle samplers, a CAMM, a RAMS, and a 30°C dried TEOM monitor, were used for real-time (1 h basis) determination of PM2.5 mass concentrations in both Houston and Seattle. For 24 h integrated mass measurements, an R&P Partisol-Plus PM2.5 sequential sampler was deployed in Houston and an URG mass aerosol speciation sampler (MASS) was deployed in Seattle. summarizes the configuration of the PM mass samplers used in this study.
Table 1 Sampler configuration of continuous and integrated samplers used for PM2.5 mass measurements in Houston and Seattle
Continuous Ambient Mass Monitor
The CAMM developed at the Harvard School of Public Health was used to collect particles on a short time (typically 30–60 min) basis. It minimizes volatilization and absorption artifacts due to changes in concentration and composition during sampling (CitationBabich 2000). The CAMM in this study was a commercial unit supplied by Thermo Andersen (Smyrna, GA, USA). It has now been withdrawn from the market. The measurements of the system are based on the determination of the increase in pressure drop across a Teflon membrane filter (FluoroporeTM) during particle sampling. The monitor consisted of a WINS impactor/inlet to remove particles larger than 2.5 m, a diffusion dryer to remove particle-bound water, a filter tape to collect particles, a filter tape transportation system to allow unassisted sampling, and a data acquisition and control unit. For each sampling period (1 h in this study), a new segment of the filter tape was exposed to the air stream so that particles would remain close to equilibrium with the sample air during their collection. The air flow is as low as 0.3 l/min. The full description of this process can be found in CitationBabich (2000).
Real-Time Total Ambient Mass Sampler
The RAMS developed by Brigham Young University was also used to determine total (nonvolatile + semivolatile) PM2.5 mass. It is based on diffusion denuder and dual TEOM monitor technology (CitationEatough 1999, Citation2001, Citation2003). The RAMS consisted of a combined impactor–particle concentrator inlet to separate PM2.5 from coarse particles; two triethanolamine (TEA)-coated annular denuders (University Research Glass) to remove O3 and NO2; a Nafion dryer (Perma Pure Inc., Model PD-750-1) to remove gas-phase water; a charcoal-impregnated multiparallel filter (CIF) diffusion denuder to remove gas-phase semivolatile organic material, inorganic gases (HNO3, NH3, SO2), and oxidants (O3 and H2O2); a second TEA-coated annular denuder to remove any NO2 formed from NO on the CIF denuder; a second Nafion dryer to remove gas-phase water from the sampled air stream; a sandwich filter containing a Teflon coated (Rupprecht & Patashnick (R&P) TX40) particle collection filter followed by a charcoal-impregnated glass (CIG) fiber filter (Schleicher and Schuell, Dassel, Germany) to collect any semivolatile species lost from the particles during sampling; and a TEOM monitor to measure total mass of collected PM2.5 (CitationPang 2002).
Among total air flow (16.7 l/min), a flow of 5 l/min was used for particle collection, which splits into two 2.5 l/min streams for particle and active blank measurements after the first Nafion dryer. The face velocity at the filter was 24 cm s−1. Each sample flow was passed through a CIF denuder, the second TEA-coated annular denuder, and a Nafion drier in turn, and then the sample was monitored with a separate TEOM. The blank system corrects for any gas-phase species that will be absorbed by the CIG of the TEOM “sandwich filter” but that are not removed by the various denuders and dryers.
One of the parameters that needs to be measured in the RAMS is the efficiency of the concentrator. An in-line filter was placed in the major flow from the concentrator. The amount of sulfate on this filter, as well as on the filter in the “blank” side of the RAMS, were measured by leaching the filters and ion chromatography (IC). In Houston, the particle concentrator in the RAMS was found to have an unusably high collection efficiency of over 90%. Compared to the concentrator efficiency (ranging 50–70%) obtained in the previous studies (CitationEatough 1999; CitationModey 2001), this value appeared to be anomalous. The Seattle value of the concentrator was about 65%, which is consistent with the range typically observed (D. Eatough, private communication). If the Seattle concentrator efficiency was applied to the Houston data, the Houston RAMS value would be increased by a factor of 1.52 (i.e., the ratio of the original Houston concentrator efficiency to the Seattle concentrator efficiency). Thus, in this article the adjusted values of the mass will be given for the Houston data.
Tapered Element Oscillating Microbalance
The TEOM (R&P, Inc.), one of the continuous-measurement instruments, is now widely used and well known. The 30°C dried TEOM consisted of a sharp-cut cyclone PM2.5 inlet (CitationKenny 2000), a Nafion dryer that allows conditioning of the PM sample stream at a lower humidity level maintained by a SES (CitationMeyer 2000), and a 47 mm Teflon filter system with the TEOM sensor. A 3.0 l/min filter flow was used for particle collection, and the face velocity was 37.7 cm s−1. The measurement principle of the instrument is described in detail elsewhere (CitationPatashnick and Rupprecht 1991). Since semivolatile PM will evaporate at default temperature, 50°C, the operating temperature was set to 30°C in this study and a Nafion dryer was added after the inlet to reduce RH enough so that water vapor will not adsorb on the particles during sample collection.
Partisol-Plus PM2.5 Sequential Sampler
Integrated PM2.5 mass were measured daily for 24 h using a Partisol-Plus R&P 2025 PM2.5 sequential sampler in Houston. In this study, PM2.5 particles passed in parallel through 47 mm Polytetrafluoroethylene (PTFE) and quartz fiber filters downstream of a PM2.5 cut-size WINS impactor inlet (CitationRussell 2004). The inlet flow rate of the sampler was 16.7 l/min. After the inlet, the air was split into separate channels with a flow rate of 8.35 l/min. Total mass was determined gravimetrically from the PTFE filters. The same filters were used for determination of elements by X-Ray Fluorescence (XRF). The quartz filters were used for organic carbon and elemental carbon (OC and EC, respectively) and ion analyses. The OC and EC collected were analyzed by the thermal optical transmittance (TOT) method. The measured PM2.5 mass was used in the comparison with the continuous mass monitors.
Table 2 Sampler characteristics and sampling information for PM2.5 speciation instruments used in the study
Mass Aerosol Speciation Sampler
An URG-MASS sampler was deployed by Washington State Department of Ecology in Seattle for integrated PM2.5 mass measurements. Sampling was made every 3 days over a 24 h period. The sampler consisted of two modules, each with a size-selective inlet and WINS PM2.5 cut-size impactor. One module was equipped with a Na2CO3-coated annular denuder to remove acidic gases in the air stream and a dual filter pack. The top 47 mm Teflon filter was analyzed for PM2.5 mass and chemical elements by XRF. The second Nylon (Nylarsorb) filter was used to collect volatile nitrate from the preceded Teflon filter. The other module was equipped with a single-filter pack with a quartz fiber filter for OC/EC and ionic species determination. A nominal air flow rate was 16.7 l/min for each module. Total PM2.5 mass determined gravimetrically from Teflon filters was used for comparison with the continuous PM2.5 mass.
Speciation Measurements
A few PM2.5 speciation measurements were carried out by other groups. summarizes instrument characteristics that the speciation samplers used. A particle composition monitor (PCM) was used for PM2.5 speciation sample collection in Houston. The PCM was equipped with 3 separate mass-flow-controlled channels for PM2.5 sampling on discrete time scales between 6 and 24 h, depending on the pollution level. The first and second channel consisted of denuders that were coated with phosphorous acid and sodium carbonate solutions to remove acidic and basic gases, a Teflon filter to collect PM mass, and a cellulose backup filter (Whatman 41) to collect semivolatile ammonium nitrate that evaporated from the preceded Teflon filter. Backup filters were also coated with the same solutions used in the denuders. In the third channel, OC and EC were caught on a quartz fiber filter (Pallflex 2500 QAT-UP), with a preceding XAD-4 denuder for semivolatile organic gases removal and a XAD-4 quartz backup filter for semivolatile organic compounds volatilized from particles on the quartz filter (CitationBaumann 2003). The PCM sampler provided the artifact-free OC concentrations.
A particle-into-liquid-sampler (PILS) coupled with an IC was also used to measure PM2.5 nitrate and ammonium every 15 min in Houston. Particles are exposed to a saturated vapor to grow to large drops that are easily collected (CitationWeber 2001). The resulting liquid containing dissolved aerosol components is analyzed by IC. Water vapor supersaturation is achieved by turbulently mixing ambient air with the stream. A PM2.5 size cut inlet and denuders coated with sodium carbonate and citric acid were used in the PILS (Lee 2001).
A particle concentrator, Brigham Young University organic sampling system (PC-BOSS) described by CitationEatough (1999) and CitationObeidi and Eatough (2002), was used to collect 24 h integrated samples in Seattle. The PC-BOSS is a combination of a BOSS (CitationTang 1994) and a Harvard particle concentrator (CitationSioutas 1994). Ambient particles of less than 2.3 μm aerodynamic diameter were sampled using a Bendix cyclone at an inlet flow of 150 l/min (Chan and Lippmann 1971) followed by a virtual impactor particle concentrator. The minor flow (25% of the inlet flow) containing concentrated particles entered the BOSS diffusion denuder to remove gas-phase organic compounds O3, HNO3, and SO2. The major flow gases and particles <0.1 μm. The denuder is followed by two parallel filter packs. One filter pack contained a 47 mm prefired quartz filter (Pallflex) followed by a 47 mm charcoal-impregnated cellulose filter (Schleicher and Schuell) to determine fine particulate carbonaceous materials and nitrate, including semivolatile organic material or nitric acid lost from the particles during sampling. The other filter pack contained a 47 mm Teflon (Gelman Zeflour) filter followed by a 47 mm Nylon (Gelman Nylasorb) filter to determine fine particulate mass, sulfate, and nitrate, including any nitrate lost from particles. Carbonaceous particles were analyzed by temperature- programmed volatilization (TPV; CitationTang 1994), and ions were analyzed by IC.
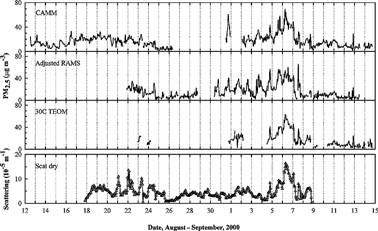
Figure 1 Variation of hourly PM2.5 mass collected using continuous instruments and scattering coefficients measured in Houston, TX. The RAMS values plotted were adjusted by a factor of 1.52.
Light scattering was measured using integrating nephelometers (Radiance Research, M903) with and without a Nafion dryer at the Houston and Seattle sites. The nephelometers were lightweight, low-power instruments designed for portable operation as well as general environmental monitoring. They measured the light-scattering extinction using an optical filter at a wavelength of 530 nm. Data from the nephelometer with a dryer was used in Houston since the nephelometer without a dryer malfunctioned.
Meteorological data such as ambient temperature (T) and relative humidity (RH) were also obtained by other groups who were collecting the integrated PM2.5 mass data.
Table 3 Mean concentration (μg m−3) of valid hourly PM2.5 mass during the measurement period
RESULTS AND DISCUSSION
Intercomparison of Continuous PM2.5 Mass
shows the hourly variation of PM2.5 mass from the continuous methods and the scattering coefficients in Houston (LaPorte site), along with T and RH. RAMS values plotted are the adjusted ones by a factor of 1.52. There were two periods of high PM2.5 concentration events during the measurement period: during 16–24 August and 4–7 September. The arithmetic mean mass concentrations of the CAMM, adjusted RAMS, and 30°C TEOM during the August event period were not highly different from the average mass concentrations of the sampler over the whole period, whereas those during the September event period were 29.8 μg m−3, the same for the three methods, which were higher than the average mass concentrations shown in . In particular, the peaks during 4–7 September 2000 resulted from marsh fires that occurred on 4 September near Beaumont, TX about 95 km northeast of the LaPorte site.The highest PM2.5 mass concentrations were observed on 6 and 7 September, with an hourly maximum of 69 μg m−3 for the CAMM, 66 μg m−3 for the adjusted RAMS, and 63 g m−3 for the 30°C TEOM. The adjusted RAMS showed the maximum concentration several hours later than the other two. The reason for the lag of the adjusted RAMS mass is not obvious. These concentrations were about 3–4 times higher than the average mass over the whole period. The increased PM concentrations during the day were likely influenced by the transport of fire emissions from Beaumont to the site area. The dried nephelometer tracked well with the mass variation, with the exception of spikes around 21–24 August.
Figure 2 Variation of hourly PM2.5 mass collected using continuous instruments and scattering coefficients measured in Seattle, WA.
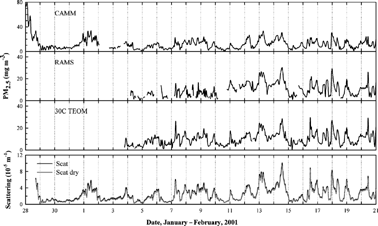
depicts variation of hourly PM2.5 mass concentrations in Seattle (Beacon Hill site). Compared to the Houston results, hourly variation of PM2.5 mass concentrations showed good correspondence to one another. In addition, the two nephelometer results showed good covariance. The RH in Seattle had a larger oscillation than in Houston and was generally higher when PM mass increased.
After quality control, a total of 204 sampling intervals could be used for each method for intercomparison of the continuous methods in Houston, and a total of 362 intervals were available from each method for the intercomparison in Seattle. The average PM2.5 mass concentrations at both sites for the contemporaneous samples are given in . For the Houston data, the adjusted RAMS values are given here and the original values can be calculated by dividing by 1.52.
To examine the agreement in measurements of the three samplers statistically, an analysis of variance (ANOVA) was carried out for PM2.5 mass concentrations of pairs of the samplers at each site. In Houston, the average mass concentrations of those 204 contemporaneous samples were 20.4 μg m−3 for the CAMM, 18.5 μg m−3 for the adjusted RAMS, and 18.6 μg m−3 for the 30°C TEOM. The CAMM showed the highest mass concentration, with a value about 9% higher than the TEOM or the adjusted RAMS. The ANOVA results showed no significant difference between each pair of the mass at a confidence level of 0.05. This result suggests that all instruments have the similar performance in measurement of the variation of PM2.5 mass in the air. In Seattle, the average PM2.5 mass for the 362 contemporaneous samples was 12.2 μg m−3 for the CAMM, 10.5 μg m−3 for the RAMS, and 11.2 μg m−3 for the 30°C TEOM. From the results of the ANOVA, the mean of the PM sample sets were not significantly different between the CAMM and 30°C TEOM, or between the RAMS and 30°C TEOM. However, there was a significant difference observed in the mean of the CAMM and RAMS PM2.5 mass. The CAMM showed a concentration 16% higher mass than the RAMS and 9% higher mass than the 30°C TEOM, while the RAMS mass was about 7% lower than the 30°C TEOM mass.
In order to further consider the agreement in PM2.5 mass concentrations between pairs of the continuous samplers for the contemporaneous 1 h average data points of 204 in Houston and of 362 in Seattle for periods when all instruments were running simultaneously, a linear regression analysis was performed. Since the continuous methods have their own measurement uncertainties, a more complete Deming least-squares linear regression was used, rather than using a regular least-squares linear regression, to obtain the best estimate of the regressions. A standard least-squares linear regression accounts only for uncertainties in the y variable and assumes no errors in the x variable (i.e., x is a controlled variable). Use of a standard least-squares approach can lead to significant error in the estimated functional relationship between x and y when the ratio of the estimated uncertainties in x and y exceeds 0.2 (CitationCornbleet and Gochman 1979). The estimated uncertainty is expressed as a standard deviation or a variance. The Deming linear regression approach (CitationDeming 1943) solves the linear least-squares problem by minimizing the weighted sum of the squares, S, where S is defined as
To avoid errors in the regression due to outliers, we excluded from the regression data points whose perpendicular distance (Rp ) from the regression line exceeded four times the standard error (SE) of the estimate as recommended by the literature (CitationCornbleet and Gochman 1979; CitationTurpin and Huntzicker 1995). We used a Microsoft Excel add-in program, Analyze-It version 1.71, which computes the Deming linear regression solution.
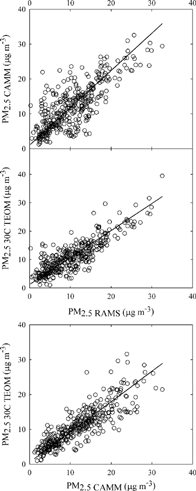
illustrates agreement in PM2.5 mass concentrations between pairs of the continuous samplers in Houston. Deming slopes and intercepts are summarized in . Only one outlier (i.e., a value with Rp > 4SE) was removed from the regression between the CAMM and adjusted RAMS PM2.5 mass. Slope increased a little higher than that obtained when outlier was included, but intercept was slightly lower, and regression error was lower when outlier was removed. The CAMM PM2.5 mass showed a good correlation with the adjusted RAMS PM2.5 mass, with a slope of 0.93 ± 0.03, an intercept of 3.14 ± 0.74 μg m−3, and an R 2 of 0.81. A similarly strong correlation was also found between the 30°C TEOM and adjusted RAMS PM2.5 mass, with a slope of 0.92 ± 0.03, an intercept of 1.5 ± 0.8 μg m−3, and an R 2 of 0.80. The best agreement was observed between the 30°C TEOM and CAMM PM2.5 mass concentrations, showing a slope of 1.01 ± 0.03, an intercept of −1.9 ± 0.8 μg m−3, and an R 2 of 0.83.
Figure 3 Intercomparison of 1 h average continuous PM2.5 mass measurements in Houston. The solid lines are the Deming linear regression fits in .
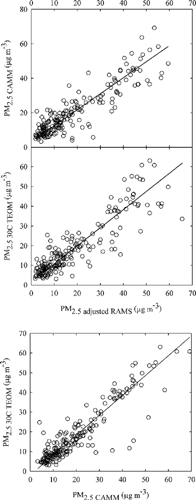
Table 4 Deming slopes and intercepts for PM2.5 mass between pairs of the continuous mass samplersFootnote a
depicts Deming linear correlations between PM2.5 mass concentrations for pairs of the continuous samplers in Seattle. One data point was removed from the regression due to outlier based on the criteria (i.e., Rp > 4SE) set earlier. A moderate correlation was observed between PM2.5 mass measurements of the pair of the CAMM and RAMS with a slope of 1.07 ± 0.05, an intercept of 1.03 ± 0.55 μg m−3, and an R 2 of 0.61. A good correlation was achieved between the 30°C TEOM and RAMS PM2.5 mass with a slope of 0.95 ± 0.03, an intercept of 1.24 ± 0.38 μg m−3, and an R 2 of 0.72. A higher correlation but lower regression slope were estimated for PM2.5 mass between the 30°C TEOM and CAMM, showing a slope of 0.87 ± 0.03, an interception of 0.55 ± 0.37 μg m−3, and an R 2 of 0.74.
Figure 4 Intercomparison of 1 h average continuous PM2.5 mass measurements in Seattle. The solid lines are the Deming regression fits in .
Comparing mass concentrations with scattering coefficients in Houston (not shown), the 30°C TEOM had the best correlation with the dried nephelometer (R 2 = 0.95). The correlation observed between other continuous PM2.5 methods (the CAMM and adjusted RAMS) and the nephelometer with a dryer was lower than that with the 30°C TEOM, having R 2 values of 0.40 for the CAMM and 0.41 for the adjusted RAMS. The coefficients with a dryer agreed very well with scattering coefficients without the dryer in Seattle. The correlation coefficient (R 2) was 1.00 for the valid 603 samples, with a slope of 0.94 ± 0.00. This investigation was not performed in Houston, since the nephelometer without a dryer condensed water in it because of the lower temperature inside the trailer as compared to the ambient temperature. Correlation R 2 of scattering coefficients with PM2.5 mass was 0.84 with the CAMM, 0.72 with the RAMS, and 0.80 with the 30°C TEOM.
Effects of Water Vapor and Temperature
The effects of air temperature and water vapor on the observed PM2.5 mass concentrations were examined between pairs of measurements. The ambient RH and T during the study period in Houston was 22–99% (average = 70%) and 21–40°C (average = 29°C), respectively. The ambient RH and T in Seattle were 39–100% (average = 80%) and −1.6–12°C (average = 4.9°C), respectively.
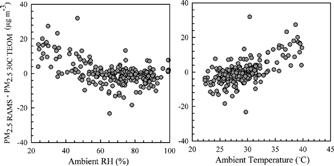
shows the difference between the 30°C TEOM and adjusted RAMS PM2.5 mass concentrations with RH and T in Houston. There was a clear trend of decreasing mass difference between the adjusted RAMS and 30°C TEOM up to 60% RH and then little difference after that. For other pairs of instruments such a trend was not found. This difference may be associated with ammonium nitrate loss and water vapor. Ammonium nitrate has a deliquescent relative humidity (DRH) of 61.8% at 298 K (CitationSeinfeld and Pandis 1998). At the DRH or higher RH, the ammonium nitrate salt exists as a saturated salt solution. However, as the RH decreases below the DRH, all the water in the particle needs to evaporate to maintain thermodynamic equilibrium, and ammonium nitrate particles become solid state. At higher temperature and lower RH, ammonium nitrate is easily evaporated into gaseous nitric acid and ammonia (CitationSeinfeld and Pandis 1998). This difference of PM2.5 mass may have resulted from the loss of water and ammonium nitrate in particles on the TEOM filter. Even at 30°C, the TEOM could still lose particulate nitrate. This seems plausible because the variation between the two instruments essentially increased at higher temperature as shown in .
In Seattle, no clear trends were found between the difference of the paired PM2.5 mass concentrations with RH or T (not shown). A higher loss of semivolatile materials might be expected in winter for the 30°C TEOM since the 30°C TEOM heats the sample filter significantly in winter. The difference between the filter (i.e., 30°C) and ambient temperatures is much greater than in the summer and can result in the loss of semivolatile materials. Based on the 24 h integrated speciation data that were measured by the PC-BOSS, the mass fraction of particulate nitrate lost from the front filter was less than 1% of the total PM mass (sum of volatile and nonvolatile compounds measured by the PC-BOSS). It indicates that the loss of semivolatile ammonium nitrate would not be large for the Seattle PM2.5 mass, and thus such a relationship with mass difference and RH as was seen in Houston was not likely to be seen in Seattle.
Thus, material likely to be lost in the 30°C TEOM is semivolatile organic matter. Semivolatile organic compounds measured using the PC-BOSS contributed about 14% to total PM mass of the PC-BOSS. Therefore, the 30°C TEOM may lose some of semivolatile organic matter because of heating. The CAMM and RAMS collect samples at the ambient temperature. The RAMS is more aggressive in removing water from the aerosol stream in that it has two sets of Nafion driers and the sheath air is dried to a very low absolute humidity. Thus, the small difference between the CAMM and the RAMS may be differences in the amount of remaining particle-bound water.
Continuous and Filter Measurements of PM2.5 Mass
Hourly average PM2.5 mass concentrations of each of the CAMM, RAMS (adjusted RAMS in Houston), and 30°C TEOM were converted to 24 h average values in order to compare with the integrated 24 h averaged PM2.5 mass concentrations in both Houston and Seattle. The integrated PM2.5 mass concentrations were determined using a R&P Partisol-Plus PM2.5 speciation sampler in Houston and using a URG MASS PM2.5 speciation sampler in Seattle.
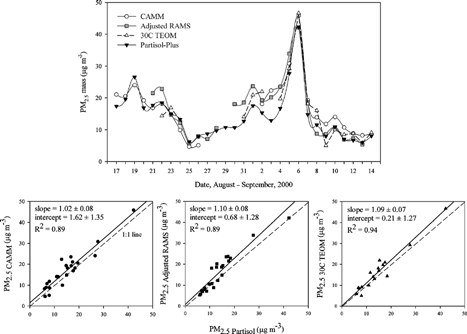
shows the 24 h average PM2.5 mass concentrations of the three continuous samplers and the integrated sampler in Houston during the study period. The three continuous PM2.5 mass samplers showed a good agreement with the integrated Partisol PM2.5 mass sampler. The mean value of the 24 h average PM2.5 mass concentrations was 17.0 ± 9.1 μg m−3 for the CAMM, 16.0 ± 9.4 μg m−3 for the adjusted RAMS, 16.4 ± 10.1 μg m−3 for the 30°C TEOM, and 14.2 ± 8.0 μg m−3 for the integrated Partisol. The 24 h averaged continuous PM2.5 mass concentrations were about 1.8–2.8 μg m−3 higher than that of the integrated PM2.5 mass concentration. Linear regression between the continuous and integrated PM2.5 mass values are also presented in . The slopes of the regression lines are close to 1.0 and the correlation coefficients are greater than 0.9, suggesting good agreement. Although there was good agreement observed between the continuous PM2.5 mass concentrations and the integrated PM2.5 mass concentrations as seen in , most of the data points were distributed above the 1:1 line. It shows that in Houston, the integrated PM2.5 mass sampler underestimated the ambient PM2.5 mass concentrations compared to the continuous PM2.5 mass samplers.
Figure 6 Comparison of 24 h average continuous PM2.5 mass and integrated PM2.5 mass concentrations in Houston: Daily variation of PM2.5 mass (top) and standard linear regressions (bottom). The solid lines are the standard regression fits.
The mass difference between the 24 h average continuous PM2.5 mass concentration and 24 h integrated PM2.5 mass concentration is likely to result from the loss of semivolatile material such as ammonium nitrate and organic compounds from particles on the integrated PM filter. The contribution of volatile ammonium nitrate and OC to the PM2.5 mass difference was estimated by concentrations of ammonium, nitrate, and OC measured using the Partisol PM2.5 speciation sampler, the PILS, and the PCM. The Partisol filters were analyzed for ionic species, carbonaceous species, and tracer metals. The mass balance of the Partisol PM2.5 species are given in with the average PILS and PCM speciation data.
Table 5 Average concentration (μg m−3) of PM2.5 mass and species measured from the continuous and integrated samplers in Houston
Good agreement was observed between the PILS SO4 2− (y) and Partisol SO4 2− (x) concentrations, with a slope of 1.00 ± 0.05, an intercept of −1.90 ± 0.33 μg m−3, and R 2 = 0.94. Based on this agreement between the two methods, average concentrations of particulate ammonium and nitrate from the PILS were used to estimate the loss of ammonium and nitrate from the Partisol speciation sampler. The average concentrations of particulate ammonium and nitrate from the PILS were 1.7 ± 0.9 μg m−3 and 0.4 ± 0.2 μg m−3, respectively. The average concentrations of ammonium and nitrate from the Partisol were 1.4 ± 1.1 μg m−3 and 0.3 ± 0.1 μg m−3, respectively. The average concentrations of ammonium and nitrate from these two samplers appeared to be similar, and only a small difference of concentration (∼0.4 μg m−3) was observed. The concentration of volatile OC was determined from the relationship between the Partisol and PCM OC concentrations. Since the PCM sampler has a backup quartz filter to collect semivolatile organic compounds lost from the preceding quartz filter, total OC measured by the PCM was calculated in the sum of concentrations of particulate OC and semivolatile organic compounds. The PCM total OC concentrations showed a good agreement with the Partisol OC concentrations. The regressions between the PCM total OC (y) and Partisol OC (x) are slope = 1.37 ± 0.1, intercept = 0.17 ± 0.42 μg m−3, and R 2 = 0.90. The average concentration of the PCM total OC was 4.54 ± 3.55 μg m−3, which is about 1.3 μg m−3 higher than that of the Partisol OC, 3.24 ± 2.42 μg m−3. As a result, the sum of the concentration differences for OC and ammonium nitrate turns out to be approximately 1.7 μg m−3, which comes up to 60–94% of the PM2.5 mass difference, assuming the mass difference of PM2.5 between the continuous samplers and the integrated sampler was attributed to the loss of semivolatile ammonium nitrate and organic compounds from the integrated PM mass.
depicts variation of the 24 h averaged continuous PM2.5 mass concentrations and the 24 h integrated PM2.5 mass concentrations in Seattle. Since the integrated PM2.5 mass samples were taken every third day, only a small number of the integrated mass concentrations were available for comparison. Thus, the comparison between the continuous and integrated PM2.5 mass concentrations is only discussed briefly. Except a few data points, the continuous PM2.5 mass concentrations tracked the integrated PM2.5 mass concentrations well. The mean 24 h average PM2.5 mass concentrations of each of the samplers was 11.0 ± 4.8 μg m−3 for the CAMM, 10.0 ± 4.4 μg m−3 for the RAMS, 10.2 ± 3.9 μg m−3 for the 30°C TEOM, and 10.8 ± 5.4 μg m−3 for the integrated MASS. Since comparable PM mass concentrations were observed and there are no continuous speciation data, the contributions of semivolatile materials were not considered for the Seattle PM2.5. The linear regression calculations show that the CAMM PM2.5 mass concentration was in good agreement with the MASS PM2.5 mass concentration with a slope of 0.80 ± 0.14, an intercept of 3.5 ± 1.5 μg m−3, and an R 2 of 0.87. A lower but still good correlation was observed between the RAMS and integrated MASS PM2.5 mass concentrations. The slope was 0.92 ± 0.24, the intercept was 0.2 ± 2.8 μg m−3, and R 2 was 0.78. Very good agreement was observed between the 30°C TEOM and the integrated MASS PM2.5 mass concentrations, showing a slope of 0.93 ± 0.08, an intercept of 1.2 ± 0.9 μg m−3, and an R 2 of 0.97.
Figure 7 Comparison of 24 h average continuous PM2.5 mass and integrated PM2.5 mass concentrations in Seattle: Daily variation of PM2.5 mass (top) and standard linear regressions (bottom). The solid lines are the standard regression fits.
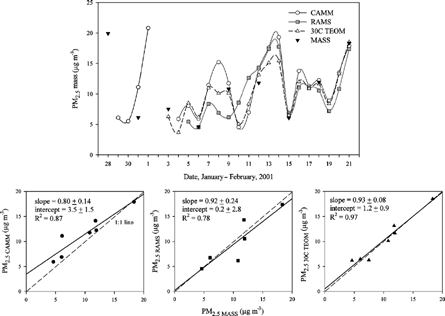
One possibility for the comparability of the continuous mass measurements to the integrated mass values is that volatilization of semivolatile materials from the integrated PM2.5 is expected to decrease during the colder winter period. Similar trends were seen in studies of CitationLong (2003). Although there were no major high concentration events from semivolatile organic compound sources (e.g., wood smoke) in Seattle during the study period because of relatively mild temperatures, the Seattle PM2.5 mass had a significant amounts of semivolatile organic compounds, as was noted previously. The colder winter temperatures resulted in greater retention of semivolatile organic materials in the integrated MASS sample. The CAMM collects samples without heating the filters (i.e., at ambient temperature), and thus all of the values are higher than the integrated MASS measurements in . The 30°C TEOM is expected to lose some semivolatile organic materials when it heats the samples, and thus a higher level of loss of semivolatile organic materials can occur, which may lead to lower ambient mass concentration measurements similar to the integrated MASS monitor values after the samples have been equilibrated at 23°C for 24 h. The RAMS is expected to measure total ambient mass including semivolatile materials, and thus the total mass by the RAMS should be higher than or equal to the mass by the MASS. However, shows that some of the RAMS mass concentrations were less than the integrated ambient PM mass and were somewhat noisier than the measurements from the other two continuous monitors, resulting in a lower correlation coefficient.
CONCLUSION
The performance of PM2.5 measurements of real-time continuous CAMM, RAMS, and 30°C TEOM was evaluated by cross comparison between the samplers in Houston during summer and in Seattle during winter. Hourly variation of PM2.5 mass measurements by all three samplers agreed well in general, suggesting a good utilization of the samplers as ambient mass monitors. The 1 h average PM2.5 mass concentrations among the continuous samplers were not significantly different in Houston, but a statistically significant difference was found in Seattle between the CAMM and the RAMS PM2.5 mass. The intercomparison of continuous PM2.5 mass concentrations showed a reasonably good agreement between the measured mass of the samplers.
Difference of PM2.5 mass was observed between the adjusted RAMS and 30°C TEOM mass in Houston with temperature and RH. This mass difference was likely to result from loss of ammonium nitrate from particles on the 30°C TEOM filter. While the loss of semivolatile ammonium nitrate was not large, a loss of semivolatile organic compounds was observed in Seattle.
Although the 24 h average continuous PM2.5 mass concentrations showed good agreement with the integrated 24 h PM2.5 mass concentrations in Houston, the continuous PM mass monitors measured consistently higher PM2.5 mass concentrations than the integrated sampler in Houston. The results suggest that the integrated PM2.5 mass sampler underestimated ambient PM2.5 mass concentrations, presumably because of the loss of semivolatile materials from particles on the integrated sample filters.
The observed PM2.5 mass concentrations were more comparable in Seattle between the continuous and integrated PM mass samplers because of decreased volatilization of semivolatile organic compounds from the integrated filters at the colder temperatures. However, the results show that the 30°C TEOM still could lose semivolatile organic compounds, and some of the RAMS mass concentrations were low with respect to integrated ambient PM2.5 mass. The loss of semivolatile materials from the filter in the 30°C TEOM system suggests that the use of a heated TEOM represents a problem for total ambient PM2.5 mass measurements.
Acknowledgments
This work was supported by the United States Environmental Protection Agency under cooperative agreement CR827591. The authors greatly appreciated access to the field measurement data from Michael Bergin (Georgia Institute Technology), Matthew Fraser (Rice University), James Frost (Washington Department of Ecology), and Delbert J. Eatough (Brigham Young University). The authors also appreciate the helpful communications with Doh-Won Lee about the PM mass data used in this study.
Notes
a The value was adjusted by multiplying a factor of 1.52 to the original RAMS values.
a Uncertainties are 95% confidence intervals. The error is the standard error of the regression. R 2 is the correlation coefficient and N is the number of samples.
REFERENCES
- Allen , G. , Sioutas , C. , Koutrakis , P. , Reiss , R. , Lurmann , F. W. and Roberts , P. T. 1997 . Evaluation of the TEOM Method for Measurement of Ambient Particulate Mass in Urban Areas . J. Air Waste Manage. Assoc. , 47 : 682 – 689 . [CSA]
- Babich , P. , Wang , P.-Y. , Allen , G. , Sioutas , C. and Koutrakis , P. 2000 . Development and Evaluation of a Continuous Ambient PM2.5Mass Monitor . Aerosol Sci. Technol. , 32 : 309 – 324 . [CROSSREF]
- Baumann , K. , Ift , F. , Zhao , J. Z. and Chameides , W. L. 2003 . Discrete Measurements of Reactive Gases and Fine Particle Mass and Composition during the 1999 Atlanta Supersite Experiment . J. Geophys. Res. , 108 : 8416 [CROSSREF]
- Chan , T. and Lippmann , M. 1977 . Particle Collection Efficiencies of Air Sampling Cyclones: An Empirical Theory . Environ. Sci. Technol. , 11 : 377 – 382 . [CROSSREF]
- Chung , A. , Chang , D. P. Y. , Kleeman , M. J. , Perry , K. D. , Cahill , T. A. , Dutcher , D. , McDougall , E. M. and Stroud , K. 2001 . Comparison of Real-Time Instruments Used to Monitor Airborne Particulate Matter . J. Air Waste Manage. Assoc. , 51 : 109 – 120 . [CSA]
- Cornbleet , P. J. and Gochman , N. 1979 . Incorrect Least-Squares Regression Coefficients in Method-Comparison Analysis . Clin. Chem. , 25 : 432 – 438 . [PUBMED] [INFOTRIEVE]
- Daum , P. H. An overview of TEXAQS 2000 . Proceedings of American Meteorological Scoiety 82nd Annual Meeting . January 13–17 2002 . Orlando, FL : Fourth Conference on Atmospheric Chemistry .
- Deming , W. E. 1943 . Statistical Adjustment of Data , New York : John Wiley & Sons .
- Eatough , D. J. , Obeidi , F. , Pang , Y. , Ding , Y. , Eatough , N. L. and Wilson , W. E. 1999 . Integrated and Real-Time Diffusion Denuder Sampler for PM2.5 . Atmos. Environ. , 33 : 2835 – 2844 . [CROSSREF]
- Eatough , D. J. , Eatough , N. L. , Obeidi , F. , Pang , Y. , Modey , W. and Long , R. 2001 . Continuous Determination of PM2.5Mass, Including Semi-Volatile Species . Aerosol Sci. Technol. , 34 : 1 – 8 .
- Eatough , D. J. , Long , R. W. , Modey , W. K. and Eatough , N. L. 2003 . Semi-Volatile Secondary Organic Aerosol in Urban Atmospheres: Meeting a Measurement Challenge . Atmos. Environ. , 37 : 1277 – 1292 . [CROSSREF]
- Kenny , L. C. , Gussman , R. and Meyer , M. 2000 . Development of a Sharp-Cut Cyclone for Ambient Aerosol Monitoring Applications . Aerosol Sci. Technol. , 32 : 338 – 358 . [CROSSREF]
- Lee , Y.-N. , Song , Z. , Liu , Y. , Daum , P. , Weber , R. , Orsini , D. , Naulainen , N. , Hubbe , J. and Morris , V. Aerosol Chemical Characterization on Board the DOE G1 Aircraft Using a Particle-Into-Liquid-Sampler During the TEXAQS 2000 Experiment . Proceedings of American Meteorological Society 82nd Annual Meeting . January 13–17 2002 . Orlando, FL : Fourth Conference on Atmospheric Chemistry .
- Long , R. W. , Eatough , N. L. , Mangelson , N. F. , Thompson , W. , Fiet , K. , Smith , S. , Smith , R. , Eatough , D. J. , Pope , C. A. and Wilson , W. E. 2003 . The Measurement of PM2.5, Including Semi-Volatile Components, in the EMPACT Program: Results from the Salt Lake City Study . Atmos. Environ. , 37 : 4407 – 4417 . [CROSSREF]
- Meyer , M. B. , Patashnick , H. , Ambs , J. L. and Rupprecht , E. 2000 . Development of a Sample Equilibration System for the TEOM Continuous PM Monitor . J. Air Waste Manage. Assoc. , 50 : 1345 – 1349 . [CSA]
- Modey , W. K. , Pang , Y. , Eatough , N. L. and Eatough , D. J. 2001 . Fine Particulate (PM2.5) Composition in Atlanta, USA: Assessment of the Particle Concentrator-Brigham Young University Organic Sampling System, PC-BOSS, during the EPA Supersite Study . Atmos. Environ. , 35 : 6493 – 6502 . [CROSSREF]
- Obeidi , F. and Eatough , D. J. 2002 . Continuous Measurement of Semi-volatile Fine Particulate Mass in Provo, Utah . Aerosol Sci. Technol. , 36 : 191 – 203 . [CROSSREF]
- Pang , Y. , Eatough , N. L. , Modey , W. K. and Eatough , D. J. 2002 . Evaluation of the RAMS Continuous Monitor for Determination of PM2.5Mass Including Semi-Volatile Material in Philadelphia, PA . J. Air Waste Manage. Assoc. , 52 : 563 – 572 . [CSA]
- Patashnick , H. and Rupprecht , E. G. 1991 . Continuous PM-10 Measurements Using the Tappered Element Oscillating Microbalance . J. Air Waste Manage. Assoc. , 41 : 1079 – 1083 .
- Russell , M. , Allen , D. T. , Collins , D. R. and Fraser , M. P. 2004 . Daily, Seasonal, and Spatial Trends in PM2.5Mass and Composition in Southeast Texas . Aerosol Sci. Technol. , 38 ( S1 ) : 14 – 26 . [CROSSREF] [CSA]
- Seinfeld , J. H. and Pandis , S. N. 1998 . Atmospheric Chemistry and Physics , New York : John Wiley & Sons .
- Sheppard , L. , Levy , D. and Checkoway , H. 2001 . Correcting for the Effects of Location and Atmospheric Conditions on Air Pollution Exposures in a Case-Crossover Study . J. Exposure Analysis and Environ. Epi. , 11 : 86 – 96 . [CROSSREF] [CSA]
- Sioutas , C. , Kotrakis , P. and Oslon , B. 1994 . Development and Evaluation of a Low Cutpoint Virtual Impactor . Aerosol Sci. Technol. , 21 : 223 – 235 . [CSA]
- Tang , H. , Lewis , E. A. , Eatough , D. J. , Burton , R. M. and Farber , R. J. 1994 . Determination of the Particle Size Distribution and Chemical Composition of Semi-Volatile Organic Compounds in Atmospheric Fine Particles . Atmos. Environ. , 28 : 939 – 947 . [CROSSREF]
- Turpin , B. J. and Huntzicker , J. J. 1995 . Identification of Secondary Organic Aerosol Episodes and Quantification of Primary and Secondary Organic Aerosol Concentrations during SCAQS . Atmos. Environ. , 29 : 3527 – 3544 . [CROSSREF]
- Weber , R. J. , Orsini , D. , Daun , Y. , Lee , Y.-N. , Klotz , P. J. and Brechtel , F. 2001 . A Particle-into-liquid Collector for Rapid Measurement of Aerosol Bulk Chemical Composition . Aerosol Sci. Technol. , 35 : 718 – 727 . [CROSSREF]