Abstract
Controlling the emission of submicron particles of toxic metals in a combustion system poses a challenge. One possible mechanism for removing these fine particles is through intercoagulation with coarse particles. A bimodal lognormal model was applied to investigate the impact of intercoagulation rate on the size distributions of fine-mode aerosols. Fine-mode particle removal time was found to depend strongly on the number concentration of coarse-mode particles, but it was independent on the number concentration of fine-mode particles. An increase of geometric standard deviation of fine-mode particles from 1 to 1.6 significantly increased the dimensionless removal time 27 times. On the contrary, an increase of the deviation of coarse-mode particles in the same range only decreased 3% of the dimensionless removal time. The variation of geometric mean size ratio, meanwhile, had only insignificant effects on dimensionless removal time. For a constant mass concentration, removal time decreased as geometric standard deviation narrowed and mean size of coarse mode decreased. Fine-mode particles ultimately approached monodisperse when the dominant mechanism was intercoagulation; meanwhile, coarse-mode particles approached the asymptotic shape because intracoagulation was the dominant mechanism. The results show that on a constant mass basis, monodisperse coarse-mode particles with a high number concentration are the optimal condition for enhanced removal of fine-mode particles through intercoagulation.
NOMENCLATURE
| b 0 | = |
b 0 = 0.633 + 0.092 σ g 2 − 0.022 σ g 3 |
| c | = |
coarse mode (subscript) |
| co | = |
continuum regime (subscript) |
| fm | = |
free molecule regime (subscript) |
| d p | = |
particle size (μ m) |
| d pg | = |
geometric mean size (μ m) |
| f | = |
fine mode (subscript) |
| K 0 | = |
initial intercoagulation rate (#/s) |
| k B | = |
Boltzmann's constant (dyne cm/K) |
| k 1,k 2 | = |
order of moment, 3 and 6 |
| n | = |
aerosol number concentration distribution function |
| N | = |
total number concentration (#/cm3) |
| M k | = |
kth moment of aerosol size distribution |
| m | = |
mass (g) |
| t | = |
time (s) |
| T | = |
temperature (K) |
| t 1/2 | = |
particle half-life time (50 wt% removal, s) |
| t r | = |
particle removal time (99.99 wt% removal, s) |
| 0 | = |
initial condition (subscript) |
| β | = |
collision frequency function |
| λ | = |
mean free path of air (μ m) |
| μ | = |
gas viscosity (g/sec/cm) |
| ρ p | = |
particle density (g/cm3) |
| σ g | = |
geometric standard deviation |
| τ s | = |
fine-mode particle scavenge characteristic time (s) |
INTRODUCTION
Heavy metal emission from combustion sources is of great concern because of its toxicity to human health and the environment. It is well known that metal particles from combustion are enriched in the submicron regime (CitationLinak and Wendt 1993; CitationBiswas and Wu 1998). Unfortunately, submicron particles show poor capture efficiency in traditional particulate control devices (CitationFlagan and Seinfeld 1988). Sorbent injection is one promising technique to control these fine metal emissions. In recent years, various studies have been conducted that use mineral sorbents to capture heavy metals (CitationLinak et al. 1995; CitationVenkatesh et al. 1996; CitationMahuli et al. 1997; CitationIida et al. 2004).
Sorbent particles injected into combustion systems are expected to adsorb heavy metals on the surface chemically. As these sorbent particles are typically in the supermicron range, the metal–sorbent product can be collected easily using traditional particulate control devices. In addition to chemical adsorption, other mechanisms such as nucleation and coagulation are also present in the system. At combustion source, removal of metal vapor by condensation so that fine-mode particle formation by vapor nucleation can be suppressed is the preferred mechanism. CitationRodriguez and Hall (2003) developed an aerosol dynamic model based on a hybrid sectional model to study condensational removal of heavy metals from exhaust gases onto sorbent particles. Comparison of their model results with experimental data (CitationRodriguez and Hall 2001) showed good agreement. Alternatively, after gas stream exits the combustion zone, coagulation with coarse-mode sorbent particles can be a possible mechanism to scavenge fine-mode particles. CitationFriedlander et al. (1991) studied the scavenging of a coagulating fine aerosol by a coarse particle mode. They derived a simple analytical criterion for competing processes such as coagulation (intracoagulation) and diffusion (intercoagulation). Based on the assumption of monodisperse nucleated fine particles and entrained ash as the coarse particles, the criterion provides a convenient tool for estimating the importance of these competing processes. In real system, however, polydisperse fine particles are present and supermicron sorbent particles are injected. While important, there has been no modeling study about the role of coagulation in sorbent injection technique. The understanding of the evolution of fine-mode particle size distribution (PSD) in such a system is of fundamental importance, although it is seldom studied. Thus, a model to simulate the aerosol dynamics in the system can help understand the dynamic interactions between metals and sorbents as well as implement the sorbent injection technique to real system.
There are several aerosol models available to describe aerosol dynamic processes such as moment (CitationFrenklach and Harris 1987; CitationWhitby 1979), continuous (CitationTsang and Brock 1982), and sectional (CitationGelbard and Seinfeld 1980; CitationLandgrebe and Pratsinis 1990; CitationWu and Biswas 1998). These models were categorized by their mathematical size distribution function. CitationWhitby et al. (1991) and CitationWilliams and Loyalka (1991) reviewed these models in more detail. Among them, aerosol moment model is one of the most commonly applied models because of its flexible model structure and low computational cost. Assuming a unimodal log-normal aerosol size distribution, CitationLin and Biswas (1994) developed a model to study metallic particle formation and growth dynamic during incineration. CitationWu and Biswas (2000) also used a unimodal lognormal size distribution model to evaluate the effects of chlorine on the evolution of lead aerosol size distribution. However, such models may not be appropriate for a metal–sorbent system because of the extreme differences between their particle sizes. Metals alone in a combustion system eventually form submicron particles through nucleation, condensation, and coagulation. The presence of supermicron sorbent particles may suppress the formation of submicron metal particles through intercoagulation and condensation, which are the key parameters for the effectiveness of the sorbent technique (CitationBiswas and Wu 1998). Consequently, a bimodal lognormal model will more pertinently represent a metal–sorbent system.
conceptually depicts the two types of coagulation, i.e., intercoagulation and intracoagulation, in a system consisting of bimodally distributed particles. Nucleation of vaporized metal compound results in nano-size particles in the fine mode. Eventually they can grow to the submicron regime by coagulation and condensation. However, their typical concentration and short residence time in combustion system do not allow these fine particles to grow to the supermicron regime by intracoagulation alone (CitationFriedlander et al. 1991; CitationBiswas and Wu 1998). Many measurements of metal compounds emissions have confirmed that metals are enriched in submicron (CitationBacci et al. 1983; CitationLyyranen et al. 1999; CitationOsan et al. 2000). When coarse sorbent particles are introduced into the system, fine-mode particles coagulating with the sorbent particles are then transformed into the supermicron regime, which can easily be collected by conventional particulate control devices. As shown, intercoagulation plays the key role in removing fine-mode particles, and therefore its effect on fine-mode particle size distribution was the focus of this work.
In this study, the modal aerosol dynamic (MAD) model (CitationWhitby and McMurry 1997) based on the lognormal moment model that has a multimodal structure and low computation cost was adopted. The evolution of fine-mode particles due to coagulation with coarse-mode particles (sorbent) was investigated. The effects of size distribution parameters such as number concentration, geometric standard deviation, and geometric mean diameter on intercoagulation were evaluated.
METHODOLOGY
Model Description
The development of the MAD model adopted in this study was provided in detail in CitationWhitby and McMurry (1997) and thus is not repeated here. Only intercoagulation, which was the main mechanism to be investigated, is discussed as follows. The intercoagulation rate for the fine mode is
and for the coarse mode is
where subscript k is order of moment (0, 3 or 6), and subscripts f and c stand for fine and coarse, respectively. Once these moments are determined, the key size distribution parameters can then be determined according to
Intercoagulation to remove fine particles is the key mechanism in this study. Thus, the time needed to remove fine-mode particles is of interest. The fine-mode particle scavenge characteristic time is defined as follows:
where N f0 is the initial total number concentration of fine-mode particles and K o is the initial intercoagulation rate. Once K o is calculated, the fine-mode particle scavenging characteristic time can be easily estimated with the initial fine-mode concentration. The analytical solution (CitationWhitby et al. 1991) for the intercoagulation rate of the 0th moment for the continuum regime can be expressed by
and for the free molecule regime by
With emission control in mind, in this work the fine-mode particle removal time (t r ) is defined as the time to remove 99.99 wt% of fine-mode particles. The dimensionless removal time can then be defined as t r /τ s .
Simulation Conditions
Intercoagulation rate depends on the size distributions of both modes. Hence the effect of number concentration, geometric standard deviation, and geometric mean size of each mode on intercoagulation rate was investigated. In this simulation, an isothermal condition was assumed. It should be noted that a nonisothermal condition may yield different results. summarizes all the simulation conditions. In the first set of simulation, the number concentration of each mode was varied, and the corresponding size distribution and removal time of the fine mode were determined. In the second set, the effects of the geometric standard deviations of coarse mode and fine mode were studied. In the third set, the impact of varying the geometric mean size of each mode on intercoagulation rate was evaluated. The final set of simulations was carried out to determine the optimal sorbent particle size distribution that could enhance the intercoagulation rate when the mass loading of sorbent particles was fixed.
Table 1 Simulation conditions for studying the effects of intercoagulation on fine mode particle removal
RESULTS AND DISCUSSION
Number Concentration
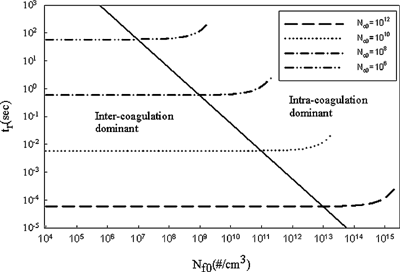
Since coagulation rate depends on number concentration, intercoagulation is expected to play a key role in determining the fine particle removal. shows the simulation results of set I. As shown, for the same initial fine-mode number concentration, the removal time strongly depends on coarse-mode number concentration. It decreases as coarse-mode number concentration increases. However, for a fixed coarse-mode number concentration the removal time is not affected by the change of fine-mode number concentration unless the fine-mode number concentration is ten times more than the coarse-mode number concentration. Careful examination of the coagulation rates (intra-and inter-) shows that when the fine-mode intracoagulation rate is much faster than the intercoagulation rate, the growth of fine-mode particles decreases its number concentration, which subsequently yields a low intercoagulation rate and therefore a longer removal time. Thus, the graph can be divided into two regions by the solid line as shown in : intercoagulation dominant and intracoagulation dominant. In short, the coarse-mode number concentration clearly plays a major role in determining fine-mode removal time when intercoagulation is the dominant mechanism; on the other hand, fine-mode number concentration must be considered to estimate t r when intracoagulation is the dominant mechanism.
Figure 2 Fine-mode removal time as a function of fine-mode number concentration for various coarse mode number concentrations. The solid line connects points where N f0/N c0 = 10.
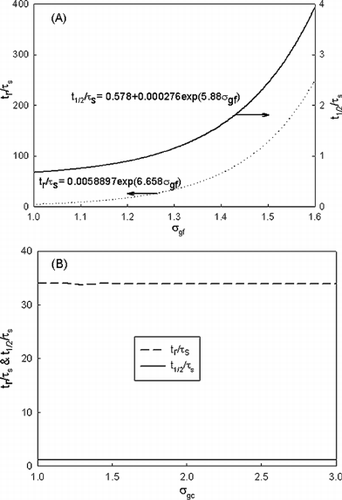
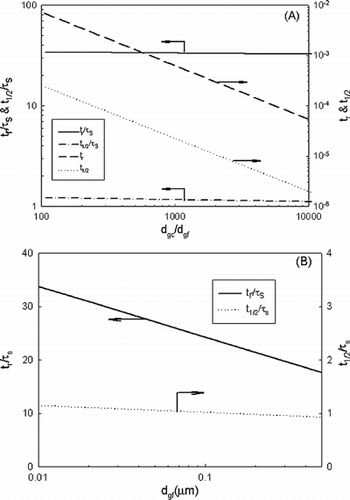
These results were further presented by dimensionless removal time (t r /τ s ) as a function of normalized number concentration N f0/N c0 in a. As shown, all data merge into one line, thus yielding a useful tool for estimating the dimensionless removal time as
Figure 3 centering Dimensionless removal time as a function of (a) normalized number concentration and (b) normalized mass or volume concentration.
for geometric standard deviation of fine mode and coarse mode of 1.3 and d gf = 0.01 μ m. Hence, as long as τ s is determined for a given system (Equation (Equation6)), the corresponding removal time (t r ) can be estimated accordingly. Since the prior study (CitationFriedlander et al. 1991) used half-life time (t 1/2), the results for t 1/2/τ s are also included in the plot. The corresponding equations are
Since in practical applications mass loading is of interest, b shows dimensionless removal time as a function of normalized mass/volume. The same trend observed in a exists. Dimensionless removal time is independent on normalized mass or volume until it exceeds 10− 8.
Geometric Standard Deviation
It is well known that the geometric standard deviation of a log-normally distributed unimodal aerosol approaches 1.35 and 1.32 in the free-molecule regime and the continuum regime, respectively, when coagulation is the dominant mechanism (CitationPratsinis 1988). Similarly, this applies to intracoagulation for multimodal aerosols. However, the effects of intercoagulation on the geometric standard deviations of multimode aerosol have never been investigated before. These effects were studied as set II, and the results are presented in for the size distributions at various times. In this simulation condition (with sorbent application in mind) intercoagulation was dominant for fine mode. As shown, fine mode became narrower when its mode size shifted toward the bigger size. It should be emphasized that the increase of mode size was not due to intracoagulation. Under the intracoagulation dominant case, there was no discernible change in the fine-mode size distribution during the simulation time (0.1 s; also marked in ). Rather, it resulted from the faster scavenging of the smaller sizes of the fine mode due to intercoagulation. Intercoagulation rate is dependent on collision frequency function, which strongly depends on particle size difference, and it increases as the size difference increases. Consequently, the smaller size of the fine-mode aerosol is scavenged much faster then the larger size in the same mode. Therefore, when intercoagulation is the dominant mechanism, σ gf (fine-mode geometric standard deviation) ultimately approaches monodisperse (i.e., σ gf = 1). Meanwhile, intracoagulation was dominant for the coarse-mode since the addition of fine-mode moments to the coarse-mode is rather insignificant. Although σ gc (coarse-mode standard deviation) did not change in the short period (fine-mode removal time) shown in the figure, it will finally approach the asymptotic value (1.32, not shown in ).
Figure 4 centering Evolution of fine-mode (σ gf0 = 1.4) and coarse-mode (σ gc0 = 1.3) particle size distributions by intercoagulation to reach removal time (t r = 0.106 s).
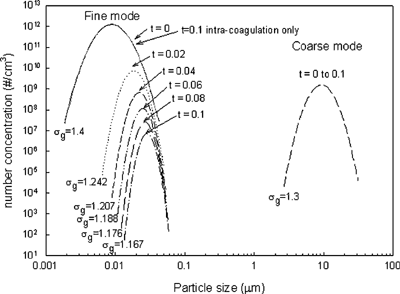
a shows dimensionless removal time and half-life time (note: two y axes) as a function of σ gf , and b shows the results for varying σ gc . When σ gf changed from 1 to 1.6 dimensionless removal time increased drastically from 9.3 to 250, demonstrating the sensitivity to the change of the fine-mode distribution. A larger σ gf implies more mass in the larger size part. The corresponding lower intercoagulation rate for the larger size resulted in a longer removal time, as discussed earlier. Similar to the previous set, an equation can be obtained as a useful tool for estimating the dimensionless time due to the change of σ gf as
Mean Size Difference
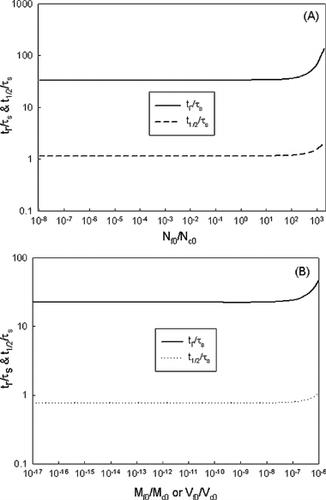
If particle size difference is large its collision frequency function is large, which enhances coagulation. a shows the effects of mean size difference. As shown, when the mean size difference between fine mode and coarse mode increased, the removal time decreased accordingly. This clearly shows that large mean size sorbent (with same number concentration) will enhance intercoagulation rate due to the large cross-section surface area for coagulation. When the results are presented in the dimensionless form as shown in a, even though the mean size difference increased, the dimensionless removal time showed only 3–7% difference for two orders of magnitude of size ratio. The reason for the insignificant difference is due to the increase of fine-mode particle scavenging characteristic time for increased mean size difference.
Figure 6 (a) Dimensionless removal time and removal time as a function of d gc /d gf . (b) Dimensionless removal time as a function of fine-mode mean size.
In addition to size difference, fine-mode mean size also affects the dimensionless removal time. Their relationship is plotted in b. A linear relationship in log graph is observed, which can be expressed as
Considering the combined effects of number concentration, geometric standard deviation, and mean size, the following final form can be used to estimate the removal time and half-life time for N f0/N c0 < 10:
For N f0/N c0 ≥ 10 the removal time and half-life time are as follows:
Application to Sorbent Injection
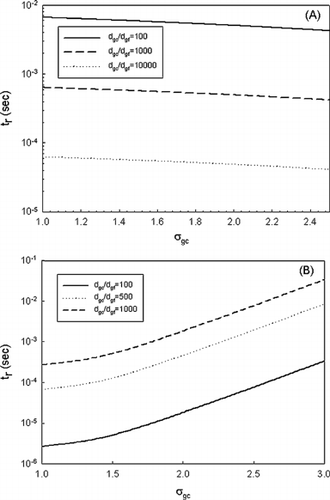
For many practical applications such as sorbent injection for toxic metal removal, minimizing the mass loading while having good removal efficiency is desired, as cost consideration is always important to the feasibility of the technology. With the same total mass concentration, there are several combinations of size distribution parameters (number concentration, geometric mean size, and geometric standard deviation). In other words, the optimal conditions based on mass concentration will be different from those obtained for number concentration. a shows the removal time for various combinations of σ gc and d gc /d gf based on the same number concentration. b, on the other hand, shows the results based on the same mass concentration. As discussed earlier, a high number concentration of coarse mode and a wide mean size difference allow for a short removal time (shown in a). However, different patterns were observed for the results based on the same mass concentration (b). As shown, a narrow size deviation and a small coarse-mode mean size had the shortest removal time. Careful examination of the size distribution parameters reveals that under the same mass concentration a smaller mean size and a narrower geometric standard deviation are translated into a higher number concentration, which is favorable for coagulation. In summary, for practical application monodisperse sorbent particles with a mean size close to 1 μ m and a number concentration of more than 107 #/cm3 are the most effective. Our finding was also applied to an experimental study conducted by CitationLinak et al. (2003). Kaolinite sorbent was used to reduce fine particle
Figure 7 (a) Removal time as a function of coarse-mode standard deviation and d gc /d gf with the same number concentration (1010/cm3) and (b) the same mass concentration (10 μ g/cm3).
formation. A 35% reduction of metal compounds in the fine mode was observed. Condensation was reported to be the responsible mechanism, while coagulation was said to have no effect on the reduction. The sorbent feed concentration was 680 mg/m3 with a mean size of 1.4 μ m, while the fine particle feed concentration rate was 83 mg/m3, with a residence time of 4.1s. shows the size distribution parameters used in the calculation and the estimated removal times. Three cases based on the same mass concentration were studied. For case I, d gf was assumed to be 0.01 μ m, and both geometric standard deviations were assumed to be 1 for the most effective condition. Equations (19) and (20) were used to calculate the dimensionless removal time and half-life time, and the fine-mode particle scavenge characteristic time was calculated using Equation (Equation7). The resultant removal time and half-life time were 8.1 × 1085 and 8.6 × 1034 s, respectively, clearly showing that coagulation could not work for their system because these values were much greater than the residence time (4.1 s). Case II was to simulate sorbent injection at a later stage where intracoagulation had lowered the fine-mode number concentration (using measured PSD of fine mode without sorbent that is reported in CitationLinak et al. 2003). If the same amount of sorbent was injected at this stage, its corresponding removal and half-life times were 2,094 and 263 s, both still much longer than the residence time. Other conditions (e.g., larger σ g ) will only result in a longer removal time. Hence, the operating condition in their system clearly was ineffective in removing the fine-mode aerosol by intercoagulation. However, if the sorbent feed was increased two orders higher (68,000 mg/m3, Case III), the removal and half-life times were down to 2.65 and 0.12 s, which were then shorter than the residence time. Using the formula developed in this work, the reason why coagulation was not important in their study was clearly seen and the criteria for effective removal by intercoagulation can be established clearly.
Table 2 Size distribution parameters and removal time for CitationLinak et al. (2003)
Analysis was then carried out for a flue gas desulfurization (FGD) system (CitationHarries et al. 1993) that used slurry droplet injection. For this case, particulate matters which are common components of flue gas also can potentially be removed by intercoagulation. summarizes the operating conditions and the corresponding droplet size distribution. The particle size distribution of particulate matter (CitationBacci et al. 1983) is also listed. Particulate matter and slurry mass ratio is about 1.07 × 10− 6. However, the large slurry droplet (bigger than 500 μ m) with monodisperse assumption results in a very low number concentration (2.3 × 102/cm3) that requires an extremely long residence time to remove particulate matter in the flue gas effectively. If the droplet mean size is 10 μ m instead and the standard deviation is 1.3, its number concentration is 2.1 × 107 #/cm3, and the corresponding particle removal time is only 0.5 s. The analysis demonstrates that fine particulate matter can potentially be removed using a typical mass loading in an FGD system, but the selection of particle size and number concentration is very critical to accomplishing the goal.
Table 3 Operating parameters of a FGD system and particle size distribution from a power plant
CONCLUSIONS
Sorbent injection is one promising method to control the emission of submicron metal compounds from the combustion system. Under this bimodally distributed condition, intercoagulation can be a key mechanism for effective sorbent injection.
In this work, the effects of intercoagulation on removing fine-mode aerosol were studied. A high number concentration of coarse mode, a large mean size difference, and a narrow geometric standard deviation of fine mode were found to favor a higher intercoagulation rate. The removal time can also be expressed in dimensionless form. Two key parameters that affect the dimensionless removal time are the geometric standard deviation of the fine mode (when the normalized fine mode concentration is less than 10) and the geometric mean size of fine mode. The assumption of monodisperse particles results in an underestimate of the scavenging time. The developed formula can be used as a convenient tool to estimate the removal time once the particle scavenge characteristic time is determined.
When intercoagulation is the dominant mechanism for removing fine-mode particles, its geometric standard deviation approaches monodisperse. For coarse-mode particles, the geometric standard deviation approaches the asymptotic value because intracoagulation is still the dominant mechanism. With regard to sorbent application where minimal mass loading is desired, a narrow size deviation and a small mean size of the coarse mode yield the optimal effectiveness. The reason is due to the corresponding high number concentration, which is the critical parameter to intercoagulation. Using the formula developed in this work the criteria for effective removal of fine-mode aerosol can be clearly established.
Acknowledgments
The authors would like to acknowledge Dr. E. R. Whitby for his insightful advice on modeling.
REFERENCES
- Bacci , P. , Monte , M. D. , Longhetto , A. , Piano , A. , Prodi , F. and Redaelli , P. 1983 . Characterization of the Particulate Emission by a Large Oil Fuel Fired Power Plant . J. Aerosol Sci. , 14 : 557 – 572 .
- Biswas , P. and Wu , C. Y. 1998 . Control of Toxic Metal Emissions from Combustiors Using Sorbents: A Review . J. Air Waste Manage. Assoc. , 48 : 113 – 127 . [CSA]
- Flagan , R. C. and Seinfeld , J. H. 1988 . Fundamentals of Air Pollution Engineering. , pp. 433 – 471 . Englewood Cliffs, NJ : Prentice Hall .
- Frenklach , M. and Harris , S. J. 1987 . Aerosol Dynamics Modeling Using the Method of Moments . J. Colloid Interf. Sci. , 118 : 252 – 261 .
- Friedlander , S. K. , Koch , W. and Main , H. H. 1991 . Scavenging of a Coagulating Fine Aerosol by a Coarse Particle Mode . J. Aerosol Sci. , 22 : 1 – 8 . [CROSSREF] [CSA]
- Gelbard , E. and Seinfeld , J. H. 1980 . Simulation of Multicomponent Aerosol Dynamics . J. Colloid Interface Sci. , 78 : 485 – 501 .
- Harries , R. R. 1993 . Process Modeling for Wet Limestone Flue Gas Desulphurization . IChemE Sym. Ser , 131 : 167 – 181 .
- Iida , K. , Pierman , J. , Tolaymat , T. , Townsend , T. and Wu , C. Y. 2004 . Control of Heavy Metal Emissions and Leaching from Incineration of CCA-Treated Woods Using Mineral Sorbents . J. Env. Eng. , 130 ( 2 ) : 184 – 192 . [CROSSREF]
- Landgrebe , J. D. and Pratsinis , S. E. 1990 . A Discrete-Sectional Model for Particulate Production by Gas-Phase Chemical-Reaction and Aerosol Coagulation in the Free-Molecular Regime . Colloid Interf. Sci. , 139 : 63 – 86 . [CROSSREF]
- Lin , W. Y. and Biswas , P. 1994 . Metallic Particle Formation and Growth Dynamics during Incineration . Combust. Sci. Technol. , 101 : 29 – 43 .
- Linak , W. P. , Miller , C. A. , Santoianni , D. A. , King , C. J. , Shinagawa , T. , Wendt , J. O. L. , Yoo , J. I. and Seo , Y. C. 2003 . Formation of Fine Particles from Residual Oil Combustion: Reducing Nuclei Through the Addition of Inorganic Sorbent . Korean J. Chem. Eng. , 20 ( 4 ) : 664 – 669 .
- Linak , W. P. , Srivastava , R. K. and Wendt , J. O. L. 1995 . Sorbent Capture of Nickel, Lead, and Cadmium in a Laboratory Swirl Flame Incinerator . Combustion Flame , 100 : 241 – 250 . [CROSSREF] [CSA]
- Linak , W. P. and Wendt , J. O. L. 1993 . Toxic Metal Emissions from Incineration: Mechanisms and Control . Prog. Energy Combust. Sci. , 19 : 145 – 185 . [CROSSREF]
- Lyyranen , J. , Jokiniemi , J. , Kauppinen , E. I. and Joutsensaari , J. 1999 . Aerosol Characterization in Medium-Speed Diesel Engines Operating with Heavy Fuel Oils . J. Aerosol Sci. , 30 : 771 – 784 . [CROSSREF]
- Mahuli , S. , Agnihotri , R. , Chauk , S. , Ghosh-Dastidar , A. and Fan , L.-S. 1997 . Mechanism of Arsenic Sorption by Hydrated Lime . Environ. Sci. Technol. , 31 ( 11 ) : 3226 – 3231 . [CROSSREF] [CSA]
- Osan , J. , Torok , S. , Fekete , J. and Rindby , A. 2000 . Case Study of the Emissions from a Heavy-Oil-Fueled Hungarian Power Plant . Energy Fuels , 14 : 986 – 993 . [CROSSREF] [CSA]
- Rodriguez , A. and Hall , M. J. 2003 . The Simulation of Condensation Removal of a Heavy Metal from Exhaust Gases onto Sorbent Particles . Waste Manage. , 23 ( 6 ) : 493 – 502 . [CROSSREF]
- Rodriguez , A. and Hall , M. J. 2001 . Removal of an Airborne Low Volatility Metal under Fuel-Rich and Fuel-Lean Condition through Condensation onto Soot and/or Sorbent Particles . Waste Manage. , 21 ( 6 ) : 589 – 607 . [CROSSREF]
- Pratsinis , S. E. 1988 . Simultaneous Nucleation, Condensation and Coagulation in Aerosol Reactors . J. Colloid Interface Sci. , 124 ( 2 ) : 416 – 427 . [CROSSREF]
- Tsang , T. H. and Brock , J. R. 1982 . Aerosol Coagulation in the Plume from a Cross-Wind Line Source . Atmos. Environ. , 16 : 2229 – 2235 . [CROSSREF]
- Venkatesh , S. , Fournier , D. J. Jr. , Waterland , L. R. and Carroll , G. J. 1996 . Evaluation of Mineral-Based Additives as Sorbents for Hazardous Trace Metal Capture and Immobilization in Incineration Processes . Haz. Waste. Haz. Mat. , 13 ( 1 ) : 73 – 94 . [CSA]
- Whitby , K. T. 1979 . Lumped Mode Aerosol Growth Model , Minneapolis, MN : Particle Technology Laboratory Publication #395, Mechanical Engineering Department, University of Minnesota .
- Whitby , E. R. and McMurry , P. H. 1997 . Modal Aerosol Dynamics Modeling . Aerosol Sci. Technol. , 27 ( 6 ) : 673 – 688 .
- Whitby , E. R. , McMurry , P. H. , Binkowski , F. and Shankar , U. 1991 . Modal Aerosol Dynamics Modeling EPA report for contract No. 68-017365
- Williams , M. M. R. and Loyalka , S. K. 1991 . Aerosol Science: Theory and Practice , New York : Pergamon Press .
- Wu , C. Y. and Biswas , P. 2000 . Lead Species Aerosol Formation and Growth in Multicomponent High Temperature Environments . Env. Eng. Sci. , 17 ( 1 ) : 41 – 60 .
- Wu , C. Y. and Biswas , P. 1998 . Study of Numerical Diffusion in a Discrete-sectional Model and Its Application to Aerosol Dynamics Simulation . Aerosol Sci. Technol. , 29 ( 5 ) : 359 – 378 .